Abstract
Mineralization density and collagen fibre orientation are two aspects of a bone's microstructural organization that influence its mechanical properties. Previous studies by our group have demonstrated a distinctly non-random, though highly variable, spatial distribution of these two variables in the human femoral cortex. In this study of 37 specimens, these variables are examined relative to one another in order to determine whether regions of bone demonstrating higher or lower mineralization density also demonstrate a prevalence of either transversely or longitudinally oriented collagen fibres. An analysis of rank-transformed collagen fibre orientation (as determined by circularly polarized light) and mineralization density (as determined by backscattered electron microscopy) data sets demonstrated that areas of low mineralization density (predominantly in the anterior-lateral cortex) tended to correspond to regions of higher proportions of longitudinally oriented collagen fibres. Conversely, areas of higher mineralization density (postero-medially) tended to correspond to regions of higher proportions of transversely oriented collagen fibres. High variability in the sample led to generally low correlations between the two data sets, however. A second analysis focused only on the orientation of collagen fibres within poorly mineralized bone (representing bone that was newly formed). This analysis demonstrated a lower proportion of transverse collagen fibres in newly formed bone with age, along with some significant regional differences in the prevalence of collagen fibres of either orientation. Again high variability characterized the sample. These results are discussed relative to the hypothesized forces experienced at the midshaft femur.
Keywords: bone microstructure, collagen fibre orientation, femur, human, mineralization density, image analysis
Introduction
The mechanical properties of bone are influenced by a variety of material and structural properties that include not only how much bone tissue is present in a given cross-section, but also how that tissue is organized, in terms of bone vs. space, amount of mineral and orientation of its collagen component. All of these aspects of a bone's structure contribute to the quantity and quality of bone tissue, and resultant macrostructural properties of the bone organ. However, little is understood about how different aspects of the bone's microstructure may relate to one another, as few studies have incorporated multiple microstructural features into a single analysis. With more researchers advocating an increasingly integrative approach to the study of bone biology (e.g. Martin, 1993; Seeman, 1997), it is important to continue to explore these variables, and their relationship to one another and to clinical measures of bone status. Their combined study will enhance our understanding of their individual and compensatory influences on bone strength, and their contribution to the aging process in terms of changes in the quality, quantity and distribution of bone.
In this study, we examine two aspects of bone's microstructure – collagen fibre orientation and mineralization density – that have been shown to have a strong influence on the mechanical properties of bone (Martin & Ishida, 1989; Martin & Boardman, 1993), and have a distinctly non-random, though highly variable, spatial distribution in the femoral cortex (Goldman et al. 2003a,b). Both of these microstructural properties play an important role in bone quality, and both influence the mechanical properties of bone.
Bone that is more heavily mineralized has increased breaking stress (Vose & Kubala, 1959; Currey, 1969), although when too mineralized, the bone can become brittle (Currey, 1969; Bonfield & Clark, 1973). As newly formed bone accumulates mineral with time, the most recently deposited bone will generally be less mineralized than bone deposited months or years earlier (although a variety of disease states can affect the mineralization process; see Grynpas, 1993). Therefore, areas that have a higher remodelling rate would be expected to have a higher percentage of bone that is still poorly mineralized, whereas regions with low remodelling rates would be expected to have a higher percentage of high mineralization bone (Boivin & Meunier, 2002).
Different orientations of collagen fibres are thought to be advantageous for resisting mechanical forces. Regions of a bone cortex where collagen is preferentially laid down in a more transverse orientation may be better able to resist compressive forces. Regions of a bone cortex where collagen is preferentially laid down in a more longitudinal orientation are thought to be better able to withstand tensile forces (Ascenzi & Bonucci, 1967, 1968; Riggs et al. 1993a).
Mineralization density and collagen fibre orientation have rarely been studied together in humans relative to the mechanical properties of a bone cortex. In a study of the human femur, Portigliatti-Barbos et al. (1983) suggested that regions of the bone believed to be predominantly loaded in tension (anteriorly and laterally) were more likely to induce remodelling, and therefore demonstrate lower mineralization density. These authors found that these same poorly mineralized cortices also showed a higher proportion of longitudinally oriented lamellae. Conversely, the regions believed to be associated with compression (postero-medial and medial) showed the highest mineralization density and the highest proportion of transverse collagen fibres. These data were based on a sample of two male individuals, both between the ages of 40 and 50 years.
Vincentelli (1978) used microradiography and linearly polarized light to determine whether recent (low mineralization) remodelling events tended to show preferred collagen fibre orientations in adult human tibia (n = 55). The author found that collagen fibres in bone that was still poorly mineralized were more frequently transverse in orientation in older individuals, and longitudinally oriented in younger individuals, indicating an age-related change in the predominant direction that collagen fibres are deposited. Although Vincentelli's study did not examine regional differences across the cortex in the distribution of transverse vs. longitudinal collagen fibre orientation in this recently remodelled bone, it did illustrate how examination of bone of particular mineralization densities could be used to examine age-related variation in predominant collagen fibre orientation.
In the current study, we specifically aim to test these hypothesized relationships between collagen fibre orientation and mineralization density reported in the studies above. To test the hypothesis of Portigliatti-Barbos et al. (1983), collagen fibre orientation and mineralization are examined in terms of their distribution relative to one another, and results are discussed relative to the expected bending forces experienced at the femoral midshaft. The antero-lateral cortex (assumed to be experiencing increased tension) would be expected to have lower mineralization density and higher proportions of longitudinal lamellae, relative to other cortices. The postero-medial cortex (assumed to be predominantly experiencing compression) would be expected to have higher mineralization density and higher proportions of transverse lamellae. To test the hypothesis of Vincentelli (1978), the distribution of collagen fibre orientations within bone of low mineralization density was examined to determine if older individuals showed increased proportions of transversely oriented lamellae in recently formed bone relative to young individuals. In addition, we hypothesized that because more incompletely mineralized bone is likely to have been laid down during a time relatively close to an individual's death, and therefore represents a relatively narrow window of bone deposition time, specifically examining the proportions of low mineralization bone in conjunction with their predominant collagen fibre orientations would provide a better understanding of the biomechanical adaptation of a given bone cortex than an analysis of each variable alone. Therefore, regional variations in the proportion of transversely oriented collagen fibres were examined in regions of recently deposited bone for each age group included in the study.
