Abstract
This study investigated age and sex differences in patterns of porosity distribution in the midshaft of the human femur. Cross-sections were obtained from 168 individuals from a modern Australian population. The sample comprised 73 females and 95 males, aged between 20 and 97 years. Microradiographs were made of 100-µm sections and pore and bone areas were determined using image processing software. Initially the sample was divided by age: young (20–44 years), middle (45–64 years) and old (65+ years), but it was found that analysis on the basis of the ratio of medullary area to total subperiosteal area gave clearer results. The cortex was divided into three rings radially and into octants circumferentially and the porosity of each segment was calculated. Results showed that a pattern with raised porosity in the posterior and anterolateral regions, and with greater porosity in the inner parts of the cortex, becomes more pronounced with age. In males this pattern develops steadily; in females there are much greater differences between the middle and older groups than earlier in life. The patterns observed are consistent with progressive bone loss occurring along a neutral axis of the cortex where bending stress is lowest and the mechanical advantage of the bone is least.
Keywords: bone aging, cortical bone, human femur, osteoporosis, sex differences
Introduction
Longer average lifespans and altered lifestyles appear to be contributory factors to the increasing prevalence of osteoporosis, particularly in developed countries (Boereboom et al. 1992; Cooper et al. 1992; Melton, 1993). This condition, with its associated risk of hip fracture, affects men as well as women (Seeman, 1999) in ever greater numbers, generating worldwide concern. As it is mainly cortical bone loss that is implicated in hip fractures (Johnston et al. 1985; Bell et al. 1999a), an understanding of how this loss occurs (i.e. how porosity develops and leads to cortical thinning) is vital. There is great individual variation in the rate and extent of cortical bone loss (Feik et al. 1997; Bell et al. 1999a; Stein et al. 1999), meaning that large numbers of subjects need to be examined over a considerable segment of the lifespan for meaningful results to be obtained. Porosity (Schaffler & Burr, 1988; McCalden et al. 1993; Yeni et al. 1997 Yeni et al. 1998), as well as geometric parameters (Ruff & Hayes, 1988; Simmons et al. 1991; Myers et al. 1993), affects the mechanical properties of bone. Cortical porosity influences femoral bone strength (Wall et al. 1979; Dickenson et al. 1981), and porosity changes in the femur account for 76% of the variance in age-related decline in strength (McCalden et al. 1993). Fracture toughness (Yeni et al. 1997 Yeni et al. 1998), stiffness and the elastic modulus of cortical bone are all affected by changes in porosity (Schaffler & Burr, 1988). The effects of porosity have been studied not only in the midshaft of the femur but also in the femoral neck where regional differences in porosity were found between fracture cases and age-matched controls (Bell et al. 1999b).
Early studies of porosity (Jowsey, 1960; Atkinson, 1965; Martin et al. 1980; Martin & Burr, 1984) showed that porosity distribution within the cortex was non-uniform, increasing from the periosteal to the endosteal. The manual counting methods employed placed limitations on the size of the area of cortex examined and the number of subjects used. A recent study (Bousson et al. 2001), using semi-automated techniques, examined the regional distribution of porosity but only in the anterior femoral cortex. Their sample was large (163 individuals), covered almost the entire lifespan (11–96 years) but comprised mainly manual workers (farmers and craftspeople) who had died almost a century ago and whose remains were exhumed 5–10 years after burial. Stein et al. (1999) determined the porosity of entire transverse sections of the femoral midshaft in 96 specimens from a modern urban population. However, they did not look at how the porosity was distributed around the cortex. We used material from the same collection to study age and sex differences in total cortical bone loss, i.e. intracortical plus medullary (Feik et al. 1997). This was followed by a study of the regional variations in cortical modelling to determine how the geometry of the mid-femur alters with age in both sexes as bone loss progresses (Feik et al. 2000). The current study is a logical follow-on from this earlier work with the aim of determining if intracortical porosity development can be related to the modelling changes we have observed previously.
The aim was to determine the distribution of porosity (percentage of cortical bone area occupied by pores of > 400 µm2) in both sexes across the adult lifespan in a large sample from a modern, urban, Caucasian population. Entire transverse sections of the femoral midshaft, which were divided into octants circumferentially and into three rings of equal width radially, were examined to try to gain an understanding of the principles underlying age-related bone loss.
Materials and methods
Bone specimens (n = 170) were collected at the Victorian Institute of Forensic Medicine, Melbourne, Australia, the majority between 1990 and 1993, and a smaller subsample in 1998. The sample was almost exclusively Anglo-Celtic, as judged by names, from people who had died suddenly with no known diseases directly affecting their bones. Information on the age, sex, supine length, weight and, in almost every case, the cause of death was available. Details of the sample, divided into three age groups, are presented in Table 1. Specimens were sawn by mortuary staff from the midshaft of the femur and fixed in 70% ethanol. Only in the subsample (20 specimens) collected later was the orientation of the specimens, all obtained from the right femur, recorded. The specimens were cleaned manually and transverse sections, ∼300 µm thick, were cut from the femoral blocks using a Leitz 1600 sawing microtome (Leitz, Wetzlar, Germany). Planoparallel sections with a nominal thickness of 100 µm were obtained from these by lapping on 1200-grade wet and dry carborundum paper.
Table 1.
