Abstract
The teleost backbone consists of amphicoelous vertebrae and intervertebral ligaments, both of which include notochord-derived structures. On the basis of a sequential developmental study of the vertebral column of Atlantic salmon (Salmo salar L.) from the egg stage up to early fry stage (300–2500 day-degrees) we show that the vertebral body consists of four layers or compartments, two of which are formed through mineralization of preformed collagenous tissue (the notochordal sheath and the intervertebral ligament) and two of which are formed through ossification. The three inner layers have ordered lamellar collagen matrixes, which alternate perpendicularly from layer to layer, whereas the outer layer consists of cancellous bone with a woven matrix. The bone layers also differ in osteocyte content. In this study we describe the structural details of the layers, and their modes of formation. The results are compared with previous descriptions, and possible phylogenetic implications are discussed.
Keywords: arcocentrum, Atlantic salmon, autocentrum, chordacentrum, development, teleost, vertebral body
Introduction
The backbone, the hallmark of the vertebrate clade, consists in teleosts of amphicoelous vertebrae and intervertebral ligaments, both of which include notochord-derived structures (François, 1966; Laerm, 1976), which, compared with those of birds and mammals, differentiate at a later stage. In the Atlantic salmon, as in other teleosts, the notochord develops into a prominent and specialized hydroskeleton that sustains the locomotion of the larvae after hatching. The notochord of the Atlantic salmon consists of a stratified epitheloid tissue that is enclosed by a thick acellular fibrous sheath. The sheath consists of a thin external elastic membrane with a high content of elastin, covering a thicker collagenous layer.
Although teleost vertebrae exhibit variation in both development and morphology, they have been described as being derived from four major elements, the chordacentrum, the autocentrum, the cartilages of the neural and haemal arches, and the arcocentrum (Arratia et al. 2001). The chordacentra are the first structures in the vertebral body to develop, and they appear as metameric mineralized zones within the notochordal sheath. In Atlantic salmon and zebrafish the notochord seems to generate the segmental pattern of the vertebral column through formation of the chordacentra, which thus may play a key role in further development of the vertebral bodies by patterning the sclerotome (Grotmol et al. 2003, 2005; Fleming et al. 2004). The chordacentrum acts as a foundation for the initial layer of perinotochordal bone, the autocentrum, which is formed by sclerotomal osteoblasts through direct ossification. The arcocentrum originates from direct ossification on the surfaces of the cartilages of the neural and haemal arches, and in some species, this bone may interconnect between the cartilages, and may fuse with the autocentrum (Arratia et al. 2001). Furthermore, the cellularity of vertebral bone varies, and in advanced teleosts the vertebrae may be devoid of osteocytes (Weiss & Watabe, 1979; Ekanayake & Hall, 1987).
In teleosts, a number of details concerning how the various structural elements are integrated and contribute to the functional properties of the vertebral column remain to be elucidated. By studying the development of the vertebrae in the Atlantic salmon, our objective was to elucidate the formation and structure of the individual components of the vertebral bodies, such as the chordacentrum, arcocentrum and autocentrum, and how these are integrated. In the teleost group there are at least five patterns of formation of the vertebral centrum (Arratia, 1991). A detailed anatomical description of the salmonid vertebra, including its ontogeny, may contribute to the comparison of these structures of both fossils and extant species, aimed to elucidate phylogeny.
Materials and methods
Stock maintenance and sampling
Eggs, larvae and juveniles of the Atlantic salmon (Salmo salar L.) were collected from a local commercial hatchery, where they had been held in a flow-through system. At the hatchery the water temperature was kept at 8.0 °C throughout the egg stage, and after hatching, when half of the yolk had been consumed the temperature was raised to 8.5 °C. At start-feeding the temperature was raised to 15.0 °C linearly over a period of 2 days. The fish were fed a commercial feed ad libitum. Developmental stages were classified by day degrees (d°), which are defined as the sum of daily mean ambient water temperatures (°C) for each day of development. Hatching occurred at around 500 d°, while first feeding commenced towards the end of the yolk-sac period, at approximately 870 d°. Developmental stages up to 2500 d° were analysed sequentially. In addition, samples of salmon at the smolt stage (∼100 g) were examined. Samples of 20 fish were collected at intervals of 2 days and brought to the laboratory in bags of oxygenated water. Before the preparative procedures, the fish were anaesthetized with 5% benzocain dissolved in water.
Histology
Specimens for methacrylate embedding were fixed by immersion in a mixture of 10 mL 10% formaldehyde (fresh from paraformaldehyde), 10 mL 25% glutaraldehyde, 20 mL 0.2 m cacodylate buffer and 60 mL phosphate-buffered saline (PBS), and the pH adjusted to 7.35. The larger specimens were decalcified in buffered formic acid for 5–7 days, depending on size. The decalcified specimens were rinsed in PBS and dehydrated in ethanol (50, 70 and 96%), before being embedded in Technovit 7100 (Heraeus Kulzer GmbH & Co., Germany). Sections 1–2 µm in thickness were stained with toluidine blue.