Materials and methods
Specimen collection
Midshaft femur blocks of 37 individuals collected from the Victorian Institute of Forensic Medicine, Melbourne, Australia, were used in this analysis. This sample represents individuals of known age, sex, height, weight and cause of death. The individuals used in this study represent a subset of the same histological sections examined in Goldman et al. (2003a,b) for variability in collagen fibre orientation and mineralization density. The sample was divided into three age groups, each containing roughly equal numbers of males and females: younger (25–44 years, n = 11), middle (45–64 years, n = 11) and older (65+ years, n = 15), as described in Goldman et al. (2003a). Further information on this sample can be found in Bertelsen et al. (1995), Feik et al. (1996, 2000) and Goldman (2001).
In 11 individuals (five aged 25–40 years, six aged 60+ years) the femur block was removed from the right side, 1 cm superior to the measured mid-point of the femur. Orientation was not recorded at the time of collection in the remainder of the samples and thus specimens were oriented in the medio-lateral plane based on the disposition of microstructural and anatomical features as described in Goldman (2001).
Sample preparation
Bone blocks of approximately 0.5 cm in thickness were removed from the samples and stored in 70% ethanol, prior to cleaning and dehydration in preparation for embedding in a poly methyl methacrylate (PMMA) and styrene mixture according to procedures described in Goldman et al. (1999). PMMA-embedded blocks were sectioned using a method developed to allow thin sections of 100 µm thickness to be imaged by both light and scanning electron microscopy (Goldman et al. 1999). Specimens were polished using a 0.01-µm alumina slurry on a Texmet pad (Buehler Ltd, Lake Bluff, IL, USA) to minimize surface topography. After all light microscopy imaging procedures were completed, specimens were carbon coated using a flash-evaporation process (Emitech, Inc.), in order to make them electrically conductive for backscattered electron microscopy (BSE-SEM) analysis, according to procedures detailed in Goldman et al. (2003b).
Image acquisition
Images of entire midshaft femur cross-sections, suitable for determining collagen fibre orientation (see Bromage et al. 2003), were obtained using a using Leica DMRX/E Universal Microscope configured with circularly polarized light filters (CPLMs) and an automated high-resolution Martzhauser x–y stage. Tiled grey-level images (each 1024 × 768, field width = 2.2 mm) were transferred to a Leica Quantimet High Resolution Image Analysis System (Q600) via a Kodak Megaplus CCD camera. Lighting was standardized through the use of a neutral density filter, the grey level of which was checked and reset to a predetermined setting prior to imaging each specimen. Tiled images were automatically montaged using a dedicated software program developed in our laboratory using Visual Basic 6.0 (Microsoft) and Leadtools Imaging 16/32 ActiveX v. 10 (LEAD Technologies, Inc., Charlotte, NC, USA). These methods, including details on procedures used to mask background (non-bone) regions, are detailed in Goldman et al. (2003a).
Information on mineralization densities was obtained by BSE-SEM imaging of the same specimens using an LEO S440 scanning electron microscope (LEO, Cambridge, UK). Digital grey-level images (each of the same field width as collected during CPLM imaging) were collected at a 15-mm working distance, 20-kV accelerating voltage and approximately 750-pA beam current. LEO Stage Scan software was used to provide automated tiling of BSE-SEM images across the entire bone cross-section. Following the method of Boyde et al. (1995), halogenated dimethacrylate standards were used to monitor the stability of the electron beam during the imaging run and to correct for any errors due to drift in the system that would increase grey-level variability between images (Boyde & Jones, 1996; Boyde et al. 1998). Details on the methodology for normalizing the images, as well as other methodological details, can be found in Goldman et al. (2003b). After normalization, tiled images were automatically montaged, as above.
Rotational alignment with CPLM images would have been very time consuming during live imaging with the BSE-SEM, so it was approximated (usually within 3°) by roughly aligning a TEM grid marker (mounted into place in the upper left-hand corner of the slide, in the first image frame of the montage) to the previously obtained same field of view CPLM image. More precise alignment was achieved post image collection by overlaying the two montages in Adobe Photoshop using the Layering Function, and rotating the SEM image until alignment was achieved (Goldman, 2001).
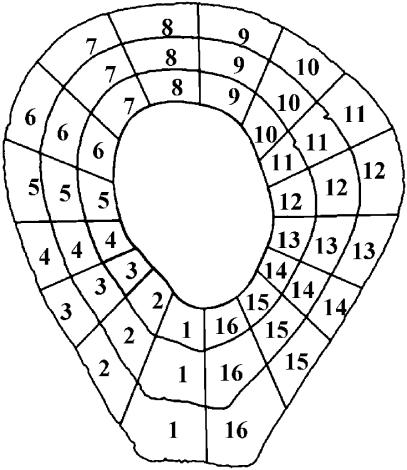
Each grey-level montage was automatically divided into segments using an Optimas Image Analysis (v. 6.5, Media Cybernetics, Inc.) software macro described in Feik et al. (2000) and Goldman (2001) (see Fig. 1). Within each segment, calculations included the number of pixels of each grey level (of a total of 255 grey levels), as well as the mean grey-level value relative to the total area of the segment (not taking into account grey levels of 0, which would indicate regions of non-bone, i.e. marrow spaces, Haversian canals, resorption spaces). This procedure produced data sets of average mineralization density and average collagen fibre orientation for each of the segments. These data were used to examine the relationship between the two variables (using Spearman's correlations on ranked data – see data analysis section below).
Fig. 1.
Using a customized macro, cortex was divided into 16 radial sectors and three rings (periosteal, mid cortex and endosteal), for a total of 48 segments. Only data from the outermost 16 sectors (periosteal ring) were included in the current analysis. All sections were oriented so that medial is to the left and posterior is towards the bottom when viewed on screen or print.
