Abstract
The anuran pelvic girdle is unique among all amphibians in that its acetabular portion is located far posterior to the sacrum, lateral to the postsacral (= caudal) vertebral column, which is reduced to a single rod-like element called the urostyle. This situation in the adult is strikingly different not only from that in ancestral temnospondyls but also in other modern amphibians. Because there is no fossil that would document this evolutionary anatomical modification except for Triadobatrachus, the only data may be inferred from development in modern anurans. We chose seven anuran species (belonging to the genera Discoglossus, Bombina, Pelobates, Bufo, Rana and Xenopus), representing the principal locomotory types (saltation, swimming, crawling and burrowing). Development of the pelvic girdle was studied on cleared and stained whole mounts and partly on serial histological sections. The basic developmental pattern was similar in all species: the pelvis on both sides develops from two centres (puboischiadic and iliac, respectively). The ilium then extends vertically towards the sacral vertebra and later rotates posteriorly so that ultimately the acetabulum is lateral to the tail (= urostyle). Only minor deviations from this pattern were found, mainly associated with differences in water and terrestrial dwelling.
Keywords: Anura, development, evolution, pelvic girdle, postcranial skeleton
Introduction
The anuran pelvis, although retaining the basic triradiate pattern of temnospondyl amphibians, deviates from this original pattern in that the ilium is elongated into a shaft located horizontally and parallel to the urostyle. A comparatively well-developed iliac shaft was already present in the Early Triassic proanurans Triadobatrachus and Czatkobatrachus (Rage & Roček, 1989; Evans & Borsuk-Białynicka, 1998) but it has never been recorded in any temnospondyl amphibian. It may be inferred from the nearly completely articulated skeleton of Triadobatrachus that the characteristic shape of the anuran ilium evolved by elongation of the bone posteriorwards from the level of the ilio-sacral articulation.
Two interesting questions concerning the anuran pelvis arise, namely why the ilium expanded posteriorly so that the acetabular joint is located lateral to the former tail, and why the ischium and pubis were so strongly reduced (the latter even remains cartilaginous in the majority of anurans). It has been suggested that these changes were associated with saltatory locomotion (Noble, 1931; MacBride, 1932; Gadow, 1933; Reig, 1957; Eaton, 1959; Gans & Parsons, 1966). Yet Triadobatrachus, the ilia of which were already moderately elongated, was surely not yet capable of jumping (Roček & Rage, 2000). Griffiths (1963) suggested that the peculiar morphology of the anuran pelvis evolved due to a swimming mechanism. However, it is hard to imagine why swimming would be associated with shortening of the presacral column only in frogs, whereas in other water-dwelling amphibians, such as newts, this part of the skeleton remained unaffected. It should also be noted that the posterior shift of the acetabular region preceded reduction of the tail; the comparatively late origin of the urostyle is not only evidenced by Triadobatrachus but also by its clear segmentation in metamorphosing tadpoles.
As fossils documenting the temnospondyl–salientian transition are scarce, and because they can provide only limited information concerning the origin of the anuran pelvis, this incompleteness in the palaeontological record may be supplemented by data gained from developmental morphology. Developmental processes, if properly assessed, may provide a useful tool for explanation of evolutionary transformations. Therefore, we decided to study the development of the pelvis and associated structures in various modern anurans in order to recognize some general developmental patterns. We believe that this can help to complete information on the evolutionary transformation of the pelvis, which is now available only from comparison of the terminal developmental stages of adult temnospondyls and anurans.
In order to interpret a developmental sequence correctly as an evolutionary one, it is necessary to discern which features of the development reflect the temnospondyl–salientian evolutionary transition and which are mere adaptations to a particular mode of anuran locomotion. Locomotion in anurans involves not only saltation (e.g. in Rana, Discoglossus), but also swimming (in permanent water-dwellers such as Pipidae, Palaeobatrachidae) and crawling (e.g. in Bufo). Burrowing (e.g. in Pelobates) is also a type of activity which is performed by the hind limbs.
Therefore, we followed development of the pelvis and associated structures (sacrum, urostyle and ilio-sacral articulation) in each representative of the anuran locomotory types; we then tried to find coincidences between anatomical peculiarities and a particular locomotory type (although it is known that species with, for instance, the same ilio-sacral articulation morphology may show significant differences in types of movement; see Emerson, 1979), and finally we tried to deduce some anatomical and functional evolutionary sequences that occurred between temnospondyls and their anuran descendants.
Materials and methods
For our study we chose Discoglossus pictus and both European species of Bombina, which represent a lineage of the Discoglossidae, whose roots may be found among Middle Jurassic anurans (Evans et al. 1990). This lineage involves both aquatic/terrestrial (Discoglossus) as well as predominantly aquatic (Bombina) forms. Mostly terrestrial toads, which move by crawling rather than by saltation, are represented by Bufo bufo. A similar type, but capable of burrowing with the help of hind legs, is Pelobates fuscus. Terrestrial frogs with extremely well-developed saltatory locomotion are represented by Rana dalmatina. Permanent water-dwellers are represented by Xenopus laevis. Therefore, four principal locomotory types of frogs are represented – terrestrial saltatory, terrestrial crawler, terrestrial burrower and permanent water-dweller. To avoid duplicating their descriptions, we give a full description of the development of Discoglossus pictus, which may be considered phylogenetically the most promitive among them and, to a certain degree, a model species; descriptions of other species are restricted to differences from this model. Developmental series were obtained from laboratory breeding in the Department of Zoology, Charles University, Prague.
Specimens used in this study were staged according to the normal table of X. laevis recognizable by external criteria (Nieuwkoop & Faber, 1967), although considerable variation exists in the appearance of internal characteristics (see discussion in Roček, 2003). Clearing and staining of the whole mounts followed methods used by Wassersug (1976), with slight modifications of concentrations and timing. We investigated 27 cleared and stained specimens of X. laevis (stages 52–66), 130 specimens of R. dalmatina (stages 47–66), 95 specimens of Bufo bufo (stages 50–66), 112 specimens of P. fuscus (stages 44–66), six specimens of D. pictus (stages 52–66), 72 specimens of Bombina bombina (stages 50–66) and 110 specimens of Bombina variegata (stages 50–66).
Some stages of Discoglossus were also prepared as three-dimensional (3D) software reconstructions from histological sections, in order to investigate the muscular context of the pelvis. For this purpose we used 3D Studio MAX 5.0; smoothing was performed in BoneViewer, a program specially written for this purpose by Ysoft Co., Czech Republic.
All the material is deposited in the Department of Zoology, Charles University, Prague.
Results
Discoglossus pictus
The earliest structure of the pelvic girdle and associated parts of the vertebral column is a pair of neural arches of the sacral (i.e. 9th) vertebra, adjacent to the dorsolateral surface of the notochord, which may be recognized as early as stage 50 (see also Table 1). At stage 52 the neural arches of the sacral vertebra grow dorsally over the neural tube, whereas the first (and sometimes also tiny rudiments of the second) postsacral neural arches (Fig. 1H) appear simultaneously with the earliest cartilaginous rudiment of the femur. The hypochord also appears at this stage, closely following the appearance of the second postsacral vertebra. At stages 52–55, the hypochord extends between the levels of both postsacral (i.e. the 10th and 11th) vertebrae (Fig. 1J), but later (at stage 57) it expands anteriorly, so that it reaches beneath the posterior part of the sacral vertebra (Fig. 1K). Its posterior expansion is only moderately delayed, so that in stage 58 it reaches beyond the level of the most posterior extent of the fused neural arches (Fig. 1L). Its posterior end is slightly declined from the notochord and is oval, instead of semicircular, in cross-section. Because constriction of the notochord proceeds in an anterior–posterior direction, owing to developing vertebral centra, the anterior end of the hypochord approaches the bases of the sacral neural arches in stage 58, whereas it still occupies its original position posteriorly (Fig. 1L). The ventral extended parts of the postsacral neural arches fuse with each other on either side in stage 57, thus forming a pair of cartilaginous strips adjacent dorsolaterally to the notochordal sheaths (Fig. 1M,1P). From these strips the neural arches of two, occasionally three, postsacral vertebrae protrude dorsally; in stage 59 (Fig. 1M), the neural arches of the 1st postsacral vertebra do not fuse with one another, whereas the sacral vertebra is already complete dorsally. Dorsal ends of the postsacral neural arches on both sides expand anteriorly and posteriorly (Fig. 1L), which ultimately results in formation of permanent (anteriorly) or temporary (posteriorly) foramina for spinal nerves. The thickened posterior end of the hypochord approaches the fused neural arches, in accordance with reduction of the notochord.
Table 1.
