Abstract
Adhesion molecules are important in supporting axonal regeneration. Qualitative studies have described increased expression of neural cell adhesion molecule (NCAM) and N-cadherin in models of nerve injury allowing active regeneration. In this study we have used quantitative immunohistochemistry to compare expression of NCAM and N-cadherin after nerve injury either with active regeneration (crush) into the distal stump or without (axotomy and capping). Quantification was performed 15 days after axotomy in proximal and distal stumps. Quantification after crush either proximal, distal or within the crushed area was performed at 2, 7, 15 and 30 days after injury. Axotomy induced increases in expression in proximal stumps between two and three times those in uninjured nerves for both molecules. In distal stumps, N-cadherin levels increased seven-fold, yet NCAM levels did not exceed control values. After crush, NCAM immunoreactivity increased in the crushed area and distal stump in contrast to axotomy and NCAM-positive axons co-localized with PGP9.5. N-cadherin levels in the distal stump increased above control levels, but the magnitude of the increase seen after crush was different to those seen after axotomy. In conclusion, increased expression of adhesion molecules, particularly NCAM, in the distal stump of injured nerves is dependent upon the presence of regenerating axons.
Keywords: injury, N-cadherin, NCAM, regeneration
Introduction
Many factors determine whether regenerating fibres of the peripheral nervous system will re-innervate their target organs and of particular importance are the interactions between axons and glia via cell contact. Neural cell adhesion molecule (NCAM) and N-cadherin from the cadherin superfamily are candidates for these cell surface interactions.
In sciatic nerves of developing mice, NCAM is expressed on fasciculating axons and non-myelinating Schwann cells (Nieke & Schachner, 1985; Martini & Schachner, 1986). However, with myelination this molecule is down-regulated except on non-myelinating Schwann cells and their associated small-calibre axons (Nieke & Schachner, 1985; Martini & Schachner, 1986; Mirsky et al. 1986). In a mouse sciatic nerve injury model, Schwann cells become immunoreactive for NCAM 2 weeks after transection (Nieke & Schachner, 1985; Martini & Schachner, 1988). Northern blot analysis of rat sciatic nerve distal stump also shows an up-regulation of NCAM-specific mRNAs between 6 and 10 days after injury (Tacke & Martini, 1990b). NCAM may mediate axon–axon interactions (Stallcup & Beasley, 1985) and axon–Schwann cell interactions (Mirsky et al. 1986; Seilheimer et al. 1989).
Members of the cadherin superfamily may also be important in interactions between Schwann cells and axons. N-cadherin is a Ca2+-dependant intercellular adhesion molecule identified on growth cone surfaces in vitro (Bixby et al. 1988; Letourneau et al. 1990; Honig & Kueter, 1995). In vivo, N-cadherin expression has been documented on normal unmyelinated fibres and regenerating fibres of the chicken peripheral nervous system (Shibuya et al. 1995). N-cadherin expression was location specific; intense immunoreactivity was identified on the plasma membrane surface of axon–axon and axon–Schwann cell contacts, but not where axons or Schwann cells were in contact with basal lamina (Shibuya et al. 1995). There is also evidence that sorting of axons into different pathways may depend upon cadherin expression (Redies, 2000) such as during brain development (Redies et al. 1993).
In cultured systems NCAM and N-cadherin may promote neurite outgrowth (Bixby et al. 1988; Doherty & Walsh, 1989) in some cases on the surfaces of Schwann cells (Bixby et al. 1988), and recent studies suggest that NCAM may act differentially from other adhesion modules during this process (Takei et al. 1999).
Previous experiments have documented adhesion molecule expression in a qualitative manner; therefore, it has been difficult to compare expression in models of sciatic nerve injury either in the presence or in the absence of active regeneration into the distal stump. In this study we have assessed expression of adhesion molecules using quantitative immunohistochemistry in axotomized and crushed rat sciatic nerve; this has allowed us to delineate the relative roles of these molecules proximal and distal to the site of injury as separate anatomical entities. In addition, our model of axotomy prevented regeneration into distal stumps in contrast to crush injury, which allowed us to clarify the difference in expression patterns that occur upon contact with the distal stump.
Materials and methods
Animals and surgical procedure
All procedures were carried out in compliance with the UK Animals (Scientific Procedures) Act 1986. Six- to 8-week-old male Sprague–Dawley rats, weighing approximately 250 g were anaesthetized with a halothane and oxygen mixture. For axotomy experiments, the left sciatic nerve was exposed and divided 5 cm distal to the sciatic notch using sharp microscissors. The cut nerve ends were then inserted into a short sterile blind ended silicon tube, anchored in place with a single 9/0 ethilon™ epineural suture (Ethicon, Johnson and Johnson, Belgium) and buried to protect the nerve ends and prevent regeneration into the distal stump. For crush experiments, after exposure the nerve was crushed 5 cm distal to the sciatic notch using a 3-mm bulldog straight vascular clamp for 30 s (in contrast to previous crush models using 0.5-mm watchmakers forceps). The crush site was marked with an epineural suture. Two days (n = 6), 7 days (n = 6), 15 days (n = 6) and 30 days (n = 6) after crush and 15 days (n = 6) after axotomy, the animals were killed using a rising concentration of carbon dioxide. The axotomized, crushed and contralateral normal sciatic (control) nerves were then harvested, fixed in 4% (w/v) paraformaldehyde (Sigma), rinsed in 0.01 m PBS containing 15% (w/v) sucrose (Sigma) and 0.1% (w/v) sodium azide (Sigma) and processed for morphological analysis.