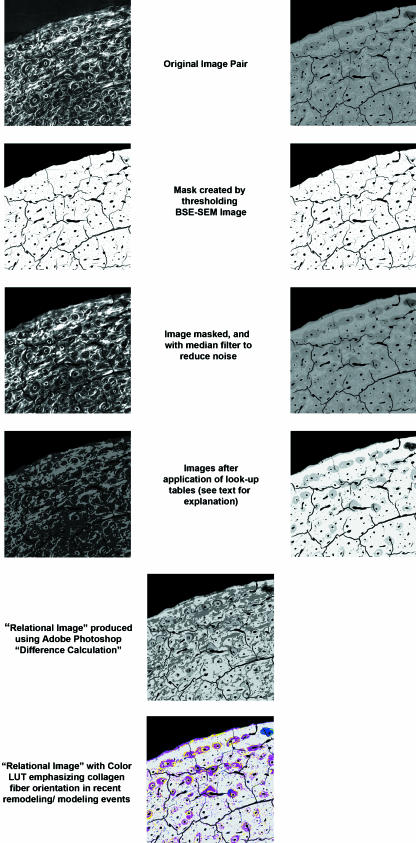
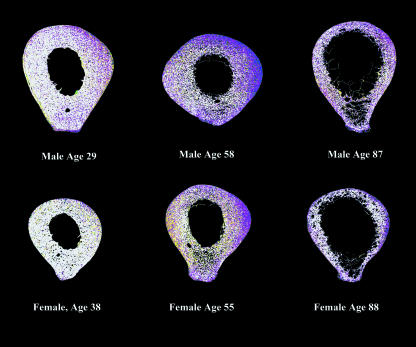
An additional image analytical procedure was carried out to produce ‘relational images’ in order to examine preferred collagen fibre orientation within areas of recently formed, poorly mineralized, bone (this method was described in Goldman et al. 2000 as ‘Procedure 2’). Paired CPLM and BSE-SEM montages from each individual were superimposed and aligned in Adobe Photoshop, and each image was then saved to a separate file (in some cases this was best accomplished by dividing the montage into four or six sections and aligning each separately). This allowed for a more precise alignment than otherwise possible – this was necessary owing to the lower accuracy of the BSE-SEM automated stage in specimen tiling.
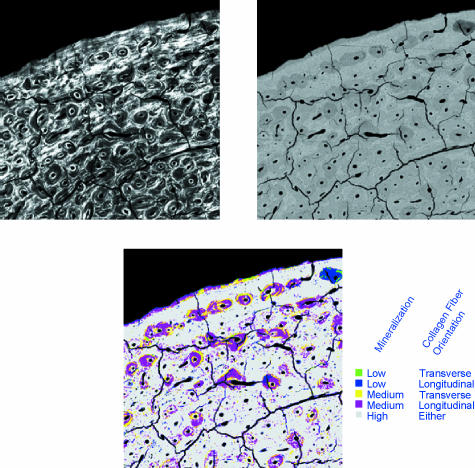
To create a ‘relational’ data set, a colour look-up table (LUT) was applied to divide the CPLM image into two grey-level bins: bright pixels (≥ 128) vs. dark pixels (< 128). An LUT applied to the BSE-SEM image allowed the recognition of five degrees of mineralization of bone tissue, as shown in Fig. 2. The two LUT images were then compared mathematically using the ‘difference’ calculation within Adobe Photoshop (providing an absolute value difference between the images), producing a new synthetic greyscale image. An LUT with colours specifically chosen to emphasize regions of newly formed bone with preferred longitudinal or transverse collagen was then applied, producing a ‘relational image’ (Fig. 3). The relative proportion of transverse collagen fibres within the lowest mineralization bone (corresponding to BSE-SEM grey-level values below 96 and coloured green and blue in the relational image – see Fig. 3) was calculated as the ‘relational value’ for each segment (using the same macro as described previously, see Fig. 1). This image analysis method could not distinguish between poorly mineralized areas of bone organized into Haversian systems vs. those with circumferential lamellar bone – both are included in the analysis below.
Fig. 2.
Illustration of image analytical steps required for creating relational images. Explanation of colour look-up table produced for relational image is provided in Fig. 3. Field width of each image = 3.75 mm.
Fig. 3.
Explanation of colour look-up table used for identifying either transverse or longitudinal collagen fibre orientations within poorly mineralized bone. Field width of each image = 3.75 mm.
Data analysis
Only microstructural data from bone located closest to the periosteum (Ring 1, Fig. 1) were included in this study, as previous microstructural analyses indicated that this bone showed the most significant differences between sectors, illustrative of a patterning of these variables around the cortex (Goldman et al. 2003a,b). In addition, bone from the periosteal ring would be expected to be of more importance for resisting bending forces as it is located furthest from the centroid, or neutral axis.
Rank-transformed mean grey-level data for mineralization and collagen fibre orientation data sets were used to determine whether significant relationships exist between the two variables in any location of the cortex. In addition, line plots were created for variables against their mean ranks to visualize the correspondence between them. Ranked data were grouped into five categories from low to high values and statistically compared using Spearman's rank correlations for each age group (analysed by sector pairs). Sexes were pooled for this analysis because of the small sample size.
Analysis of the relational values was accomplished using non-parametric statistics, as the data were not normally distributed. Specifically, Kruskal–Wallis anova was used to test for significant differences in the distributions of incompletely mineralized, newly formed bone of either preferred collagen fibre orientation between sexes, age groups and regions of the cortex. When significant results were identified, Mann–Whitney tests were used to test for significance between pairwise groupings.
Results
Correlations between collagen fibre orientation and mineralization data sets
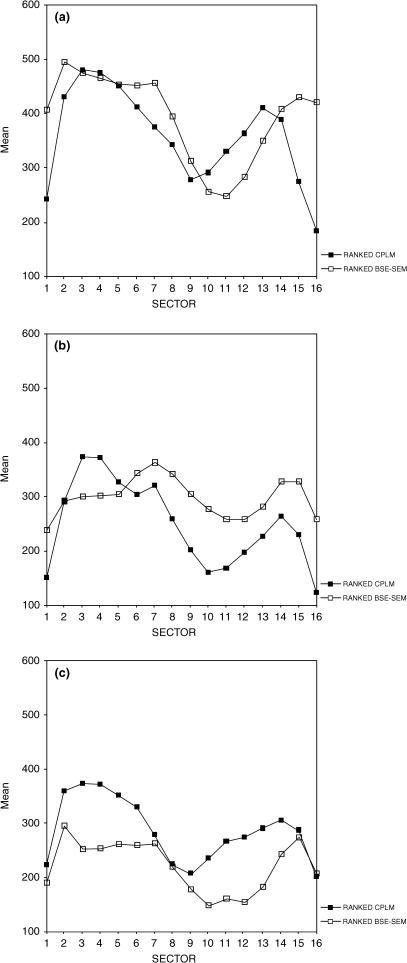
Descriptive statistics for collagen fibre orientation and mineralization density mean grey values (presented by sex for each age group) are provided in Table 1. For each age group, line plots of ranked values of each variable demonstrate that average collagen fibre orientation and degree of mineralization are distributed such that postero-medial and medial aspects tend to display the highest average grey-level values for both microstructural variables, whereas anterior and antero-lateral aspects tend to have lower average grey-level values for both microstructural variables (Fig. 4). In the youngest age group, these relationships could be demonstrated with weak but significant positive correlations between the ranked data sets in postero-medial and lateral cortices (Table 2) However, in the middle age group, medial and antero-medial cortices showed a weak but significant negative relationship (highly mineralized and greater proportion of longitudinal lamellae). In the oldest age group, antero-lateral and lateral cortices did show a weak but significant positive correlation between the data sets.
