Abstract
Cell proliferation is a key factor in sex determination where a size increase relative to the XX gonad is one of the first signs of testis differentiation. Moreover, proliferation of Sertoli cells during development is important in building up the stock of supporting cells necessary for subsequent successful fertility. Because proliferation is such an essential part of testis development, the hypothesis under long-term investigation is that it is under fail-safe control by multiple alternative growth factors. This study was undertaken to investigate the role of glial cell-derived neurotrophic factor (GDNF) on developing mouse Sertoli cells in vitro. Sertoli cells, isolated from mouse embryos at three stages of testis development, were maintained for 2–7 days in vitro (div) in the presence or absence of GDNF at 1, 10 and 100 ng mL−1. Overall the presence of extracellular matrix gel had little effect on proliferative activity, but encouraged expression of the epithelial phenotype. A statistically significant difference in proliferation, assessed by immunocytochemical staining for proliferating cell nuclear antigen, was seen with GDNF at embryonic day (E)12.5 after 2 div (at both 10 and 100 ng mL−1, P < 0.001) and 7 div (at both 10 and 100 ng mL−1, P < 0.05); at E13.5 after 3 div (at both 10 and 100 ng mL−1, P < 0.05) and at E14.5 after 7 div (100 ng mL−1, P < 0.01), compared with controls cultured without growth factor. In conclusion, GDNF stimulates mitosis throughout this critical developmental window. The in vitro approach used here is a useful adjunct to the knockout mouse model and has been applied to show that GDNF exerts a proliferative effect on developing mouse Sertoli cells.
Keywords: cell proliferation, culture, cytokines, sex differentiation, testis cord formation
Introduction
Glial cell line-derived neurotrophic factor (GDNF), a distant relative of the transforming growth factor-β superfamily, was first identified by its ability to support embryonic midbrain neurons in vitro (Lin et al. 1993). Related factors now grouped in the GDNF family are neurturin, persephin and artemin; their receptors are complexes that include a ligand-binding component in the form of an extracellular protein bound to the plasma membrane by a glycosylphosphatidylinositol anchor: GDNF family receptor α1–4 (GFRα1–4) (Massagué, 1996). As GFRα1 lacks a transmembrane and cytoplasmic region it is unable to signal on its own and requires a signalling component, the tyrosine kinase receptor Ret (Massagué, 1996). Since its initial discovery GDNF has been found to have biological importance in several non-neuronal organ systems during development.
In a wide-ranging study of GDNF family factors and their receptors in the developing and mature mouse, Golden et al. (1999) found both GDNF and neurturin expression in the developing urogenital system. Moderate GDNF labelling was first detected in the urogenital ridge on embryonic day (E)10 with low-level labelling of both GFRα1 and Ret receptors. As the ovary and testis differentiated, labelling for GDNF was retained in both sexes at E14, but a sex difference was apparent in receptor expression. Although both sexes showed moderate expression of the Ret receptor and low-level expression of GFRα2, the GFRα1 receptor was expressed with a high level of intensity in the testis, but was undetectable in the ovary. By E16, GDNF was highly expressed in the testis, but expression of its receptor was now undetectable. In the adult mouse testis both GFRα1 and GRFα2 were strongly expressed in the seminiferous epithelium, though Ret and GDNF were undetectable.
Similarly, in the rat, GDNF mRNA expression in the testis was found to increase during development, reaching a peak at postnatal day (P)7 (Trupp et al. 1995). The level of GDNF mRNA subsequently decreased in the testis during the second and third weeks of postnatal life and was lowest in adult testis. Trupp and co-workers also found expression of GDNF mRNA in an established mouse Sertoli cell line, TM4 cells, suggesting that Sertoli cells may be the source of GDNF mRNA in the testis.
