Abstract
The aim of this study was to estimate the number and volume distribution of islets of Langerhans in post-weaning young Wistar rat pancreas and their variation with age. Four groups of six normal Wistar Kyoto rats, at 3, 6, 9 and 12 weeks of age, were used. The whole pancreas was weighed (W), fixed in buffered formaldehyde and embedded in JB4 resin, and 1.5-µm serial sections were obtained. A fraction of whole tissue was obtained in accordance with the multistage fractionator principle and used to estimate total number of islets (Nis). Volume fraction (Vf) of islets and volume-weighted mean volume (Vv) of islets were estimated using point-counting and point-sampled intercept methods, respectively. The numbers of islets increased steadily with age (P < 0.001), whereas the volume-weighted mean volume of individual islets was not significantly different among all age groups of rats (P > 0.05). There was a strong positive correlation between total islet number and islet mass (r = 0.96, P = 0.001), and between volume fraction and islet mass (r = 0.969, P = 0.001). However, there was a weak positive correlation between volume fraction and volume-weighted mean islet volume (r = 0.6, P = 0.002) in the age window investigated. These findings indicate that there was an increase in the number and volume fraction of islets with age in post-weaning young rats but that individual islet volume did not change significantly. It appears the mechanism of alteration in islet morphology in the rat is mainly by the formation of new islets while keeping their individual volume distribution unchanged.
Keywords: islet volume, multistage fractionator
Introduction
The total mass of pancreatic islet cells is a critical factor in the regulation of glucose homeostasis. The total islet-cell mass consists of a dynamic cell population that either expands or declines to adapt to altered physiological conditions. Because β cells are the most abundant cell type in the endocrine pancreas, changes in their biological dynamics are the most important factors that determine islet morphology. Previous studies in the growing pancreas have shown a significant increase in β-cell mass with age (Weir et al. 1990; Bonner-Weir, 1994; Pick et al. 1998). The total β-cell mass and the body weight in rats are linearly correlated after the first month of life, thus indicating an adaptive capacity of β cells (Montanya et al. 2000).
Although changes in β-cell mass have been studied for many years, questions still remain regarding the variation in total islet number and volume in different physiological and experimental situations. It is generally accepted that in the fetal period, most β cells in the pancreas are formed by differentiation from precursor cells and that after birth, most new β cells result from replication of pre-existing β cells (Hellerstrom et al. 1988). In the post-natal period therefore the balance between β-cell replication and death would be a major determining factor in the number, volume and mass of islets. In rodents, β-cell replication is high during late gestation, is reduced in the neonatal period after weaning, and as reported previously (Swenne, 1983; Finegood et al. 1995) there are no major changes after 30–40 days of age.
In theory, an increase in total islet mass or volume can result from an increase in the total number of pancreatic islets and/or an increase in the size of pre-existing islets. The optimal approach to describe the relationship between these two parameters in different situations would be to determine the absolute distribution of islets with respect to both number and size. Investigators have evaluated islet morphology by means of the two-dimensional (2D) mean profile area of islets and referred to this as a parameter of ‘mean islet size’ (Tse et al. 1990).
In this study, we used a design-based stereological method to estimate the volume and number distribution of whole islets in the pancreas of young rats (age 21–84 days). This method is robust in that it is unbiased and provides 3D characterization of the islets. The volumetric parameter estimated is the volume-weighted mean islet volume of the pancreas using the point-sampled intercept method. By definition, the volume-weighted mean islet volume is the mean islet volume if islets are weighted proportional to their volume. This parameter can be estimated without assumptions regarding the shape of the islets and provides unbiased information of 3D size, in contrast to the commonly used 2D estimates of mean islet profile area. In a situation in which the volume of an object is proportional to the biological function of that object, then the volume-weighted mean volume can also be said to be a function-weighted mean volume. Its efficiency has previously been demonstrated when evaluating nuclear enlargements and tumour malignancy (Nielsen et al. 1986; Ladekarl, 1998). The fractionator (Gundersen, 1986) is an unbiased and efficient method to estimate any additive geometric quantity – such as number, length, surface area or volume – defined on a bounded object. The number distribution is estimated using the multistage fractionator method.
The volume-weighted mean volumes were determined by systematic random sampling (Michel & Cruz-Orive, 1988) using the stereological method of point-sampled intercepts according to Gundersen & Jensen (1985). The benefit of this method is that it is assumption-free and unbiased but design-based and therefore highly efficient (Mayhew & Gundersen, 1996).