Table 1.
Descriptive statistics
| Collagen fibre | Mineralization | Relational data† | |||||
|---|---|---|---|---|---|---|---|
| n* | Mean | SE | Mean | SE | Mean | SE | |
| Age Group 1 | |||||||
| Female | 80 | 109.81 | 3.75 | 142.50 | 0.80 | 33.02 | 1.84 |
| Male | 96 | 117.11 | 2.58 | 143.04 | 0.61 | 25.86 | 1.57 |
| Age Group 2 | |||||||
| Female | 96 | 104.04 | 2.28 | 137.00 | 0.46 | 27.90 | 1.00 |
| Male | 80 | 86.56 | 2.10 | 140.53 | 1.13 | 24.47 | 1.05 |
| Age Group 3 | |||||||
| Female | 112 | 99.98 | 1.71 | 137.16 | 0.56 | 24.39 | 1.06 |
| Male | 128 | 102.87 | 2.12 | 133.82 | 0.59 | 19.95 | 0.88 |
Collagen fibre orientation and mineralization density data were calculated from mean grey-level values per sector (on a 0–255 scale).
Each individual is represented by data from 16 sectors.
Calculated as the percentage area of newly formed bone of transverse collagen fibre orientation (bone coloured as green in the relational LUT) relative to the total area of newly formed bone.
Fig. 4.
Line plots showing the average rank by sector for collagen fibre orientation (filled squares) and mineralization (unfilled squares) for each age group (A = Younger, B = Middle, C = Older). Posterior sectors 1, 16; Postero-medial sectors 2, 3; Medial sectors 4, 5; Antero-medial sectors 6, 7; Anterior sectors 8, 9; Antero-lateral sectors 10, 11; Lateral sectors 12, 13; Postero-lateral sectors 14, 15.
Table 2.
Spearman's rank correlatons for ranks of collagen fibre orientation and mineralization data sets
| Location | Correlation coefficient | Significance (two-tailed) |
|---|---|---|
| Age Group 1 (n = 11) | ||
| Postero-medial (sectors 2,3) | 0.475 | 0.026 |
| Medial (sectors 4,5) | 0.396 | 0.068 |
| Antero-medial (sectors 6,7) | −0.115 | 0.610 |
| Anterior (sectors 8,9) | 0.078 | 0.728 |
| Antero-lateral (sectors 10,11) | 0.142 | 0.529 |
| Lateral (sectors 12,13) | 0.484 | 0.022 |
| Postero-lateral (sectors 15,16) | 0.350 | 0.111 |
| Posterior (sectors 16,1) | 0.126 | 0.577 |
| Age Group 2 (n = 11) | ||
| Postero-medial (sectors 2,3) | −0.028 | 0.903 |
| Medial (sectors 4,5) | −0.504 | 0.017 |
| Antero-medial (sectors 6,7) | −0.452 | 0.035 |
| Anterior (sectors 8,9) | 0.167 | 0.459 |
| Antero-lateral (sectors 10,11) | −0.361 | 0.099 |
| Lateral (sectors 12,13) | −0.155 | 0.490 |
| Postero-lateral (sectors 15,16) | −0.309 | 0.162 |
| Posterior (sectors 16,1) | −0.256 | 0.250 |
| Age Group 3 (n = 15) | ||
| Postero-medial (sectors 2,3) | −0.040 | 0.832 |
| Medial (sectors 4,5) | 0.213 | 0.259 |
| Antero-medial (sectors 6,7) | 0.190 | 0.315 |
| Anterior (sectors 8,9) | 0.421 | 0.021 |
| Antero-lateral (sectors 10,11) | 0.401 | 0.028 |
| Lateral (sectors 12,13) | 0.209 | 0.268 |
| Postero-lateral (sectors 15,16) | 0.041 | 0.831 |
| Posterior (sectors 16,1) | −0.354 | 0.055 |
Values in bold type are significant at P < 0.05.
Analysis of relational values
Data were examined following the production of a relational data set for which only poorly mineralized bone was considered, in order to see whether predominant collagen fibre orientation differed between ages, sexes or regions of recently deposited bone matrix (Fig. 5). Descriptive statistics (presented by sex for each age group) are provided in Table 1. Population means for the relational values (% transversely oriented lamellae within newly formed bone, corresponding to areas coloured ‘green’ in the relational image, Fig. 3) were relatively low (mean ± SD = 25.41 ± 12.73%). This was not surprising given our previous study of collagen fibre orientation (Goldman et al. 2003a), which showed that these data tended to be skewed towards darker values (longitudinal fibres) – so that the percent area of longitudinal fibres was higher than that of transverse collagen fibres, even in a relatively ‘bright’ area. This would suggest that even if a cortex did not contain a majority of fibres that were transverse in orientation, but merely a high percentage of these fibres relative to other regions, this cortex could be considered relatively ‘bright’.
Fig. 5.
Examples of relational montages. See Fig. 3 for explanation of colour look-up-table.
Age Group 1
In this age group, females showed significantly higher relational values (i.e. higher proportions of transversely oriented lamellae in their recently deposited bone) than males (P = 0.006). No significant differences were identified between individual sectors in the relational values when sexes were considered separately, but when pooled, significant differences were identified between sectors (P = 0.003). Postero-medial and medial aspects of the bone cortex in this youngest age group tended to contain significantly higher proportions of transversely oriented collagen fibres in recently formed bone than the opposing anterior and lateral aspects, and in some cases, posterior aspects. These results are summarized in Table 3.
Table 3.