Sex distribution of the study sample (grouped by age)
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Age group (years) | n | Mean | SD | % of sample | n | Mean | SD | % of sample |
| Young (20–44) | 28 | 30.1 | 7.6 | 37.3 | 27 | 32.4 | 7.6 | 28.4 |
| Middle (45–64) | 14 | 53.5 | 6.5 | 18.7 | 25 | 54.5 | 5.5 | 26.3 |
| Old (65 +) | 33 | 80.9 | 8.2 | 44.0 | 43 | 77.9 | 7.6 | 45.3 |
| Total | 75 | 56.8 | 24.3 | 95 | 58.8 | 20.5 | ||
Microradiography and image acquisition
The sections were microradiographed using a Matchlett Laboratories OEG X-ray tube with a copper target operated at 25 kV and 10 mA. The film used was Kodak SO-343 at a distance of 195 mm from the target. The microradiographs were mounted on glass slides and masked with black tape to define the borders and to control scattered light. An array of contiguous monochrome images from entire cross-sections was recorded by tiling on a computer-controlled x–y stage (Prior ProScan) fitted to a Leitz Dialux 20 microscope. The camera used was a Diagnostic Instrument's Spot 2 working at a resolution of 1315 × 1033 pixels. The image processing software used was Optimas (Media Cybernetics, Inc., Silver Spring, MD, USA) and data were recorded using Microsoft Excel. The field of view of the camera, using a ×1 microscope objective (Leitz PL 1/0.04) and matching condenser, was ∼3.5 × 2.5 mm and most sections were contained within a rectangle of 30 × 35 mm, so that approximately 180 frames were needed to cover each specimen. Frame boundaries matched to a precision of 1 µm. In addition to the images of the bone, a bright-field image was acquired with a neutral density filter and this was used to correct for variations in illumination.
Montage reconstruction and data accrual
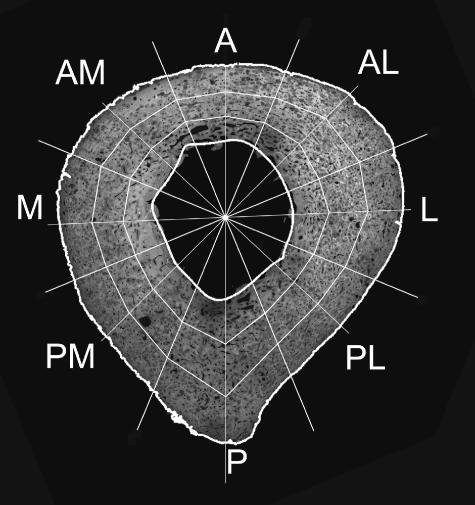
The images making up a single cross-section were combined into a montage (Fig. 1) using Optimas macro language software. In these montages the posterior surface of the femur was identified by the linea aspera and the medial and lateral aspects were distinguished by the disposition of macro- and microstructural features as observed in the small subsample where the orientation was known. The background and voids (pores) in the bone appeared black. All lighter tones represented bone; the variations in brightness were due to different degrees of mineralization or slight differences in section thickness. This necessitated an adjustment of the threshold brightness level for each section, which was usually approximately 50 (in a range of 0–255, where 0 is black) but varied somewhat from section to section. Based on this threshold the outlines of the periosteal and endosteal boundaries of the bone were extracted automatically using the Optimas image-processing package. Having isolated the periosteal outline, Optimas calculated the location of the centroid and this value was recorded. The endosteal outline was reconstructed from a series of Fourier shape descriptors truncated at the eighth harmonic, as described in detail in an earlier paper (Feik et al. 2000). The total subperiosteal area (TSPA) and cortical area (CA) (as total foreground) were acquired and the medullary area (MA) calculated from these measurements. Following the automatic location of the outlines of the bone surfaces, they were displayed on the computer screen as overlays on the image (Fig. 1). The operator then marked a point in the centre of the linea aspera, and a line joining this point to the opposite periosteal surface, passing through the centroid, was used to define the anteroposterior (A–P) axis. At this stage of processing the data were in a series of Microsoft Excel spreadsheets and contained the x,y coordinates of the linea aspera marker, the centroid of the periosteum and the centroids, and areas (in mm and mm2, respectively) of all pores > 400 µm2 (Stein et al. 1999). Scatter plots created in Excel were used to check for, and remove, occasional spurious pore measurements that were due to image noise (mostly dust specks).
Fig. 1.
Subdivisions of the cortex. A, anterior; AL, anterolateral; L, lateral; PL, posterolateral; P, posterior; PM, posteromedial; M, medial; AM, anteromedial. The three rings shown will be referred to as periosteal, midcortical and endosteal.
The cleaned data (from around 4000–5000 pores in each specimen) were reduced to average values of porosity in anatomically defined subregions of the cortex (Fig. 1) using software written for Matlab V6.1 software (The MathWorks Inc., Natick, MA, USA). This software defined the boundaries shown in Fig. 1 in a polar coordinate system having its origin at the centroid of the periosteum. The original arbitrary Cartesian coordinates of all pore centroids were transformed into the same polar coordinate system. The total area of each segment of the cortex was calculated and the areas of all pores with a centroid falling within the area were summed.