It has been suggested that the cell mass of islets present in the pancreas, particularly that of β cells, plays an essential role in determining the amount of insulin that is secreted, and can be regulated to maintain euglycaemia in various metabolic conditions (Weir et al. 1990; Bonner-Weir, 1994). Thus the aim of the study was to estimate the number and volume distribution of islets in the rat pancreas and to correlate these parameters with the age. We also wanted to determine if there was any correlation between volumetric and numeric parameters of islets within the lifespan window studied.
Materials and methods
Animals
The morphological changes of pancreatic islets in the developing pancreas were studied in male Wistar Kyoto rats. The animal experiments were conducted in accordance with the Sultan Qaboos University medical research and ethics committee (MREC) Animal Care and Use guidelines. The animals were housed in cages in the same stable with standard rat diet and water ad libitum. The day of birth was taken as day 0, and weaning was performed at day 20. A total of 24 rats were divided into four groups of six animals; the first group was killed 1 day post-weaning (21 days old), the second group at 22 days post-weaning (42 days old), the third group at 43 days post-weaning (63 days old) and the fourth group at 64 days post-weaning (84 days old).
Tissue processing
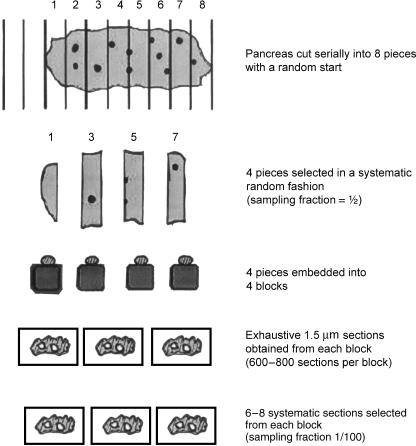
Pancreases were removed from the concavity of the duodenum, weighed, and fixed at room temperature in 2.5% glutaraldehyde, 0.8% paraformaldehyde and 0.025% CaCl2 in 0.1 m sodium cacodylate buffer (pH 7.4) for 24 h; after fixation each pancreas was cut into eight pieces (approximately 1 mm3). Four pieces were selected in a systematic random manner (Gundersen & Jensen, 1987) (e.g. samples 3, 5, 7, 1). The samples taken from each animal were dehydrated in ascending concentrations of alcohol and embedded into blocks with JB4 resin kit (Polysciences Inc., Warrington, PA, USA). From each sampled block of tissue, exhaustive 1.5-µm serial sections were obtained. Representative sections (every 50th) were selected and stained with toluidine blue and observed on a Leica microscope fitted with a digital camera. Digital micrographs obtained were analysed using stereology software (Histometrix) installed on an IBM-compatible personal computer.
Stereology and sampling scheme
Estimating total number of islets
Each pancreas was cut into eight pieces and four pieces were selected (sampling fraction ssf1 = ½). Each sample was embedded into a block. From each block, exhaustive 1.5-µm serial sections were obtained. From the stack of sections the maximum diameter of islets was measured. This diameter was used in determining the separation between sampled sections so that the overlap of islet profiles in sampled sections is minimized. Using this information every 100th section was selected (ssf2 = 1/100). Six to eight sections were obtained from each block (Fig. 1). From each section the number of profiles of islets of Langerhans was counted (Q).
Fig. 1.
Sampling protocol used in the multistage fractionator. Each pancreas is cut in to eight pieces, of which four are selected and embedded into four blocks. Serial sections were obtained from each block and 6–8 sections systematic randomly selected.
The number of islets Nis in each pancreas from six sections is given by
Estimating volume-weighted mean islet volume
From the sampled sections of the pancreas from each animal, random test points were thrown. Those points that fall on the islets’ profile (p) were counted and the remaining points on the rest of the section (P) also counted. The volume fraction (Vf) of islets on all section was obtained from
Those points that fall on the islets’ profile were also used to estimate the volume-weighted mean islet volume using the point-sampled intercept method (Gundersen, 1986). Briefly, a line is drawn in an isotropic direction passing through the test point to the boundary of the islet profile. The length l of the line (intercept) is measured. Each intercept was cubed (l3) for each islet and the mean of all values in each section was multiplied by π/3 to obtain an estimate of volume-weighted mean islet volume (Vv):
Estimating number-weighted mean islet volume
To estimate the number-weighted mean volume VN(i) of islets, first the numerical density NV(i) was estimated. This was carried out first by serially sectioning each block and selecting every 50th section. A total of 12 sections were selected from each block. Six pairs of 1.5-µm sections 50 slices apart (distance between section pairs = 75 µm) were selected and used to estimate NV(i) using the dissector method (Pakkenberg & Gundersen, 1988). Briefly, a low-power digital image of the pancreas was obtained from each of the pair of sections. These images were loaded into stereology software (Histometrix MIL6, Kinetic Imaging Ltd) and used to estimate NV(i) using the equation
where, a/f is the area of the sampling frame (calculated by the software = 3.45E08 µm2), h is the distance between sections = 75 µm, and Σ Q− is the total number of islet profiles on one section but not on the ‘look-up’ section.