Specimens for paraffin embedding were fixed by immersion in buffered 4% formaldehyde, decalcified (as described above), rinsed in PBS, dehydrated in ethanol, embedded and sectioned at 5 µm. To demonstrate elastin, deparaffinized sections were stained for 30 min with Verhoeff's haematoxylin, rinsed in dH2O and differentiated in 2% ferric chloride for 2 min. Sections were subsequently rinsed in dH2O and treated with 5% sodium thiosulphate for 1 min. They were then dehydrated, cleared and mounted in Mountex (Histolab, Gothenburg, Sweden).
Digital micrographs were acquired with a ProgRes C14 camera (Jenoptik GmbH, Jena, Germany) on an Olympus Vanox AHBT3 microscope (Olympus, Tokyo, Japan), and processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA, USA).
Morphometry
Osteocyte counts (cells per mm2 of bone) were performed on semithin sections of methacrylate-embedded tissue in individual bone layers within a total area of approximately 0.3 mm2 in each of four vertebrae (V30) from four different fish (stage: 2100 d°). The dimensional proportions of the vertebral bodies and the bone layers within the vertebrae were measured at several different developmental stages. For the morphometry, Image-Pro 3.0 software (Media Cybernetics Inc., Silver Spring, MD, USA) was employed.
Transmission electron microscopy (TEM)
Specimens for TEM were fixed and decalcified in the same way as the tissues embedded in methacrylate. They were then rinsed in PBS and in dH2O, before post-fixation in 1% OsO4. Specimens were dehydrated in ethanol (70, 90 and 100%) and embedded in Epon 812 (Fluka Chemie AG, Switzerland). Ultrathin sections were placed on grids, contrasted with uranyl acetate and lead citrate, and viewed in a Phillips CM 200 transmission electron microscope.
Scanning electron microscopy (SEM)
For specimens of vertebral bone, the midregion of the vertebral column (V25 → V31) was heated in water for 15 min at 50 °C, and the surrounding musculature was removed by dissection. Vertebrae were sectioned medially, horizontally and along the transversal plane. Samples were post-fixed in 1% OsO4 for 1 h and rinsed in dH2O, before dehydration in acetone (50, 70 and 100%). Specimens were dried to the critical point, coated with gold–palladium and viewed in a Jeol 6400 scanning electron microscope. X-ray microanalysis was performed with a NORAN Voyager system (Altran, Paris, France).
Whole mounts
Parallel samples of whole specimens were stained with Alcian blue for visualization of cartilage, and with Alizarin red for displaying bone matrix (Taylor & van Dyke, 1985). Specimens were fixed by immersion for 48 h in buffered 4% formaldehyde, pH 7.4. Some samples were stained in 0.1 mg mL−1 Alcian blue in 1 : 4 glacial acetic acid/95% ethanol (pH 4.5) for 16–24 h, according to size. They were then bleached in 0.3% H2O2 in 1% KOH for 1–4 h. The soft tissue was removed for 5–14 days in an enzyme/buffer solution containing 0.4 mg mL−1 trypsin (Sigma, St Louis, MO, USA), proteinase K (Merck, Darmstadt, Germany), pronase (Merck) and lipase (Sigma). Parallel samples were transferred to the enzyme/buffer solution without staining in Alcian blue. They were then washed in buffer, and bleached as described above, before staining with Alizarin red S in 1% KOH for 24–48 h. All specimens were then dehydrated and stored in glycerol.
X-ray microcomputed tomography (micro-CT)
Samples were prepared from newly killed fresh specimens, by dissection of the midregion of the vertebral column. For stable positioning, each specimen was carefully placed in air in the coned part of a 1.5-mL polypropylene Eppendorf tube. To prevent shrinkage movements during scanning due to drying, a small drop of PBS that was not in contact with the sample had been added to the tubes. Scanning was performed in a microcomputed tomography system (SkyScan 1072, SkyScan NV, Aartselaar, Belgium), according to the manufacturer's instructions. The X-ray source was run at 74 kV, and the data sets were acquired with a voxel side length of 5.8 µm. Stacks of tomographic images were reconstructed from primary shadow images using SkyScan software. From these image stacks, three-dimensional (3D) digital models of mineralized tissue were constructed by image thresholding and surface rendering employing Imaris 4.0.5 software (Bitplane AG, Zurich, Switzerland). Images of the 3D reconstructions were processed using Imaris and Adobe Photoshop 7.0.
Results
In order to improve clarity, the main findings are schematically illustrated in Fig. 1, to which the reader may refer when examining the original data.
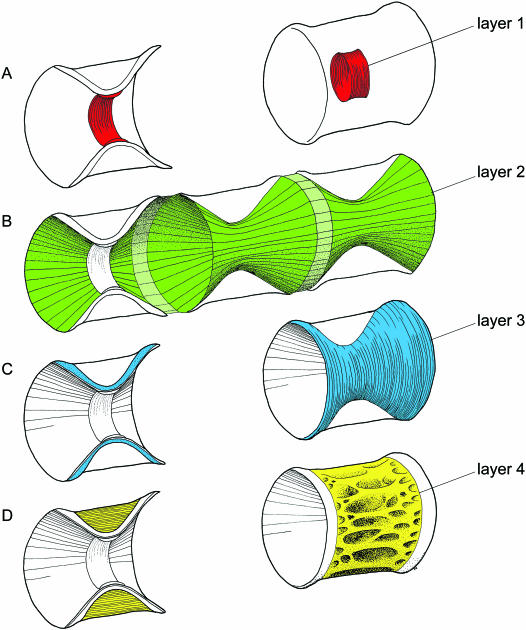
Fig. 1.