Developmental chronology of the anuran pelvic skeleton expressed in Nieuwkoop & Faber (1967) stages
| Developmental event | Discoglossus pictus | Bombina bombina | Bombina variegata | Bufo bufo | Pelobates fuscus | Rana dalmatina | Xenopus laevis |
|---|---|---|---|---|---|---|---|
| SV: neural arches appear | 50–51 | 51 | 51–53 | 50 | 50 | 50–51 | 52 |
| SV: neural arches fuse with one another | 56–57 | 57 | 57 | 58 | 54 | 55–56 | 57 |
| SV: neural arches begin to ossify | 56–57 | 58 | 55 | 56 | 55 | 55–56 | 58 |
| SV: neural arches completely ossified | 58–66< | 66 | 66< | 65 | 62–63 | 66 | 62–63 |
| SV: centrum appears | 55 | 55 | 55 | 56 | 52 | 54–55 | 58 |
| SV: complete cartilaginous centrum | 56–57 | 58 | 57 | 57 | 56 | 57 | 59 |
| SV: centrum begins to ossify | 58–66< | 58 | 56–57 | 58 | 56 | 57 | 59 |
| SV: centrum completely ossified | 58–66< | 66< | 66< | 66 | 62–63 | 66< | 66< |
| SV: diapophyses appear | 55–56 | 56 | 55 | 56 | 53 | 55–56 | 57–58 |
| SV: final shape of diapophyses | 58–66< | 62 | 61 | 64 | 63 | 61 | 62–63 |
| SV: diapophyses begin to ossify | 58–66< | 62 | 58–59 | 58 | 56 | 61 | 59 |
| SV: diapophyses completely ossified | 58–66< | 66< | 66< | 66 | 62–63 | 66< | 66 |
| U: neural arches of 10th vertebra appear | 52 | 52 | 53 | 54–56 | 51–52 | 53–55 | 52 |
| U: neural arches of 10th vertebra fuse | 56–57 | 57 | 57–58 | 59–62 | 57 | 62 | 58 |
| U: centrum of 10th vertebra appears | 56 | 56 | 55 | 58 | 57–58 | 56 | 62 |
| U: diapophyses of 10th vertebra appear | 56–57 | 57 | 55 | 59–62 | 58 | – | 62 |
| U: 10th vertebra begins to ossify | 56–57 | 58 | 55–56 | 57 | 56 | 56 | 59–60 |
| U: neural arches of 11th vertebra appear | 52 | 56 | 54 | 57 | 53 | 55–56 | 52 |
| U: 11th vertebra begins to ossify | 58–66< | 59 | 56 | 62 | 62–63 | 61 | 59–60 |
| U: neural arches of 12th vertebra appear | 56–57 | 56 | 55–56 | 58 | 53 | 56 | 53 |
| U: 12th vertebra begins to ossify | 58–66< | 66 | 66< | 66< | 63–66< | 61 | 64–66 |
| U: neural arches of 13th vertebra appear | 56–57 | 58 | 58 | 58 | – | – | 54–57 |
| U: sacro-urostylar articulation develops | 56–57 | 58 | 58 | 58–59 | 61–62 | 61 | 59–60 |
| U: 10th and 11th vertebrae fuse | 56–57 | 56 | 57–58 | 58 | 60–61 | 56 | 58 |
| U: 11th and 12th vertebrae fuse | 56–57 | 57 | 57 | 57 | 53–54 | 56 | 62 |
| U: 12th and 13th vertebrae fuse | 56–57 | 58 | 58 | 58 | – | – | 63 |
| U: hypochord appears | 52 | 55 | 54–55 | 55–56 | 53 | 55 | 53 |
| U: hypochord fuses with neural arches | 58–66< | 66 | 63 | 65–66 | 64–66< | 66 | 66 |
| U: ossification complete | 58–66< | 66< | 66< | 66< | 63–66< | 66< | 62–66< |
| IL: ilia appear | 54 | 54 | 54 | 54 | 53 | 54–55 | 54 |
| IL: rotation begins | 55 | 57 | 57 | 57 | 55 | 55 | 55 |
| IL: ossification of ala begins | 55–56 | 56 | 55–56 | 56 | 56 | 55–56 | 57–58 |
| IL: ilio-sacral contact established | 66 | 58 | 57–58 | 58–59 | 62–63 | 57 | 60 |
| IL: ossification of corpus ilii begins | 57–58 | 58 | 60 | 58–59 | 64 | 58–61 | 61–62 |
| IL: ossification complete | 58–66< | 66< | 66< | 66 | 66–66 | 66< | 66–66< |
| IS: ischia appear | 54 | 55 | 54 | 54 | 53 | 55 | 53 |
| IS: ischia in touch | 56–57 | 57 | 57–58 | 56 | 56 | 57 | 59 |
| IS: full contact of medial surfaces | 58–66< | 63 | 64–66 | 58–59 | 65 | 63 | 63 |
| IS: ossification begins | 58–66< | 66 | 66 | 64 | 62–63 | 63 | 59 |
| IS: ossification complete | 58–66< | 66< | 66< | 66 | 63–66< | 66< | 66–66< |
| P: pubes appear | 54 | 55 | 54 | 54 | 53 | 54–55 | 53 |
| P: pubes fuse with ilia | 54 | 55 | 54 | 54 | 53 | 55 | 53 |
| P: pubes in touch | 57–66< | 58 | 62–63 | 58 | 62–63 | 61 | 62 |
| P: full contact of medial surfaces | 58–66< | 63 | 66 | 62 | 65 | 63 | 63 |
| P: praepubes (epipubes) appear | 56–57 | 56 | – | – | – | – | 59 |
| P: ossification of pubes begins | – | – | – | – | – | – | 63 |
| P: ossification of pubes complete | – | – | – | – | – | – | 66–66< |
Abbreviations: SV, sacral vertebra; U, urostyle; IL, ilium; IS, ischium; P, pubis; 66< indicates post-metamorphic stages.
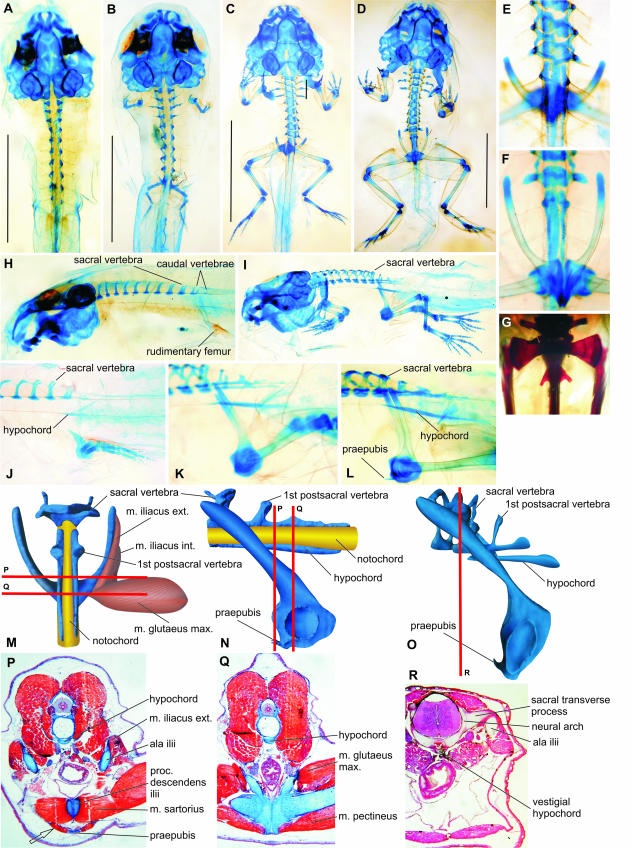
Fig. 1.
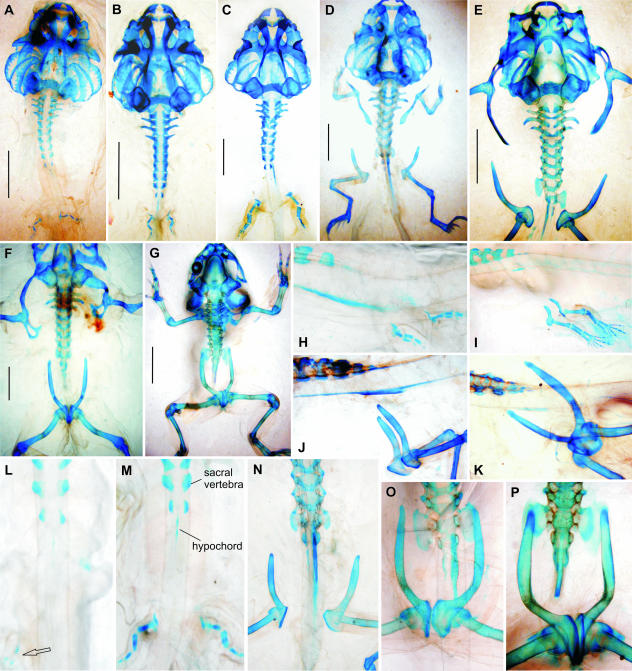
Discoglossus pictus. Note early fusion of both halves of the pelvis (B) and presence of the praepubis (L,P). (A–H) Stage 52; (B–J) stage 55; (C, E, I, K) stage 57; (D, F, L1) stage 58; (G) adult. (A–D, E, G, M in dorsal view; F, ventral view; H–L, N, O in left lateral view). (M) 3D model of pelvis and of some pelvic muscles at stage 59. (N) 3D model of pelvis at stage 59. (O) 3D model of pelvis at stage 66. (P, Q) Frontal sections at the levels indicated in M and N; m. rectus abdominis attached to the praepubis is marked by an arrow. (R) Frontal section at the level indicated in O. Scale bars in A–D, 5 mm.