Tissue processing and analysis
The specimens were blocked in OCT compound (BDH Laboratory Supplies, Poole, UK) with a piece of rat liver marking the proximal end of the nerve to identify the orientation of each sample. In crushed nerves a piece of rat liver also marked the crushed site. Cryostat sections (15 µm) were cut longitudinally, and collected on Vectabond-coated slides (Vector, UK) and then dried overnight at 37 °C. The sections were permeabilized with 0.1% (w/v) Triton-X, washed in PBS (5 min) and blocked with 1% (w/v) normal goat and horse serum for 1 h. Sections were incubated overnight with a primary antibody against all NCAM isoforms (rabbit polyclonal, dilution 1 : 500, Chemicon, UK), N-cadherin (rabbit polyclonal, dilution 1 : 20, Santa Cruz Biotechnology, Inc, USA), S-100 (mouse monoclonal 1 : 600, Affiniti, UK, or rabbit polyclonal 1 : 1200 DAKO, Denmark) and PGP9.5 (mouse monoclonal 1 : 2000, Biogenesis, UK, or rabbit polyclonal 1 : 1200 Affiniti, UK). Sections were washed in PBS (2 × 5 min) and incubated with polyclonal goat anti-rabbit FITC (1 : 100, Vector Laboratories, USA) for adhesion molecules; sections stained with S-100 or PGP9.5 were incubated with either polyclonal goat anti-rabbit Cy™ 3 (1 : 100, Amersham Biosciences, UK) or monoclonal goat anti-mouse Cy3 (1 : 100, Amersham Biosciences, UK) for one hour before a final rinse in PBS (2 × 5 min) and mounting in Vectashield to minimize fading of fluorescence (Vector Laboratories, USA).
Imaging, quantification and morphometric analysis
Specimens were examined with an Olympus BH60 microscope (Olympus, Japan) and high-definition monochrome images were captured with a digital camera (Spot™, Diagnostic Instruments Inc., USA). All images for quantification were taken in monochrome to minimize loss of signal. To minimize decay of fluorescence, image capturing was performed within 48 h of immunostaining. The image capture and exposure times were determined for each antibody in a pilot experiment and then standardized.
The histological area of image acquisition was standardized as follows. In normal sciatic nerve, a point equating to the midpoint of the nerve was identified and captured sequentially across the nerve width at × 40 magnification. In the axotomized sciatic nerve the most proximal front was first identified. From this point, an area was located in the nerve stump corresponding to three microscopical fields of view (∼2 mm) proximal to the regeneration front (×20 magnification). A similar procedure was used for the distal stump. Images were then captured sequentially across the nerve width at × 40 magnification. In the crushed sciatic nerve images were taken at three fields of view proximal and distal to the crush site and also at the centre of the crushed zone at × 40 magnification. In all sections adjacent fields for each image were captured ensuring that there was no overlap or duplication of the measured area. For each captured image the areas of positive staining were edited above the threshold levels to exclude background noise. Image-Pro Plus® software (Media Cybernetics, USA) was used to measure the overall intensity of positively stained areas. Intensity per unit area (arbitary units) was then calculated from these values and used as a relative indicator of adhesion molecule expression.
Statistical analysis
The Sigmastat® software program (SPSS Science, USA) was used for statistical analysis. Kruskal–Wallis one-way analysis of variance on ranks was used to analyse the median values of intensity/unit area of adhesion molecule expression in normal, crushed and cut nerves. A multiple comparisons test (Dunn's test) was performed to compare results of experimental and control nerve groups. Data were analysed on ranks as they did not conform to a normal distribution. The Mann–Whitney Rank Sum test was used to compare median values between the crushed and axotomy experiments.
Results
Immunostaining
NCAM and N-cadherin are expressed at low levels in uninjured nerves
In normal sciatic nerves, NCAM and N-cadherin immunostaining was present at low levels (Fig. 1A, D). Positive staining was seen in some areas co-localized with S-100, suggesting a close association with Schwann cells. Areas of NCAM-positive adhesion molecule staining without co-localization with S-100 may indicate the presence of these molecules on axons (Fig. 2A). The pattern of N-cadherin expression differs from that of NCAM in that it resembled more closely S-100 expression and may therefore be localized predominantly in association with Schwann cells (Fig. 2B).
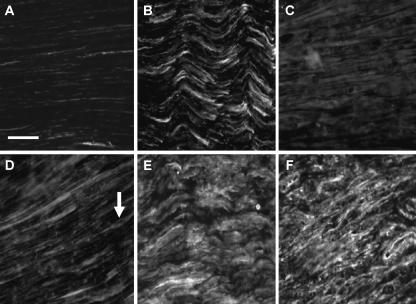
Fig. 1.