Schematic illustrations of the layers within the vertebral body of the Atlantic salmon. The layers are displayed both in medial section and within the whole vertebra. The main direction of the collagen fibres within the matrix of each layer is indicated by lines. (A) Layer 1 (chordacentrum), which forms through mineralization of the notochordal sheath, is shown in red. (B) Layer 2 is a continuous, thin layer that encases the entire notochord. The vertebral part of the layer is shown in green, and the part that participates in forming the intervertebral ligament is in light green. (C) Layer 3 is shown in blue, and makes up the bulk of the laminar bone within the amphicoel. (D) Layer 4 is shown in yellow, and consists of cancellous bone, mainly with an interconnected mesh of longitudinal and transverse trabeculae.
The first segmental structures to form are the cartilages of the neural and haemal arches
The developmental progression of perichordal segmental skeletal structures was initiated at approximately 300 d° through formation of the sclerotome-derived neural and haemal arch cartilages (Fig. 2A). The basal part of the cartilages was situated directly on the external elastic membrane of the notochord. At approximately 650 d°, osteoblasts and perichondral bone could be observed on the surface of the cartilages, and eventually the neural and haemal arches with associated structures, such as zygapophyses, were formed.
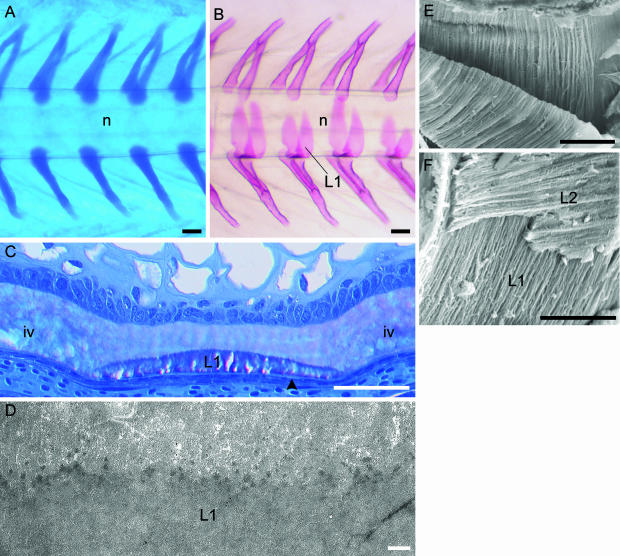
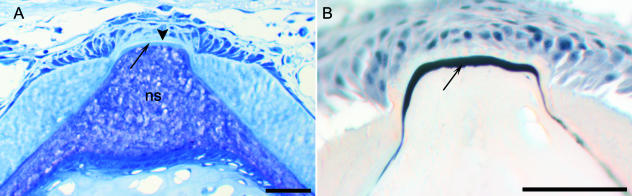
Fig. 2.
Development of the vertebral column of salmon. Formation of the neural and haemal arch cartilages and chordacentra (layer 1 of the vertebral body). (A,B) Micrographs of the caudal region of whole specimens. (A) Alcian blue staining showing the paired neural and haemal arch cartilages, which are situated directly on the external elastic membrane of the notochord. The notochord (n) is indicated. Developmental stage: 400 d°. (B) Alizarin red staining displaying layer 1 (L1) that develops as chordacentra within the notochordal sheath, from the ventral midline. Eventually this layer forms complete mineralized rings that increase in width in both cranial and caudal directions. Thus during early developmental stages, the vertebrae are cylindrical and acquire an amphicoelous shape as the layers external to the chordacentra are formed. The notochord (n) is indicated. Stage: 680 d°. (C) Methacrylate-embedded tissue stained with toluidine blue. Longitudinal section of the ventral part of the notochord. At this stage (700 d°) the notochordal sheath is divided into vertebral and intervertebral (iv) regions. In the vertebral regions layer 1 (L1) (chordacentra) are located in the external half of the notochordal sheath, covered by the external elastic membrane (arrowhead). (D) TEM micrograph of a longitudinal section of the notochordal sheath with a chordacentrum (L1). Stage: 700 d°. At the border between the chordacentrum and the remaining inner non-mineralized part of the notochordal sheath, multifocally distributed electron-dense areas are found. These may constitute initial centra of mineralization. (E) SEM micrograph of a chordacentrum that has been cut longitudinally and torn in two in order to expose the internal matrix structure. The collagen fibres are arranged in parallel cords (d about 1 µm), which run circumferentially in relation to the notochord. (F) Relation between layer 1 (L1) and layer 2 (L2), which both have ordered collagen matrixes that are orientated perpendicular to each other. Scale bars (A,B) 100 µm; (C) 50 µm; (D) 1 µm; (E) 50 µm; (F) 25 µm.