Data analysis
The definition of Laval-Jeantet et al. (1983) was adopted for intracortical porosity, i.e. the percentage of cortical bone occupied by vascular and resorption cavities. Knowing the location and the area of each pore we could determine the porosity distribution throughout the whole cross-section both radially and circumferentially, i.e. in each octant and ring. Initially we analysed the data by age, dividing the sample into three age groups (Table 1), as used by Goldman et al. (2003a,b) in their studies on bones from the same collection. Differences between region mean porosities were assessed using one-way ANOVA followed by Tukey's HSD tests where multiple comparisons were being made. This analysis of the results showed that an age-based division of the sample leads to difficulties with the female subjects in that, in some regions of the cortex, porosity values overlap between the middle and old age groups (see Fig. 2f). We therefore decided to use an additional grouping, which we thought might provide a clearer picture of the pattern of bone loss as porosity increases. We have shown previously (Feik et al. 1997) that by far the largest contribution to the change with age in the amount of bone present in the mid-femoral shaft came from an increase in MA. The change in the intracortical component, although statistically significant, was small and appeared to play little part in the increased total subperiosteal porosity (TSPP) that occurred with age. However, great variability in the development of TSPP in elderly individuals of similar chronological age was seen. Stein et al. (1999) also demonstrated that age explained little of the interindividual variation in intracortical porosity. We considered therefore that a measure based on cross-sectional geometry, such as the ratio of MA to TSPA, might be a better indicator of the ‘biological age’ of a bone than is chronological age. We used this measure to divide the study sample into three groups with low, middle and high MA/TSPA ratios (Table 2) based on the tertile values reported by the statistical package SPSS (SPSS Inc., Chicago, IL, USA) for the distribution. In this way, we examined the regional distribution of intracortical porosity within each of these ‘biologically similar’ (at least in terms of bone loss) groups.
Fig. 2.
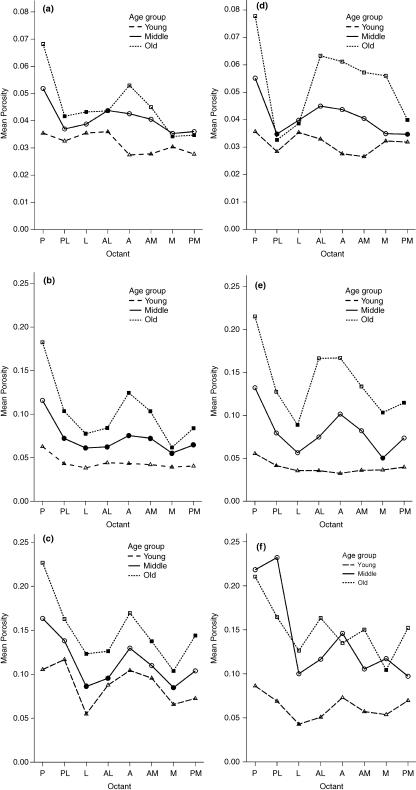
Porosity distribution around the cortex, with specimens grouped by age: (a–c) males, (d–f) females. The upper pair is for the periosteal ring (note different vertical scales), the middle pair for the midcortical and the lower pair for the endosteal ring. Solid symbols indicate significant difference at P = 0.05 from the posterior octant within each group.
Table 2.
Sex distribution of the study sample (grouped by MA/TSPA ratio)
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | n | Mean | SD | % of sample | n | Mean | SD | % of sample |
| Low (0–0.219) | 21 | 0.177 | 0.010 | 28.0 | 35 | 0.180 | 0.028 | 36.8 |
| Middle (0.219–0.315) | 22 | 0.256 | 0.030 | 29.3 | 39 | 0.258 | 0.026 | 41.1 |
| High (0.315 +) | 32 | 0.423 | 0.099 | 42.7 | 21 | 0.384 | 0.051 | 22.1 |
| Total | 75 | 95 | ||||||
Results
As shown in Table 1, the old group (65+ years) comprised the largest fraction (∼45%) of the study sample in both sexes. However, there were proportionally more young females than males (∼37% vs. 28%) in the sample. When grouped by MA/TSPA ratios (Table 2) the numbers and sex distribution in the groups altered markedly. The largest fraction of the sample with a high ratio, i.e. a relatively large medullary area, was found among the females, comprising ∼43% of the total female sample, a very similar proportion to that occupied by the old when grouped by age (∼44%). The males differed in that those with the greatest bone loss, i.e. a high MA/TSPA ratio, comprise the smallest fraction (∼22%) of the males in the study sample. In the males the greatest number of cases was found in the middle group, i.e. those with intermediate bone loss; the fraction occupied by these (∼41%) was only slightly lower than that occupied by the old (∼45%) when grouped by age.
When the regional porosity distribution between the three age groups was examined a clear delineation between the groups was evident in the males. When the three rings were combined the lowest porosity and the least differentiation between regions was found in the young, ranging from ∼6.5% in the posterior octant to ∼4.5% in the lateral octant. In the middle group not only were the porosity levels generally higher (∼6–11%) but a clear pattern of differing porosity levels among the octants emerged. The posterior octant was most porous, followed by the anterolateral, with the lateral and medial being comparable and showing the least porosity. In the old group porosity levels ranged from ∼7 to 16% and the same regional pattern was observed although greatly accentuated. In the females grouped by age a more complex picture emerged. The young group showed less regional differentiation than the equivalent male group. The difference in porosity levels was also greater between the young (∼4–6%) and the middle (∼7–14%) groups in females than it was in males. The most noticeable difference between the sexes when grouped by age was the loss of differentiation between the middle and old groups and the broader spread of porosity into the anterior octant in old females.
Grouping the sample by MA/TSPA ratio and combining the three rings removed the overlap between groups and provided a clearer picture of the pattern of change in regional porosity distribution, highlighting the sex differences that occur with progressive bone loss. The overall porosity distribution in males in the three MA/TSPA groups was very similar to that seen in males grouped by age; however, the patterns were somewhat accentuated with the former grouping. In females the differences between the age and MA/TSPA groupings were marked. The low and the middle groups showed very similar porosity levels (∼2% difference at most) in contrast to that seen when grouped by age. The high group was clearly differentiated from the middle group, showed much higher porosity levels (∼9% to > 19%) and showed marked regional porosity differences.