Immunofluorescent staining of NCAM (A–C) and N-cadherin (D–F) in normal rat sciatic nerve (A,D), and proximal (B,E) and distal stumps (C,F) after axotomy. Monochrome images were taken for quantification in order to minimize loss of signal intensity. Images were taken ∼2 mm proximal or distal to the injury site. Low levels of staining are seen in all normal nerve sections (A,D). N-cadherin stains structures that resemble Schwann cells with slender processes originating from a more densely stained cell body (arrow). Fifteen days after axotomy there appears to be an increase in staining intensity for NCAM (B) and N-cadherin (E). A similar increase in expression is seen in distal stumps for N-cadherin (F), although NCAM levels do not appear to stain as intensely (C). Scale bar, 50 µm (objective × 40). NB. Increased exposure times were used for normal nerve sections to allow staining to be adequately visualized.
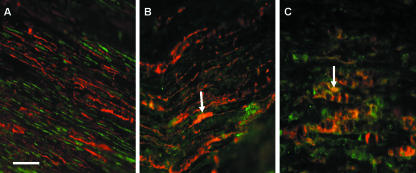
Fig. 2.
Immunofluorescent double-staining with S-100 (red fluorescence) and either NCAM (A), N-cadherin (B) in normal rat sciatic nerve or N-cadherin in the distal stump after axotomy (C) (all adhesion molecules show green fluorescence). NCAM staining in normal nerve sections does not appear to co-localize with S-100, unlike N-cadherin (arrow in B) (orange/yellow staining areas). In the distal stump after axotomy N-cadherin staining co-localizes with S-100 (arrow in C), indicating that Schwann cells may be responsible for the high levels of expression of N-cadherin in distal stumps. Scale bar, 50 µm (objective × 40). NB. Increased exposure times were used for normal nerve sections to allow staining to be adequately visualized.
Axotomy induces changes in expression
After axotomy, expression of both adhesion molecules appeared to increase in proximal stumps as compared with control nerve (Fig. 1B, E). An increase in expression was seen for N-cadherin in distal stumps (Fig. 1F) yet not for NCAM (Fig. 1C). In the distal stump, N-cadherin appeared to be closely associated with Schwann cells, with a good co-localization of staining with S-100 for N-cadherin (Fig. 2C) but not for NCAM (data not shown). N-cadherin staining co-localized with S-100 showed the typical Schwann cell morphology seen during Wallerian degeneration (Fig. 2C).
NCAM expression after crush parallels regeneration into distal stumps
After crush injury the overall staining pattern was similar to that after axotomy. NCAM staining in particular appeared to follow the course of regenerating axons as they passed through the crushed zone and into the distal stump. In the crushed zone after 2 days (Fig. 3A) a low level of PGP9.5 staining is present, representative of degenerating axons, yet unrecognizable from PGP9.5 staining in uninjured nerves due to damage caused by crush. After 7 days regenerating axons positively stained for PGP9.5 can been seen in the most proximal part of the crushed zone co-localized with NCAM (Fig. 3B). In the distal stump 15 days after crush injury, few regenerating axons are seen (Fig. 3C) as compared with the appearance of PGP9.5 staining in normal nerves (data not shown) and there is also minimal NCAM staining. After 30 days the distal stump contains axons positively stained for both PGP9.5 and NCAM (Fig. 3D).
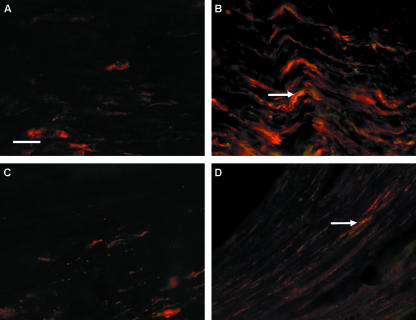
Figure. 3.
Immunofluorescent double-staining with PGP9.5 (red fluorescence) and NCAM (green fluorescence). In the centre of the crushed zone after 2 days (A), PGP9.5 expression is minimal and undistinguishable from PGP9.5 staining in uninjured nerve due to the physical destruction caused by nerve crush. However, after 7 days (B) PGP9.5-positive staining is seen corresponding to regenerating axons co-localizing with NCAM staining (arrow) (orange/yellow staining areas). In the section of nerve distal to the crush injury after 15 days (C) there is little PGP9.5 staining, indicating regenerating axons may not have crossed the crushed zone; however, in the same region after 30 days (D) some regenerating axons appear to be present. Again NCAM appears to be expressed in co-localization with PGP9.5 in the distal zone 30 days after crush (arrow in D) (orange/yellow staining areas). Scale bar, 50 µm (objective × 40).