Simultaneously with the development of the postsacral vertebral column, the hind limb develops towards its distal end. The tibia and fibula appear shortly after the femur. Only when the astragalus and calcaneus are formed, and when the neural arches of the 11th vertebra and of the hypochord may be recognized (at stage 54), do the earliest rudiments of the pelvis appear. As the early development of the pelvis is very fast and because the number of investigated specimens in these crucial developmental stages was limited, we were not able to decide which pelvic element appeared as the very first. In Bombina, which is closely related to Discoglossus, although affected by heterochrony (retarded skeletogeny), the ilium appears first (see below). By the end of stage 54 the complete vertical pelvic rod is present, and the ilium begins to expand dorsally into a shaft (ala ossis ilii) in the direction of the sacral vertebra. Moderate rotation of the pelvis starts as soon as stage 55 (Fig. 1J), still before the ilia reach the level of the sacral neural arches at stages 56–57 (Fig. 1I,K,L). The iliac shafts retain their nearly vertical position until stage 58 (Fig. 1L). The rotation accelerates at stage 59 (Fig. 1N), but the ultimate position of the pelvis is reached only in fully grown adults, whereas at the end of metamorphosis (stage 66) the angle of the iliac shafts is still about 45° (Fig. 1O). Both sacral diapophyses develop independently on the neural arches (the line of contact between both cartilages my be still discerned by the end of metamorphosis; see Fig. 1R). Both halves of the pelvic girdle, which are associated with rudiments of each extremity, begin to develop separately from one another, but come into contact with each other as early as stage 57, although the line of coalescence may be discernible until the end of metamorphosis. They establish contact first in the posterior part, by the ischia, then by the pubes. The praepubes appear in stages 56–57 (Fig. 1L) as a symmetrical pair of cartilages protruding anteriorly from the pubes (Fig. 1L,N–P). Both pubes and praepubes remain cartilaginous in adults; the definitive morphology of the ilia develops from stage 59, when the dorsal margin of the iliac shaft expands as a thin crista made first of perichondrium (Fig. 1P) that later ossifies. The ultimate morphology of the ilia is undoubtedly formed in association with large muscles. For instance, the dorsal crista on the iliac shaft and the tuber superius, which develop from the perichondrium, are areas of attachment for the iliacus externus and gluteus maximus muscles, respectively (Fig. 1P,Q).
The ilio-sacral articulation develops at about stage 60 when the dorsal portion of the iliac shaft comes into contact with the lower surface of the developing sacral diapophysis (Fig. 1R). The ilia may not reach anteriorly far beyond the sacral diapophyses, which suggests that they may slide forwards and backwards over the sacral diapophyses to a limited extent (Fig. 1D,F,G).
Simultaneously with the development and positional changes of the pelvis, the neural arches of further vertebrae develop (12th and sometimes also 13th; rudimentary arches may be recognizable as dorso-lateral extensions from the surface of the notochord). The neural arches grow towards each other below and above the spinal nerves, to make a posteriorly tapering structure pierced by one or more spinal foramina. Only later do the neural arches expand dorsally over the neural tube so that both halves gradually fuse together; simultaneously, the hypochord approaches their lateral sides because of reduction of the notochord, so that all these three elongated parts give rise to the urostyle. The urostyle of the adult retains its articulation (bicondylar, procoelous) with the sacral vertebra, and develops a pair of vestigial diapophyses declined posteriorly (Fig. 1G); a pair of spinal foramina is also preserved. The attachment of the urostyle to the sacral vertebra is movable in the adult.
Bombina variegata and B. bombina
Pelvic development in both European species of Bombina is similar to that in Discoglossus. The sacral neural arches appear at stages 51–53, and the earliest appearance of the 1st postsacral was recorded at stage 53. The very first rudiment of the pelvis is the ilium, which appears simultaneously (at stage 54) with the astragalus and calcaneus in both Bombina species (Figs 2H and 3D). It is located vertically, meeting the proximal end of the femur, which is in a horizontal position (Fig. 2H). Soon afterwards (stage 55), another chondrification centre appears below the proximal end of the femur (Fig. 3E) and fuses with the former (Fig. 3F); the latter may be identified as the ischium. Although the rate of development of the posterior limbs is similar to that in Discoglossus, development of the 2nd postsacral vertebra and of the hypochord is slightly delayed in Bombina. In addition, the hypochord appears at stages 54–55, before the 2nd postsacral may be recognized (Fig. 2A,I,J,M). The shift of the anterior end of the hypochord towards the bases of the sacral neural arches (Fig. 2K) occurs at stage 60, because of gradual constriction of the notochord in an anterior–posterior direction.
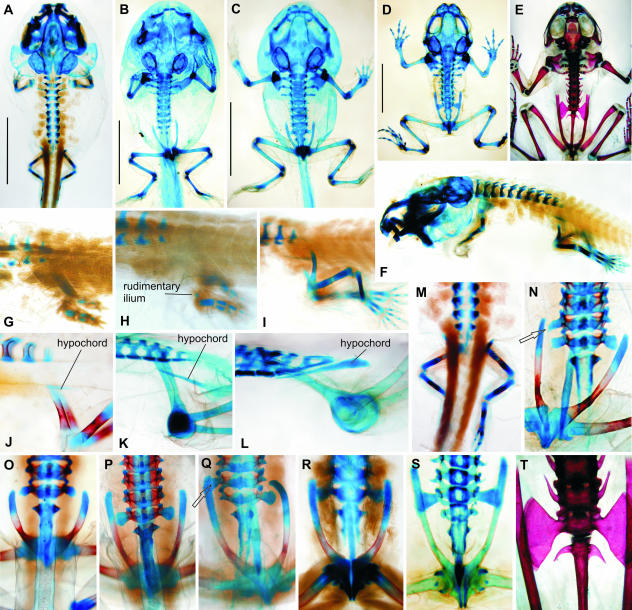
Fig. 2.
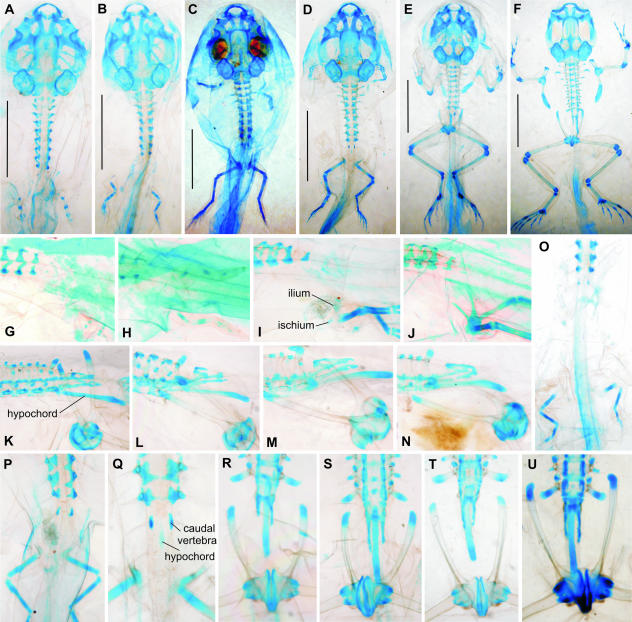
Bombina variegata. Note body outlines in B and C, and occurrence of abnormalities. (A, F, I, M) Stage 56; (B, J, O, P, Q) stage 58 (asymmetrical expanded diapophysis on presacral vertebra substituting the sacral diapophysis is marked by an arrow in Q); (C) stage 61; (D, L, S) stage 66; (E) adult; (G) stage 53; (H) stage 54; (K) stage 60; (N) stage 57; (R) stage 59; (T) adult (A–E in dorsal view; F–L in left lateral view; M–P in dorsal view; Q–T in ventral view). Scale bars in A–D, 5 mm.
Fig. 3.
Bombina bombina. Note asymmetrical development of the presacral, sacral and caudal transverse processes (L, M, P, Q, S). (A, I, J) Stage 58; (B, O) stage 64; (C, P, Q, R) stage 66; (D) stage 54; (E) stage 55; (F) stage 55; (G) stage 55–56; (H) stage 56; (K) stage 60; (L–N) stage 62; (S, T) adult (A–C, L–T in dorsal view; E, F in ventrolateral view; D, G–I in dorsolateral view). Disarticulation of the hypochord from vertebral column is an artefact. Scale bars in A–C, 5 mm.
