Abstract
Piecemeal degranulation (PMD) has been recognized in two cases of human pheochromocytoma from the adrenal medulla, which were studied by transmission electron microscopy. Tumour pheochromocytes presented a highly characteristic cytoplasmic admixture of normal resting granules, swollen granules with eroded matrices and enlarged empty containers. Chromaffin granules that appeared to be normal and altered granules maintained their individual structure, and did not fuse with each other or with the plasma membrane. In accordance with the currently accepted model for granule discharge during PMD, electron-dense or clear vesicles 30–150 nm in diameter were seen either attached to the surface of chromaffin granules and the plasma membrane or free in the cytosol. This is the first description of PMD in human adrenal chromaffin cells and, in addition, is the first report of PMD in tumour secretory cells. These findings add further to the concept that PMD may have a broader spectrum of expression than hitherto recognized.
Keywords: cell secretion, chromaffin granules, pheochromocytoma, piecemeal degranulation, transmission electron microscopy
Introduction
In the early 1970s, Dvorak and co-workers identified a novel secretory pathway in guinea-pig and human basophils (Dvorak et al. 1973, 1974, 1976). They coined the term ‘piecemeal degranulation’ (PMD) to describe this unique pattern of release events, because granule-stored material presented partial losses and characteristic erosive features when viewed by electron microscopy. This degranulation pattern was soon recognized and extensively investigated in mast cells from different species, including humans (reviewed in Dvorak, 1991), and was subsequently identified in human and guinea-pig eosinophils (Dvorak et al. 1992; Erjefalt et al. 1998; Karawajczyk et al. 2000).
Diagnostic criteria for PMD were based upon precise ultrastructural changes (Dvorak, 1991, 1994). Activated granules in PMD do not fuse with each other and do not open to the cell exterior. They characteristically maintain their individual structure and exhibit partial or complete loss of secretory contents. Partially empty granules show focal pieces or packets of lost granule particles, whereas empty granules appear as large cytoplasmic containers. Altered granules are characteristically intermingled with resting, unstimulated granules that have a normal ultrastructural appearance. In addition, cells undergoing PMD exhibit a rich supply of membrane-bound, cytoplasmic vesicles 30–150 nm in diameter. Most vesicles appear electron-lucent although a variable proportion contain particles similar in structure, size and electron-density to those making up the cytoplasmic granules. Vesicles may be either free in the cytoplasm or fused with the plasma membrane or the perigranular membrane in a process of attaching to or budding from the granules.
These ultrastructural determinations have suggested a basic mechanism for granule content release during PMD. The discharge mechanism would imply formation of vesicles that shuttle back and forth between the granules and the plasma membrane (Dvorak et al. 1996; Dvorak, 1998). Vesicles containing pieces of granule contents would bud from the perigranule membrane, move through the cytoplasm and fuse with the plasma membrane. Parallel formation of endocytotic vesicles from the plasma membrane would fuse with the granule membrane (Dvorak, 1994). If the rate and amount of vesicular traffic are balanced, granule containers empty in a piecemeal fashion but maintain a constant size. If, on the other hand, the inward flow of endocytotic vesicles exceeds the outward flow of exocytotic vesicles, granule chambers become enlarged. The latter event is more frequently observed.
We recently identified PMD morphologies in cell types other than basophils, mast cells and eosinophils, namely the endocrine cells of the human and murine gastro-intestinal tract and the chromaffin cells of the mouse and rat adrenal medulla (Crivellato et al. 2002, 2003b, 2004). No report has yet appeared concerning PMD in tumour secretory cells. In the present study, we analysed the fine morphology of such a degranulation route in human pheochromocytoma, a rare neoplasia of the adrenal medulla (Erlandson, 1994; Lack, 1997).
Materials and methods
Processing for light and electron microscopy
Two patients, one male (aged 28 years) and one female (aged 64 years), were studied. Classic clinical symptoms were noted, consisting of crises of paroxysmal hypertension, headache, palpitation and anxiety. Increased urinary levels of catecholamines and catecholamine metabolites were found. Pre-operative localization of tumour masses was accomplished by computed tomography. Both patients showed enlarged right adrenal glands.