Quantification
Quantification after axotomy: NCAM and N-cadherin
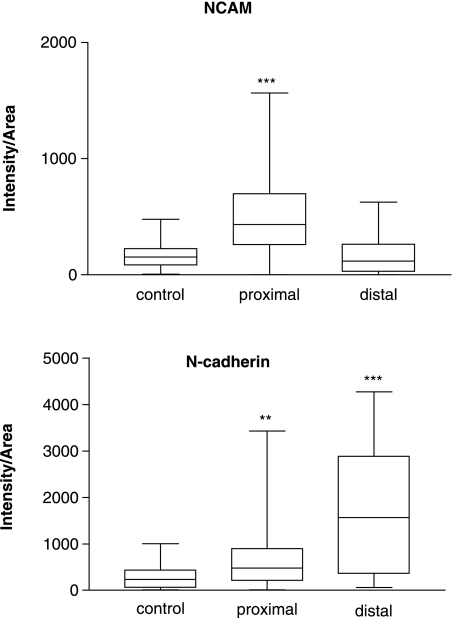
In this study we have used intensity of immunostaining expressed per unit area (arbitary units – IA) as a measure of adhesion molecule expression. Quantification in this manner allows a comparison of relative levels after both axotomy in which regeneration into the distal stump was prevented in comparison with crush injury where active regeneration into the distal stump occurs. Two weeks after axotomy the levels in the proximal stumps increased above control levels (Fig. 4, Table 1) for both N-cadherin (P < 0.01) and NCAM (P < 0.001). In distal stumps, N-cadherin levels (P < 0.001) were again elevated, but NCAM levels were similar to control levels (P > 0.05) (Fig. 4, Table 1).
Fig. 4.
Box and whisker plot showing quantification (median and 25–75 percentile range) of NCAM and N-cadherin expression in normal rat sciatic nerves and in proximal and distal nerve stumps at 15 days after axotomy (n = 6 for each group). **P < 0.01 or ***P < 0.001 when proximal or distal stump is compared with control. anova, Dunn's multiple comparisons test.
Table 1.
Absolute values [median (25–75%)] for intensity/area measured for immunostaining of adhesion molecules after axotomy.
| Axotomy | Normal nerve | Proximal stump | Distal stump |
|---|---|---|---|
| NCAM | 152 (82–225) | 432 (259–699) | 117 (28–263) |
| N-cadherin | 231 (61–433) | 479 (217–887) | 1562 (377–2797) |
Quantification after nerve crush: NCAM
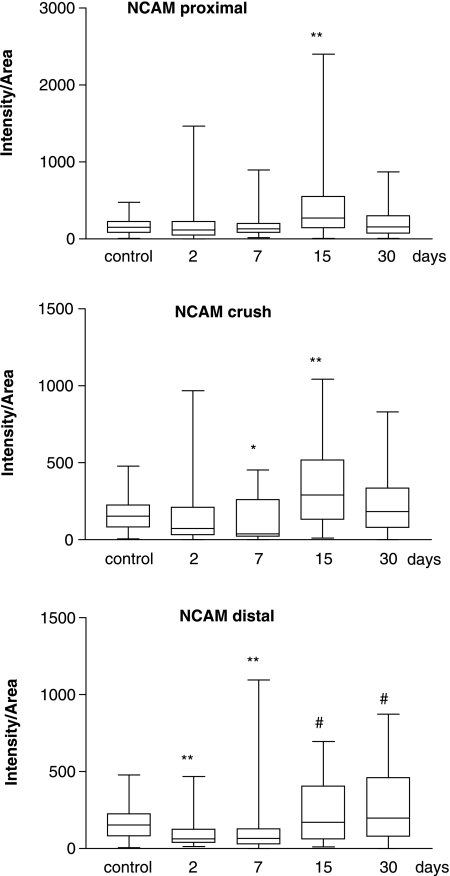
In the three anatomical sites studied, median expression values of both adhesion molecules peaked 15 days after crush injury. However, their level of expression over time was varied (Figs 5 and 6). NCAM expression (Fig. 5, Table 2) proximal to the crush site did not increase significantly until 15 days after injury (P < 0.01), declining towards control levels by 30 days. In the crushed zone, expression levels decreased compared with control nerve at 7 days (P < 0.05) but by 15 days after injury levels were significantly elevated (P < 0.01), again returning to control values by 30 days. Distal to the site of crush the pattern of NCAM expression was quite different to that in either the proximal or the crushed zones. After 2 days, levels of NCAM decreased significantly (P < 0.01) and remained below control levels at 7 days (P < 0.01). No statistically significant increase in expression was found when results 15 and 30 days after crush were compared with control levels (P = 0.158). However, if these results are compared with the low levels reached 2 days after injury it is clear that an increase in expression had occurred (P < 0.001). When NCAM levels in the proximal stump are compared with those in the distal stump 15 days after injury similar levels of expression were achieved (P > 0.05).
Fig. 5.
Box and whisker plot showing quantification (median and 25–75 percentile range) of NCAM expression, proximal (top) and distal (bottom) to the crushed site and also within the crushed area (middle) at different time points after injury. *P < 0.05 or **P < 0.01 when median values are compared with control using Dunn's multiple comparisons test. #P < 0.001 when median values 15 and 30 days after injury are compared with values 2 days after injury.
Fig. 6.