The iliac shafts reach the level of the notochord during stage 58 (Fig. 2J), when the 2nd caudal vertebra is still absent. They begin to rotate at stage 57, and their position at the end of metamorphosis (stage 66) is the same as in Discoglossus (Fig. 2L). The tips of the iliac shafts ultimately reach the level of the last presacral vertebra, and the line interconnecting both tips is approximately the axis of iliac rotation. In normal development the shafts adjoin the developing sacral transverse processes (whose development begins at stage 57) ventrally and, owing to the position of the rotation axis, the anterior ends of the shafts reach over the anterior margins of sacral diapophyses (Fig. 2P, S). However, there is some variation in the development of the ilio-sacral articulation, which may include, in addition to the sacral diapophysis, the transverse process of the most posterior presacral vertebra (Fig. 2N), or may entirely substitute the sacral diapophysis, which is not developed (Fig. 2Q). Nevertheless, in both species of Bombina, normal development results in a considerable shift of the pelvis anteriorly so that the tips of the ilia reach anteriorly beyond the level of the sacral vertebra (Fig. 2T), and sometimes even to the level of the second presacral vertebra (Fig 2S, T and 3N, P–R). Consequently, the postsacral part of the vertebral column located between both iliac shafts is relatively short and may be elongated by the inclusion of additional postsacral vertebrae (symmetrically or asymmetrically) so that in completely developed adults the anterior tips of the shafts extend only moderately over the sacral diapophyses (compare Fig. 3T with Fig. 2T).
The pelvic development is completed (with the exception of the ilio-sacral articulation) as early as stage 58, before the front legs become apparent externally (Fig. 2B). There is, however, considerable variation in ossification degree: whereas in some individuals the ilia begin to ossify in very early stages (as early as stage 58; see Fig. 2J), the whole skeleton in others may remain cartilaginous even when their metamorphosis is completed (Fig. 2L).
Bufo bufo
The earliest rudiments of the pelvis appear at stage 54, as in Discoglossus. However, there is noticeable retardation in the development of the caudal skeleton, because neither the postsacral vertebrae nor the hypochord are developed at this stage (Fig. 4A). A short hypochord is present at stage 56 (Fig. 4F), but sometimes is still barely discernible (Fig. 4K). The ossification of the long bones starts as early as stage 57 (Fig. 4B,L), when cartilaginous neural arches of the 1st postsacral vertebra begin to develop. The neural arches of the 1st postsacral vertebra begin to ossify soon afterwards (stage 58), though before they fuse with one another above the neural tube (Fig. 4H,M). In addition, further development of the postsacral vertebral column is very slow – the pair of rudimentary (but already partly ossified) neural arches of the 1st postsacral (10th) vertebra fuses with the minute cartilaginous rudiments of the 2nd postsacral (11th) vertebra, so that an oval spinal foramen arises at stage 63 on either side (Fig. 4I). The hypochord is still entirely cartilaginous at stage 63 and is well separated from postsacral neural arches, although the sacral and praesacral vertebrae have well-developed and largely ossified arches (Fig. 4I). Development in the concluding stages of metamorphosis mainly concerns the hypochord, which fuses dorsolaterally with the extended ventral bases of the neural arches, and the degree of ossification; at stage 66 (Fig. 4J,P) the ossification of the pelvis and corresponding part of the vertebral column is nearly complete, although the ischia are still separated from the ilia by cartilage (Fig. 4P).
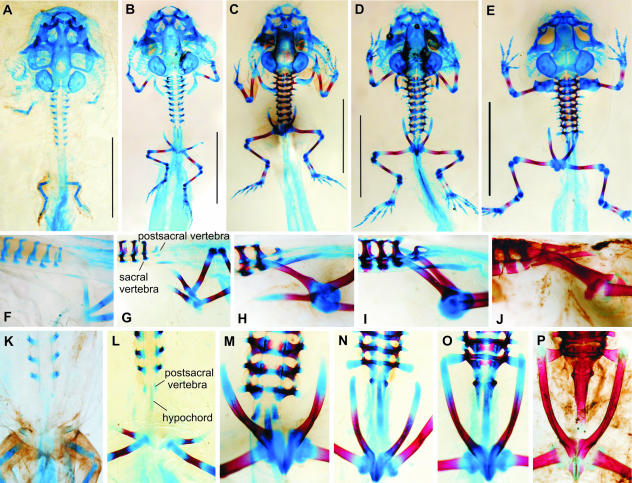
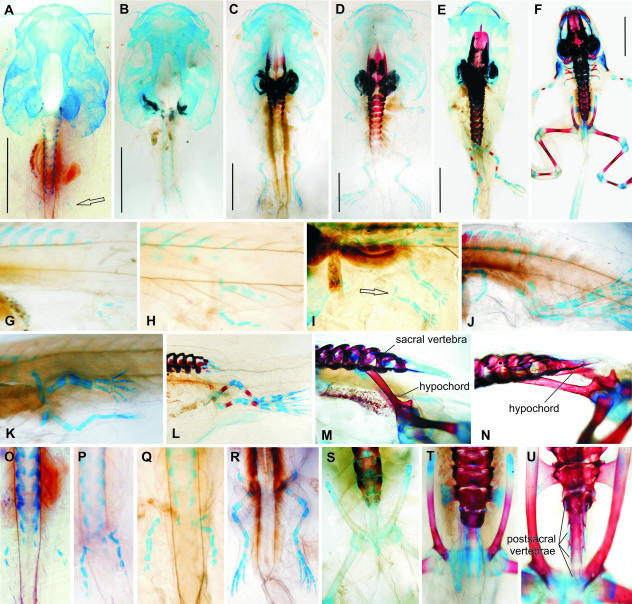
Fig. 4.
Bufo bufo. Note the early onset of ossification (B, L) and retardation in development of the urostyle. (A, K) Stage 56; (B, G, L) stage 57; (C, H, M) stage 58; (D) stage 58–59; (E, N) stage 62; (F) stage 56; (I, O) stage 63; (J, P) stage 66 (A–E in ventral view; F–J in left lateral view; K–P in dorsal view). Scale bars in A–E, 5 mm.
The sacral diapophyses begin to develop at stage 58 (Fig. 4M) and attain their ultimate size at stage 62 (Fig. 4N). Although during the rotation of the ilia their tips reach as far as the level of the praesacral vertebrae (Fig. 4M), the ilio-sacral contact is established (Fig. 4O) with their anterior ends (stage 63).
Pelobates fuscus
Figure 5(A,H,L) shows the rudimentary hind limbs far behind the level of the sacral vertebra. The first postsacral (10th) vertebra appears shortly after the sacral and nearly simultaneously with the hypochord (stage 53). Both units (axial skeleton and posterior extremity) develop independently for a long period. Although the quickly developing pelvic girdle with the extremity soon moves (at stage 56) beneath the hypochord (Fig. 5J), the iliac shafts are still short and thus widely separated from the sacral vertebra. Nearly the whole posterior rotation of the ilia proceeds far from the sacral diapophyses (which are already developing at stage 56;Fig. 5N). The ilio-sacral contact is established only at stage 63 (Fig. 5O). In addition, both halves of the pelvic girdle remain widely separated for a long time, coming into contact with each other only at stage 62 (Fig. 5F). The hypochord and the neural arches of the two postsacral elements are separate from each other, and they fuse together only at stage 65 (Fig. 5P). The urostyle involves two postsacral vertebrae and develops an immovable articulation with the sacral vertebra (later both coalesce with each other). The urostyle is comparatively short and does not reach the level of fused ilia.
Fig. 5.
Pelobates fuscus. Note the distance between the early rudiments of the posterior limbs and the sacral vertebra (A, L) and late fusion of both halves of the pelvis (E, N). Note also asymmetric development of the sacrum (E). (A, B, H, L, M) Stage 53; (C, I) stage 54; (D, J, N) stage 56; (E) stage 60–61; (F, K) stage 62; (G, P) stage 65 (rudimentary femur, tibia and fibula are marked by an arrow); (O) stage 63 (A in ventral view; B–F in dorsal view; H–K in left lateral or ventrolateral views; L–P in ventral view). Scale bars A–F 5 mm.
Rana dalmatina
The neural arches of the sacral (9th) vertebra appear at stages 50–51. The axial skeleton consisting of nine pairs of neural arches persists until stage 55. The 1st postsacral vertebra may still be absent (Fig. 6A,B) or may be present as a pair of tiny cartilaginous rudiments. The first rudiment of the hind limb appears at stage 54, beginning with the femur, then extending distally as tibia and fibula and astragalus and calcaneus (at stage 55; Fig. 6A,B,O). The ilia appear at stage 55, soon followed by the ischia (Fig. 6I). The hypochord appears at stage 56, simultaneously with the second postsacral vertebra; at this stage the iliac shafts extend dorsally to almost reach the notochord (Fig. 6J). The ilia continue their dorsal expansion so that their tips reach the level of (but do not contact with) the developing sacral diapophyses (Fig. 6K). It is only at this stage (stage 62) that the iliac shafts begin to rotate posteriorly. During the rotation the tips of the iliac shafts never exceed the level of the sacral diapophyses anteriorly (Fig. 6R–Fig. 6T). The ilio-sacral articulation is established later than in stage 66, only after completion of metamorphosis (Fig. 6U).