Several growth factors have been implicated in events such as cell survival, cell proliferation and cell–cell interactions at different stages of gonadal development and differentiation (for a review see Mackay, 2000). What role might neurotrophic factors play in testis morphogenesis? Various roles have been indicated for nerve growth factor (NGF) and other neurotrophins in the developing testis. For example, mice carrying a null mutation for the NGF receptor trkA and for the neurotrophin 3 receptor trkC show a reduced number of seminiferous cords at E14 and a reduction in germ cell number at P19 compared with wild-type mice, indicating roles in both cord formation and germ cell survival (Cupp et al. 2002). The low-affinity neurotrophin receptor p75 may function in cell migration, cell invasiveness and apoptosis (Hempstead, 2002) and it has been shown to be expressed by the mesenchymal precursors of peritubular smooth muscle cells as they migrate into the mouse testis from the mesonephros (Campagnolo et al. 2001). Cupp et al. (2003) have also shown that mouse Sertoli cells express neurotrophin 3 as a chemotactic agent for migrating mesonephros cells. Ingress and differentiation of peritubular myoid cells is a key event in testicular morphogenesis, because the Sertoli cells and peritubular cells co-operate to produce the basal lamina, which stabilizes testicular cord structure (Tung et al. 1984). In the rat, inhibition of trk receptors prevented cord formation (which in this species occurs normally at E14) in explanted E13 testes (Levine et al. 2000a). As in the mouse and human (Robinson et al. 2003), Levine et al. (2000a) demonstrated a role for neurotrophins in epithelial–mesenchymal interactions in the developing rat testis. Produced by epithelial Sertoli cells, NT3 interacts with mesenchymal cells expressing p75 first (E13) in the mesonephros and later (E14) around testicular cords. However, this study also showed that NT3 and trkC knockout mice had normal cord morphology, suggesting the operation of a fail-safe mechanism, with compensation between different neurotrophin ligands, receptors and/or growth factors. Similarly, despite evidence of GDNF expression in the developing testis, three papers from independent laboratories published simultaneously in Nature in 1996 reported normal gonadal development in transgenic mice defective in GDNF (Moore et al. 1996; Pichel et al. 1996; Sánchez et al. 1996).
In order to resolve this apparent contradiction and to clarify the role of GDNF in testis morphogenesis the present study was undertaken. Previous work from our laboratory has established a method for isolating and culturing developing mouse Sertoli cells (Mackay et al. 1999). This has recently been applied to investigate their production of anti-Müllerian hormone and effects on developing ovaries in co-culture (Mackay et al. 2004) and to demonstrate that fibroblast growth factor 9 (FGF9) exerts a proliferative effect on developing Sertoli cells in vitro (Willerton et al. 2004). Our method will now be applied to examine the role of GDNF on developing mouse Sertoli cells, isolated at key stages in differentiation – E12.5 (when Sertoli cells first aggregate to form cords), E13.5 (when male germ cells enter mitotic arrest and peritubular myoid cells differentiate) and E14.5 (when testicular cords are stabilized) – and maintained in vitro.
Materials and methods
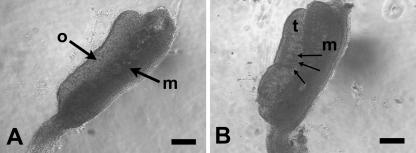
Pregnant CBA strain mice, maintained on a reverse lighting regime, were killed by CO2 asphyxiation under Schedule 1 of the Home Office Regulations at three time-points of gestation: E12.5, E13.5 and E14.5, as estimated from the day of finding a vaginal plug (day 0 of pregnancy). Embryos were removed to Hanks’ buffered salt solution (HBSS) (Gibco) supplemented with 1% sodium bicarbonate and staged according to Theiler (1972); any whose developmental age differed from its littermates was discarded. At E12.5 the testis can be distinguished from the ovary by its vascularity, including the presence of a peripheral blood vessel and by a striped appearance due to testicular cords (Fig. 1). Testes were separated from mesonephroi, and the right and left testis from each individual was transferred to different Eppendorf tubes containing Hanks’ buffer, so that resulting control and experimental cultures contained tissue from the same individuals, thus reducing variation.
Fig. 1.
Phase contrast micrograph of ovary (A) and testis (B) isolated from E12.5 mouse embryos. Abbreviations: o = ovary, t = testis, m = mesonephros. Arrows in B indicate blood vessels between testicular cords and testicular cords. Scale bars, 200 µm.
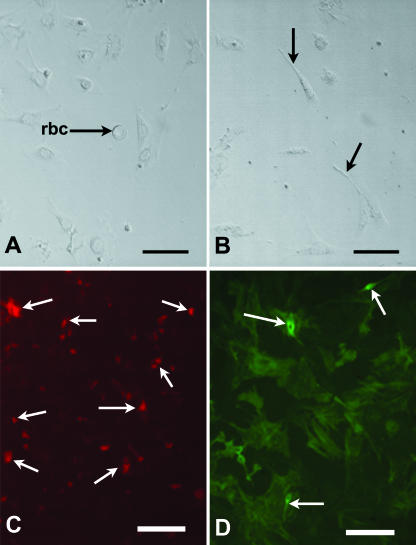
Sertoli cells were isolated using our established method by enzyme treatment (trypsin, 0.025%; collagenase, 0.25%), mechanical disruption and differential centrifugation steps (Mackay et al. 1999). A series of centrifugations was carried out at 1000, 2000, 3000 and 4000 r.p.m. (equating to relative centrifugal forces of 62, 250, 560 and 1000 g, respectively) for 1 min each in a Microcentaur. The supernatant was removed between each centrifugation and cultured in uncoated wells to observe the types of cells removed during the isolation steps (Fig. 2A,B). The pellet was re-suspended with fresh medium. After the final suspension in Dulbecco's minimal essential medium (DMEM), two drops of cell suspension and Trypan blue solution (Sigma, cat. no. 73K2420) were transferred to a haemocytometer to assess the number of cells present. Total cell number and the number of dead cells (which failed to exclude Trypan blue) were recorded to calculate the percentage of live cells. Cell viability was found to be typically approximately 95%. Cells were then plated out into 96-well cell culture cluster plates (Corning Inc.) at a density of 3.5 × 105 mL−1 and cultured in the wells either coated or uncoated with ECM gel (Sigma Aldrich cat. no. E1270).