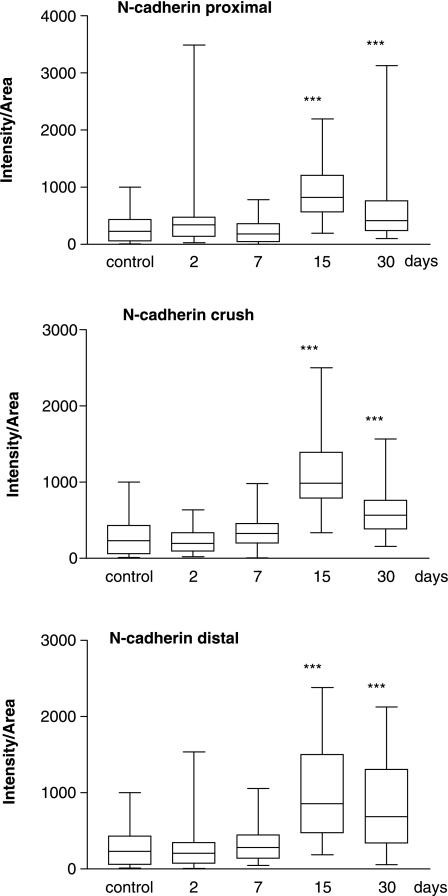
Box and whisker plot showing quantification (median value and 25–75 percentile range) of N-cadherin expression, proximal (top) and distal (bottom) to the crushed site and also within the crushed area (middle) at different time points after injury. ***P < 0.001 when median values are compared with control nerve using Dunn's multiple comparisons test.
Table 2.
Absolute values [median (25–75%)] for intensity/area for immunostaining of adhesion molecule expression after crush
| Crush | Normal nerve | 2 day | 7 day | 15 day | 30 day |
|---|---|---|---|---|---|
| NCAM (proximal) | 152 (82–225) | 115 (52–219) | 130 (84–203) | 271 (443–554) | 155 (73–295) |
| NCAM (crush) | 152 (82–225) | 74 (30–201) | 40 (21–262) | 291 (131–517) | 185 (78–305) |
| NCAM (distal) | 152 (82–225) | 64 (38–126) | 66 (30–129) | 171 (65–403) | 199 (87–454) |
| N-cadherin (proximal) | 231 (61–433) | 341 (134–475) | 185 (45–361) | 825 (564–1210) | 413 (238–763) |
| N-cadherin (crush) | 231 (61–433) | 197 (93–336) | 327 (196–458) | 985 (788–1395) | 690 (339–1308) |
| cadherin (distal) | 231 (61–433) | 210 (71–350) | 280 (136–448) | 855 (475–1502) | 567 (380–762) |
Quantification after nerve crush: N-cadherin
N-cadherin levels appeared to show a similar expression pattern in the three sites studied (Fig. 6, Table 2). Maximal levels were reached at 15 days and the increases were all of a similar magnitude (control: 231.2 IA, proximal: 825 IA, crush: 985.4 IA, distal: 855.2 IA; P < 0.001) Levels at 2 and 7 days did not increase above control values in any of the three areas examined. By 30 days levels appeared to be declining but in all cases were still higher than control values.
Comparison of quantified values after crush and axotomy: NCAM and N-cadherin
Fifteen days after injury, NCAM levels measured in the proximal stumps after axotomy were higher (Fig. 4) than after crush (Fig. 5) (axotomy: 431.8 IA, crush: 270.6 IA; P = 0.008), yet in distal stumps levels measured in crushed nerve were higher (axotomy: 117.1 IA, crush: 171.3 IA; P = 0.013). N-cadherin levels proximal to injury sites were higher after crush than axotomy (Fig. 6) (axotomy: 479 IA, crush: 825 IA; P ≤ 0.001), yet they were higher after axotomy than crush distal to the injury (axotomy: 1562 IA, crush: 855.2 IA; P = 0.05) (Fig. 4).
Discussion
In this study we have quantified the expression of two adhesion molecules by measuring intensity of immunostaining. A low level of expression of NCAM and N-cadherin in normal sciatic nerves contrasted with axotomy-induced increases in expression for these molecules in the proximal stump and for N-cadherin but not NCAM in the distal stump. Crush injury induced high levels of both adhesion molecules after 15 days, irrespective of their location relative to the site of crush, and generally maintaining higher levels than control after 30 days. Crush and axotomy produced differences in both the pattern and the magnitude of expression relative to the site of injury and which may be attributable to regeneration into the distal stump. To our knowledge this is the first study comparing the relative roles of these two adhesion molecules, thought to be important in nerve regeneration, by quantification of expression. Hence, we were able to define the pattern and magnitude of adhesion molecule expression after injury and during regeneration.
Consistent with previous findings (Daniloff et al. 1986; Mirsky et al. 1986; Cifuentes-Diaz et al. 1994) we have identified a low level of expression of NCAM and N-cadherin in normal sciatic nerves. NCAM has been shown to be down-regulated as myelination occurs (Rieger et al. 1986) and hence low levels are seen in the sciatic nerve with mixed myelinated and non-myelinated fibres. It has been proposed that N-cadherin has a role in the stabilization of myelin sheaths based on its distribution in normal nerves (Cifuentes-Diaz et al. 1994). In our study, N-cadherin staining appeared prominent on Schwann cell processes, which may corroborate this role.