At pathological examination, lesions consisted of round to oval, apparently encapsulated adrenal tumours measuring 3–5 cm in diameter. They were well demarcated by surrounding adrenal tissue. The cut surface was pale grey with some haemorrhagic spots. Multiple bioptic specimens were taken from both tumour areas and surrounding normal medullary tissue, and processed for both conventional histopathological examination and transmission electron microscopy. This last procedure briefly consisted in cutting tumour material into 1-mm3 pieces. They were immediately immersed in a mixture of aldehydes containing 2% paraformaldehyde, 2.5% glutaraldehyde, 0.025% CaCl2 in 0.1 m sodium cacodylate buffer, pH 7.4, and held for 3 h at 4 °C. Samples were washed in the same buffer, post-fixed in 1% buffered osmium tetroxide for 4 h at 4 °C, and then dehydrated in a graded ethanol–propylene oxide series prior to infiltration and embedding in Epon. Plastic 1-µm sections were stained with toluidine blue and examined by light microscopy. Ultrathin sections (70–80 nm) were contrasted with uranyl acetate and lead citrate, and examined in a Philips CM 12 electron microscope at 80 kV.
Quantitative evaluation of electron microscopic data
To determine whether PMD is a significant event in tumour pheochromocytes, we performed a series of morphometric comparative analyses with non-tumour chromaffin cells. These were located in the normal medulla surrounding the tumour mass. Ultrastructural quantitative investigations included determination of the frequency of (1) resting granules, (2) altered granules (granules with reduced or mobilized components) and (3) empty containers, in either tumour or normal chromaffin cells. In addition, the number of small, membrane-bound vesicles (30–150 nm in diameter) was evaluated in the cytoplasm of both tumour and non-tumour pheochromocytes. Fifteen tumour pheochromocytes and 15 normal chromaffin cells from each patient were selected at random and photographed at ×8000 and ×30 000 magnification. Enlarged prints (18 × 24 cm) were analysed for morphometric evaluation. The number of resting granules, altered granules and empty containers was counted in the group of 60 low-magnification micrographs and expressed as a percentage of the total number of granules/containers. The number of 30–150-nm cytoplasmic vesicles was evaluated in the group of 60 high-magnification micrographs for each printed cell and related to the net cytoplasmic area (expressed in square micrometres) of the corresponding cell. The net cytoplasmic area was calculated using a BDS-Image software package system interfaced with a Macintosh computer, which allowed subtraction of the areas occupied by chromaffin granules, nucleus, Golgi region and mitochondria from the total cytoplasmic area of a given cell. Vesicle frequency in tumour pheochromocytes and normal chromaffin cells was eventually expressed as the average number of vesicles per net cytoplasmic area.
Data were expressed as mean ± SD and analysed statistically using Student's t-test. Values of P < 0.05 were considered statistically significant. The software package SPSS (Statistical Package Social Sciences, SPSS Inc., Chicago, IL, USA) was used for data analyses.
Results
Histologically, tumours consisted of cords or nests or alveolar clusters of large polyhedral pheochromocytes, lined by a fine fibro-vascular stroma rich in reticulin. Tumour cells contained abundant basophilic, granular cytoplasm. Nuclei were vesicular, round to oval, with coarsely clumped chromatin and a distinct nucleolus. Pleomorphism was marked and some mitoses were also recognizable.
Under the electron microscope, neoplastic pheochromocytes presented as large, polyhedral cells arranged in solid nests separated by delicate sinusoids. Each cell contained an oval, slightly indented nucleus rich in loosely arranged chromatin. The abundant cytoplasm was filled by numerous pleomorphic, partially empty, electron-dense granules.