The development of the vertebral bodies is initiated within the notochord
The primordia of the vertebral bodies, the chordacentra, were formed at approximately 680 d° as ring-shaped acellular mineralized zones within the notochordal sheath (denoted layer 1). The first chordacentra appeared in the region beneath the dorsal fin, and from here successive chordacentra developed in both cranial and caudal directions. The mineralization process for each chordacentrum started along the ventral midline, on the inside of the external elastic membrane, and proceeded towards the dorsal side in a bilateral, symmetrical manner, finally forming a complete cylinder (Figs 1A and 2B,C). The thickness of the chordacentra, when initially formed, was approximately half that of the notochordal sheath (Fig. 2C). Except for the most cranial and caudal chordacentra, the mean cranio-caudal length and diameter were approximately 95 µm and 420 µm, respectively. The chordacentra were electron-dense, and the collagen fibres were organized in a circular fashion, following their cylindrical shape (Fig. 2D–F).
The notochord is first encased by a layer of longitudinally orientated collagen fibres
At approximately 800 d°, a thin continuous layer of cranio-caudally orientated collagen fibres (layer 2) surrounded the notochord, in both the vertebral and the intervertebral regions (Figs 1B and 3A). In later stages the longitudinal sectional profile of the layer was tapered, being thinnest in the vicinity of the chordacentra and thickest where it formed the external part of the intervertebral ligament. The fibroblasts in the intervertebral regions were also orientated cranio-caudally, and the collagen matrix produced by these cells made up the layer. As the vertebrae increased in size, the collagen fibres within layer 2 remained generally longitudinally orientated, and made up the entire internal surface of the amphicoel, following its inner contour (Fig. 3B–D). The layer was devoid of cells, and increased only to a thickness of 3–4 µm at the cranial and caudal rims of the vertebrae at 2500 d°.
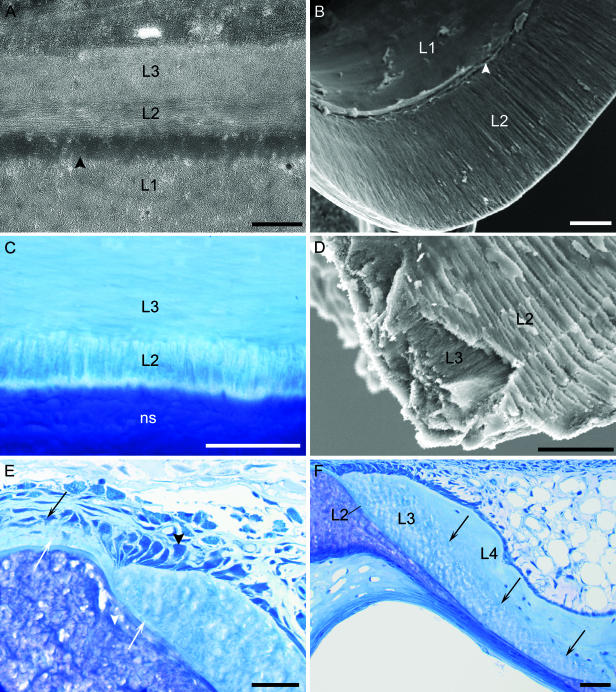
Fig. 3.
The structure of vertebral tissue around the notochord. (A) TEM micrograph of a longitudinal section of the midregion of a vertebra from a specimen at 800 d°. Layer 2 (L2) is formed on the outside of the chordacentrum (L1) and the external elastic membrane of the notochord (arrowhead). This layer is thin and contains longitudinally orientated collagen fibres. Layer 3 (L3) is formed outside layer 2, and its matrix consists of collagen fibres that are orientated perpendicularly to those of layer 2, running circumferentially around the notochord. (B) SEM image of the inner aspect of chordacentrum (L1) and layer 2 from a specimen at 1600 d°. Layer 2 (L2) makes up the inner wall of the conical parts of the amphicoel. Note the cranio-caudal orientatation of the matrix structure. In the midregion of the vertebra, layer 2 is deposited externally to the chordacentrum. The external elastic membrane is indicated by an arrowhead. (C) Light micrograph of a transverse section of a vertebra stained with toluidine blue from a salmon smolt (100 g). Layer 2 (L2) consists of a thin layer of mineralized collagen fibre bundles, here seen in cross-section. Layer 2 is situated between the notochordal sheath (ns) and layer 3 (L3), which is characterized by matrix collagen fibres that run circumferentially around layer 2. (D) SEM micrograph of a fracture at the rim of the amphicoel (inner aspect). The matrix structure within layer 2 (L2) and layer 3 (L3) is lamellar, and the collagen fibres within each of the layers are orientated perpendicularly to each other. Note that layer 2 consists of cylindrical bundles of collagen arranged in bands. (E,F) Light micrographs of longitudinal sections stained with toluidine blue showing the rim of the amphicoel and the intervertebral region at two stages of development. (E) Stage: 2000 d°. Layer 2 is continuous and connects vertebral and intervertebral regions (white arrows). This layer is deposited by fibroblasts (black arrow) as the intervertebral ligament, and is successively mineralized when covered with osteoid from osteoblasts (black arrowhead) forming layer 3. These osteoblasts are polarized and orientated circumferentially along the rim of the amphicoel. (F) Stage: 2000 d°. Both layer 2 (L2) and layer 3 (L3) are tapered, being thickest towards the rim of the amphicoel where they are formed, and thinnest at the middle of the vertebra. The osteocyte content of layer 3 is clearly lower than in layer 4 (L4). The border between these layers is indicated by arrows. Scale bars (A) 1 µm; (B–F) 50 µm.