Figure 2(a–f) depicts the age-grouped regional porosity distribution in each ring separately. The posterior octant always showed the highest level of porosity. Solid symbols denote octants where the porosity differed significantly (P = 0.05) from the posterior octant. The trends were similar to that observed when all the rings are combined in that porosity levels seemingly increase as age increases. In all age groups porosity increased from the periosteal to the endosteal. As porosity increases, whether by age grouping or radial location in the cortex, the regional differentiation between octants becomes more clearly defined. It is least obvious in the periosteal rings and most marked in the endosteal. However, a grouping based on chronological age fails to distinguish between fast and slow bone losers because in Fig. 2(f), which depicts the endosteal ring in females, the middle and old groups cannot be separated.
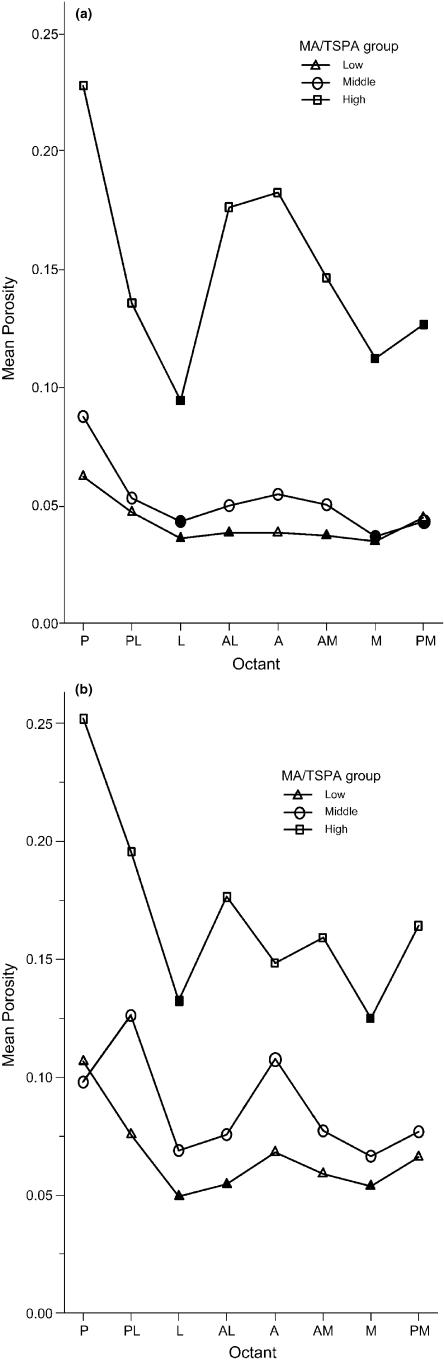
When the above analysis was performed with an MA/TSPA grouping replacing that based on age the patterns of regional porosity distribution found were similar in all except the midcortical and endosteal rings in females. Figure 3(a,b) show the latter two instances for comparison with Fig. 2(e,f). In the midcortical ring (Fig. 3a) the porosities in the low and middle MA/TSPA groups are very similar and quite clearly separated from the high group. This differs from the age grouping (Fig. 2e) where the porosities in the three groups show a more uniform progression of bone loss. Differences between the groupings (Fig. 2f vs. Fig. 3b) are even more pronounced in the endosteal ring where again there is a clear differentiation between the low/middle and the high MA/TSPA groups whereas the age grouping, as described above, shows no separation between the middle and old groups, which overlap in a seemingly haphazard fashion.
Fig. 3.
Porosity distribution around the cortex, with specimens grouped by MA/TSPA ratio. Solid symbols indicate significant difference at P = 0.05 from the posterior octant within each group. Graphs are for females only: (a) data from the midcortical and (b) data from the endosteal rings. (a) and (b) should be compared with Fig. 2(e) and (f), respectively.
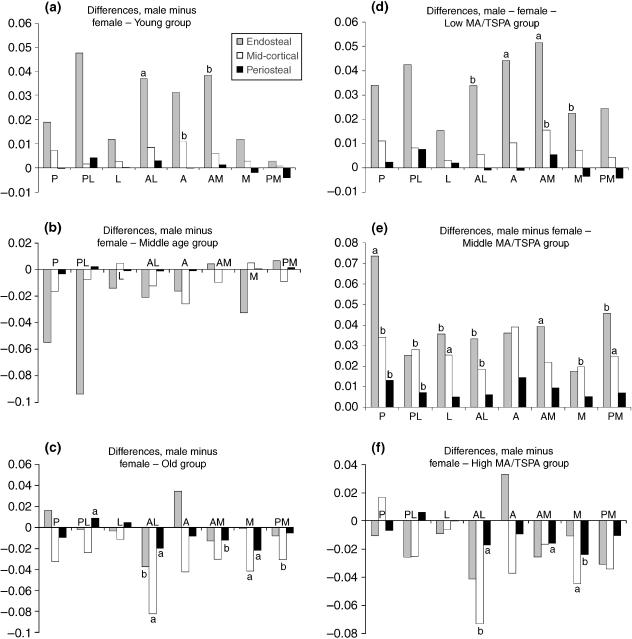
Figure 4 (a–c grouped by age and d–f by MA/TSPA ratio) presents the differences in porosity (in percentage points) between males and females for all subdivisions of the cortex. The porosity difference (male minus female) is generally greatest where porosity levels are highest, in particular in the endosteal ring and anterior region. In the young (and low MA/TSPA group) the differences between the sexes are the same for either grouping with the males being more porous in most regions. In the middle groups the picture differs greatly between the two types of grouping, with the age-based analysis (Fig. 4b) showing the females as more porous than the males (although the differences do not reach significance at P = 0.1) whereas when grouped by MA/TSPA ratio (Fig. 4e) the reverse is seen. The males are shown to be more porous with half of the differences being significant at P = 0.1 and three at P = 0.05. In the old (and high MA/TSPA) the patterns shown by the two groupings are again very similar, as they were in the young (and low MA/TSPA). However, with only a few exceptions the females are more porous than the males.