By using the estimates of NV(i) and Vf the number-weighted mean volume VN(i) of islets was obtained:
where Vf is the volume fraction of islets and NV(i) is the numerical density of islets.
Data analysis
Data are reported as mean ± SEM. Comparison of means was done using the analysis of variance (ANOVA) test followed by Dunn's multiple comparison post-hoc test. The selected criterion for statistical significance was when the two-tailed P-value was less than 0.05. Correlation analysis based on Pearson's correlation coefficient r was carried out on the paired data of volume fraction of islets and volume-weighted mean islet volume, and the total number of islets and islet mass. The relationship between age and morphometric parameters estimated was also investigated in a linear regression model based on the least-squares fit.
Results
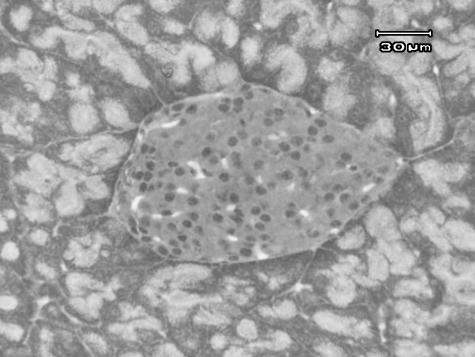
The appearance of the islets is shown in Fig. 2 surrounded by acini of exocrine pancreas. Numerous capillaries can be seen between the endocrine cells. Islets were found to have an average maximum diameter of 100–150 µm. The mean mass of pancreas in different age groups is given in Table 1.
Fig. 2.
Micrograph of an islet of Langerhans within exocrine pancreas.
Table 1.
Mean (±SD) pancreatic parameters in post-weaning and young adult Wistar rats
| Age group (weeks) n = 6 | Pancreas mass (mg) | Islet mass (mg) | Number density Nv (µm−3) | Number-weighted volume VN (µm3) | Volume-weighted volume Vv (µm3) | Coefficient of variation of volume distribution (CV) |
|---|---|---|---|---|---|---|
| 3 | 325.8 ± 3.2 | 18.25 ± 0.18 | 1.884E-08 ± 0.12E-08 | 3.048E06 ± 0.19E-06 | 3.49E06 ± 0.05E06 | 0.38 |
| 6 | 395.5 ± 18.8 | 32.39 ± 1.5 | 2.604E-0 ± 0.08E-08 | 3.21E06 ± 0.17E-06 | 3.55E06 ± 0.16E06 | 0.32 |
| 9 | 454.5 ± 15.7 | 41.4 ± 1.4 | 3.140E-08 ± 0.18E-08 | 2.98E06 ± 0.18E06 | 3.8E06 ± 0.14E06 | 0.52 |
| 12 | 554.8 ± 17.4 | 60.95 ± 1.9 | 3.633E-08 ± 0.16E-08 | 3.11E06 ± 0.23E06 | 3.95E06 ± 0.06E06 | 0.52 |
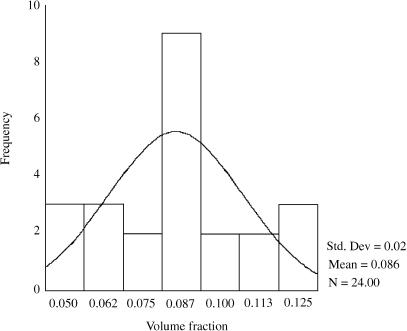
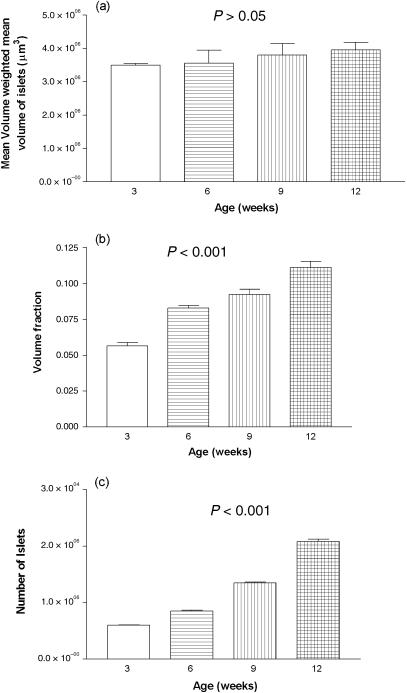
The frequency distribution of standardized residuals of the volume fraction is shown in Fig. 3. It indicates that the data are approximately normally distributed. Volume-weighted mean volume of islets was 3.49E106µm3± 0.58E106 (SEM) in 21-day-old (3-week) old rats and 3.95 E106 µm3 ± 0.06E106 (SEM) in 84-day-old (12-week) rats, representing an increase of 11.6% (P > 0.05) (Fig. 4a).