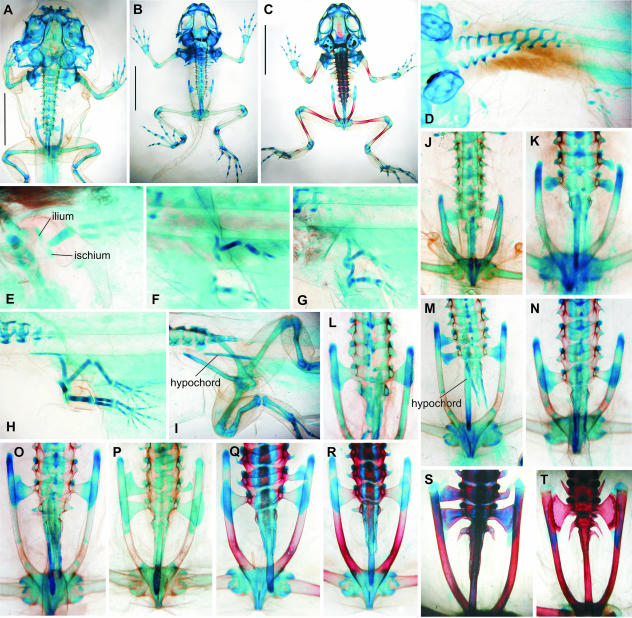
Fig. 6.
Rana dalmatina. Note retardation of the development of the caudal skeleton, as in Bufo, and late constitution of the ilio-sacral articulation. (A, B, I, O, P) stage 55; (C, J) stage 55–56; (D, E, Q) stage 57; (F, K, S) stage 62; (G) stage 54; (H) stage 54–55; (L) stage 63; (M) stage 63–64; (N, U) stage 66; (R) stage 61; (T) stage 64 (A–F in dorsal view; G–N in left lateral view; O–U in dorsal view). Scale bars in A–F, 5 mm.
Development of the postsacral vertebral column is delayed; both neural arches of the postsacral vertebra fuse with one another above the neural tube, but their bases remain paired and only moderately expand posteriorly. This situation (including independent hypochord) remains unchanged through the end of metamorphosis (Fig. 6U). The firm synchondrotic articulation of the iliac shafts with the sacral diapophyses develops only in fully grown adults.
Xenopus laevis
The sacral vertebra appears at stage 52, closely followed by the 1st postsacral (later at stage 52), rarely also with a tiny asymmetrical rudiment indicating early development of the 11th vertebra. Hind limbs appear at stage 53 (Fig. 7A,G,O); they are located closely behind the level of the first postsacral vertebra. The earliest rudiment of the pelvic girdle may be discerned at stage 53, below the proximal end of the femur; during stage 54 another ossification centre appears, slightly elongated and vertical in position, above its proximal end (Fig. 7H). The former may be identified as the ischium, the latter as the ilium. Both remain separated from one another, even as the ilium begins to expand dorsally (stage 55; Fig. 7I). The iliac shafts still retain their nearly vertical position at stage 60, when they begin to ossify in their middle parts (Fig. 7L). Subsequent rotation of the pelvis and ossification of the ilium and femur at stages 62 and 63 are accompanied by their anterior shift (Fig. 7M,T). This, together with the developing sacral diapophyses (at stage 63), results in the formation of the ilio-sacral articulation, in which the middle part of the iliac shaft comes into contact with the lower surface of the sacral diapophysis; consequently, the shafts extend over the anterior edge of the sacral diapophyses at stage 66 (Fig. 7N,U).
Fig. 7.
Xenopus laevis. The pelvis develops below the sacral and praesacral vertebrae, which is probably due to sliding function of the ilio-sacral articulation in adults of water-dwellers (T, U). Note also the segmentation of the urostyle (U). (A, G, O) Stage 53 (rudiments of the posterior limb marked by arrow); (B, H, P) stage 54; (C, J, R) stage 57–58; (D) stage 59; (E, L, S) stage 60; (F, M, T) stage 63; (I) stage 55 (bipartite rudiment of the pelvic girdle marked by arrow); (K) stage 58; (N, U) stage 66; (Q) stage 57 (A, C–F in dorsal view; B in ventral view; G–N in left lateral or ventrolateral views; O, S–U in dorsal view; P–R in ventral view). Scale bars in A–F, 5 mm.
The postsacral part of the vertebral column remains arrested in its development (only two pairs of small neural arches being present) until stage 60, when the sacral vertebra and whole presacral vertebral column is already extensively ossified (Fig. 7L). It is only at stage 62 when the hypochord appears (only in a single specimen among all investigated), whereas at stage 63 it was found in all others, in most of them being partly ossified (in the anterior part). Notably, the pubes may begin to ossify at stage 63 but it is not until the end of metamorphosis (and sometimes even later) that they are completely ossified. At stage 63, as well, the neural arches on each side fuse into a pair of elongated structures pierced by spinal foramina; both these structures are interconnected only at the level of the first postsacral, which is ossified, the more posterior parts (still cartilaginous) being separated from each other as well as from the hypochord (Fig. 7M,T). The identity of four postsacral vertebrae may still be recognized in the nearly completely ossified urostyle (stage 66), as preserved paired chondrifications separating spinal foramina (Fig. 7U). The urostyle then coalesces with the sacral vertebra to form a single unit in the adult.
Discussion
It appears that in all anurans the earliest rudiments of the pelvic girdle are present in a form of two chondrification centres. According to Green (1931), the ventral rudiment of the early anuran pelvis is the pubo-ischiadic portion and the dorsal rudiment is the iliac portion. From this interpretation it may be deduced that the ventral portion is homologous with the pubo-ischiadic plate of amphibian piscine ancestors (exemplified by Eusthenopteron), from which the ilium expanded only later during the transition on to dry land (although the pelvic element of this Devonian fish was also interpreted as consisting of the pubic and iliac regions, with the ischiadic part absent; compare Jarvik, 1980 and Andrews & Westoll, 1970).
These two parts (iliac and pubo-ischiadic) arise independently from each other (Fig 3E and 7I), either at the same time or one shortly after the other (either with the ilium preceding the pubis–ischium, or vice versa; this seems to be a matter of individual variation). It may be of some interest that in further development, the middle part of the ilium ossifies earlier than the ischium; this suggests that the ossification sequence within the pelvic region does not reflect the evolutionary sequence. This seems to be confirmed by the considerable intraspecific variation in the rate of ossification (compare Fig. 2J and 2L).
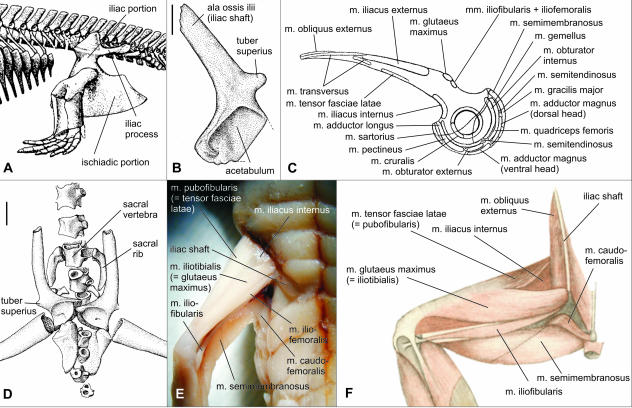
A striking feature of the development of the anuran pelvis is that although the ilium is originally in a vertical position, it soon begins to rotate posteriorly, and ultimately it attains a position that is nearly parallel with the caudal part of the vertebral column. It is obvious that the vertical position corresponds to the situation in early amphibians, in which the blade-like part was perpendicular to the vertebral column (Jarvik, 1996; Fig. 8A). The prominent rod-like iliac process (‘postiliac process’) located close to the acetabulum and directed posteriorly is also present in other primitive amphibians such as Acanthostega, Tulerpeton and Whatcheeria (Lebedev & Coates, 1995; Coates, 1996; Bolt & Lombard, 2000; Smithson, 2000), in some anthracosaurs (Smithson, 2000) and in the oldest microsaurs (Carroll, 2000). This slender outgrowth is located at the same place as the tuber superius in anurans, in which its lateral surface serves as an insertion area for the m. glutaeus maximus (Fig. 8C). In early development of the anuran pelvis, the tuber superius is directed posteriorly as in the early amphibians; only later does it attain the dorsal position as a consequence of the rotation of the pelvis. One may suppose that the developmental rotation of the pelvis reflects the evolutionary sequence of anatomical interstages during the temnospondyl–anuran transition.
Fig. 8.