Fig. 4.
Differences (percentage points) between porosities in males and females (males minus females) in all subdivisions: (a) Young (20–44 years), (b) middle age (45–64 years), (c) old (65 and older), (d) low MA/TSPA, (e) middle MA/TSPA, (f) high MA/TSPA. Text annotations on the graphs: a indicates significance at P = 0.05, and b at P = 0.1.
Discussion
In this study we determined the regional porosity distribution in the femoral mid-diaphysis comparing trends between the sexes as age and bone loss progressively increase. Complete cross-sections were divided circumferentially into octants and the cortex partitioned radially into three rings of equal width. We grouped the sample into three age ranges and also into three groups based on MA/TSPA ratio as the latter measure provided a clearer picture of the sex differences in particular instances. In females grouped by MA/TSPA there was clear separation between the low/middle groups and the high group that was not evident when the sample was grouped by age. Males, however, whether grouped by age or MA/TSPA ratio, showed a similar pattern of a more uniform change with progressive bone loss. For all ages and both sexes the posterior octant was consistently the most porous in any one specimen (range ∼3.5 to ∼26%), followed by the anterior (range ∼3 to ∼19%), with the medial and lateral octants (range ∼3 to ∼13%) showing the lowest porosity levels. However, these regional differences in porosity distribution only became marked as porosity levels increased, i.e. they were most obvious in the endosteal ring in the old or high MA/TSPA groups and barely discernible in the periosteal ring in the young or low MA/TSPA groups. The midcortical ring in the MA/TSPA grouping showed the clearest differentiation between the sexes in patterns of bone loss. Across all groups and cortical regions the anterolateral octant has the greatest number of significant sex differences in porosity. In summary, our study of the mid-diaphyseal femoral cortex has shown clear and predictable patterns of porosity changes with increasing ‘biological age’ and distinct sex differences in this process, a process that ultimately leads to cortical thinning.
We were uniquely advantaged in this study in that we had access to a large collection of complete cross-sections of the femoral midshaft covering the entire adult lifespan of both sexes. The bones were well characterized, and details such as the age, supine length, weight and in most cases the cause of death (from autopsy reports) of each subject were known. The sample derived from a largely Anglo-Celtic population in a prosperous, modern, urban setting. The porosity results obtained therefore should be widely applicable to other urbanized Western countries because the prevalence of osteoporosis in Melbourne resembles that in similar communities in Europe and the USA (Seeman et al. 1993). Automatic image analysis techniques were used as much as possible to ensure reproducibility and to decrease subjectivity during data collection. Acquisition of bone outlines and the frequently problematic endosteal surface, subdivision of the cortex circumferentially and radially and the location and area of each pore were all automated. However, some steps such as adjusting the threshold brightness level of each section and marking the centre of the linea aspera (which determined the A–P axis) were carried out by the operator and hence were potentially variable. This was minimized by using one operator for all specimens.
The study was of necessity cross-sectional and the age changes reported may be subject to cohort bias due to secular and lifestyle changes between generations, a problem that is unavoidable in a study of this kind. The cross-sectional nature of the study, compounded by the great individual variation, also makes it impossible to determine precisely the sequence and timing of how increased porosity levels lead to cortical thinning in any given individual. The orientation of the sections, apart from a subsample of 20 in which the precise orientation was known, was determined by observation of structural features. If the linea aspera could not be precisely identified the specimen was eliminated. Distinguishing the medial from the lateral posed the greatest problem and again one operator was used for all determinations and in cases of uncertainty the specimen was not used. The image resolution that we used was such that only pores of > 400 µm2 were recorded, thus excluding osteocytic lacunae and canaliculi. However, this may not be important as Haversian/Volkmann's canals are the major contributors to intracortical porosity (Martin, 1984) and show age-related porosity changes whereas the porosity of osteocytic lacunae appears to be independent of age (Wang & Ni, 2003).
The concept of ‘biological age’, as opposed to chronological age, is commonly used in developmental studies of children (Baer, 1977) with ‘skeletal age’ being an important and easily assessable component of this determination (Pyle et al. 1971; Roche et al. 1988). This concept, however, is not nearly as widely used in bone aging studies in adults where chronological age is usually used as the basis for comparison between supposedly similar groups. Yet Hough (1998) showed in menopausal women that, although the rate of bone loss varied greatly, it was symmetrically distributed. This implies that post-menopausally, for a particular chronological age, a bone could display a range of appearances varying with the turnover rate. A similar conclusion can be drawn from studies attempting to determine age at death using morphometric methods. From these studies it is generally acknowledged that the relationship between chronological age and bone appearance is imprecise, with variations up to a decade either side of the actual age being the norm obtained by these methods (Thomas et al. 2000). Many studies (Atkinson, 1965; Feik et al. 1997; Stein et al. 1999; Bousson et al. 2001) have drawn attention to the great individual variation in bone loss with age. All of this argues for the use of biological age when comparing adults as well as children. Biological age, measured by hand radiography, was in fact used in a recent study (Karasik et al. 2004) of American adults in which 57% of the variation in biological age was attributed to genetic factors. We explained our reasons for using MA/TSPA groupings as well as age in the Materials and Methods section. The use of this ratio, which indicates cumulative bone turnover, appears warranted as a much clearer pattern of developing porosity was detected in some regions of the cortex. This again points to the potential usefulness of using biological markers rather than chronological age alone for comparative purposes.