Fig. 3.
Frequency distribution of standardized residuals of the volume fraction data indicating that it is fairly normally distributed.
Fig. 4.
Bar graph of (a) volume-weighted mean islet volume, (b) volume fraction and (c) total number of islets and their variation with age.
A summary of the volume distribution, total pancreatic mass and total islet mass is presented in Table 1. Numerical density Nv rose from 1.88E10−8 µm−3 ± 0.12E10−8 (SEM) in 21-day-old (3-week) rats to 4.89E10−8 µm−3 ± 0.17E10−8 (SEM) in 84-day-old (12-week) rats, an increase of 61.5% (P < 0.05). Number-weighted mean volume of islets was 3.04E106 µm3 ± 0.19E106 (SEM) in 21-day-old (3-week) rats and 2.27E106 µm3 ± 0.07E106 (SEM) in 84-day-old (12-week) rats, representing an decrease of 25.3% (P < 0.05). The coefficient of variation for the frequency distribution of islet volumes is also given in Table 1. Total pancreatic mass increased from 325.8 mg ± 3.2 (SEM) in 3-week-old rats to 554.8 mg ± 17.4 (SEM) in 12-week-old rats, an increase of 70% (P < 0.001). Mean islet mass increased from 18.25 mg ± 0.18 (SEM) in 3-week-old rats to 60.95 mg ± 1.9 (SEM) in 12-week-old animals, an increase of 233% (P < 0.001) (Table 1).
The mean volume fraction of islets in 21-day-old (3-week) rats was 0.0565 ± 0.002. This had progressively increased to 0.111 ± 0.004 in 84-day-old (12-week) rats, an increase of 49.5% (P < 0.001) (Fig. 4b). The number of islets increased steadily from 6.017E103 ± 0.1E103 (SEM) in 21-day-old (3-week) rats to 2.081E104 ± 0.43E104 (SEM) in 84-day-old (12-week) rats, an increase of 71.1% (P < 0.001) (Fig. 4c). The islet mass (Vf islets × weight of pancreas) in 21-day-old (3-week) rats was 18.25 mg ± 0.18 (SEM) and 68.95 mg ± 1.9 (SEM) in 84-day-old (12-week) rats, an increase of 75.5% (P < 0.001).
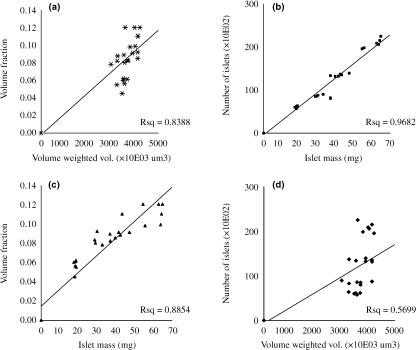
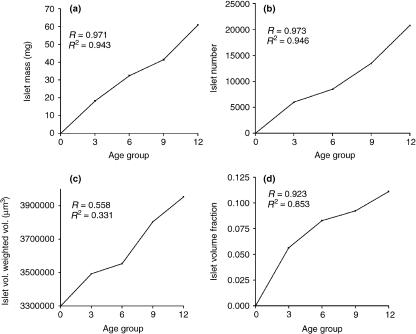
There was a weak positive correlation between volume fraction and volume-weighted mean islet volume (r = 0.6, P = 0.002). However, there was a strong positive correlation between total islet number and islet mass (r = 0.96, P = 0.001) and between volume fraction and islet mass (r = 0.969, P = 0.001) in the age window investigated (Fig. 5). Linear regression analysis scatter is shown in Fig. 6 It indicates high correlation between observed and predicted values in islet number, islet mass and volume fraction (R > 0.9; R2 > 0.9) but not volume-weighted mean volume (R < 0.6; R2 < 0.5).