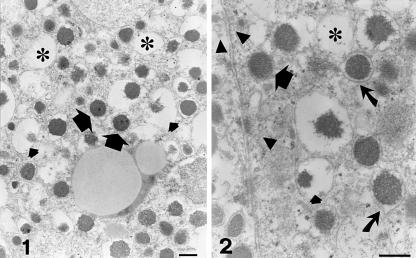
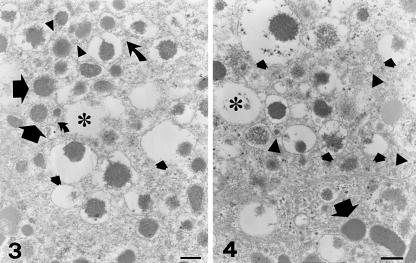
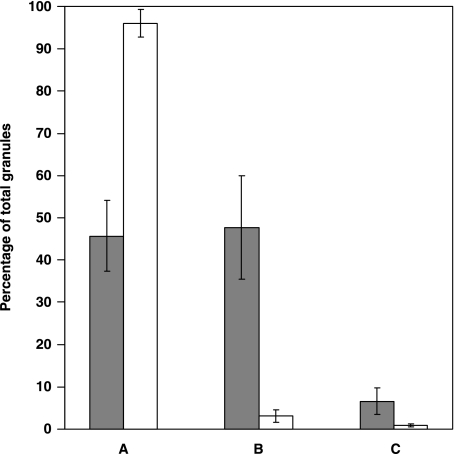
Fine details of chromaffin granules in tumour pheochromocytes were recognized at higher magnification. The most striking finding was the close admixture of resting unaltered granules, dilated non- fused partially empty granules, and empty containers (Figs 1–4). Resting granules accounted for 45.7 ± 8.4% of total granule repertoire (Table 1) and were identified by their electron-dense content and closely adhering limiting membrane. Enlarged, haloed granules (47.7 ± 12.2% of the total granules) showed variable reduction of their secretory material, which appeared irregularly eroded and was often pushed to the granule periphery (Figs 1–4). Some altered granules presented irregular, budding protrusions of their limiting membrane (Figs 2 and 3). A total of 6.6 ± 3.2% of chromaffin granules had transformed into large, empty or partially empty, membrane-bound containers (Figs 1–4).
Figs 1 and 2.
Electron micrographs showing features suggestive of piecemeal degranulation in human tumour pheochromocytes. A characteristic admixture of resting granules (large arrows), haloed granules with ‘piecemealed’ or dissolved matrices (small arrows) and empty containers (asterisks) is observable. Numerous electron-dense and electron-lucent vesicles (arrowheads in Fig. 2) are free in the cytoplasm or attached to the plasma membrane. Some granules present irregular, budding projections of their limiting membranes (curved arrows in Fig. 2). Scale bars, 0.2 µm.
Figs 3 and 4.
Chromaffin granules exhibit a highly characteristic polymorphism and are not fused with each other. Resting, normal granules (large arrows) are mixed with empty containers (asterisks) and dilated granules with evidence of reduced or mobilized components (small arrows). Curved arrows in Fig. 3 point to perigranule membrane budding. A rich population of membrane-bound, clear or electron-dense vesicles 30–150 nm in diameter is visible close to granules (arrowheads). Scale bars, 0.2 µm.
Table 1.
Quantitative morphometric analysis in tumour and normal pheochromocytes
| Tumour pheochromocytes | Normal pheochromocytes | |
|---|---|---|
| Normal resting granules | 45.7 ± 8.4 (a)* | 96 ± 3.2 (a) |
| Altered ‘haloed’ granules | 47.7 ± 12.2 (a)* | 3.1 ± 1.4 (a) |
| Empty containers | 6.6 ± 3.2 (a)* | 0.9 ± 0.3 (a) |
| Vesicle density | 1.22 ± 0.15 (b)* | 0.56 ± 0.06 (b) |
Percentage of total granules.
Vesicles per net cytoplasmic area.
Significantly different from control, P < 0.01.
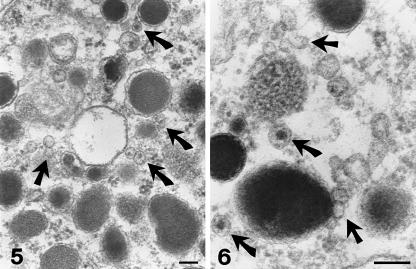
In the cytoplasm of these cells we observed membrane-bound vesicles, 30–150 nm in size (Figs 2–6). Quantitative morphometric analysis indicated a vesicle density of 1.22 ± 0.15 µm−2. Most vesicles were electron-lucent (Figs 2, 3, 5 and 6) but some exhibited an inner electron-dense core (Figs 4–6). The content of these latter vesicles consisted of particles similar in structure and density to those making up the chromaffin granules (Figs 5 and 6). Vesicles were either free in the cytoplasm (Figs 4–6) or attached to granules (Figs 3–6) and the plasma membrane (Fig. 2).
Figs 5 and 6.
At higher magnification, small electron-lucent vesicles (straight arrows) or vesicles containing pieces of dense material (curved arrows) are seen attached to chromaffin granules or free in the cytosol. Scale bars, 0.1 µm.