Fig. 2.
(A,B) Phase contrast micrographs of cells from E12.5 supernatant 1 (A) and supernatant 2 (B) after 2 div. Note red blood cell (rbc) in A, cells with peritubular phenotype (arrows) in B. Scale bars represent 20 µm. (C,D) Immunolocalization of AMH (C) and α-SMA (D) in E13.5 Sertoli cells cultured for 7 div in the presence of 10 ng mL−1 GDNF. Arrows indicate areas of positive staining. Scale bars, 100 µm.
Success of Sertoli cell isolation procedures was monitored by immunocytochemistry (see below for method) for cell markers: AMH for Sertoli cells, smooth muscle α-actin for presumptive peritubular cells (Tung & Fritz, 1990), showing that cultures were at least 85% pure (Fig. 2C,D). A range of GDNF concentrations (Sigma: G1401) were tested: 1, 10 and 100 ng mL−1. All cultures were incubated for 2, 3 or 7 days in 5% CO2, 95% air at 37 °C, monitored on a daily basis using an Olympus 1 ×50 inverted microscope with Hoffmann modulation contrast (Olympus Optical Co., Europa GmbH). Reasons for culture period variations were as follows: cultures established from E14.5 testes were incubated for 7 days only, to determine whether proliferation was possible in Sertoli cells isolated from established testicular cords. Cells isolated at earlier developmental stages (E12.5, E13.5) were cultured for two periods to monitor possible changes in proliferative activity over time in culture. These additional periods were 2 days for cells isolated at E12.5, but 3 days for cells isolated at E13.5 cultures, as the latter took up to 24 h longer to attach. A photographic record was kept using an Olympus DP50 digital camera and DP-Soft software. Medium was changed every 48 h. At the end of the culture period, cells were fixed in 4% paraformaldehyde for immunocytochemistry.
Immunocytochemical analysis was carried out using a variety of primary antibodies at various working concentrations. The following antibody dilutions were used: anti-proliferating cell nuclear antigen (PCNA) (Santa Cruz: SC-56) at 1 : 100; anti-GFR-α1 (Santa Cruz: SC-10716) at 1 : 200; anti-AMH (Santa Cruz: SC-6886) at 1 : 50, as a marker for Sertoli cells (Mackay et al. 2004), and anti-smooth muscle α-actin antibody (Sigma cat. no. A-2547) at 1 : 50 dilution, as a specific marker of peritubular myoid cells (Tung & Fritz, 1990). Both diaminobenzidine tetrahydrochloride (DAB) (Vector Laboratories: SK-4100) and various fluorochromes were used for identification of the antigen–antibody complexes. The secondary fluorescent antibodies used reflected the species in which the primary antibodies were raised: rhodamine-conjugated anti-goat IgG (Stratech Scientific Ltd: 705-295-147) was used in the case of AMH, FITC-conjugated donkey anti-mouse IgG (Stratech Scientific Ltd: 715-095-150) in the case of α-smooth muscle actin. They were added in a dilution of 1 : 200 to cultured cells for 1 h, and then cultures were directly viewed and photographed by fluorescent microscopy. As far as possible both negative controls (in the absence of the primary antibody) and positive controls (tissues known to express the antigen under investigation) were included.
As PCNA is synthesized during mitosis at G1 and S phase of the cell cycle, it is routinely used as a mitotic marker (Kurki et al. 1988). For proliferation assessment, cells strongly positive for PCNA were identified as mitotic; positive cells were counted and expressed as a percentage of the total cell number for both the controls and the experimental cultures. A total of 3–8 fields per well were counted to establish the mean total of cells per well and the mean percentage of proliferating cells. Experiments were repeated at least three times each. One-way analysis of variance (anova) was performed, followed by a Kruskal–Wallis post test and a Dunn's multiple comparisons test, using GraphPad InStat (version 3.00 for Windows 95, GraphPad Software Inc., San Diego, CA, USA) to analyse any effects on the percentage of proliferative cells present.