Fig. 5.
Correlation scatter between paired data: (a) Vf and Vv, (b) Nis and W, (c) Vf and W, and (d) Nis and Vv. Notice weak correlation between Vv and other parameters.
Fig. 6.
Regression model plot between age and (a) islet number, (b) islet mass, (c) volume-weighted mean islet volume, and (d) volume fraction. Notice poor model fit between age and volume-weighted mean islet volume in (c).
Discussion
The mass of pancreatic islets is dynamic and can be modified to maintain normoglycaemia in response to changes in metabolic demand. Islet mass depends on, among other factors, the changes in α- and β-cell formation, individual cell size and rate of cell death. The balance between these elements determines whether islet mass is increased, remains stable or is reduced. In this study, we determined islet mass and number from 3 to 12 weeks of life in the Wistar rat strain. In addition, we also estimated the volume fraction of islets within the whole pancreas, and the volume-weighted mean volume of individual islets at 3-week intervals within the same age window. The results indicate that the cell mass of pancreatic islets increases with age throughout young adult life. This increase is probably due to an increase in the mass of β cells because they are the predominant cell type in the islet (Leeson et al. 1998). Their apoptosis is also known to be a major determinant of the dynamics of islet mass in normal rat pancreas in the neonatal and immediate post-partum periods (Scaglia et al. 1995, 1997), during which increased apoptosis has been shown to determine the remodelling of β-cell mass. At a macroscopic level, this increase in islet mass could possibly be due to an increase in the number of islets rather than size of pre-existing islets, as evidenced by an increase in volume fraction, while the mean volume of individual islets remained fairly constant. Previous studies on β-cell replication rates have shown that β-cell birth rate is about 3–4% in post-weaning normal rats, which then declines to between 1 and 3% in young adult rats (Swenne, 1983; Montanya et al. 2000). These replication rates correlate very closely if extrapolated to the number distribution in this study (assuming that β cells account for most of the changes in islets). The increase in islet mass with age reported here is in accordance with earlier studies on cellular dynamics of islets in young adult rodents (Hellman, 1959; McEvoy, 1981).
The increasing volume fraction of islets with age indicates the addition of new islet tissue to the existing tissue. This increase could be primarily due to (among other reasons) a specific increase in β-cell mass and surrounding connective tissue. However, the individual islet volume in three dimensions (volume-weighted mean volume) appeared not to have changed significantly with age even though the overall volume fraction (and therefore presumably the total volume in three dimensions) of islets had. One possible explanation for this phenomenon is that the size of individual islets is predetermined and once that size limit is reached, it stops growing. New islets can then be formed from the existing ones by budding off ‘daughter’ islets, which then continue to grow until they also reach the maximum possible size and the process then repeats itself. This suggestion is supported by the lack of strong correlation between volume fraction and volume-weighted mean volume of islets as well as between islet number and volume-weighted mean volume. In this way the number of islets, volume fraction and mass will progressively increase while individual islet volume will remain relatively constant. Another possible explanation could be that once the islet reaches the predetermined size, it undergoes some sort of fission whereby the islet disintegrates (undergoes fission) into smaller islets that will then continue to grow until they reach the maximum size and the process repeats itself. In this scenario, there will be a huge variation between the number and weighted volume which was observed here. It seems plausible to suggest that a combination of ‘fission’ and ‘budding off’ is taking place during islet growth in the rat pancreas.
An increase in Nv with age is indicative either of an increase in islet number accompanied by a comparatively smaller increase in the whole pancreas volume, or shrinkage of pancreas volume. The progressive increase in islet mass observed makes the former possibility most plausible. Number-weighted volume is smaller compared to Vv and appears to show a downward trend with age. This decrease in number-weighted volume with age suggests that the rate of formation of new, smaller islets from existing ones decreases as age advances. This also seems to tally with the observation that the volume-weighted volume does not change significantly as many of the new islets reach a steady predetermined size. However, the coefficent of variation for the frequency distribution of islets appeared to be increasing with age. It is possible that as age advances beyond the window studied here, the variation will probably sharply decline provided no new islets are formed and existing islets all reach a steady size. In that case the number-weighted volume will be nearly equal to the volume-weighted volume.