The main portion of the amphicoel consists of a bone with collagen orientated concentrically around the notochord
In the region of the chordacentrum, osteoblasts condensed outside layer 2, and deposited osteoid with concentric collagen fibres orientated circularly (layer 3), thus encompassing layer 2 and the notochord (Figs 1C and 3A,C–E). The mineralization of layer 2 in the vertebral regions appeared to result from this osteoblast activity forming layer 3. Both these layers developed into the compact bone of the amphicoel (Fig. 3A,C,E). Layer 3 made up the bulk of this compact lamellar bone, and was deposited by a dense population of osteoblasts, mainly located along the cranial and caudal rims of the vertebrae (Fig. 3E). The osteoblasts were up to 20 µm tall, had narrow bases with finger-like extensions, and were polarized along the circumference of the rim of the amphicoel. At the transition between the intervertebral and vertebral regions, osteoblasts were close to the external surface of the intervertebral ligament, and X-ray microanalysis indicated that the mineralization of the ligament took place in this zone. The osteocyte content of the bone of layer 3 was low, approximately 35 cells per mm2. The layer was thin in the midregion of the vertebrae, and increased in thickness towards the cranial and caudal rims, being about 70 µm thick at 2500 d° (Fig. 3F). At this stage, the length and diameter of the vertebral body were equal (approximately 1050 µm), thus giving a conical angle within the amphicoel of about 45°.
The cancellous bone surrounding the amphicoel has distinct morphology
At approximately 1000 d°, deposition of cancellous bone (layer 4) was initiated on the lateral sides of the vertebra, eventually occupying the external concavity of the amphicoel. Although the collagen fibres mainly followed the spatial pattern of the trabeculae, the matrix had a more woven and less dense appearance than that of layers 2 and 3 (Fig. 4A,B). Within layer 4, the first structures to appear were two, and soon after two more, lateral trabeculae, which formed straight plates orientated along the cranio-caudal axis (Fig. 4C). From about 1300 d° short interconnecting transverse trabeculae were formed. As the vertebrae grew, the trabeculae branched to form a complex lattice delimiting a labyrinthine system of intercommunicating spaces, mainly filled with adipose tissue (Figs 1D and 4A,D–F). On the apical ridges of the trabeculae, dense populations of large osteoblasts were observed, whereas in the periost of the horizontal surfaces the osteoblasts had a squamous morphology (Fig. 4E). In layer 4, the density of osteocytes was about ten times higher than in layer 3, with approximately 350 cells per mm2 (Figs 3F and 4A). The bone of the haemal and neural arches had similar properties to layer 4, and in the tail region of the trunk, where the arches were fused to the vertebral body (holospondyli), no demarcation between these structures was observed within the bone.
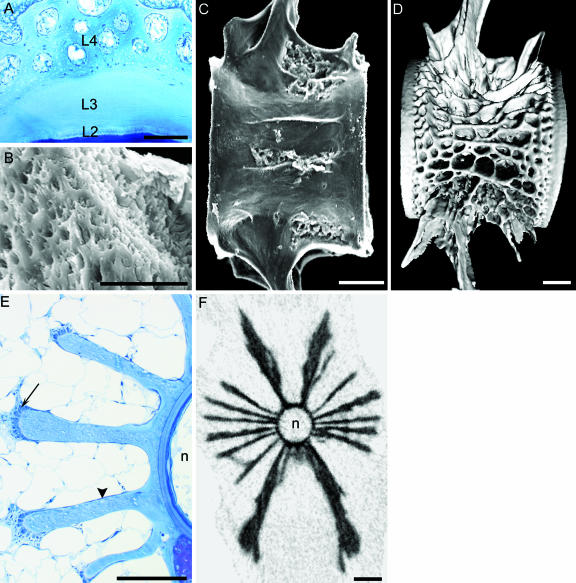
Fig. 4.