Mann–Whitney tests for relational data
| Group 1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 1 | ||||||||||||||||
| 2 | 0.013 | |||||||||||||||
| 3 | 0.002 | n.s. | ||||||||||||||
| 4 | 0.013 | n.s. | n.s. | |||||||||||||
| 5 | 0.034 | n.s. | n.s | n.s | ||||||||||||
| 6 | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||
| 7 | n.s. | 0.013 | 0.002 | 0.010 | 0.019 | n.s. | ||||||||||
| 8 | n.s. | n.s. | 0.010 | 0.040 | n.s. | n.s. | n.s. | |||||||||
| 9 | n.s. | 0.010 | 0.002 | 0.008 | 0.008 | n.s. | n.s. | n.s. | ||||||||
| 10 | n.s. | 0.016 | 0.001 | 0.007 | 0.010 | n.s. | n.s. | n.s. | n.s. | |||||||
| 11 | n.s. | 0.016 | 0.040 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| 12 | n.s. | n.s. | 0.013 | 0.034 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||
| 13 | n.s. | n.s. | 0.040 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||
| 14 | n.s. | n.s. | 0.023 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| 15 | n.s. | 0.034 | 0.008 | 0.034 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| 16 | n.s. | 0.016 | 0.001 | 0.013 | 0.034 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Group 2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 1 | ||||||||||||||||
| 2 | n.s. | |||||||||||||||
| 3 | n.s. | n.s. | ||||||||||||||
| 4 | n.s. | n.s. | n.s. | |||||||||||||
| 5 | n.s. | n.s. | n.s | n.s | ||||||||||||
| 6 | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||
| 7 | n.s. | n.s. | n.s. | n.s | n.s. | n.s. | ||||||||||
| 8 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||
| 9 | n.s. | n.s. | n.s. | n.s | n.s. | n.s. | n.s. | n.s. | ||||||||
| 10 | n.s. | n.s. | 0.000 | 0.019 | 0.023 | n.s. | n.s. | n.s. | n.s. | |||||||
| 11 | n.s. | n.s. | 0.010 | 0.023 | 0.047 | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| 12 | n.s. | n.s. | 0.034 | n.s | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||
| 13 | n.s. | n.s. | 0.040 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||
| 14 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| 15 | n.s. | n.s. | n.s. | n.s | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| 16 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
Data are presented with sexes pooled. No significant differences were found in Age Group 3.
Values in normal type are significant at P < 0.05.
Values in bold type are significant at P < 0.01.
n.s. = not significant.
Age Group 2
In this age group, females again showed higher proportions of transversely oriented lamellae in their recently deposited bone than males, significant at P < 0.05 (P = 0.034). With sexes considered separately, only females showed significant differences between sectors of the cortex in relational values. Owing to small sample size, it was not possible to use Mann–Whitney tests to determine which sector pairs differed. With sexes pooled, differences between sectors were significant at P < 0.05. Mann–Whitney tests demonstrated a few significant differences (P < 0.01) between postero-medial (sector 3) and antero-lateral sectors reminiscent of Age Group 1 (see Table 3).
Age Group 3
In this age group, females again showed significantly higher proportions of transversely oriented lamellae relative to males in their recently deposited bone (P = 0.001). However, no significant differences were found in proportions of transverse lamellae in recently formed bone between individual sectors, with sexes pooled or separated.
Variation between age groups
Young females were found to have significantly (P = 0.001) higher relational values than older age group females. Young and middle and middle and older age groups were not significantly different from one another. Young and middle aged males were found to have significantly (P = 0.005 and 0.001, respectively) higher relational values than older age group males, although young and middle aged males did not differ significantly. These results indicate a decrease in transverse lamellae deposited in newly formed bone with age.
Discussion
Distributions of two aspects of a bone's organization, mineralization density of the matrix and preferred collagen fibre orientation, were considered relative to one another in an effort to understand better how the tissue-level organization of bone might correlate to its mechanical properties. The first analysis presented in this paper tests the hypothesis of Portigliatti-Barbos et al. (1983) concerning the relationship between collagen fibre orientation and mineralization density in the human femur, by examining correlations between two data sets originally analysed separately in Goldman et al. (2003a,b). A second analysis was then carried out, in which a new data set was produced, specifically targeting preferred collagen fibre orientation in regions of newly formed bone matrix (poorly mineralized bone). By isolating bone deposited during a relatively narrow window of time prior to the individual's death, the regional distribution of both collagen fibre orientation could be better appreciated.
The comparison of rank-transformed data sets of collagen fibre orientation and mineralization density demonstrated few significant results. On average, areas of low mineralization density (antero-lateral and lateral; posterior) tended to correspond to regions of higher proportions of longitudinally oriented collagen fibres. Areas of high mineralization density (medial and postero-medial cortices) tended to correspond to regions of higher proportions of transversely oriented collagen fibres, as shown in Fig. 4. Moreover, previous analyses of these two data sets individually (Goldman et al. 2003a,b) demonstrated a significant, non-random pattern for each of these two variables that would seemingly support such trends – in both cases postero-medial and medial sectors were significantly brighter (i.e. more transversely oriented lamellae and highly mineralized bone) than antero-lateral and lateral sectors (most apparent in the youngest age group, more variable in older age groups). In this analysis, however, only a few sector pairs showed significant positive correlations (in the youngest and oldest age groups) between data sets, and these correlations were relatively weak. Moreover, a weak, but significant, negative correlation was detected in the middle age group along the medial cortex, which would not support our hypothesis. To some extent, the small sample size available in this study hampered our analysis and it is possible that with a larger sample size, our results would improve. However, considering the large degree of variation identified in other studies of the microstructure of this population with larger sample sizes (e.g. Feik et al. 1997; Goldman et al. 2003a), it is possible that a simple correlation between these two variables may not be detectable at the population level. Rather, we think it is important to understand some of the possible complexities in the relationship that might contribute to the relative lack of correlation demonstrated in this study.