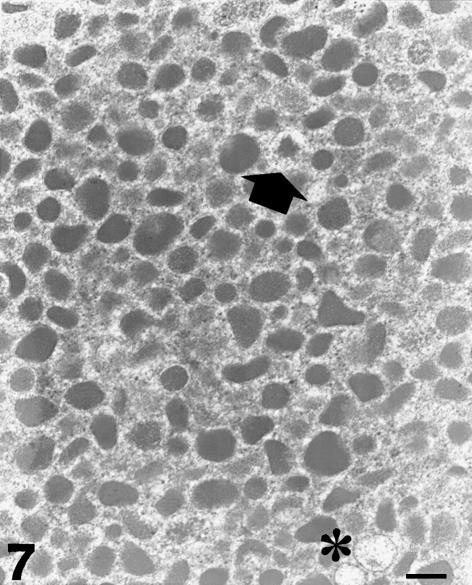
Normal pheochromocytes exhibited a less pleomorphic repertoire of secretory granules (Fig. 7). The number of intact granules was greatly increased in comparison with tumour pheochromocytes (96 ± 3.2%, P < 0.01). Enlarged, haloed granules with mobilized material and empty containers were still observable in non-tumour chromaffin cells, but their frequency was significantly reduced in comparison with tumour pheochromocytes (3.1 ± 1.4 and 0.9 ± 0.3%, respectively; P < 0.01 in both cases) (Fig. 8). In addition, the number of small, membrane-bound cytoplasmic vesicles identified in normal pheochromocytes was again significantly lower compared with quantitative figures obtained in tumour chromaffin cells (0.56 ± 0.06 µm−2; P < 0.01).
Fig. 7.
Electron micrograph of a normal pheochromocyte. The population of secretory granules is less pleomorphic and consists mostly of electron-dense structures with closely adhering limiting membranes. A haloed granule with ‘piecemealed’ matrix (arrow) and two empty containers (asterisk) are visible. Scale bar, 0.3 µm.
Fig. 8.
Graph highlighting the differences in granule features between tumour (shaded columns) and normal (open columns) pheochromocytes. (A) Resting granules, (B) altered ‘haloed’ granules and (C) empty containers. Each column represents the mean ± SD. Each value in tumour pheochromocytes is significantly different from control (P < 0.01).
Discussion
Pheochromocytoma is a rare tumour arising from chromaffin cells of the adrenal medulla. Its great clinical importance stems from the hypertensive effects of catecholamine release from neoplastic cells, along with its association with multiple endocrine neoplasia and other familial syndromes (Gallimore, 1992; Erlandson, 1994; Lack, 1997; Dickersin, 2000).
This study demonstrates that tumour pheochromocytes in two cases of human pheochromocytoma express ultrastructural features highly representative of PMD. All changes diagnostic for PMD (Dvorak, 1991, 1994) were recognizable. Granules often show variable reduction in their content. They present eroded components that are often pushed to the granule periphery. It has been suggested that this eccentric ultrastructural morphology depends upon noradrenaline content (Tannenbaum, 1970; Gómez et al. 1991). Granules also undergo dilation, to form large containers and vacuoles. In addition, some exhibit budding projections of their limiting membranes. Notably, altered haloed granules do not fuse with each other or with the plasma membrane and are always intermingled with normal, resting granules (Dvorak, 1991). Cytoplasmic changes are also highly diagnostic and consist of smooth, membrane-bound, electron-lucent or electron-dense vesicles 30–150 nm in diameter that are either free in the cytoplasm or attached to granules and plasma membranes.
Although exocytosis has long been considered the main route of granule discharge in chromaffin cells, the present data in human pheochromocytes and previous observations in mouse and rat chromaffin cells (Crivellato et al. 2003b, 2004) suggest that PMD may provide an alternative mechanism of catecholamine release. PMD has been interpreted as a long-lasting and discrete secretory process, as compared with exocytosis (Dvorak, 1991). This concept derives from the observation that the expression of PMD in basophils and mast cells is largely related to chronic conditions such as protracted inflammatory diseases and tumours whereas anaphylactic degranulation, which is a rapid explosive secretory event, is largely associated with IgE-dependent immediate hypersensitivity reactions (Dvorak et al. 1976). We have previously proposed that PMD in adrenal chromaffin cells may provide a finely controlled mechanism of hormone discharge by granule deposits (Crivellato et al. 2003a). Indeed, transfer to the cell exterior of small amounts of secretory material through an outward flow of loaded vesicles would allow a subtle modulation of catecholamine secretion.