Results
Purity of Sertoli cell cultures: by monitoring cell markers AMH (for Sertoli cells) and α-smooth muscle actin (for peritubular cells)
Immunopositive cells for AMH were demonstrated in all culture regimes (with or without ECM gel, with or without exogenous GDNF at the doses tested). Staining was cytoplasmic and granular-like, indicative of hormone storage vesicles in the developing Sertoli cells (Fig. 2C). Staining was strongest in cells within cord-like aggregations. A few spindle-shaped, elongated, peritubular-like cells stained positively for alpha-smooth muscle actin in all culture conditions (Fig. 2D). These two results confirmed that the cultures were at least 85% pure for Sertoli cells. Both fluorescent staining of AMH and α-SMA was absent in all negative controls.
Culture morphology
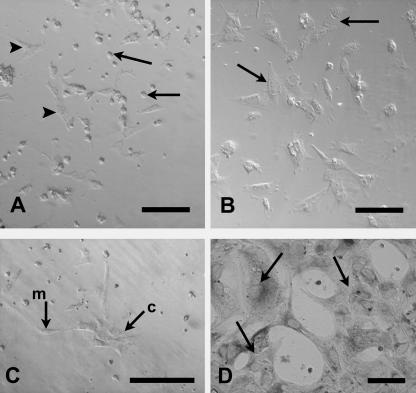
For the first 24 h in culture, cells remained rounded as on seeding. However, after 24 h the embryonic Sertoli cells in all experiments appeared to be flattening down and attaching to the substrate (Fig. 3A), especially at the periphery of the well. After 36 h, increased proliferation was evident. Cells isolated at E13.5 and cultured in ECM-coated wells formed obvious cord-like aggregations after 48 h (Fig. 3B), and by 72 h cell number was greatly increased, almost forming a monolayer. When cells were isolated at the earlier stage of E12.5 aggregation of Sertoli cells, with apposition of cell membranes at more than one surface, was observed after only 1 day in vitro (div) (Fig. 3C), resulting in cord-like clusters in some wells (Fig. 3D). Cells appeared less densely packed in cultures without GDNF or in ECM-coated wells, where they generally showed a relatively slower proliferation compared with those cells cultured without gel. However, on ECM gel more cord-like aggregations were evident.
Fig. 3.
Morphology of cultured Sertoli cells. (A) After 1 div some E13.5 cells without GDNF remain rounded (arrows) whilst others are flattening to the uncoated substrate (arrowheads). (B) E13.5 cells cultured on ECM gel substrate align together (arrows) and begin to form cord-like aggregations after 2 div without GDNF. (C) E12.5 cells cultured on ECM gel substrate without GDNF, 1 div. Note cord-like cluster of cells at c, with apposition of cell membranes; other cells remain mesenchymal (m). (D) Cord-like aggregations of cells (arrows) seen after 2 div on ECM gel and PCNA immunolabelling. Scale bars represent 100 µm (A,B), 50 µm (C,D).
PCNA expression in cultured embryonic Sertoli cells
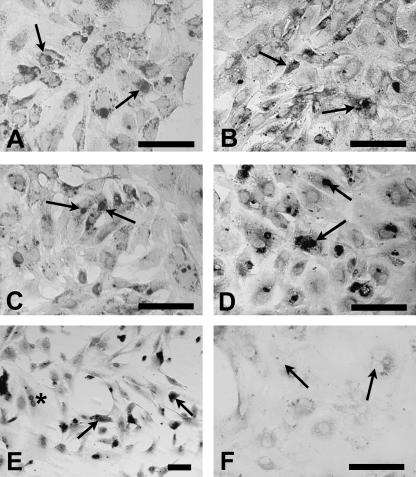
Cells labelled with anti-PCNA antibody were evident in all culture wells (Fig. 4). Only cells showing nuclear staining (see Fig. 4A–E) were counted as being in the mitotic state, because the presence of nuclear staining indicates that cells are at G phase of mitotic division. Nuclear staining was absent in preparations omitting the primary antibody as a negative control (Fig. 4F).
Fig. 4.
(A–D) Immunolocalization of PCNA in E13.5 Sertoli cells cultured for 3 div in the absence or presence of ECM gel or GDNF. Arrows indicate positive nuclear staining. (A) Cells cultured without GDNF on ECM gel-coated substrate. (B) Cells cultured without GDNF on uncoated substrate. (C) Cells cultured with 10 ng mL−1 GDNF on ECM gel-coated substrate. (D) Cells cultured with 10 ng mL−1 GDNF on uncoated substrate. (E) Immunolocalization of PCNA in E14.5 Sertoli cells cultured on ECM gel-coated substrate for 7 div; note cell division in progress (asterisk). (F) Immunolocalization of PCNA in E13.5 Sertoli cells cultured with 10 ng mL−1 GDNF on uncoated substrate for 3 div; negative control with omission of primary antibody. Note absence of immunostaining in nuclei (arrows). Scale bars, 50 µm.