In this study, we based our hypothesized relationship between these two variables on the assumption that bending was the force that would be predominantly influencing the microstructure at the femoral midshaft (after Blaimont, 1968; Amtmann, 1971; Pauwels, 1980). Moreover, our hypothesis assumed, based on the predominant literature, and the present understandings of the relationship between bone curvature and bending forces (i.e. Biewener, 1991), which cortices would be predominantly loaded in compression vs. tension. In reality, the loading situation of the femoral diaphysis is likely to be far more complex than assumed in this analysis. If we were to have considered that bending forces in the femur change during different phases of gait, either shifting in location or by producing bending and counter bending along the same plane (see Paul, 1966, 1971; Duda et al. 1997), or if we were to take into account the important effects of muscle pull (i.e. the tensor fasciae latae via the ilio-tibial band) on reducing bending in the femoral diaphysis (i.e. Rybicki et al. 1972; Pauwels, 1980), the relationships between mineralization density and collagen fibre orientation might be more complex than originally hypothesized. By assuming a more complex model of midshaft femoral loading, we might have expected a more medio-laterally symmetrical microstructure, with bone on either cortex showing a compromise in architecture to withstand all forces best. The microstructural variation encountered, and the unanswered questions engendered by this study, suggests that some of these additional factors need to be considered in future studies seeking to understand the relationship between microstructure and mechanical loading.
Adding to the complexity of interpreting the relationship between these two variables is the fact that the above analysis included all of the bone in a given cortex, including that formed by both modelling and remodelling processes. In an adult sample, we would expect that much of the primary circumferential bone formed by the modelling process would have been laid down at an earlier growth period in the individual's life, and therefore be more heavily mineralized. Areas of the cortex experiencing higher rates of bone turnover would have less of this highly mineralized primary bone tissue remaining, with more of its cortex occupied by poorly mineralized osteonal bone formed by the remodelling process. These differences in bone turnover rates between cortices would have consequences for the distribution of both mineralization density and collagen fibre orientation in a given individual, particularly in younger individuals in which more circumferential lamellar bone would probably remain. Moreover, bone formed by modelling processes during growth may not have been deposited in response to the same mechanical loads that would have existed on these cortices closer to the time of death of the individuals, owing to complexities of cortical drift and of the growth process itself (see Riggs et al. 1993b; Martin et al. 1996). Additional studies need to be completed in which these two different tissue types are analysed separately.
In our second analysis, we attempted to target packets of bone of low mineralization – bone that was likely to be more recently formed. [In most instances, this recently formed bone would have represented bone formed through remodelling (osteonal bone). However, our methods could not distinguish between newly formed primary vs. osteonal bone, so both were included in this analysis.] By examining these areas separately, we hoped to eliminate the problems of including older, highly mineralized primary bone formed during growth and development, and provide a more informative age-based assessment of collagen alignment in the femur, as well as a better understanding of the relationship of collagen fibre orientation and mineralization density to hypothesized loading patterns at the midshaft femur.
In each of the three age groups, significant differences in relational values between locations of the cortex were found, with some postero-medial sectors having significantly higher proportions of transversely oriented lamellae than either antero-lateral and posterior sectors. These were most pronounced in the youngest age group, though middle and older age groups showed the same trends, but with fewer significant results. The cortices that demonstrated significant increases in transversely oriented lamellae (along the medial side) are the same regions that demonstrated significantly higher average mineralization densities (Goldman et al. 2003b), and that have been hypothesized to be experiencing high compression (postero-medial cortex). This result would support previous studies in humans (Portigliatti-Barbos et al. 1983) and studies utilizing strain-gauged animal models (Skedros et al. 1997, 2001; Takano et al. 1999) suggesting a relationship between mineralization density (and, by extension, remodelling rate) and collagen fibre orientation. However, a study of the horse radius by Riggs et al. (1993b) found an opposite relationship – low mineralization density (and increased remodelling rate) on the compressive cortex, which the authors explained by the need for re-alignment of collagen fibres from predominantly longitudinal fibres deposited during growth to predominantly transverse and oblique orientations required of the adult for resistance to compression. These results were corroborated by Mason et al. (1995) for the horse radius and by Skedros et al. (1996) for the horse metacarpal. In the present study, only adult humans were examined. This relationship should be studied further in juvenile samples to see how the relationship between mineralization density, collagen fibre and loading may be complicated by the growth and development process. More specifically, focusing on preferred collagen fibre orientation and mineralization with reference to osteon population density, and hence remodelling rate, should also help to elucidate these relationships.
Age and sex differences in the orientation of collagen fibres in newly formed bone were also demonstrated in this study. In each age group, females showed a higher proportion of transversely oriented lamellae in newly formed bone than males. This result supports similar trends seen in the analysis of collagen fibre orientation on the whole bone cortex of this sample (Goldman et al. 2003a), though in that study, these differences were only significant along the periosteal portion of the bone cortex in the middle age group. These results suggest that bone formed during adulthood (largely due to the turnover process) is being preferentially laid down transversely in females relative to males throughout the adult age range. This difference has not been demonstrated in any previous studies, and should be studied further using a larger sample size – one in which regional variations in this distribution can be investigated more closely, and in which osteonal bone is exclusively isolated.
One surprising finding of this analysis was that the proportion of newly formed bone with transversely oriented collagen fibres decreased with age. This is contrary to previous studies in which transversely oriented lamellae were found to increase in proportion with age (Amprino & Bairati, 1936; Smith, 1960; Vincentelli & Evans, 1971), even when only recently formed secondary osteons were considered (Vincentelli, 1978). These results also seem to contradict the collagen fibre orientation study previously completed on this sample (Goldman et al. 2003a), which found an increase in transverse collagen fibres between the middle and older age groups, at least among males.
One possible explanation is that these results are due to a sampling error owing to the tendency for collagen fibre orientation data to be generally skewed towards more longitudinal lamellae (Puustjärvi et al. 1999; Goldman et al. 2003a). Regions experiencing very high bone turnover would have a larger number of pixels represented in the analysis, and potentially a greater skew towards longitudinal lamellae. Alternatively, these results could be a true reflection of the remodelling process: as we age, there will be an increase in longitudinal lamellae within osteonal bone formed to replace pre-existing predominantly transverse circumferential lamellae formed by the modelling process (following the hypothesis of Riggs et al. 1993b– see above). This hypothesis can be tested in the future by manually selecting areas of recently deposited secondary osteonal bone relative to recently deposited primary bone. In addition, specifically studying the collagen fibre orientations in highly mineralized bone (i.e. those regions coloured grey in the relational LUT that were not examined in the current study) can help determine whether there is any preferential retention of transversely oriented bone with age despite higher proportions of longitudinally oriented secondary osteonal bone.