The formation and structure of the cancellous bone (layer 4) that forms the outer layer of the vertebral body. (A) Micrograph of a transverse section of a vertebra embedded in methacrylate and stained with toluidine blue, smolt stage. Layer 4 (L4) consists of cancellous bone with cavities, and is characterized by a woven collagen matrix and a high osteocyte content. This layer is formed outside layers 2 (L2) and 3 (L3). Layer 2, which is the thinnest layer of the amphicoel, has a lamellar structure and is acellular. Layer 3, which makes up the bulk of the compact lamellar bone of the amphicoel, has very low osteocyte content. (B) SEM micrograph of the cancellous bone in layer 4. The matrix structure is porous, non-laminar and woven. (C,D) Images of holospondylous vertebrae from the caudal region of the trunk. (C) SEM micrograph of a vertebra from a specimen at 1000 d°. The first structures to form within layer 4 are longitudinal plate-like trabeculae on the lateral sides of the vertebra. (D) Digital 3D reconstruction of micro-CT data of a vertebra at the smolt stage. At this stage layer 4 has a cancellous structure with both longitudinal and transverse trabeculae that occupies the concavity between the biconoid surfaces of the compact bone of the amphicoel. Layer 4 grows through branching and extension of the trabecular plates. The cranial and caudal rims of the vertebra are made up of the compact bone of layers 2 and 3, and a demarcation zone between layer 3 and 4 runs parallel to the rims. The cancellous bone of layer 4 is continuous with the bone of the neural and haemal arches. (E) Transverse methacrylate section of the midregion of a vertebra, stained with toluidine blue. Stage: 2200 d°. Within layer 4 the trabeculae grow through deposition of osteoid from dense aggregations of osteoblasts on the apical surfaces (arrow). As the trabeculae extend they also grow in thickness, which results in a club-shaped cross-sectional profile. The lateral surfaces of the trabeculae are covered with osteoblasts with a squamous morphology (arrowhead). The space between the trabeculae is filled with adipose tissue. The notochord (n) is indicated. (F) Cross-section of a vertebra at the smolt stage reconstructed from the same X-ray micro-CT data-set as in D. Note the open structure of the vertebra, and at this stage there are six main trabeculae on each side in addition to the neural and haemal arches. The notochord (n) is indicated. Scale bars (A) 100 µm; (B) 50 µm; (C) 100 µm; (D) 1 mm; (E) 100 µm; (F) 1 mm.
The outer layer of the intervertebral ligament is continuous with a layer within the vertebral body
The intervertebral ligament was composed of three acellular structural components: the notochordal sheath, the elastic membrane of the notochord and an external, sclerotome-derived collagenous ligament (Fig. 5A,B). In the intervertebral regions, a thickening of both the notochordal sheath and the elastic membrane were observed in association with the formation of the chordacentra. From this point onward, up to 2500 d°, the intervertebral elastic membrane increased in thickness from about 1 to 4 µm. As mentioned above, both the fibroblasts and the collagen matrix of the sclerotome-derived collagenous part of the ligament were orientated cranio-caudally, and were continuous with layer 2 of the vertebrae (Figs 3E and 5A,B).
Fig. 5.
Structures of the intervertebral region. (A) Longitudinal methacrylate section stained with toluidine blue. The intervertebral ligament is composed of an inner notochordal sheath (ns), the external elastic membrane of the notochord (arrow), and the external collagenous ligament (arrowhead), which is an integrated part of layer 2. All these structures are acellular. (B) Longitudinal paraffin section stained for elastin (black) with Verhoeff's haematoxylin. The elastic membrane in the intervertebral region (arrow) thickens continuously during growth, and has a tapered longitudinal profile towards the vertebral region, where it is continuous with the external elastic membrane of the notochord. Scale bar, 50 µm.
Discussion
In this study we show that the amphicoelous vertebrae of the Atlantic salmon consists of four compartments, two of which are formed through mineralization of preformed collagenous tissues (the notochordal sheath and the intervertebral ligament) and two of which are formed through direct ossification. The three inner layers have ordered lamellar collagen matrixes, which alternate perpendicularly from layer to layer, while the outer layer consists of cancellous bone with a more woven matrix. The bone layers also differ in osteocyte content.
Does the vertebral body of salmonids consist solely of a chordacentrum and an autocentrum?
Within the teleost clade, the primitive vertebral structures comprise the neural and haemal arch cartilages, in addition to the chordacentrum, autocentrum and arcocentrum. Although no comprehensive comparative studies are available, there seems to be wide variations in how these components are organized among teleost orders. In primitive groups such as the salmonids, all the components are present, whereas in advanced groups, some of the structures are reduced (Arratia et al. 2001). The vertebral body of salmonids has been described as consisting of a chordacentrum and an autocentrum, where the autocentrum includes both the compact layer of the amphicoel and the external cancellous ornamentation, while the arcocentrum only participates in forming the neural and haemal arches (Arratia et al. 2001) (Fig. 6A). However, our results indicate that the vertebral body of the Atlantic salmon has a more complex structure, comprising a chordacentrum and three distinct layers external to the notochord (Fig. 6B). Layer 2, the innermost of these layers, is an acellular continuous sheath of cranio-caudally orientated collagen fibres that encases the entire notochord, and thus alternates between a mineralized and non-mineralized form in the vertebral and intervertebral regions, respectively. The lack of descriptions of this layer in other teleost species makes comparative considerations difficult, and whether this layer thus belongs to the autocentrum proper or constitutes a phylogenically distinct structure remains to be elucidated.
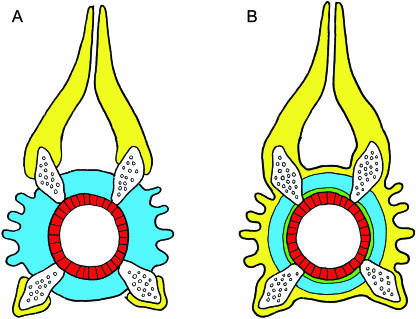
Fig. 6.