Cultures established at E12.5
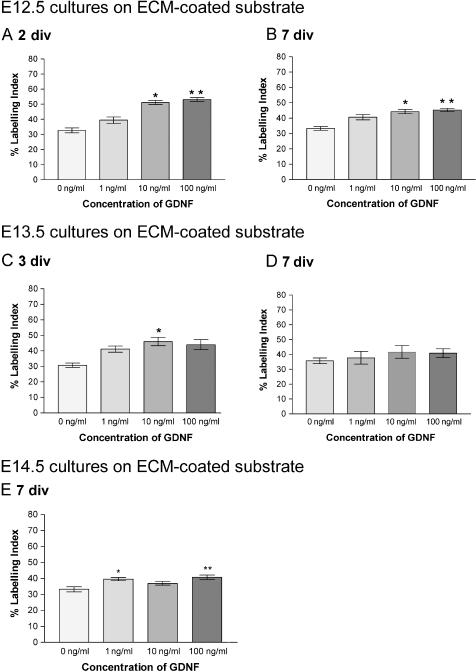
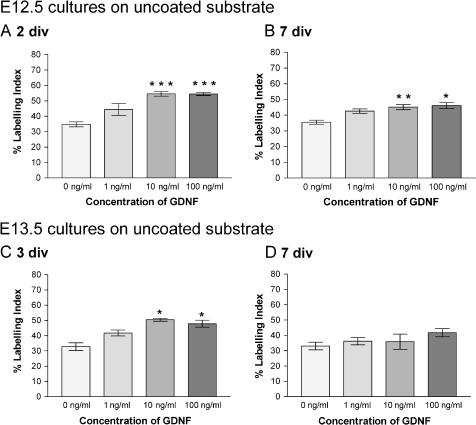
Counts were made in all experimental conditions for cells isolated at E12.5, and the percentage of proliferating cells (labelling index, LI) was quantified (Figs 5 and 6). After both 2 and 7 div, experimental wells containing GDNF doses of 10 and 100 ng mL−1 showed a significantly (P < 0.05) higher LI compared with cultures without growth factor supplementation (Figs 5A,B and 6A,B). The effect of GDNF on cell proliferation was highly significant when cells were cultured for 2 div without ECM gel (P < 0.001) (Fig. 6A).
Fig. 5.
Mean percentage of PCNA-positive Sertoli cells cultured on ECM gel-coated substrate: (A) E12.5 cells cultured for 2 div; (B) E12.5 cells cultured for 7 div; (C) E13.5 cells cultured for 3 div; (D) E13.5 cells cultured for 7 div; (E) E14.5 cells cultured for 7 div. Data were analysed using Kruskall–Wallis analysis and Dunn's post hoc analysis. *Significantly different from 0 ng mL−1 GDNF cultures, P < 0.05; **significantly different from 0 ng mL−1 GDNF cultures, P < 0.01.
Fig. 6.
Mean percentage of PCNA-positive Sertoli cells cultured on uncoated substrate: (A) E12.5 cells cultured for 2 div; (B) E12.5 cells cultured for 7 div; (C) E13.5 cells cultured for 3 div; (D) E13.5 cells cultured for 7 div. *Significantly different from 0 ng mL−1 GDNF cultures, P < 0.05; **significantly different from 0 ng mL−1 GDNF cultures, P < 0.01; ***significantly different from 0 ng mL−1 GDNF cultures, P < 0.001.
Cultures established at E13.5
All cultures with GDNF had a higher LI (Figs 5C,D and 6C,D) compared with cultures without exogenous growth factor added. However, the significantly higher levels (P < 0.05) evident at 3 div in the presence of ECM gel were not maintained after 7 div (Figs 5D and 6D). In cultures without ECM gel, a similar effect was noted in that levels were significantly higher (P < 0.05) at 3 div with doses of 10 and 100 ng mL−1 (Fig. 6C) but by 7 div the effects had disappeared (Fig. 6D).
Cultures established at E14.5
Sertoli cells cultured with DMEM alone and GDNF were immunopositive for PCNA (Fig. 4E). An increase in LI in cultures with GDNF, compared with controls without, was evident after approximately 72 h. At 7 div differences between cultures without exogenous growth factor and those with added GDNF at 1 ng mL−1 (P < 0.05) and 100 ng mL−1 (P < 0.01) were statistically significant (Fig. 5E). Interestingly, with the 10 ng mL−1 concentration the increase in LI was not significant.