We have designed our study to maximize the changes in adhesion molecule expression because 2 weeks after transection Schwann cells become immunoreactive for NCAM (Martini & Schachner, 1988). Therefore, we chose to examine and quantify adhesion molecule expression at this time point. In previous studies of axotomy the proximal and distal stumps have been approximated (Daniloff et al. 1986; Martini & Schachner, 1988). However, in our experiments we chose to examine the effect of isolating and capping the proximal and distal stumps and so preventing active regeneration into the distal stump as this would more closely mimic long-gap injury as seen in a clinical setting. In crush injury there is preservation of the basal lamina through which active regeneration into the distal stump may occur. Previous studies of adhesion molecule expression have documented the presence of an inflammatory cap at the distal tip of the regenerating stump where inflammatory cells express cell adhesion molecules (Martini & Schachner, 1988). Therefore, we elected to quantify adhesion molecule levels at a set distance from either the distal point of the axotomized nerve stump or the border of the crushed zone in crushed nerves.
Expression of NCAM and N-cadherin in proximal stumps after axotomy
Two weeks after axotomy, regenerating axons are present in the proximal stump, accompanied by Schwann cells, which have proliferated (Ide, 1996) and subsequently migrated along the regenerating axons, attaching and ensheathing them (Torigoe et al. 1996). NCAM may play a role in the interactions between Schwann cells and axons (Seilheimer & Schachner, 1988) and it is expressed by Schwann cells after injury (Martini & Schachner, 1988). Our results showing increased NCAM expression in proximal stumps after injury are consistent with the observations of Daniloff et al. (1986). Similarly, N-cadherin has been shown to participate in the interactions between Schwann cells and axons (Shibuya et al. 1995) and to be important for neurite outgrowth in vitro on the surfaces of Schwann cells (Bixby et al. 1988; Letourneau et al. 1990). Again our results are consistent with a functional role for N-cadherin in the outgrowth of regenerating neurons from proximal stumps due to the high expression seen in both axotomy and crush models. However, previous studies of N-cadherin expression after injury produce conflicting results either in line with our findings (Shibuya et al. 1995) or showing no overall increase in expression (Cifuentes-Diaz et al. 1994).
Expression of NCAM and N-cadherin in distal stumps after axotomy
In distal stumps during Wallerian degeneration Schwann cells are devoid of contact from axons and they proliferate forming bands of Büngner within basal lamina tubes. In previous studies on mice it has been shown that Schwann cells re-express NCAM on their surfaces after denervation when they are in close contact with each other (Martini & Schachner, 1988). However, the extent to which this re-expression occurs compared with normal nerves has not previously been quantified.
NCAM expression in distal stumps after axotomy was not significantly increased above control levels in our study. Previous experiments suggested weak staining for NCAM in mouse and chicken sciatic nerve 3 days after axotomy and a more intense staining by 10 days (Daniloff et al. 1986). It is possible that NCAM levels in the distal stump may peak at 10 days and begin to decline by 15 days, accounting for the discrepancy observed in our findings. However, in our study axotomized nerve stumps were isolated, threreby preventing any axonal regeneration into the distal stump, in contrast to previous studies in which axotomized stumps were in close proximity with possible axonal re-growth into the distal stump. Regenerating axons may not affect Schwann cell proliferation in rat distal stumps in vivo (Siironen et al. 1994) in contrast to that seen in vitro (Dent et al. 1992), and therefore the increase in NCAM expression in distal stumps seen in experiments by Daniloff et al. (1986) may be attributed to NCAM-positive axons regenerating into the distal stump. Increased NCAM mRNA has been observed by Northern blotting in distal stumps of mouse sciatic nerves after axotomy (Tacke & Martini, 1990a); however, it was unclear whether the production of NCAM mRNA originated from Schwann cells or inflammatory cells, or indeed whether active regeneration into the distal stump was permitted. In addition, a rise in NCAM mRNA would not necessarily lead to increased NCAM protein expression.
Nine days after axotomy N-cadherin mRNA is up-regulated in the distal stumps of mouse sciatic nerves during Wallerian degeneration, especially by myelinating Schwann cells after myelin degradation (Padilla et al. 1999). This is consistent with our findings showing that N-cadherin levels dramatically increased in distal stumps 15 days after axotomy. It is also interesting to note that the high expression levels in distal stumps seen here might correlate with the results of recent studies in the chicken CNS, which found evidence that N-cadherin, in combination with other cadherins, may be important in targeting axons to specific fibre tracts in the optic tectum (Treubert-Zimmermann et al. 2002).