Regression analysis modelling reveals a very good model fit for islet number, volume fraction and mass with age but not for volume-weighted mean volume. If this model holds true in all ages within the window of 3–12 weeks, then any of the above three parameters (dependent variable) can be reliably predicted by the equation
In summary, we have found that in post-weaning and young adult rats the volume-weighted mean volume of individual islets does not vary significantly with age although the volume fraction increases with age. Total islet number and mass show a linear increase with age and correlate in a linear fashion with one another. This study provides a focused perspective for understanding the dynamics of pancreatic islets in young adult and post-weaning animals.
References
- Bonner-Weir S. Regulation of pancreatic β-cell mass in vivo. Recent Prog. Horm. Res. 1994;49:91–104. doi: 10.1016/b978-0-12-571149-4.50008-8. [DOI] [PubMed] [Google Scholar]
- Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of b-cell mass in the growing rat pancreas: estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. Stereological estimation of the volume-weighted mean volume of arbitrary particles observed on random sections. J. Microsc. 1985;138:127–142. doi: 10.1111/j.1365-2818.1985.tb02607.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG. Stereology of arbitrary particles: a review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hellerstrom C, Swenne I, Andersson A. Islet replication and diabetes. In: Lefebvre PJ, Pipeleers DG, editors. The Pathology of the Endocrine Pancreas in Diabetes. Berlin: Springer-Verlag; 1988. pp. 141–170. [Google Scholar]
- Hellman B. The volumetric distribution of the pancreatic islet tissue in young and old rats. Acta Endocrinol. 1959;31:91–106. doi: 10.1530/acta.0.xxxi0091. [DOI] [PubMed] [Google Scholar]
- Ladekarl M. Objective malignancy grading: a review emphasizing unbiased stereology applied to breast tumors. APMIS Suppl. 1998;79:1–34. [PubMed] [Google Scholar]
- Leeson TS, Leeson CR, Paparo AA. The digestive system In Text and Atlas of Histology. Philadelphia: W.B. Saunders Co; 1998. pp. 471–472. [Google Scholar]
- Mayhew TM, Gundersen HJG. ‘If you assume you can make an ass out of u and me’: a decade of the dissector for stereological counting of particles in 3D space. J. Anat. 1996;188:1–15. [PMC free article] [PubMed] [Google Scholar]
- McEvoy RC. Changes in the volumes of the α-, β-, and δ-cell populations in the pancreatic islets during the postnatal development of the rat. Diabetes. 1981;30:813–817. doi: 10.2337/diab.30.10.813. [DOI] [PubMed] [Google Scholar]
- Michel RP, Cruz-Orive LM. Application of the Cavalieri principle and vertical sections method to lung; estimation of volume and pleural surface area. J. Microsc. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Montanya E, Nacher V, Biarnes M, Soler J. Linear correlation between β-cell mass and body weight throughout the lifespan in Lewis rats: role of β-cell hyperplasia and hypertrophy. Diabetes. 2000;49:1341–1346. doi: 10.2337/diabetes.49.8.1341. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Colstrup H, Nilsson T, Gundersen HJG. Stereological estimates of nuclear volume correlated with histopathological grading and prognosis of bladder tumor. Virchows Arch. B Cell Pathol. 1986;52:41–54. doi: 10.1007/BF02889949. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Total number of neurons and glial cells in human brain nuclei estimated by the dissector and fractionator. J. Microsc. 1988;150:1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Pick A, Clark J, Kubstrup C, et al. Role of apoptosis in failure of beta cell mass compensation for insulin resistance and β-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of β cell mass in the post-partum rat pancreas. Endocrinology. 1995;136:5461–5468. doi: 10.1210/endo.136.12.7588296. [DOI] [PubMed] [Google Scholar]
- Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- Swenne I. Effects of aging on the regenerative capacity of the pancreatic beta cell of the rat. Diabetes. 1983;32:14–19. doi: 10.2337/diab.32.1.14. [DOI] [PubMed] [Google Scholar]
- Tse EO, Gregoire FM, Reusens B. Changes of islet size and islet size distribution resulting from protein malnutrition in lean (Fa/Fa) and obese (fa/fa) Zucker rats. Obes. Res. 1990;5:563–571. doi: 10.1002/j.1550-8528.1997.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S, Leahy JL. Islet mass and function in diabetes and transplantation. Diabetes. 1990;39:401–405. doi: 10.2337/diab.39.4.401. [DOI] [PubMed] [Google Scholar]