(A) Resconstruction of the pelvis in the early amphibian Ichthyostega from the Middle/Late Devonian in left lateral view (from Jarvik, 1996). (B) Left ilium of the pro-anuran Czatkobatrachus from the Early Triassic in presumably original position; left lateral view; scale bar, 1 mm (from Evans & Borsuk-Białynicka, 1998). (C) Left ilium of the modern anuran Rana with indication of muscle origins; left lateral view (from Gaupp, 1896). (D) Pelvis of the pro-anuran Triadobatrachus massinoti from the Early Triassic in ventral view. Both ilia are disarticulated and exposed in lateral aspect. Note presence of free sacral and first postsacral ribs; scale bar, 5 mm (from Rage & Roček, 1989). (E) Superficial thigh muscles in the neotenous tailed amphibian Ambystoma in dorsolateral view. (F) Superficial thigh muscles of Rana in dorsal view (from Gaupp, 1896). Note similar muscle patterns in E and F, although orientation of the iliac shaft is markedly different.
The question arises as to whether thigh muscles were affected by the iliac rotation. The pattern of anuran thigh musculature is rather uniform, although some limited variation may be observed (e.g. Dunlap, 1960; Griffiths, 1963; Limeses, 1964; Cannatella, 1985). The tuber superius is always a place of origin of the m. glutaeus maximus (inserting on to the inner surface of the fascia lata on the dorsal surface of the distal end of the femur) and of two others originating by a single tendon in adults, immediately posterior to the former (Gaupp, 1896; Fig. 8C): m. iliofibularis (inserting on the inner posterior surface of the fibular head of the tibiofibula) and m. iliofemoralis (inserting on the inner surface of the femur).
Because the origin of the m. glutaeus maximus in frogs is located dorsally and anteriorly from the acetabulum, this muscle is principally a flexor of the thigh (together with m. tensor fasciae latae and m. cruralis), but it is also responsible for extension in the knee joint (it is inserted on to the inner surface of the fascia lata extending over the knee joint). Because of positional changes of the ilium in the temnospondyl–anuran transition, it was excluded from the group of thigh extensors (principally mm. semimembranosus, gracilis major et minor, and adductor magnus) taking part in saltation and swimming. Another change associated with the elongation and posterior rotation of the iliac shaft was a shift of the origin of the tensor fasciae latae muscle (pubofibularis muscle in tailed amphibians) from the pubis onto the iliac shaft (Fig. 8C), which, together with the positional change of the glutaeus maximus muscle, improved the efficiency of these thigh flexors. No other substantial change in the arrangement of the thigh muscles occurred as a consequence of re-orientation of the iliac shaft (compare Fig. 8E and 8F). M. iliofibularis remained a flexor of the knee joint (acting similarly to the sartorius on the ventral side of the thigh), thus not belonging among those muscles responsible for jumping. M. iliofemoralis, originating together with m. iliofibularis, is a thin muscle acting parallel with iliacus internus, i.e. as the flexor of the thigh.
Apparently the original thigh muscle pattern of the temnospondyls was a satisfactory prerequisite for later saltation and swimming of the anurans (even burrowing did not affect the proximal hind-limb muscles, although it causes a decrease in jumping ability; Emerson, 1976). In frogs, both types of locomotion and all types of functional patterns of the thigh and ilio-sacral musculatures are largely the same (Emerson & De Jongh, 1980), although it seems that swimming by ‘jumping’ movements was acquired only secondarily – there would not be a need to change the anatomical pattern associated with swimming by means of lateral body undulations if there was not an intermediate terrestrial stage between the temnospondyls and anurans. In this context, the posterior rotation and elongation of the iliac shaft was an evolutionary process (compare in Fig. 8A, 8B and 8C) that was not accompanied by profound changes in the thigh musculature pattern. Besides, the fact that in frogs the tuber superius became an insertion area exclusively for flexors suggests that the tuber is not associated with jumping as is sometimes believed. It may rather be a vestigial iliac process that was developed for insertion of iliocaudal muscles in swimming tailed amphibians. This would also explain the considerable size of the tuber superius in proanuran Triadobatrachus and Czatkobatrachus (Rage & Roček, 1989; Evans & Borsuk-Białynicka, 1998; Fig. 8B), although they were still not capable of jumping, and its comparatively small size in the earliest frog Prosalirus (Jenkins & Shubin, 1998) and in good jumpers such as Rana.
Although the basic developmental pattern of the pelvic skeleton is similar in the anuran species studied here, there is some positional, chronological and structural variation, as well as variation in ossification rate. In Discoglossus and Bombina, the pelvis develops beneath the first postsacral (10th) vertebra, the ilium expands nearly vertically, and only when reaching the level of the dorsal surface of the notochord does it begin to rotate. The same holds true for Xenopus and also for Ascaphus (Van Dijk, 1959, 1960); early in development their pelvises lie close behind the level of the sacral vertebra. In contrast, in Bufo, Rana and especially in Pelobates the pelvis arises far posterior, behind the level of anterior postsacrals and hypochord. Only when the ilia begin to expand dorsally is the whole pelvis shifted anteriorly. In Pelobates, moreover, this isolated development of the pelvis from the axial skeleton is accompanied by an extremely long period of independent development of both halves of the pelvis (until both ilia attain nearly their ultimate size;Fig. 5O). Positional relations between the developing pelvis and axial skeleton may be associated with the type of ilio-sacral articulation in the adult. If the ilia reach anteriorly over the fluted sacral diapophyses, which seems to be the case with permanent or prevailing water-dwellers Bombina, Xenopus (Ridewood, 1897; Van Dijk, 2002) and Palaeobatrachus (judging from specimens in which imprints of cartilaginous vertebral arches were preserved; Roček, 2003), their pelvis develops closely behind the sacral region. Both halves of the pelvis come in contact rather soon. In Discoglossus, which is both aquatic and terrestrial, the pelvis develops in a similar way as in water-dwelling Xenopus and Bombina, but adaptation for the terrestrial way of life is given by another type of the ilio-sacral articulation that is attained only in fully grown adults – the tips of the ilia become movably attached to the sacral diapophyses (they allow lateral swing or slewing; Whiting, 1961; Emerson, 1982).
The pubis, which was ossified in adults of early amphibians, and which was indistinguishable from the other two parts of the pelvis (except in some specimens of Ichthyostega; Jarvik, 1996), was separated by sutures in more advanced forms (e.g. in the Permian Archeria; Romer, 1957). However, it remained cartilaginous in many primitive amphibians. The pubis is developmentally arrested at the cartilaginous stage in the anurans too, with the exception of the Pipidae, Ascaphidae and Leiopelmatidae. In Xenopus, we observed the beginning of ossification of the pubis in metamorphosis (stage 63), but apparently there is some variation in the timing of ossification of this element (Trueb & Hanken, 1992). The pubis also ossified in Ascaphus (Ritland, 1955; Van Dijk, 1955), and in Leiopelma, where it may also be represented by calcified cartilage, or it may be cartilaginous throughout life (Stephenson, 1952; Ritland, 1955).
Ossification seems to be a matter of considerable variation even within a single species. Nevertheless, a comparatively early onset of ossification was noticed in Xenopus (as observed by Mookerjee, 1931).
Remarkable elements adjacent to both pubes are the praepubes (‘epipubes’) of Discoglossus, some Bombina bombina specimens, Ascaphus, Leiopelma (Van Dijk, 1955), Xenopus (De Villiers, 1925) and Pseudohymenochirus (Cannatella, 1985). De Villiers (1925) and Van Dijk (1959) followed the development of the praepubes in Xenopus and Ascaphus, respectively, and stated that they were strips of cartilage developed on the inner margin of the m. rectus abdominis, arising independently from the pubes.De Villiers (1934) concluded that these strips are chondrifications of the linea alba and that their synchondrotic continuity with the pubes is secondary. Their significance remains obscure (see Van Dijk, 1955, for a review of the literature). We first found cartilaginous rudiments of the praepubes in Discoglossus and Bombina bombina in stage 56, which corresponds to Ascaphus whose development was investigated from histological sections (Van Dijk, 1959).
Formation of the urostyle in the course of development attracted some interest because this structure is one of the characteristic features of the Anura. The number of postsacral (caudal) vertebrae and their developmental sequence may be deduced from the gradual appearance of the neural arches and, to a lesser extent (because of their later obliteration), also from the foramina for spinal nerves. It consists of four caudal vertebrae in Megophrys (Griffiths, 1963), three or four (10th−13th) in Xenopus (Hodler, 1949; Smit, 1953; Branham & List, 1979), three (10th−12th) in Palaeobatrachus (Špinar, 1972; Roček, 2003), three in Alytes (Hodler, 1949), three (11th−13th, and possibly also including the anterior half of the 14th vertebra) in Ascaphus Van Dijk (1960), and three in Leiopelma (Stephenson, 1951; Stephenson, 1960). It is interesting that some metamorphosing frogs (e.g. Megophrys; Griffiths, 1956, 1963) may have calcified vertebrae in their tail.