Schematic illustration of the individual layers of the vertebra, shown in transverse section. The chordacentrum is in red, the autocentrum in blue and the arcocentrum in yellow. The neural and haemal arch cartilages are indicated. (A) Figure based on Arratia et al. (2001, figs 37G and 40A), illustrating the structural organization of the vertebral body components in a salmonid. In this model the autocentrum includes both the compact lamellar bone of the amphicoel and the ornamented surface of cancellous bone. It is interesting to note that layer 2 is not included. (B) Interpretation of our data in relation to A. The main differences are the presence of layer 2, which is in green, and that the autocentrum only comprises lamellar compact bone of the amphicoel. Thus, in this model, the arcocentrum reaches around the entire vertebral body, and includes the bone of the neural and haemal arches together with the external cancellous bone layer.
Our interpretation is that layer 3 constitutes the autocentrum in the Atlantic salmon. This layer, which is characterized by a highly organized, lamellar matrix structure and a low osteocyte content, makes up the bulk of the compact bone within the amphicoel, and has thus distinct structural properties from those of the surrounding cancellous bone (layer 4). The bone of the neural and haemal arches and the cancellous bone of the vertebral body, which makes up layer 4, have a similar histological morphology, with both a woven collagen matrix and osteocyte content. This supports the notion that the cancellous bone of the vertebral body of the Atlantic salmon is a part of the arcocentrum, which thus reaches around the entire vertebral body (Fig. 6B). This organization of vertebral structures thus differs from that previously described for salmonids (Arratia et al. 2001).
The vertebrae are formed through both mineralization and ossification
The tissue of the salmon vertebrae originates from several distinct processes. The formation of the chordacentrum coincides with a segmentation of the notochord, reflected both by formation of metameric morphological patterns (Grotmol et al. 2003) and by segmental alkaline phosphatase activity within the chordoblast layer (Grotmol et al. 2005). The chordacentrum thus seems to form within the preformed notochordal sheath through mineralization of specific subpopulations of chordoblasts. Our observations indicate that layer 2 is formed by fibroblasts as the external intervertebral ligament, which is successively mineralized when covered with osteoid from osteoblasts located along the circumference of the amphicoel, forming layer 3 (Fig. 7). In contrast, the autocentrum proper (layer 3) and the cancellous part of the arcocentrum form through direct ossification.
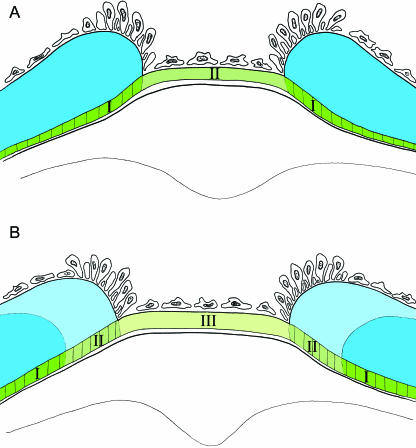
Fig. 7.
Schematic illustration of the intervertebral region at two developmental stages, demonstrating the possible mode of growth. Layer 2 is shown in different shades of green, and the vertebral part of this layer indicated by vertical lines, which is covered by layer 3, is mineralized. Layer 3 is shown in shades of blue. Osteoblasts and fibroblasts are indicated. (A) Layer 2 forms a continuous collagenous structure, which is deposited by fibroblasts in the intervertebral region. The layer consists of a mineralized (I) and a non-mineralized (II) part. (B) Later stage where the part of layer 2, which formed the intervertebral ligament (II) in A, is covered by layer 3 (light blue), and is subsequently mineralized. During this process, new non-mineralized collagen is deposited in the intervertebral region (III).
The osteoblasts of the arcocentrum and autocentrum probably have distinct functional properties
The compact bone of the amphicoel (the autocentrum or layer 3) and the cancellous bone (the arcocentrum or layer 4) are formed by distinct populations of osteoblasts. The autocentrum is deposited by osteoblasts orientated along the cranial and caudal rims of the amphicoel, whereas those forming the arcocentrum are mainly located along the distal ridges of the trabeculae. In mammals, osteoblasts with similar morphology have different molecular characteristics, depending on their location within the bone, probably reflecting a functional diversity (Candeliere et al. 2001). Teleost osteoblast cultures, derived from vertebral bone, also display lineage-specific functional properties (Pombinho et al. 2004), possibly reflecting a different compartmental origin within the vertebra. It is thus probable that the osteoblasts of the arcocentrum and autocentrum in salmon, which deposit bone with a different matrix structure and cellularity (lamellar and cancellous), also possess functional differences at the molecular level. This may reflect separate evolutionary origins of these osteoblast populations.
The open architecture of the vertebral body enables growth without need of remodelling
Our results show that the vertebral bodies of salmon, unlike those, for example, of tetrapods, are ‘open’ structures with no outer delimiting bone elements. The compact bone (layer 3) may thus grow through extension at the rims of the amphicoel, while the cancellous bone (layer 4) may grow by trabecular elongation and branching (Fig. 6). Supporting the notion of growth without remodelling is that no osteoclasts have been detected within the vertebral bodies of teleosts (Witten & Villwock, 1997). Furthermore, the utilization of preformed structures such as the notochordal sheath and the intervertebral ligament, through mineralization, may constitute an efficient and less metabolically demanding mode of growth.