Expression of NCAM and N-cadherin after crush injury and during regeneration into the distal stump
In contrast to axotomy, after crush injury the basal lamina remains intact providing guidance for active regeneration to occur. By exploiting the difference between the two models of injury we show increased NCAM expression in distal stumps of crushed nerves compared with those after axotomy. This may be attributable to the presence of regenerating fibres as they progress from the proximal stump through the crush site and enter the distal stump at different time points. In our crush injury model, by staining with PGP9.5 we demonstrate the presence of regenerating axons in distal stumps coinciding with increased NCAM levels in the distal stump, which may be attributable to increased axon–Schwann cell contacts. The presence of only a few regenerating fibres in the distal stump 15 days after crush injury is unusual as previous studies (Bajrovic et al. 2002; Kvist et al. 2003) have shown the presence of regenerating fibres in this area 6 days after crush injury. However, previous studies used a crushing instrument that gave an injury approximately 0.5 mm in length, in contrast to the 3-mm crush produced in this study; hence, the pressure produced by the instrument used in our study may be greater and more destructive. Increasing the crush area and pressure may be responsible for increasing the time taken for axons to regenerate through the injured area. In addition, in previous studies the sensory pinch test was used as an outcome measure as opposed to the PGP9.5-positive immunostaining used here, and therefore the different outcome measures may produce different results. It is also interesting that high levels of NCAM were observed proximal to the injury site after 15 days when the regeneration front would have passed through. This suggests that NCAM may persist in the axon after injury as the process of myelination, during which NCAM is down-regulated (Jessen et al. 1987), is not yet complete.
For N-cadherin a peak of expression was observed 15 days after crush in all nerve sites under study. Proximal to the crushed area this increase would correlate with recent in vitro results showing that N-cadherin has a role in directing the extension of Schwann cell processes on axons and in the initial stages of axon–Schwann cell interactions (Wanner & Wood, 2002). After 30 days the levels of N-cadherin in the proximal stump declined, although to levels still higher than controls, which may be consistent with in vitro co-culture studies in which the intensity of N-cadherin immunostaining decreased after neurites and Schwann cells made contact (Wanner & Wood, 2002). In crushed and distal segments it has been shown that dedifferentiating myelinating Schwann cells express maximum levels of N-cadherin mRNA at 9 days after transection (Padilla et al. 1999), which would corroborate our results, assuming that the regulation of cadherin mRNA and protein expression is similar. Levels in the crushed and distal regions declined after 30 days, consistent with a drop in N-cadherin mRNA at 21 days after transection that is concomitant with the establishment of bands of Büngner and an end to the period of myelin degradation (Padilla et al. 1999). It is notable that N-cadherin levels were significantly higher in distal stumps after axotomy than after crush, which correlates with in vitro studies described above (Wanner & Wood, 2002), whereby a re-establishment of axon–Schwann cell contacts may contribute to the decline in expression levels.
In summary, we have shown that quantitative immunohistochemistry can be a useful tool for the assessment of adhesion molecule expression. Here we confirm the descriptions of other groups regarding expression after injury, and by exploiting different models of injury in specific anatomical regions we also provide evidence for changes of expression induced by regeneration into the distal stump.
Acknowledgments
We wish to thank The Wellcome Trust for supporting Martin Thornton on a Research Training Fellowship.
References
- Bajrovic FF, Sketelj J, Jug M, Gril I, Mekjavic IB. The effect of hyperbaric oxygen treatment on early regeneration of sensory axons after nerve crush in the rat. J. Peripher. Nerv. Syst. 2002;7:141–148. doi: 10.1046/j.1529-8027.2002.02020.x. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Lilien J, Reichardt LF. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J. Cell Biol. 1988;107:353–361. doi: 10.1083/jcb.107.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Diaz C, Nicolet M, Goudou D, Rieger F, Mege RM. N-cadherin expression in developing, adult and denervated chicken neuromuscular system: accumulations at both the neuromuscular junction and the node of Ranvier. Development. 1994;120:1–11. doi: 10.1242/dev.120.1.1. [DOI] [PubMed] [Google Scholar]
- Daniloff JK, Levi G, Grumet M, Rieger F, Edelman GM. Altered expression of neuronal cell adhesion molecules induced by nerve injury and repair. J. Cell Biol. 1986;103:929–945. doi: 10.1083/jcb.103.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Ida JAJ, Yoshino JE. Isolated growth cones stimulate proliferation of cultured Schwann cells. Glia. 1992;5:105–111. doi: 10.1002/glia.440050204. [DOI] [PubMed] [Google Scholar]
- Doherty P, Walsh FS. Neurite guidance molecules. Curr. Opin. Cell Biol. 1989;1:1102–1106. doi: 10.1016/s0955-0674(89)80057-2. [DOI] [PubMed] [Google Scholar]