The hypochord develops simultaneously with the neural arches in an anterior–posterior direction; it is first represented by a median strip-like aggregation of fibrous connective tissue extending antero-posteriorly along the whole ventral surface of the notochord, which later (when cartilaginous 10th and 11th neural arches appear) chondrifies in its postsacral region (Mookerjee, 1931). In the Pipidae and extinct Palaeobatrachidae, the hypochord in the postsacral region ossifies independently of neural arches (Fig. 7N; see also Špinar, 1972; Roček, 2003); both coalesce with each other (and with the sacral vertebra) only in the fully grown adult. It should be noted that Hodler (1949) considered the posterior extent of the cartilaginous hypochord as a landmark indicating the posterior end of the urostyle in the adult. The caudal hypochord is interpreted as fused vestigial caudal haemal arches (basiventralia) in which segmentation vanished (Mookerjee, 1931; Mookerjee & Das, 1939); in contrast, Mahendra & Charan (1972) argued against the idea of the urostyle being formed from the caudal vertebrae.Griffiths (1963) noted, without further comment, its continuity with the first postsacral intervertebral body in Megophrys.
One of the last events in the development of the pelvis is constitution of the ilio-sacral articulation. This is due to late development of the sacral diapophyses. The sacral diapophysis takes its origin independently of the neural arch (in the present study we were able to find evidence for this on histological sections of Discoglossus, which was the only species in which we have sections through the pelvis region in all developmental stages). Ribs on the vertebrae within pectoral and even posterior praesacral regions of the vertebral column are known in adults of primitive living anurans (Ascaphus, Leiopelma, Discoglossus); in the Pipidae and Palaeobatrachidae, however, they fuse with the transverse processes only in fully grown adults (Nevo, 1968; Roček, 2003), or are present at least in early periods of larval development in Rana, Hyla and Pelobates where they later coalesce with the transverse processes (Mookerjee, 1931; Blanco & Sanchiz, 2000). As the proanuran Triadobatrachus still possessed well-developed sacral ribs (Rage & Roček, 1989; Fig. 8D), it might be supposed that anuran sacral diapophyses have the same origin.
The peculiar condition in swimming anurans (e.g. Xenopus, Palaeobatrachus, Bombina), in which the tips of their iliac shafts reach far beyond the anterior margin of the expanded and fluted sacral diapophyses, may be caused by insertion of the ilio-lumbaris muscle on the transverse processes of presacral vertebrae 4–6 (Emerson, 1982); according to different theories, this peculiarity may have had a functional role in the mechanisms of breathing, food capture or shock absorbence (Willem, 1936, 1938a,b; Palmer, 1960; Whiting, 1961; see Van Dijk, 2002, for a more comprehensive discussion).
We noticed some differences in the timing of developmental events among species (Table 1). For instance, development of the hypochord in Bombina is obviously retarded if compared with Discoglossus. The hypochord and caudal vertebrae appear comparatively late in Bufo.
As our investigation mainly concerned developmental series until stage 66, we were not able to follow later development, including the mode of ossification and fusion of the sacral diapophyses with the first postsacral or most posterior presacral diapophyses. Transverse processes from the urostyle may contribute to formation of the ilio-sacral articulation, fuse to the sacral diapophyses or replace sacral diapophyses in this function in Pelobates and Scaphiopus (Ritland, 1955).
Summary
The anuran pelvis develops from two chondrification centres on each side of the body, which arise shortly one after another and soon fuse together; they may be interpreted as the pubo-ischiadic and iliac plates, respectively. The former corresponds to the pelvis of piscine ancestors of amphibians.
Posterior rotation of the pelvis from its original vertical position, together with reduction of the tail, undoubtedly reflects evolutionary changes that occurred in temnospondyls; they resulted in functional changes of some thigh muscles, and were obviously a device for saltation and swimming.
The tuber superius on the iliac shaft is probably homologous with the iliac process of early amphibians, and is not correlated with saltation/swimming. It is a place of origin of the m. glutaeus maximus (a homologue of m. iliotibialis of tailed amphibians), which plays only a limited role (extension of knee joint) in jumping or swimming.
Development of the pelvis closely behind the level of the sacral vertebra seems to be associated with the type of ilio-sacral articulation (modified for longitudinal sliding over the sacral diapophyses in water dwellers); the pelvis of Discoglossus, which is both aquatic and terrestrial, develops in much the same way, but additional modifications occur in the adult – tips of the ilia become attached to the sacral diapophyses so that they are not capable of sliding.
Fusion of both halves of the pelvis also occurs earlier in Discoglossus, Bombina and Xenopus, before the ilia attain their ultimate size, whereas in Bufo, Pelobates and Rana they are still separated even if the ilia are of nearly definitive size.
The developmental pattern of the anuran pelvis is largely the same for all studied species. Morphological features used in taxonomy (e.g. shape of the ilium, sacral diapophyses, sacro-urostylar articulation) develop only during and after metamorphosis.
Only the more profound differences (e.g. short pelvis associated with sliding ilio-sacral articulation of water-dwellers contra long pelvis with firm but flexible articulation, or vertical rotation, of the tips of ilia with tips of sacral diapophyses in terrestrial forms) arise earlier and may be recognized by different topographic relations between the developing pelvis and axial skeleton.
In water-dwelling anurans (Bombina, Xenopus), the pelvis develops closely behind the level of the sacral region of the vertebral column, and both halves of the pelvis come into contact before the ilia reach their ultimate size.
In terrestrial anurans (Bufo, Pelobates, Rana), the pelvis develops far behind the level of the sacral region of the vertebral column, and both halves of the pelvis come into contact only when the ilia reach their ultimate size.
The ossification mode seems to be highly variable even within a single species; however, Xenopus begins to ossify noticeably earlier than in any other investigated species.
The pubis ossifies only in Xenopus (and in all other Pipidae), and in Ascaphus and Leiopelma. An ossified pubis in frogs is considered to be a primitive feature.
The praepubis (epipubis) was found only in Xenopus, Discoglossus and one individual of Bombina bombina. It is a paired chondrification arising independently of the pubis, with which it fuses only secondarily.
The urostyle develops from 3–4 pairs of neural arches representing vestigial caudal vertebrae (10th–13th, except for Ascaphus which has nine, instead of eight, praesacral vertebrae), and from the unpaired hypochord adjoining the notochord ventrally. Simultaneous with a degenerating notochord, the hypochord approaches the bases of the neural arches (which fuse with each other, so that two parallel cartilaginous rods adjoin the dorsolateral surface of the notochord), ultimately fusing with them. The hypochord may even ossify separately from other components of the urostyle (in the Pipidae and Palaeobatrachidae).
The sacral diapophyses arise independently from neural arches and may be thus interpreted as the sacral ribs.
Acknowledgments
We are grateful to Gerhard Schlosser, University of Bremen, for providing us with breeding pairs of Discoglossus pictus; Jenny Narraway, Hubrecht Laboratory, Utrecht, for the loan of a series of sectioned developmental stages of Discoglossus pictus under her care; Karel Královec, University of Pardubice, for providing us with cleared and stained mounts of Xenopus laevis; and L. Sedláčková, Masaryk University, Brno, for cleared and stained mounts of Bombina bombina and B. variegata. The work was partially funded by the Grant Agency of the Academy of Sciences of the Czech Republic (GAAV IAA3013206 to Z.R.).