The data presented here also raise the question of the significance of PMD in neoplastic cells. Our morphometric analysis indicates that the whole secretory PMD process is amplified in tumour chromaffin cells. These cells do exhibit a significantly reduced frequency of normal granules as compared with normal pheochromocytes (45.7 ± 8.4 vs. 96 ± 3.2%, P < 0.01). Moreover, tumour pheochromocytes show increased levels of both enlarged granules with mobilized content material and empty containers (47.7 ± 12.2 vs. 3.1 ± 1.4% and 6.6 ± 3.2 vs. 0.9 ± 0.3%, respectively; P < 0.01 in both cases). In addition, the number of small, membrane-bound cytoplasmic vesicles per net cytoplasmic area is again higher in tumour pheochromocytes than in normal chromaffin cells (1.22 ± 0.15 vs. 0.56 ± 0.06, P < 0.01). All ultrastructural parameters suggestive of PMD are therefore expressed at an increased level in these cells, suggesting a direct link between such a secretory process and disease pathology. Amplification of PMD events in neoplastic pheochromocytes may therefore be the expression of an impaired control of cell secretion. This is not surprising, because tumour cells often exhibit exaggerated or caricaturized functional aspects (Pierce & Speers, 1988; Ratcliffe, 1992). Based on our electron microscopic findings we suggest: (1) that classic single exocytosis is the primary mechanism responsible for secretion of catecholamines from adrenal chromaffin cells; and (2) that other modes of catecholamine release, such as PMD, may operate in these cells in response to intense stimulation, leading to a chronic release of mediators, or as a result of neoplastic transformation. These morphological data may be helpful in explaning the biochemical variability and extreme diversity of clinical manifestations in patients with pheochromocytoma, ranging from mild labile hypertension to sudden death secondary to a hypertensive crisis, myocardial infarction or cerebral vascular accident (Gallimore, 1992; Lack, 1997).
Biogenic amines are stored in chromaffin granules along with a series of hormone peptides such as opioid peptides, neuropeptide Y, neurotensin, calcitonon-gene-related peptide and adrenomedullin, which possibly concur to exert relevant clinical manifestations in pheochromocytoma patients (De Lellis et al. 1983; O'Connor et al. 1983; Lundberg et al. 1986; Pelto-Huikko, 1989; Kuch-Wocial et al. 2004). These mediators are co-released from chromaffin cells during exocytosis (O'Connor et al. 1983; Bastiaensen et al. 1988; Kobayashi et al. 2003). An interesting and still open issue is whether PMD may lead to selective mobilization of stored molecules from the granule compartment. It has been demonstrated that rat peritoneal mast cells treated with progesterone or stimulated with compound 48/80 after pretreatment with amitriptyline undergo differential release of serotonin without a comparative release of histamine, despite the fact that both amines are stored together in mast cell granules (Kraeuter Kops et al. 1990; Vliagoftis et al. 1990). This secretory process does not lead to degranulation events and is accompanied by the formation of numerous small vesicles, either empty or filled with electron-dense material, located near the granules or the plasma membrane. Kraeuter Kops et al. (1990) suggest that differential serotonin release may result from exocytosis of small transport vesicles from which histamine is excluded. Further ultrastructural immunocytochemistry and dynamic investigations will shed light upon this important issue and clarify whether cytoplasmic vesicles in chromaffin cells contain different kinds of catecholamines and medullary hormones.
In conclusion, although exocytosis has long been considered the main route of granule discharge in adrenal chromaffin cells (Coupland, 1965; Brooks & Carmichael, 1987; Carmichael et al. 1989), ultrastructural data in rodents indicate that PMD can be considered an alternative secretory pathway (Crivellato et al. 2003b, 2004). Here, we have reported on PMD in a population of tumour chromaffin cells from the human adrenal medulla. This finding is a novelty because PMD has not hitherto been recognized in neoplastic cells and provides further evidence for PMD being a general secretory pathway in granulated secretory cells involved in paracrine/endocrine functions.
Acknowledgments
This work was supported by local funds from the Ministero dell’Istruzione, dell’Università e della Ricerca, Rome, to the Department of Medical and Morphological Research, Anatomy and Pathology Sections, University of Udine.