Discussion
The purity of our cultures was assessed by immunocytochemistry for cell markers. Smooth muscle α-actin has been shown to be an excellent specific marker of peritubular myoid cells both in seminiferous tubules and in culture (Tung & Fritz, 1990). Although Tung and Fritz found that staining decreased during the first 4 days, the percentage of positive cells increased from day 6 to day 10 in vitro; in our experiments, the small percentage of cells positively stained for smooth muscle α-actin remained unaltered in cultures after 2, 3 and 7 div. Furthermore, the positively stained cells showed an elongated spindle-shaped morphology. Smooth muscle α-actin immunostaining has been used by others to identify both developing peritubular myoid cells at 72 h of culture (Campagnolo et al. 2001), and cultured adult peritubular myoid cells after 7 days in vitro (Konrad et al. 2000). The majority of cells in our cultures showed an epithelial morphology and positive immunostaining for the specific Sertoli cell marker anti-Müllerian hormone.
One of the earliest known steps in testis determination triggered by Sry expression is an increase in proliferation: quantitative studies by Mittwoch (2000) indicate that XY gonads grow faster than XX gonads prior to Sertoli cell differentiation. Indeed, this differentiation may be dependent on a critical cell number. In the mouse, the male gonad doubles its size relative to the female by increased proliferation of coelomic epithelial cells at E11.25. Immediately following the window of Sry expression, there is an 8-h period of mitosis, critical for the establishment of the male pathway (Schmahl & Capel, 2003). This increase in the developing and early postnatal testis is crucial for achieving the adult size of the testis, and for healthy functioning in the adult (Sharpe et al. 2003).
Many candidate growth factors are involved in these events, i.e. FGF9 was demonstrated to stimulate Sertoli cell proliferation (Colvin et al. 2001; Willerton et al. 2004), whilst Levine et al. (2000a) suggested that transforming growth factor-β (TGF-β) has a role in regulating the growth of embryonic rat testis. Previous studies have suggested neurotrophins are expressed at high levels in the postnatal testis (Levine et al. 2000a). There is evidence in the rat that GDNF is expressed by Sertoli cells and acts in an autocrine manner to stimulate their proliferation in the early postnatal period (Widenfalk et al. 1997; Viglietto et al. 2000). The current observations demonstrate the potential that GDNF may play a role in the embryonic testis morphogenesis. Although a limitation to the use of PCNA has been suggested by some workers, due to the fact that the protein is retained by cells for a period after division is complete, PCNA immunostaining has been shown to correlate extremely well with cell proliferation as measured by tritiated thymidine incorporation (Levine et al. 2000b), and is widely used in cultures maintained beyond 24 h, for example by Miura et al. (2004). In this study, adding GDNF to embryonic Sertoli cells in culture enhanced the proliferation of the cells, as demonstrated by the increased number of PCNA-immunopositive mitotic cells. This result indicates that GDNF has a stimulatory effect on the proliferation of Sertoli cells during mouse embryonic testis development.
All E12.5 Sertoli cell cultures and E13.5 3-day cultures showed a significantly greater proliferative activity with a dose of 10 ng mL−1 GDNF compared with cultures without growth factor. However, a similar response in positive PCNA staining was achieved using either 10 or 100 ng mL−1. Additionally, in the 3-day cultures of E13.5 Sertoli cells the effect of 100 ng mL−1 GDNF on cell proliferation was even less than with 10 ng mL−1. These results suggest that the response of E13.5 Sertoli cells to GDNF is not dose dependent over the range tested. However, E12.5 cells after 2 div showed increased proliferation with a dose increase, indicating that cell proliferation tends to be related to GDNF concentration. Despite this trend, however, statistical analysis showed there was no significant difference between different GDNF doses.
The process of seminiferous cord formation is the first morphological event that differentiates a testis from an ovary and indicates male sex determination. The results presented suggest that the cells cultured on ECM gel formed cord-like aggregations in which a more epithelial-like phenotype was expressed. These results are consistent with the previous findings that ECM gel enhances cord-like aggregation and morphological maturation of the embryonic Sertoli cells in culture (Mackay et al. 1999).
Our investigation suggests that the appearance of exogenous GDNF in Sertoli cell cultures correlated with the morphogenetic event of cord formation. The cells in the wells containing GDNF formed cord-like aggregations after 48 h in culture, and this became obvious after 3 days. In contrast, the cells cultured without GDNF were distributed randomly with fewer cord-like aggregations and with some mesenchymal cells present, although they displayed the same level of attachment to the substrate.