References
- Andrews SM, Westoll TS. The postcranial skeleton of Eusthenopteron foordi Whiteaves. Trans. R. Soc. Edinb. Earth Sci. 1970;68:207–329. [Google Scholar]
- Blanco MJ, Sanchiz B. Evolutionary mechanisms of rib loss in anurans: a comparative developmental approach. J. Morph. 2000;244:57–67. doi: 10.1002/(SICI)1097-4687(200004)244:1<57::AID-JMOR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bolt JR, Lombard RE. Palaeobiology of Whatcheeria deltae, a primitive Mississippian tetrapod. In: Heatwole H, Carroll E, editors. Amphibian Biology Vol. 4 Paleontology. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1044–1252. [Google Scholar]
- Branham AE, List JC. Development of urostyle during metamorphosis in five species of Anura. J. Morph. 1979;159:311–330. doi: 10.1002/jmor.1051590303. [DOI] [PubMed] [Google Scholar]
- Cannatella DC. A phylogeny of primitive frogs (archaeobatrachians) Lawrence: The University of Kansas; 1985. Unpublished dissertation. [Google Scholar]
- Carroll RL. Lepospondyls. In: Heatwole H, Carroll E, editors. Amphibian Biology Paleontology. Vol. 4. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1198–1273. [Google Scholar]
- Coates MI. The Devonian tetrapod Acanthostega gunnari Jarvik: postcranial anatomy, basal tetrapod interrelationships and patterns of skeletal evolution. Trans. R. Soc. Edinb. Earth Sci. 1996;87:363–421. [Google Scholar]
- De Villiers CGS. On the development of the ‘Epipubis’ of Xenopus. Ann. Transvaal Mus. 1925;11:129–150. [Google Scholar]
- De Villiers CGS. On the morphology of the epipubis, the Nobelian bones and the phallic organ of Ascaphus truei Stejneger. Anat. Anz. 1934;78:23–47. [Google Scholar]
- Dunlap D. The comparative myology of the pelvic appendage in the Salientia. J. Morph. 1960;106:1–76. doi: 10.1002/jmor.1051060102. [DOI] [PubMed] [Google Scholar]
- Eaton TH. The ancestry of the modern Amphibia. Univ. Kansas Publ. Mus. Nat. Hist. 1959;12:155–180. [Google Scholar]
- Emerson SB. Burrowing in frogs. J. Morph. 1976;149:437–458. doi: 10.1002/jmor.1051490402. [DOI] [PubMed] [Google Scholar]
- Emerson SB. The ilio-sacral articulation in frogs: form and function. Biol. J. Linn. Soc. 1979;11:153–168. [Google Scholar]
- Emerson SB, De Jongh HJ. Muscle activity at the ilio-sacral articulation of frogs. J. Morph. 1980;186:129–144. doi: 10.1002/jmor.1051660202. [DOI] [PubMed] [Google Scholar]
- Emerson SB. Frog postcranial morphology: identification of a functional complex. Copeia. 1982;1982:603–613. [Google Scholar]
- Evans SE, Milner AR, Musset F. A discoglossid frog from the Middle Jurassic of England. Palaeontology. 1990;33:299–311. [Google Scholar]
- Evans SE, Borsuk-Białynicka M. A stem-group frog from the Early Triassic of Poland. Acta Palaeontol. Pol. 1998;43:573–580. [Google Scholar]
- Gadow H. The Evolution of the Vertebral Column. Cambridge: Cambridge University Press; 1933. [Google Scholar]
- Gans C, Parsons TS. On the origin of the jumping mechanism in frogs. Evolution. 1966;20:92–99. doi: 10.1111/j.1558-5646.1966.tb03345.x. [DOI] [PubMed] [Google Scholar]
- Gaupp E. Anatomie des Frosches. Abt. 1. Braunschweig: Vieweg u. Sohn; 1896. [Google Scholar]
- Green TL. On the pelvis of the Anura: a study in adaptation and recapitulation. Proc. Zool. Soc. Lond. 1931;1931:1259–1289. [Google Scholar]
- Griffiths I. Status of Protobatrachus massinoti. Nature. 1956;177:342–343. [Google Scholar]
- Griffiths I. The phylogeny of the Salientia. Biol. Rev. 1963;38:241–292. doi: 10.1111/j.1469-185x.1963.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Hodler F. Untersuchungen über die Entwicklung von Sacralwirbel und Urostyl bei den Anuren. Ein Beitrag zur Deutung des anuren Amphibientypus. Rev. Suisse Zool. 1949;56:747–790. [Google Scholar]
- Jarvik E. Basic Structure and Evolution of Vertebrates 1. London: Academic Press; 1980. [Google Scholar]
- Jarvik E. The Devonian tetrapod Ichthyostega. Fossils Strata. 1996;40:1–213. [Google Scholar]
- Jenkins FA, Jr, Shubin NH. Prosalirus bitis (Shubin and Jenkins 1995) and the anuran caudopelvic mechanism. J. Vertebr. Paleontol. 1998;18:495–510. [Google Scholar]
- Lebedev OA, Coates MI. The postcranial skeleton of the Devonian tetrapod Tulerpeton curtum Lebedev. Zool. J. Linn. Soc. 1995;114:307–348. [Google Scholar]
- Limeses CE. La musculatura del muslo en los Ceratofrínidos y formas afines. Contrib. cient. Univ. Buenos Aires, Series Zool. 1964;1:193–245. [Google Scholar]
- MacBride EW. Some recent work on the development of the vertebral column. Biol. Rev. 1932;7:108–148. [Google Scholar]
- Mahendra BC, Charan D. Homology and functional significance of the urostyle in Rana tigrina Daud. and Bufo andersonii Bouleng. Ann. Zool. 1972;8:51–60. [Google Scholar]
- Mookerjee HK. On the development of the vertebral column of Anura. Phil. Trans. R. Soc. Lond. Series B, Biol. Sci. 1931;119:165–195. [Google Scholar]
- Mookerjee HK, Das SK. Further investigations on the development of the vertebral column in Salientia (Anura) J. Morph. 1939;64:167–209. [Google Scholar]
- Nevo E. Pipid frogs from the Early Cretaceous of Israel and pipid evolution. Bull. Mus. Comp. Zool. 1968;136:255–318. [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publishing Co; 1967. [Google Scholar]
- Noble GK. Biology of the Amphibia. New York: McGraw-Hill Book Co; 1931. [Google Scholar]
- Palmer M. Expanded ilio-sacral joint in the toad Xenopus laevis. Nature. 1960;187:797–780. doi: 10.1038/187797a0. [DOI] [PubMed] [Google Scholar]
- Rage J-C, Roček Z. Redescription of Triadobatrachus massinoti (Piveteau, 1936) an anuran amphibian from the early Triassic. Palaeontogr. A. 1989;206:1–16. [Google Scholar]
- Reig OA. Los anuros del Matildense. Acta Geol. Lilloana. 1957;1:185–297. [Google Scholar]
- Ridewood WG. On the development of the vertebral column in Pipa and Xenopus. Anat. Anz. 1897;13:359–376. [Google Scholar]
- Ritland RM. Studies on the post-cranial morphology of Ascaphus truei. I. Skeleton and spinal nerves. J. Morph. 1955;97:119–177. [Google Scholar]
- Roček Z, Rage J-C. Anatomical transformations in transition from temnospondyl to proanuran stages. In: Heatwole H, Carroll E, editors. Amphibian Biology–Paleontology. Vol. 4. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1276–1284. [Google Scholar]
- Roček Z. Larval development in Oligocene palaeobatrachid frogs. Acta Palaeontol. Pol. 2003;48:595–607. [Google Scholar]
- Romer AS. The appendicular skeleton of the Permian embolomerous amphibian Archeria. Contrib. Mus. Paleontol. Univ. Michigan. 1957;13:103–159. [Google Scholar]
- Smit AL. The ontogenesis of the vertebral column of Xenopus laevis (Daudin) with special reference to the segmentation of the metotic region of the skull. Ann. Univ. Stellenbosch. 1953;29A:79–136. [Google Scholar]
- Smithson T. Anthracosaurs. In: Heatwole H, Carroll E, editors. Amphibian Biology–Paleontology. Vol. 4. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1053–1063. [Google Scholar]
- Špinar ZV. Fossil Frogs from Central Europe. Prague: Academia; 1972. [Google Scholar]
- Stephenson EM. The vertebral column and appendicular skeleton of Leiopelma hochstetteri Fitzinger. Trans. R. Soc. N. Zeal. 1952;79:601–613. [Google Scholar]
- Stephenson EM. The skeletal characters of Leiopelma hamiltoni McCulloch, with particular reference to the effects of heterochrony on the genus. Trans. R. Soc. N. Zeal. 1960;88:473–488. [Google Scholar]
- Stephenson NG. Observations on the development of the amphicoelous frogs, Leiopelma and Ascaphus. J. Linn. Soc. Lond. (Zool) 1951;42:18–28. [Google Scholar]
- Trueb L, Hanken J. Skeletal development in Xenopus laevis (Anura: Pipidae) J. Morph. 1992;214:1–41. doi: 10.1002/jmor.1052140102. [DOI] [PubMed] [Google Scholar]
- Van Dijk E. The ‘tail’ of Ascaphus. Ann. Univ. Stellenbosch, A. 1955;31:1–71. [Google Scholar]
- Van Dijk E. On the cloacal region of Anura in particular of larval Ascaphus. Ann. Univ. Stellenbosch, A. 1959;35:169–249. [Google Scholar]
- Van Dijk E. Segmentation of the pelvic region of Anura with particular reference to the urostyle of Ascaphus. S. Afr. J. Sci. 1960;56:78–80. [Google Scholar]
- Van Dijk E. Longitudinal sliding articulations in pipid frogs. S. Afr. J. Sci. 2002;98:555–556. [Google Scholar]
- Wassersug RJ. A procedure for differential staining of cartilage and bone in whole formalin-fixed vertebrates. Stain Technol. 1976;51:131–134. doi: 10.3109/10520297609116684. [DOI] [PubMed] [Google Scholar]
- Whiting HP. Pelvic girdle in amphibian locomotion. Symp. Zool. Soc. Lond. 1961;5:43–57. [Google Scholar]
- Willem V. Observations sur les manouvres respiratoires chez. les anoures aglosses. Mém. Mus. Hist. Nat. Belg. Ser2. 1936;3:375–385. [Google Scholar]
- Willem V. L’Articulation sacro-iliaque chez quelques amphibiens anoures. Trav. Station Zool. Wimereaux. 1938a;13:749–760. [Google Scholar]
- Willem V. Nouvelles observations sur la respiration des anoures aglosses. Mém. Acad. R. Belg. (Sci) 1938b;17:3–22. [Google Scholar]