Another issue that complicates the analysis of age trends in this study is the fact that individuals were grouped based on the chronological age of the individuals. Other analyses of this same sample have demonstrated high variability in microstructural and shape-related parameters (Feik et al. 1996, 2000; Thomas et al. in press), indicative of very different rates of aging among individuals in the sample. This differentiation between ‘chronological age’ and ‘skeletal age’ may be important to consider in future analyses of variability in the microstructural properties investigated in this study.
Continued exploration of the interactions among aspects of a bone's material and structural properties using controlled samples, together with a better understanding of bone organ biomechanical properties, will certainly help to elucidate the very complex relationships identified in the present study. Clearly, very little control for individual variability in activity, nutrition or disease state could be achieved here. Moreover, we were limited by a small sample size (though significantly larger than other previous studies addressing these microstructural variables at the level of the whole bone cortex). Despite these limitations, this research does highlight the importance of incorporating multiple histocompositional variables when examining bone quality, as well as the importance of differentiating between bone tissue formed from the modelling vs. remodelling processes.
Acknowledgments
We would like to thank the mortuary staff at the Victorian Institute of Forensic Medicine, Australia, for their efforts in collecting the material used in this study. We also acknowledge Ms Rita Bruns and Ms Sherie Blackwell, University of Melbourne, for their support and contributions to specimen preparation. We thank Mr Aron Blayvas, Hunter College, for his work on creating in-house programs that were used in this project. Advice and commentary provided by Dr Mitch Schaffler and Professor Alan Boyde during the completion of the dissertation project from which this paper stems (H.M.G.),greatly improved this manuscript. Research support was provided by grants from the National Science Foundation (grant numbers: SBR-9512373 and SBR-972768) and the Louis B. Leakey Foundation.
References
- Amprino R, Bairati A. Processi di ricostruzione e di riassorbimento nella sostanza compatta delle ossa dellúomo. Z. Zellforsch. Mikrosk. 1936;24:439–511. [Google Scholar]
- Amtmann VE. Mechanical stress functional adaptation and the variation structure of the human femur diaphysis. Erg. Anat. Und. Ent. 1971;44:1–87. [PubMed] [Google Scholar]
- Ascenzi A, Bonucci E. The tensile properties of single osteons. Anat. Rec. 1967;158:375–386. doi: 10.1002/ar.1091580403. [DOI] [PubMed] [Google Scholar]
- Ascenzi A, Bonucci E. The compressive properties of single osteons. Anat. Rec. 1968;161:377–392. doi: 10.1002/ar.1091610309. [DOI] [PubMed] [Google Scholar]
- Bertelsen PK, Clement JG, Thomas CDL. A morphometric study of the cortex of the human femur from early childhood to advanced old age. Forensic Sci. Int. 1995;74:63–77. doi: 10.1016/0379-0738(95)01738-5. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Musculoskeletal design in relation to body size. J. Biomech. 1991;24(Suppl 1):19–20. doi: 10.1016/0021-9290(91)90374-v. [DOI] [PubMed] [Google Scholar]
- Blaimont P. Contribution á l'étude biomécanique du fémur humain. Acta Orthop. Belg. 1968;34:665–844. [PubMed] [Google Scholar]
- Boivin G, Meunier PJ. Changes in bone remodeling rate influence the degree of mineralization of bone. Connect Tissue Res. 2002;43:535–537. doi: 10.1080/03008200290000934. [DOI] [PubMed] [Google Scholar]
- Bonfield W, Clark EA. Elastic deformation of compact bone. J. Materials Sci. 1973;8:1590–1594. [Google Scholar]
- Boyde A, Davy KWM, Jones SJ. Standards for mineral quantitation of human bone by analysis of backscattered electron images. Scanning. 1995;17:6–7. [Google Scholar]
- Boyde A, Jones SJ. Establishment of a database for bone mineralization density using backscattered electron microscopy. Scanning. 1996;18:186–187. [Google Scholar]
- Boyde A, Compston JE, Reeve J, et al. Effect of estrogen supression on the mineralization density of iliac crest biopsies in young women as assessed by backscattered electron imaging. Bone. 1998;22:241–250. doi: 10.1016/s8756-3282(97)00275-5. [DOI] [PubMed] [Google Scholar]
- Bromage TG, Goldman HM, McFarlin SC, Warshaw J, Boyde A, Riggs CM. Circularly polarized light standards for investigations of collagen fiber orientation in bone. Anat. Rec. 2003;274B:157–168. doi: 10.1002/ar.b.10031. [DOI] [PubMed] [Google Scholar]
- Currey JD. The mechanical consequences of variation in the mineral content of bone. J. Biomech. 1969;2:1–11. doi: 10.1016/0021-9290(69)90036-0. [DOI] [PubMed] [Google Scholar]
- Duda GN, Schneider E, Chao YS. Internal forces and moments in the femur during walking. J. Biomech. 1997;30:933–941. doi: 10.1016/s0021-9290(97)00057-2. [DOI] [PubMed] [Google Scholar]
- Feik SA, Thomas CDL, Clement JG. Age trends in remodeling of the femoral midshaft differ between the sexes. J. Orthop. Res. 1996;14:590–597. doi: 10.1002/jor.1100140413. [DOI] [PubMed] [Google Scholar]
- Feik SA, Thomas CD, Clement JG. Age-related changes in cortical porosity of the midshaft of the human femur. J. Anat. 1997;191(3):407–16. doi: 10.1046/j.1469-7580.1997.19130407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feik SA, Thomas CD, Bruns R, Clement JG. Regional variations in cortical modeling in the femoral mid-shaft: sex and age differences. Am. J. Phys. Anthropol. 2000;112:191–205. doi: 10.1002/(SICI)1096-8644(2000)112:2<191::AID-AJPA6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Goldman HM, Kindsvater J, Bromage TG. Correlative light and backscattered electron microscopy of bone – Part I: specimen preparation methods. Scanning. 1999;21:40–43. doi: 10.1002/sca.4950210106. [DOI] [PubMed] [Google Scholar]