In early studies by Jowsey (1960) and Atkinson (1965) it was shown that the porosity gradient increases from the periosteal to the endosteal. However, those studies used manual counting methods and only examined limited areas of the cortex in relatively few individuals. Bousson et al. (2001), using semi-automated image analysis techniques, recorded the porosity levels in the anterior cortex of the mid-femur in 163 individuals. The results they obtained for males (range ∼2 to ∼18%) are very similar to ours (∼3 to ∼22%) (Fig. 2a–c). The range obtained for females (∼2 to ∼28%) is slightly greater than ours (∼3 to ∼24%) (Fig. 2d–f). Possible reasons for this difference, include sample population variations, and the choice of method for delineating the endosteal surface. The radial differences in porosity levels between the two studies are greatest in the endosteal subregion in females where trabecularization of the cortex is usually most pronounced.
To our knowledge, the circumferential regional differences in porosity in the mid-femoral shaft have not been reported previously. The pattern of change at the microstructural level is broadly consistent with the modelling changes reported earlier from a study of the same material (Feik et al. 2000). There we showed that apposition was least in the posterior cortex and resorption greatest in the anterior, the latter being particularly evident in older females. We have no explanation for the higher porosity levels in the posterior region in young bones; perhaps the remodelling space is greater because of higher bone turnover in an area of muscle attachment. In older individuals, as muscle strength declines (Burr, 1997; Frost, 1997) and the pull of the adductor muscles lessens (Mittlemeier et al. 1994) functional bone strain will decrease and adaptive remodelling occur (Biewener, 1991; Lanyon, 1992). This will result in greater porosity in this region. The high porosity levels in the anterior (relative to the medial and lateral) also support our previous findings (Feik et al. 2000) of cortical width reduction and anterior cortical drift as gait (Craik, 1989) and posture change with age (Sinclair & Dangerfield, 1998). The lower porosity levels in the medial and lateral octants do not surprise us either as we showed that radial widths increased in older males and females and cortical widths declined only in females, i.e. periosteal apposition, rather than endosteal resorption, predominated in this region, especially in males.
If obligatory bone loss were to occur, as purportedly occurs in women around the time of the menopause (Riggs & Melton, 1986; Pouilles et al. 1993; Rico et al. 1993), one would expect it to occur where bone strain is least, e.g. along the neutral axis of bending and in areas where its presence contributes least to overall stiffness of the bone, i.e. endosteally (Van Buskirk, 1989; Currey, 2002). Ruff & Hayes (1983, their figure 6) showed that the direction of greatest bending rigidity in a typical femoral shaft at 50% of bone length passed through the anterolateral and posteromedial. As the shaft became more circular, e.g. at 35% of bone length measured from the distal, the axis approximated the anterior and posterior more closely. We have shown previously (Feik et al. 2000) that with age the femoral midshaft became more eurycnemic approaching near circularity in the old groups (midshaft index 0.95 in both sexes), and thus a similar shift in the neutral axis may occur. The porosity patterns (see Figs 2 and 3) described in this paper are in accordance with the above observations and suggest that the direction of loading influences bone loss in this region as well as in the femoral neck (Bell et al. 1999b).
The sex differences, as described earlier, are most obvious in the midcortical ring, with the males showing a uniform progression as bone loss increases whereas in females only the group showing high bone loss is clearly distinguished from the others. This pattern, of the middle groups being most dissimilar, has been observed previously when mid-femoral geometric parameters were studied (Feik et al. 1997 Feik et al. 2000). It appears that females are relatively protected up to the menopause and then rapid bone loss occurs thereafter. Rutherford & Jones (1992) similarly found maintenance of bone in the mid-femur of females until the sixth decade. The lower levels of endosteal porosity in females in the low and middle MA/TSPA groups support the notion that females store bone endosteally during the reproductive period and lose it post-menopausally. This confirms the findings of earlier workers such as Garn (1970) who stated that females deposit more bone endosteally than males, especially during puberty. In addition, Ferretti et al. (1998) in a bone densitometry study reported greater mineral storage in premenopausal women than in age-matched men, suggesting, but not being able to prove, that the ‘surplus’ mineral was stored in the endosteal area. With the age at menopause being very variable, and possibly increased artificially by the use of hormone replacement therapy, the use of chronological age as an independent indicator of changes to bone is problematical.
A detailed knowledge of the regional porosity distribution, perhaps combined with the use of geometric parameters, may prove useful in developing alternative methods of determining age at death from skeletal remains, although the problem of wide differences between biological and chronological age remains. In a number of earlier studies (Kerley, 1965; Ahlqvist & Damsten, 1969; Kerley & Uberlaker, 1978), proposed methods of age estimation were based on the analysis of subperiosteal bone histology yet our results suggest that subject differentiation in this area is not as great as in the midcortical region, particularly the anterior octant. In future studies we will test this observation and report on pore size and density distribution within complete cross-sections of the femoral midshaft to gain a better understanding of porosity changes with age and how this may affect the mechanical properties.
Acknowledgments
We thank Professor Stephen Cordner, Director of the Victorian Institute of Forensic Medicine, and his staff for their assistance in the collection of this series of bone specimens.