In the current experiments the effect of GDNF on embryonic Sertoli cells isolated at three timepoints in development (E12.5, E13.5 and E14.5) has been investigated. The earlier timepoint was chosen as pre-Sertoli cells begin to undergo a mesenchymal–epithelial transformation and aggregate to form cord-like structures in the mouse at this time (Mackay, 2000). At E13.5 testicular cord formation is well underway; and by E14.5 pre-Sertoli cells have formed definite testicular cords surrounding centrally placed germ cells, some of which are still in mitosis (Mackay & Smith, 1989), although mitotic arrest of germ cells is said to begin at about E13.5 (McLaren & Southee, 1997). In this study, cultures from both these embryonic stages showed an obvious response to the exogenous GDNF with an increase in Sertoli cell proliferation and an enhancement of cord-like aggregations. Proliferation of cells isolated at E12.5 and E13.5 was tested shortly after their attachment (at 2 and 3 div, respectively) as well as after 7 div. Cells at E12.5 had quicker attachment and proliferation than those at E13.5 in the first 2 days of culture, indicating that the proliferative activity of Sertoli cells is higher at the time of the initial formation of testis cords before germ cells enter mitotic arrest. Current results demonstrate that E14.5 cells still undergo proliferation and are responsive to exogenous growth factor.
AMH was shown to be present after 3 days of culture, indicating that the immunopositive cells were Sertoli cells with functional maturity (Mackay et al. 2004). In this study, a high purity of Sertoli cells (more than 85%) was achieved, as demonstrated by the low percentage of immunostaining for α-SMA and morphological observations of epithelial phenotype.
Our observations using this in vitro approach demonstrate an important role for GDNF in embryonic testis development, especially during the early proliferation of developing Sertoli cells. Our results suggest that GDNF had a stimulatory effect on proliferation and cord formation of embryonic Sertoli cells isolated at E12.5, E13.5 and E14.5. The hypothesis under investigation during this work was that growth factors such as GDNF trigger events in testicular morphogenesis in which Sertoli cell interactions are likely to play a central role. Resulting signalling cascades potentially regulate cell proliferation, further differentiation, and at times, cell death pathways. Likely influences on Sertoli cells include P-Mod-S from the differentiated peritubular cells, and interactions from extracellular matrix components, such as fibronectin and laminin acting via α and β integrin subunits. Growth factor receptors are likely to be incorporated into multicomplex structures together with the integrin subunits to control morphogenesis. Our in vitro studies enable us to monitor growth factor interactions providing a useful adjunct to knockout studies in which overall development may be compromised if the gene under investigation has multiple functions. Future studies will be directed to determine the time of onset of growth factor receptor expression in vivo, and also to define precisely the time window when GDNF and other growth factors, both singly and in combination, can operate throughout critical stages of development.
Acknowledgments
We are grateful to the Wellcome Trust for their financial support (grant no. 067482), to Dr Louise Willerton for her contribution to the work and to Mr David Russell for technical assistance with immunocytochemistry.
References
- Campagnolo L, Russo MA, Puglianiello A, Favale A, Siracusa G. Mesenchymal precursors of peritubular smooth muscle cells of the mouse testis can be identified by the presence of the p75 neurotrophin receptor. Biol. Reprod. 2001;64:464–472. doi: 10.1095/biolreprod64.2.464. [DOI] [PubMed] [Google Scholar]
- Colvin J, Green RP, Schmahl J, Capel B, Ornitz D. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- Cupp AS, Tessarollo L, Skinner MK. Testis developmental phenotypes in neurotrophin receptor trkA and trkC null mutations: role in formation of seminiferous cords and germ cell survival. Biol. Reprod. 2002;66:1838–1845. doi: 10.1095/biolreprod66.6.1838. [DOI] [PubMed] [Google Scholar]
- Cupp AS, Uzumcu M, Skinner MK. Chemotactic role of neurotropin-3 in the embryonic testis that facilitates male sex determination. Biol. Reprod. 2003;68:2033–2037. doi: 10.1095/biolreprod.102.012617. [DOI] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF and GDNF family-receptor mRNA in the developing and mature mouse. Exp. Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Hempstead B. The many faces of p75NTR. Curr. Opin. Neurobiol. 2002;12:260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- Konrad L, Albrecht M, Renneberg H, Anmüller G. Transforming growth factor-β2 mediates mesenchymal–epithelial interactions of testicular somatic cells. Endocrinology. 2000;141:3679–3686. doi: 10.1210/endo.141.10.7728. [DOI] [PubMed] [Google Scholar]