- Goldman HM, Blayvas A, Boyde A, et al. Correlative light and scanning electron microscopy of bone Part II: automated image analysis. Scanning. 2000;22:337–344. doi: 10.1002/sca.4950220601. [DOI] [PubMed] [Google Scholar]
- Goldman HM. PhD Thesis. New York: City University of New York; 2001. Histocomposition and geometry at the human mid-shaft femur. [Google Scholar]
- Goldman HM, Bromage TG, Thomas CDL, Clement JG. Preferred collagen fiber orientation in the human mid-shaft femur. Anat. Rec. 2003a;272A:434–445. doi: 10.1002/ar.a.10055. [DOI] [PubMed] [Google Scholar]
- Goldman HM, Bromage TG, Boyde A, Thomas CDL, Clement JG. Intrapopulation variability in mineralization density at the human femoral mid-shaft. J. Anat. 2003b;203:243–255. doi: 10.1046/j.1469-7580.2003.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynpas M. Age and disease-related changes in the mineral of bone. Calcif. Tissue Int. 1993;53(Suppl. 1):S57–S64. doi: 10.1007/BF01673403. [DOI] [PubMed] [Google Scholar]
- Martin RB, Ishida J. The relative effects of collagen fiber orientation porosity density and mineralization on bone strength. J. Biomech. 1989;22:419–426. doi: 10.1016/0021-9290(89)90202-9. [DOI] [PubMed] [Google Scholar]
- Martin RB. Aging and strength of bone as a structural material. Calcif. Tissue Int. 1993;53:S34–S40. doi: 10.1007/BF01673400. [DOI] [PubMed] [Google Scholar]
- Martin RB, Boardman DL. The effects of collagen fiber orientation porosity density and mineralization on bovine cortical bone bending properties. J. Biomech. 1993;26:1047–1054. doi: 10.1016/s0021-9290(05)80004-1. [DOI] [PubMed] [Google Scholar]
- Martin RB, Lau ST, Mathews PV, Gibson VA, Stover SM. Collagen fiber organization is related to mechanical properties and remodeling in equine bone. A comparison of two methods. J. Biomech. 1996;29:1515–1521. [PubMed] [Google Scholar]
- Mason MW, Skedros JG, Bloebaum RD. Evidence of strain-mode-related cortical adaptation in the diaphysis of the horse radius. Bone. 1995;17:229–237. doi: 10.1016/8756-3282(95)00213-w. [DOI] [PubMed] [Google Scholar]
- Paul JP. The biomechanics of the hip joint and its clinical relevance. Proc. Roy. Soc. Med. 1966;59:943–948. [PMC free article] [PubMed] [Google Scholar]
- Paul JP. Load actions on the human femur in walking and some resultant stresses. Exp. Mech. 1971;11:121–125. [Google Scholar]
- Pauwels F. Biomechanics of the Locomotor Apparatus: Contributions on the Functional Anatomy of the Locomotor Apparatus. Berlin: Springer Verlag; 1980. [Google Scholar]
- Portigliatti-Barbos M, Bianco P, Ascenzi A. Distribution of osteonic and interstitial components in the human femoral shaft with reference to structure calcification and mechanical properties. Acta Anat. 1983;115:178–186. doi: 10.1159/000145688. [DOI] [PubMed] [Google Scholar]
- Puustjärvi K, Nieminen J, Räsänen T, et al. Do more highly organized collagen fibrils increase bone mechanical strength in loss of mineral density after one-year running training? J. Bone Miner. Res. 1999;14:321–329. doi: 10.1359/jbmr.1999.14.3.321. [DOI] [PubMed] [Google Scholar]
- Riggs C, Vaughan L, Evans G, Lanyon L, Boyde A. Mechanical implications of collagen fibre orientation in cortical bone of the equine radius. Anat. Embryol. 1993a;187:239–248. doi: 10.1007/BF00195761. [DOI] [PubMed] [Google Scholar]
- Riggs CM, Lanyon LE, Boyde A. Functional associations between collagen fibre orientation and locomotor strain direction in cortical bone of the equine radius. Anat. Embryol. 1993b;187:231–238. doi: 10.1007/BF00195760. [DOI] [PubMed] [Google Scholar]
- Rybicki EF, Simonen FA, Weis EBJ. On the mathematical analysis of stress in the human femur. J. Biomech. 1972;5:203–215. doi: 10.1016/0021-9290(72)90056-5. [DOI] [PubMed] [Google Scholar]
- Seeman E. From density to structure: growing up and growing old on the surfaces of bone. J. Bone Miner. Res. 1997;12:509–521. doi: 10.1359/jbmr.1997.12.4.509. [DOI] [PubMed] [Google Scholar]
- Skedros JG, Mason MW, Bloebaum RD. Modeling and remodeling in a developing artiodactyl calcaneus: a model for evaluating Frost's Mechanostat hypothesis and its corollaries. Anat. Rec. 2001;263:167–185. doi: 10.1002/ar.1094. [DOI] [PubMed] [Google Scholar]
- Skedros JG, Mason MW, Nelson MC, Bloebaum RD. Evidence of structural and material adaptation to specific strain features in cortical bone. Anat. Rec. 1996;246:47–63. doi: 10.1002/(SICI)1097-0185(199609)246:1<47::AID-AR6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Skedros JG, Su SC, Bloebaum RD. Biomechanical implications of mineral content and microsructural variations in cortical bone of horse elk and sheep calcanei. Anat. Rec. 1997;249:297–316. doi: 10.1002/(SICI)1097-0185(199711)249:3<297::AID-AR1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Smith JW. The arrangement of collagen fibres in human secondary osteones. J. Bone Joint Surg. 1960;42:588–605. doi: 10.1302/0301-620X.42B3.588. [DOI] [PubMed] [Google Scholar]
- Takano Y, Turner CH, Owan I, et al. Elastic anisotropy and collagen orientation of osteonal bone are dependent on the mechanical strain distribution. J. Orthop. Res. 1999;17:59–66. doi: 10.1002/jor.1100170110. [DOI] [PubMed] [Google Scholar]
- Thomas CDL, Feik SA, Clement JG. Regional variation of intracortical porosity in the mid-shaft of the human femur: sex and age differences. J. Anat. 2005;206:115–125. doi: 10.1111/j.1469-7580.2005.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentelli R, Evans FG. Relations among mechanical properties collagen fibers and calcification in adult human cortical bone. J. Biomech. 1971;4:193–201. doi: 10.1016/0021-9290(71)90004-2. [DOI] [PubMed] [Google Scholar]
- Vincentelli R. Relation between collagen fiber orientation and age of osteon formation in human tibial compact bone. Acta. Anat. 1978;100:120–128. doi: 10.1159/000144890. [DOI] [PubMed] [Google Scholar]
- Vose GP, Kubala AL. Bone strength – its relationship to z-ray determined ash content. Hum. Biol. 1959;31:261–270. [Google Scholar]