References
- Ahlqvist M, Damsten O. A modification of Kerley's method for the microscopic determination of age in human bone. J. Forensic Sci. 1969;14:205–212. [PubMed] [Google Scholar]
- Atkinson PJ. Changes in resorption spaces in femoral cortical bone with age. J. Path. Bacteriol. 1965;89:173–178. doi: 10.1002/path.1700890118. [DOI] [PubMed] [Google Scholar]
- Baer MJ. Growth and Maturation. an Introduction to Physical Development. Cambridge, MA: Howard A Doyle; 1977. [Google Scholar]
- Bell KL, Loveridge N, Power J, et al. Structure of the femoral neck in hip fracture: Cortical bone loss in the inferoanterior to superoposterior axis. J. Bone Miner Res. 1999a;14:111–119. doi: 10.1359/jbmr.1999.14.1.111. [DOI] [PubMed] [Google Scholar]
- Bell KL, Loveridge N, Power J, Garrahan N, Meggitt BF, Reeve J. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999b;24:57–64. doi: 10.1016/s8756-3282(98)00143-4. [DOI] [PubMed] [Google Scholar]
- Biewener A. Musculoskeletal design in relation to body size. J. Biomech. 1991;24(Suppl 1):19–29. doi: 10.1016/0021-9290(91)90374-v. [DOI] [PubMed] [Google Scholar]
- Boereboom F, Raymakers J, de Groot R, Duursman S. Epidemiology of hip fractures in the Netherlands: women compared with men. Osteoporosis Int. 1992;2:279–284. doi: 10.1007/BF01623183. [DOI] [PubMed] [Google Scholar]
- Bousson V, Meunier A, Bergot C. Distribution of intracortical porosity in human midfemoral cortex by age and gender. J. Bone Miner. Res. 2001;16:1308–1317. doi: 10.1359/jbmr.2001.16.7.1308. [DOI] [PubMed] [Google Scholar]
- Burr DB. Muscle strength, bone mass, and age-related bone loss. J. Bone Miner Res. 1997;12:1547–1551. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- Cooper C, Campion G, Melton LJI. Hip fractures in the elderly: a world-wide projection. Osteoporosis Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- Craik R. Changes in locomotion in the aging adult. In: Woollacott MH, Schumway-Cook A, editors. Development of Posture and Gait Across the Life Span. Columbia, SC: University of South Carolina Press; 1989. pp. 176–201. [Google Scholar]
- Currey JD. Bones: Structure and Mechanics. Princeton: Princeton University Press; 2002. [Google Scholar]
- Dickenson RP, Hutton WC, Stott JRR. The mechanical properties of bone in osteoporosis. J. Bone Joint Surg. 1981;63-B:233–238. doi: 10.1302/0301-620X.63B2.7217148. [DOI] [PubMed] [Google Scholar]
- Feik SA, Thomas CDL, Clement JG. Age-related changes in cortical porosity of the midshaft of the human femur. J. Anat. 1997;191:407–416. doi: 10.1046/j.1469-7580.1997.19130407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feik S, Thomas C, Bruns R, Clement J. Regional variations in cortical modeling in the femoral mid-shaft: sex and age differences. Am. J. Phys. Anthropol. 2000;112:191–205. doi: 10.1002/(SICI)1096-8644(2000)112:2<191::AID-AJPA6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ferretti JL, Capozza RF, Cointry GR, et al. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone. 1998;22:683–690. doi: 10.1016/s8756-3282(98)00046-5. [DOI] [PubMed] [Google Scholar]
- Frost HM. On our age-related bone loss: insights from a new paradigm. J. Bone Miner. Res. 1997;12:1539–1546. doi: 10.1359/jbmr.1997.12.10.1539. [DOI] [PubMed] [Google Scholar]
- Garn SM. The Earlier Gain and the Later Loss of Cortical Bone. Springfield, IL: Charles C Thomas; 1970. [Google Scholar]
- Goldman HM, Bromage TG, Boyde A, Thomas CDL, Clement JG. Intrapopulation variability in mineralization density at the human femoral midshaft. J. Anat. 2003a;203:243–255. doi: 10.1046/j.1469-7580.2003.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman HM, Bromage TG, Thomas CDL, Clement JG. Preferred collagen fiber orientation in the human mid-shaft femur. Anat. Rec. 2003b;272A:434–445. doi: 10.1002/ar.a.10055. [DOI] [PubMed] [Google Scholar]
- Hough S. Fast and slow bone losers – Relevance to the management of osteoporosis. Drug Aging. 1998;12(Suppl 1):1–7. doi: 10.2165/00002512-199812001-00001. [DOI] [PubMed] [Google Scholar]
- Johnston CC, Norton J, Khairi MRA. The heterogeneity of fracture syndrome in postmenopausal women. J. Clin. Endocrinol. Metab. 1985;61:551–556. doi: 10.1210/jcem-61-3-551. [DOI] [PubMed] [Google Scholar]
- Jowsey J. Age changes in human bone. Clin. Orthop. 1960;17:210–217. [Google Scholar]
- Karasik D, Hannan MT, Cupples LA, Felson DT, Kiel DP. Genetic contribution to biological aging: the Framingham Study. J. Gerontol. A Biol. 2004;59:218–226. doi: 10.1093/gerona/59.3.b218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley ER. The microscopic determination of age in human bone. Am. J. Phys. Anthropol. 1965;23:149–164. doi: 10.1002/ajpa.1330230215. [DOI] [PubMed] [Google Scholar]
- Kerley ER, Uberlaker DH. Revisions in the microscopic method of estimating age at death in human cortical bone. Am. J. Phys. Anthropol. 1978;49:545–546. doi: 10.1002/ajpa.1330490414. [DOI] [PubMed] [Google Scholar]
- Lanyon LE. The success and failure of the adaptive response to functional load-bearing in averting bone fracture. Bone. 1992;13:S17–S21. doi: 10.1016/8756-3282(92)90191-x. [DOI] [PubMed] [Google Scholar]