The mechanical properties of the vertebra
In bone, bioapatites are arranged with their axis parallel to the long axis of collagen, and the precipitation of bioapatite is likely to be controlled by a template imposed by collagen (Landis, 1996). In the Atlantic salmon the chordacentrum and the two compact lamellar layers of the amphicoel have matrixes with a perpendicular alternating collagen fibre orientatation. Stabilizing this structure are the reinforcing struts and trusses of the cancellous bone. In addition, the chordacentrum embeds the vertebrae in the notochordal sheath, while the longitudinal collagen fibres of layer 2 that encases the notochord tightly interconnect the vertebrae. How this complex vertebral structure is related to specific mechanical properties remains to be investigated. It is also interesting to note that the collagen matrix of the chordacentrum seems to run circumferentially perpendicular to the cranio-caudal axis. If this reflects the organization of the entire notochordal sheath in salmon, its structure differs from the opposite spiralling collagen fibres (geodesic pattern) found in Xenopus laevis, which is regarded as a common vertebrate trait (Koehl et al. 2000).
Acknowledgments
We gratefully acknowledge the expert help of Marine Harvest Norway AS (Tveitevågen) in rearing of the Atlantic salmon. We thank Nina Ellingsen and Teresa Cieplinska for excellent technical assistance and Irene Nesje for providing some of the material. We are also greatly indebted to Dr Phil Salmon and Dr Elke Van de Casteele at SkyScan NV, Aartselaar, Belgium, where the micro-CT scanning was performed. The Research Council of Norway funded this project.
References
- Arratia G. Caudal skeleton of Jurassic teleosts; a phylogenetic analysis. In: Chang M-M, Liu H, Zhang G, editors. Early Vertebrates and Related Problems in Evolutionary Biology. Beijing: Science Press; 1991. pp. 249–282. [Google Scholar]
- Arratia G, Schultze H-P, Casciotta J. Vertebral column and associated elements in dipnoans and comparison with other fishes: development of homology. J. Morphol. 2001;250:101–172. doi: 10.1002/jmor.1062. [DOI] [PubMed] [Google Scholar]
- Candeliere GA, Liu F, Aubin JE. Individual osteoblasts in the developing calvaria express different gene repertoires. Bone. 2001;28:351–361. doi: 10.1016/s8756-3282(01)00410-0. [DOI] [PubMed] [Google Scholar]
- Ekanayake S, Hall BK. The development of acellularity of the vertebral bone of the Japanese medaka, Oryzias latipes (Teleostei; Cyprinidontidae) J. Morphol. 1987;193:253–261. doi: 10.1002/jmor.1051930304. [DOI] [PubMed] [Google Scholar]
- Fleming A, Keynes R, Tannahill D. A central role for the notochord in vertebral patterning. Development. 2004;131:873–880. doi: 10.1242/dev.00952. [DOI] [PubMed] [Google Scholar]
- François Y. Structure et développement de la vertèbre de Salmo et des téléostéens. Arch. Zool. Exp. Gén. 1966;107:287–328. [Google Scholar]
- Grotmol S, Kryvi H, Nordvik K, Totland GK. Notochord segmentation may lay down the pathway for the development of the vertebral bodies in the Atlantic salmon. Anat. Embryol. 2003;207:263–272. doi: 10.1007/s00429-003-0349-y. [DOI] [PubMed] [Google Scholar]
- Grotmol S, Nordvik K, Kryvi H, Totland GK. A segmental pattern of alkaline phosphatase (ALP) activity within the notochord coincides with the formation of the vertebral primordia. J. Anat. 2005 doi: 10.1111/j.1469-7580.2005.00408.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl MAR, Quillin KJ, Pell CA. Mechanical design of fiber-wound hydraulic skeletons: the stiffening and straightening of embryonic notochords. Am. Zool. 2000;40:28–41. [Google Scholar]
- Laerm J. The development function and design of amphicoelous vertebrae in teleost fishes. Zool. J. Linn. Soc. 1976;58:237–254. [Google Scholar]
- Landis WJ. Mineral characterization in calcifying tissues: atomic, molecular and macromolecular perspectives. Connect. Tissue Res. 1996;34:239–246. doi: 10.3109/03008209609005267. [DOI] [PubMed] [Google Scholar]
- Pombinho AR, Laize V, Molha DM, Marques SMP, Cancela ML. Development of two bone-derived cell lines from the marine teleost Sparus aurata; evidence for extracellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res. 2004;315:393–406. doi: 10.1007/s00441-003-0830-1. [DOI] [PubMed] [Google Scholar]
- Taylor WR, van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985;9:107–119. [Google Scholar]
- Weiss RE, Watabe N. Studies on the biology of fish bone. III. Ultrastructure of osteogenesis and resorption in osteocytic (cellular) and anosteocytic (acelluar) bones. Calcif. Tissue Int. 1979;28:43–56. doi: 10.1007/BF02441217. [DOI] [PubMed] [Google Scholar]
- Witten PE, Villwock W. Growth requires bone resorption at particular skeletal elements in a teleost fish with acellular bone (Oreochromis niloticus, Teleostei: Cichlidae) J. Appl. Ichthyol. 1997;13:149–158. [Google Scholar]