- Honig MG, Kueter J. The expression of cell adhesion molecules on the growth cones of chick cutaneous and muscle sensory neurons. Dev. Biol. 1995;167:563–583. doi: 10.1006/dbio.1995.1049. [DOI] [PubMed] [Google Scholar]
- Ide C. Peripheral nerve regeneration. Neurosci. Res. 1996;25:101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, Morgan L. Myelinated, but not unmyelinated axons, reversibly down-regulate N-CAM in Schwann cells. J. Neurocytol. 1987;16:681–688. doi: 10.1007/BF01637659. [DOI] [PubMed] [Google Scholar]
- Kvist M, Danielsen N, Dahlin LB. Effects of FK506 on regeneration and macrophages in injured rat sciatic nerve. J. Peripher. Nerv. Syst. 2003;8:251–259. doi: 10.1111/j.1085-9489.2003.03021.x. [DOI] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA, Roche FK, Takeichi M, Lemmon V. Nerve growth cone migration onto Schwann cells involves the calcium-dependent adhesion molecule, N-cadherin. Dev. Biol. 1990;138:430–442. doi: 10.1016/0012-1606(90)90209-2. [DOI] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J. Cell Biol. 1986;103:2439–2448. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and myelin-associated glycoprotein) in regenerating adult mouse sciatic nerve. J. Cell Biol. 1988;106:1735–1746. doi: 10.1083/jcb.106.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR, Schachner M, Goridis C. Distribution of the adhesion molecules N-CAM and L1 on peripheral neurons and glia in adult rats. J. Neurocytol. 1986;15:799–815. doi: 10.1007/BF01625196. [DOI] [PubMed] [Google Scholar]
- Nieke J, Schachner M. Expression of the neural cell adhesion molecules L1 and N-CAM and their common carbohydrate epitope L2/HNK-1 during development and after transection of the mouse sciatic nerve. Differentiation. 1985;30:141–151. doi: 10.1111/j.1432-0436.1985.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Padilla F, Marc MR, Sobel A, Nicolet M. Upregulation and redistribution of cadherins reveal specific glial and muscle cell phenotypes during wallerian degeneration and muscle denervation in the mouse. J. Neurosci. Res. 1999;58:270–283. [PubMed] [Google Scholar]
- Redies C, Engelhart K, Takeichi M. Differential expression of N- and R-cadherin in functional neuronal systems and other structures of the developing chicken brain. J. Comp. Neurol. 1993;333:398–416. doi: 10.1002/cne.903330307. [DOI] [PubMed] [Google Scholar]
- Redies C. Cadherins in the central nervous system. Prog. Neurobiol. 2000;61:611–648. doi: 10.1016/s0301-0082(99)00070-2. [DOI] [PubMed] [Google Scholar]
- Rieger F, Daniloff JK, Pincon-Raymond M, Crossin KL, Grumet M, Edelman GM. Neuronal cell adhesion molecules and cytotactin are colocalized at the node of Ranvier. J. Cell Biol. 1986;103:379–391. doi: 10.1083/jcb.103.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilheimer B, Schachner M. Studies of adhesion molecules mediating interactions between cells of peripheral nervous system indicate a major role for L1 in mediating sensory neuron growth on Schwann cells in culture. J. Cell Biol. 1988;107:341–351. doi: 10.1083/jcb.107.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilheimer B, Persohn E, Schachner M. Neural cell adhesion molecule expression is regulated by Schwann cell–neuron interactions in culture. J. Cell Biol. 1989;108:1909–1915. doi: 10.1083/jcb.108.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya Y, Mizoguchi A, Takeichi M, Shimada K, Ide C. Localization of N-cadherin in the normal and regenerating nerve fibers of the chicken peripheral nervous system. Neuroscience. 1995;67:253–261. [Google Scholar]
- Siironen J, Collan Y, Röyttä M. Axonal reinnervation does not influence Schwann cell proliferation after rat sciatic nerve transection. Brain Res. 1994;654:303–311. doi: 10.1016/0006-8993(94)90492-8. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Involvement of the nerve growth factor-inducible large external glycoprotein (NILE) in neurite fasciculation in primary cultures of rat brain. Proc. Natl Acad. Sci. USA. 1985;82:1276–1280. doi: 10.1073/pnas.82.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke R, Martini R. Changes in expression of mRNA specific for cell adhesion molecules (L1 and NCAM) in the transected peripheral nerve of the adult rat. Neurosci. Lett. 1990a;120:227–230. doi: 10.1016/0304-3940(90)90045-b. [DOI] [PubMed] [Google Scholar]
- Tacke R, Martini R. Changes in expression of mRNA specific for cell adhesion molecules (L1 and NCAM) in the transected peripheral nerve of the adult rat. Neurosci. Lett. 1990b;120:227–230. doi: 10.1016/0304-3940(90)90045-b. [DOI] [PubMed] [Google Scholar]
- Takei K, Chan TA, Wang FS, Deng H, Rutishauser U, Jay DG. The neural cell adhesion molecules L1 and NCAM-180 act in different steps of neurite outgrowth. J. Neurosci. 1999;19:9469–9479. doi: 10.1523/JNEUROSCI.19-21-09469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe K, Tanaka HF, Takahashi A, Awaya A, Hashimoto K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp. Neurol. 1996;137:301–308. doi: 10.1006/exnr.1996.0030. [DOI] [PubMed] [Google Scholar]
- Treubert-Zimmermann U, Heyers D, Redies C. Targeting axons to specific fiber tracts in vivo by altering cadherin expression. J. Neurosci. 2002;22:7617–7626. doi: 10.1523/JNEUROSCI.22-17-07617.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB, Wood PM. N-cadherin mediates axon-aligned process growth and cell–cell interaction in rat Schwann cells. J. Neurosci. 2002;22:4066–4079. doi: 10.1523/JNEUROSCI.22-10-04066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]