References
- Bastiaensen E, De Block J, De Potter WP. Neuropeptide Y is localized together with enkephalins in adrenergic granules of bovine adrenal medulla. Neuroscience. 1988;25:679–686. doi: 10.1016/0306-4522(88)90268-0. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Carmichael SW. Ultrastructural demonstration of exocytosis in intact and saponin-permeabilized cultured chromaffin cells. Am. J. Anat. 1987;178:85–89. doi: 10.1002/aja.1001780111. [DOI] [PubMed] [Google Scholar]
- Carmichael SW, Brooks JC, Malhotra RK, Wakade TD, Wakade AR. Ultrastructural demonstration of exocytosis in the intact rat adrenal medulla. J. Electron Microsc. Technol. 1989;12:316–322. doi: 10.1002/jemt.1060120404. [DOI] [PubMed] [Google Scholar]
- Coupland RE. Electron microscopic observations on the structure of the rat adrenal medulla. I. The ultrastructure and organization of chromaffin cells in the normal adrenal medulla. J. Anat. 1965;99:231–254. [PMC free article] [PubMed] [Google Scholar]
- Crivellato E, Ribatti D, Mallardi F, Beltrami CA. Granule changes of human and murine endocrine cells in the gastro-intestinal epithelia are characteristic of piecemeal degranulation. Anat. Rec. 2002;268:353–359. doi: 10.1002/ar.10149. [DOI] [PubMed] [Google Scholar]
- Crivellato E, Nico B, Mallardi F, Beltrami CA, Ribatti D. Piecemeal degranulation as a general secretory mechanism? Anat. Rec. 2003a;274:778–784. doi: 10.1002/ar.a.10095. [DOI] [PubMed] [Google Scholar]
- Crivellato E, Nico B, Perissin L, Ribatti D. Ultrastructural morphology of adrenal chromaffin cells indicative of a process of piecemeal degranulation. Anat. Rec. 2003b;270:103–108. doi: 10.1002/ar.a.10013. [DOI] [PubMed] [Google Scholar]
- Crivellato E, Belloni A, Nico B, Nussdorfer GG, Ribatti D. Chromaffin granules in the rat adrenal medulla release their secretory content in a particulate fashion. Anat. Rec. 2004;277:204–208. doi: 10.1002/ar.a.20004. [DOI] [PubMed] [Google Scholar]
- De Lellis RA, Tischler AS, Lee AK, Blount M, Wolfe HJ. Leu-enkephalin-like immunoreactivity in proliferative lesions of the human adrenal medulla and extra-adrenal paraganglia. Am. J. Surg. Pathol. 1983;7:29–37. doi: 10.1097/00000478-198301000-00003. [DOI] [PubMed] [Google Scholar]
- Dickersin GR. Diagnostic Electron Microscopy. A Text/Atlas. 2nd. New York: Springer; 2000. [Google Scholar]
- Dvorak HF, Dvorak AM, Churchill WH. Immunologic rejection of diethylnitrosamine-induced hepatomas in strain 2 guinea pigs. Participation of basophilic leukocytes and macrophages aggregates. J. Exp. Med. 1973;137:751–775. doi: 10.1084/jem.137.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF, Mihm MC, Jr, Dvorak AM, et al. Morphology of delayed type hypersensitivity reactions in man. I. Quantitative description of the inflammatory response. LabInvest. 1974;31:111–130. [PubMed] [Google Scholar]
- Dvorak AM, Mihm MC, Jr, Dvorak HF. Degranulation of basophilic leukocytes in allergic contact dermatitis reactions in man. J. Immunol. 1976;116:687–695. [PubMed] [Google Scholar]
- Dvorak AM. Basophil and mast cell degranulation and recovery. In: Harris JR, editor. Blood Cell Biochemistry. Vol. 4. New York: Plenum Press; 1991. pp. 340–377. [Google Scholar]
- Dvorak AM, Ackerman SJ, Furitsu T, Estrella P, Lerourneau L, Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vescicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am. J. Pathol. 1992;140:795–807. [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM. Ultrastructural analysis of human basophil and mast cell recovery after secretion. Sem. Clin. Immunol. 1994;8:5–16. [Google Scholar]
- Dvorak AM, MacGlashan DW, Jr, Morgan ES, Lichtenstein LM. Vesicular transport of histamine in stimulated human basophils. Blood. 1996;88:4090–4101. [PubMed] [Google Scholar]