- Kurki P, Ogata K, Tan EM. Monoclonal-antibodies to proliferating cell nuclear antigen (PCNA) cyclin as probes for proliferating cells by immunofluorescence microscopy and flow-cytometry. J. Immunol. Methods. 1988;109:49–59. doi: 10.1016/0022-1759(88)90441-3. [DOI] [PubMed] [Google Scholar]
- Levine E, Cupp AS, Skinner MK. Role of neurotropins in rat embryonic testis morphogenesis (cord formation) Biol. Reprod. 2000a;62:132–142. doi: 10.1095/biolreprod62.1.132. [DOI] [PubMed] [Google Scholar]
- Levine E, Cupp AS, Miyashiro L, Skinner MK. Role of transforming growth factor-α and the epidermal growth factor receptor in embryonic rat testis development. Biol. Reprod. 2000b;62:477–490. doi: 10.1095/biolreprod62.3.477. [DOI] [PubMed] [Google Scholar]
- Lin L-F, Doherty J, Lile S, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Mackay S, Smith RA. Mouse gonadal differentiation in vitro in the presence of fetal calf serum. Cell Diff. Dev. 1989;27:19–28. doi: 10.1016/0922-3371(89)90041-5. [DOI] [PubMed] [Google Scholar]
- Mackay S, Booth SH, MacGowan A, Smith RA. Ultrastructural studies demonstrate that epithelial polarity is established in cultured mouse pre-Sertoli cells by extracellular matrix components. J. Electron. Microsc. 1999;48:159–165. doi: 10.1093/oxfordjournals.jmicro.a023662. [DOI] [PubMed] [Google Scholar]
- Mackay S. Gonadal development in mammals at the cellular and molecular levels. Int. Rev. Cytol. 2000;200:47–99. doi: 10.1016/s0074-7696(00)00002-4. [DOI] [PubMed] [Google Scholar]
- Mackay S, Willerton L, Ballingall CL, Henderson NJS, Smith RA. Developing mouse Sertoli cells in vitro: effects on developing ovaries in co-culture and production of anti-Mullerian hormone. Cells Tiss.Org. 2004;177:79–86. doi: 10.1159/000079183. [DOI] [PubMed] [Google Scholar]
- Massagué J. Crossing receptor boundaries. Nature. 1996;382:29–30. doi: 10.1038/382029a0. [DOI] [PubMed] [Google Scholar]
- McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev. Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Genetics of sex determination: exceptions that prove the rule. Mol. Genet. Metab. 2000;71:405–410. doi: 10.1006/mgme.2000.3075. [DOI] [PubMed] [Google Scholar]
- Miura T, Shiota K, Morriss-Kay G. A mesenchyme-free culture system to elucidate the mechanism of otic vesicle morphogenesis. J.Anat. 2004;205:297–312. doi: 10.1111/j.0021-8782.2004.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Fariñas I, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Robinson LL, Townsend J, Anderson RA. The human fetal testis is a site of expression of neurotrophins and their receptors: regulation of the germ cell and peritubular cell population. J. Clin. Endocrinol. Metab. 2003;88:3943–3951. doi: 10.1210/jc.2003-030196. [DOI] [PubMed] [Google Scholar]
- Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Capel B. Cell proliferation is necessary for the determination of male cell fate in the gonad. Dev. Biol. 2003;258:264–276. doi: 10.1016/s0012-1606(03)00122-2. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells and their relevance to disorders of testes function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Theiler K. The House Mouse Development and Normal Stages from Fertilization to Four Weeks of Age. Berlin: Springer-Verlag; 1972. [Google Scholar]
- Trupp M, Rydén M, Jörnvall H, et al. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J. Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung PS, Skinner MK, Fritz IB. Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann. NY Acad. Sci. 1984;438:435–446. doi: 10.1111/j.1749-6632.1984.tb38304.x. [DOI] [PubMed] [Google Scholar]
- Tung PS, Fritz IB. Characterization of rat testicular peritubular myoid cells in culture: α-smooth muscle isoactin is a specific differentiation marker. Biol. Reprod. 1990;42:351–365. doi: 10.1095/biolreprod42.2.351. [DOI] [PubMed] [Google Scholar]
- Viglietto G, Dolci S, Bruni P, et al. Glial cell line-derived neurotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int. J. Oncol. 2000;16:689–694. doi: 10.3892/ijo.16.4.689. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L. Neurturin and glial cell line-derived neurotrophic factor receptor beta (GDNFR-beta), novel proteins related to beta GDNF and GDNFR-alpha with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J. Neurosci. 1997;17:8506–8519. doi: 10.1523/JNEUROSCI.17-21-08506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerton L, Smith RA, Russell D, Mackay S. Effects of FGF9 on embryonic Sertoli cell proliferation and testicular cord formation in the mouse. Int. J. Dev. Biol. 2004;48:637–643. doi: 10.1387/ijdb.031778lw. [DOI] [PubMed] [Google Scholar]