- Laval-Jeantet AM, Bergot C, Carroll R, Garcia-Schaefer F. Cortical bone senescence and mineral bone density of the humerus. Calcified Tissue Int. 1983;35:268–272. doi: 10.1007/BF02405044. [DOI] [PubMed] [Google Scholar]
- Martin RB, Pickett JC, Zinaich S. Studies of skeletal remodeling in aging men. Clin. Orthop. 1980;149:268–282. [PubMed] [Google Scholar]
- Martin RB. Porosity and specific surface of bone. Crit. Rev. Biomed. Eng. 1984;10:179–222. [PubMed] [Google Scholar]
- Martin RB, Burr DB. Mechanical implications of porosity distribution in bone of the appendicular skeleton. Orthop. Trans. 1984;8:342–343. [Google Scholar]
- McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralisation and microstructure. J. Bone Joint Surg. 1993;75-A:1193–1205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- Melton LJ. Hip fractures; a worldwide problem today and tomorrow. Bone. 1993;14:S1–S8. doi: 10.1016/8756-3282(93)90341-7. [DOI] [PubMed] [Google Scholar]
- Mittlemeier T, Mattheck C, Dietrich F. Effects of mechanical loading on the profile of human femoral diaphyseal geometry. Med. Eng. Phys. 1994;16:75–81. doi: 10.1016/1350-4533(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Myers ER, Hecker AT, Rooks DS, Hipp JA, Hayes WC. Geometric variables from DXA of the radius predict forearm fracture load in vitro. Calcified Tissue Int. 1993;52:199–204. doi: 10.1007/BF00298718. [DOI] [PubMed] [Google Scholar]
- Pouilles JM, Tremollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcified Tissue Int. 1993;52:340–343. doi: 10.1007/BF00310195. [DOI] [PubMed] [Google Scholar]
- Pyle SI, Waterhouse AM, Greulich WW. A Radiographic Standard of Reference for the Growing Hand and Wrist. Cleveland, OH: Press of Case Western Reserve University; 1971. [Google Scholar]
- Rico H, Hernandez ER, Revilla M, Villa LF, Alvarez del Buergo M, Cuendo E. Bone changes in postmenopausal Spanish women. Calcified Tissue Int. 1993;52:103–106. doi: 10.1007/BF00308317. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ. Involutional osteoporosis. N. Engl. J. Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Roche AF, Chumlea WC, Thissen D. Assessing the Skeletal Maturity of the Hand–Wrist: FELS Method. Springfield IL: Charles C. Thomas; 1988. [DOI] [PubMed] [Google Scholar]
- Ruff C, Hayes WC. Cross-sectional geometry of Pecos Pueblo femora and tibiae – a biomechanical investigation I Method and general patterns of variation. Am. J. Phys. Anthropol. 1983;60:359–381. doi: 10.1002/ajpa.1330600308. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Hayes WC. Sex differences in age-related remodeling of the femur and tibia. J. Orthop. Res. 1988;6:886–896. doi: 10.1002/jor.1100060613. [DOI] [PubMed] [Google Scholar]
- Rutherford OM, Jones DA. The relationship of muscle and bone loss and activity levels with age in women. Age Ageing. 1992;21:286–293. doi: 10.1093/ageing/21.4.286. [DOI] [PubMed] [Google Scholar]
- Schaffler M, Burr D. Stiffness of compact bone: effects of porosity and density. J. Biomech. 1988;21:13–16. doi: 10.1016/0021-9290(88)90186-8. [DOI] [PubMed] [Google Scholar]
- Seeman E, Edmonds J, Kotowicz M, Nicholson G, Cumming RG, Martin TJ. The Incidence of Hip Fractures in Women and Men in Australia. Queenstown, New Zealand: Australian and New Zealand Bone and Mineral Society; 1993. p. 45. [Google Scholar]
- Seeman E. The structural basis of bone fragility in men. Bone. 1999;25:143–147. doi: 10.1016/s8756-3282(99)00117-9. [DOI] [PubMed] [Google Scholar]
- Simmons ED, Pritzker KPH, Grynpas MD. Age-related changes in the human femoral cortex. J. Orthop. Res. 1991;9:155–167. doi: 10.1002/jor.1100090202. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Dangerfield P. Human Growth After Birth. 6th. Oxford: Oxford University Press; 1998. [Google Scholar]
- Stein MS, Feik SA, Thomas CDL, Clement JG, Wark JD. An automated analysis of intracortical porosity in human femoral bone across age. J. Bone Miner. Res. 1999;14:624–632. doi: 10.1359/jbmr.1999.14.4.624. [DOI] [PubMed] [Google Scholar]
- Thomas C, Stein M, Feik S, Wark J, Clement J. Determination of age at death using combined morphology and histology of the femur. J. Anat. 2000;196:463–471. doi: 10.1046/j.1469-7580.2000.19630463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk WC. Elementary stress analysis of the femur and tibia. In: Cowin SC, editor. Bone Mechanics. Boca Raton FL: CRC Press; 1989. pp. 43–52. [Google Scholar]
- Wall JC, Chatterji SK, Jeffery JW. Age-related changes in the density and tensile strength of human femoral cortical bone. Calcified Tissue Int. 1979;27:105–108. doi: 10.1007/BF02441170. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ni Q. Determination of cortical bone porosity and pore size distribution using a low field pulsed NMR approach. J. Orthop. Res. 2003;21:312–319. doi: 10.1016/S0736-0266(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Yeni YN, Brown CU, Wang Z, Norman TL. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone. 1997;21:453–459. doi: 10.1016/s8756-3282(97)00173-7. [DOI] [PubMed] [Google Scholar]
- Yeni YN, Brown CU, Norman TL. Influence of bone composition and apparent density on fracture toughness of the human femur and tibia. Bone. 1998;22:79–84. doi: 10.1016/s8756-3282(97)00227-5. [DOI] [PubMed] [Google Scholar]