- Dvorak AM. A role for vesicles in human basophil secretion. Cell Tissue Res. 1998;293:1–22. doi: 10.1007/s004410051093. [DOI] [PubMed] [Google Scholar]
- Erjefalt JS, Andersson M, Greiff L, Korsgren M, Gizycki M, Jeffery PK, Persson CGA. Cytolysis and piecemeal degranulation as distinct modes of activation of airway mucosal eosinophils. J. Allergy Clin. Immunol. 1998;102:286–294. doi: 10.1016/s0091-6749(98)70098-3. [DOI] [PubMed] [Google Scholar]
- Erlandson RA. Diagnostic Transmission Electron Microscopy of Tumors. New York: Raven Press; 1994. pp. 615–621. [Google Scholar]
- Gallimore A. The adrenal medulla. In: McGee JO'D, Isaacson P, Wright NA, editors. Oxford Textbook of Pathology. Vol. 2b. Oxford: Oxford University Press; 1992. pp. 1987–89. [Google Scholar]
- Gómez RR, Osborne BM, Ordoñez NG, Mackay B. Pheochromocytoma. Ultrastruct. Pathol. 1991;15:557–562. doi: 10.3109/01913129109016263. [DOI] [PubMed] [Google Scholar]
- Karawajczyk M, Seveus I, Garcia R, et al. Piecemeal degranulation of peripheral blood eosinophils: a study of allergic subjects during and out of the pollen season. Am. J. Respir. Cell. Mol. Biol. 2000;23:521–529. doi: 10.1165/ajrcmb.23.4.4025. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Yanagita T, Yokoo H, Wada A. Pathophysiological function of adrenomedullin and proadrenimedullin N-terminal peptides in adrenal chromaffin cells. Hypertens. Res. 2003;26(Suppl):S71–78. doi: 10.1291/hypres.26.s71. [DOI] [PubMed] [Google Scholar]
- Kraeuter Kops S, Theoharides TC, Cronin CT, Kashgarian MG, Askenase PW. Ultrastructural characteristics of rat peritoneal mast cells undergoing differential release of serotonin without histamine and without degranulation. Cell Tissue Res. 1990;262:415–424. doi: 10.1007/BF00305238. [DOI] [PubMed] [Google Scholar]
- Kuch-Wocial A, Slubowska K, Kostrubiec M, et al. Plasma neuropeptide Y immunoreactivity influences left ventricular mass in pheochromocytoma. Clin. Chim. Acta. 2004;345:43–47. doi: 10.1016/j.cccn.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Lack EE. Tumors of the adrenal grand and extra-adrenal paraganglia. In: Rosai J, Sobin LH, editors. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1997. pp. 223–259. 285–301. [Google Scholar]
- Lundberg JM, Hökfelt T, Hemsen A, et al. Neuropeptide Y-like immunoreactivity in adrenaline cells of the adrenal medulla and in tumors and plasma of pheochromocytoma patients. Regul. Pept. 1986;13:169–182. doi: 10.1016/0167-0115(86)90224-7. [DOI] [PubMed] [Google Scholar]
- O'Connor DT, Frigon RP, Deftos LJ. Immunoreactive calcitonin in catecholamine storage vesicles of human pheochromocytomas. J. Clin. Endocrinol. Metab. 1983;56:582–585. doi: 10.1210/jcem-56-3-582. [DOI] [PubMed] [Google Scholar]
- Pelto-Huikko M. Immunocytochemical localization of neuropeptides in the adrenal medulla. J. Electron Microsc. Techn. 1989;12:364–379. doi: 10.1002/jemt.1060120409. [DOI] [PubMed] [Google Scholar]
- Pierce GB, Speers WC. Tumours as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 1988;48:1996–2004. [PubMed] [Google Scholar]
- Ratcliffe JG. Endocrine effects of tumors. In: McGee JO‘D, Isaacson P, Wright NA, editors. Oxford Textbook of Pathology. Vol. 1. Oxford: Oxford University Press; 1992. pp. 694–703. [Google Scholar]
- Tannenbaum M. Ultrastructural pathology of adrenal medullary tumors. Pathol. Annu. 1970;5:145–171. [PubMed] [Google Scholar]
- Vliagoftis H, Dimitriadou V, Theoharides TC. Progesterone triggers selective mast cell secretion of 5-hydroxytryptamine. Int. Arch. Allergy Appl. Immunol. 1990;93:113–119. doi: 10.1159/000235289. [DOI] [PubMed] [Google Scholar]