Abstract
Recently discovered endogenous opioid peptides such as nociceptin are known to modulate neurotransmitter release of primary afferent neurons (especially substance P, SP) and they have also been demonstrated in peripheral nerve fibres. The aim of this study was to investigate the opioid peptidergic innervation of the anterior eye segment and to compare it with the innervation pattern of SP in order to shed light on the functional relationship between these peptides. Anterior eye segments of 20 rat eyes were cut in a tangential plane and the sections stained with antibodies against SP, nociceptin, nocistatin, endomorphin 1 and 2, leu-enkephalin and met-enkephalin. Sections of the spinal cord or brain were used as positive controls. Numerous SP-immunoreactive nerve fibres were found in the conjunctiva, cornea, episclera, trabecular meshwork, iris and ciliary body. A weak staining for met-enkephalin and leu-enkephalin could only be found in the iris and anteriormost ciliary body. Nerve fibres immunoreactive for nociceptin, nocistatin, and endomorphin 1 or 2 could not be detected in any part of the anterior eye segment. It is tempting to speculate that the opioid peptidergic innervation of the anterior ciliary body may play a role in the modulation of intraocular inflammation.
Keywords: comea, glaucoma, iris, neuropeptides, trabecular meshwork
Introduction
In various species (rat, rabbit, guinea-pig, cat, monkey and human), the anterior eye segment is densely innervated by substance P (SP)-ergic nerve fibres (Stone et al. 1982; Stone & Kuwayama, 1985; Kuwayama & Stone, 1987; Stone, 1996). These sensory nerve fibres originate mainly from the ipsilateral trigeminal ganglion (Luhtala & Uusitalo, 1991; Elsas et al. 1994; Stone, 1996).
The main site of SP-ergic innervation is the cornea (Stone & Kuwayama, 1985; Jones & Marfurt, 1998). However, abundant SP-immunoreactive nerve fibres have also been detected in the conjunctiva (Luhtala & Uusitalo, 1991; Elsas et al. 1994), episclera Selbach et al 1998, 2004), trabecular meshwork (Laties et al. 1981; Selbach et al. 2000) and iris (Beckers et al. 1993). Recently we found SP-positive mechanoreceptor-like nerve endings in the primate trabecular meshwork which may be involved in aqueous humor outflow regulation (Selbach et al. 2000; see also Ruskell, 1976; 1994). The SP-ergic innervation of the rat eye has been investigated in several studies (Luhtala & Uusitalo, 1991; Beckers et al. 1993; Elsas et al. 1994; Jones & Marfurt, 1998; Selbach et al. 1998). However, none of these studies revealed SP-ergic nerve fibres in the trabecular meshwork of the rat.
Opioid mechanisms (opiates) appear to influence intraocular pressure, possibly through effects on outflow (Stone, 1996). There is little information about opioid-peptidergic innervation of the anterior eye segment. Only a single study has described enkephalin-immunoreactivity in iris nerves (Björklund et al. 1984). Recently discovered endogenous opioid peptides such as nociceptin, nocistatin or endomorphin 1 and 2 are known to modulate neurotransmitter release of primary afferent neurons (and especially of SP), primarily in the spinal cord, and they are believed to play a role in the modulation of pain perception (Pierce et al. 1998; Okuda-Ashitaka & Ito, 2000; Mousa et al. 2002; Ahmadi et al. 2003; Muth-Selbach et al. 2004; Sanderson Nydahl et al. 2004). However, these opioid peptides have been demonstrated not only in the central nervous system but also in peripheral nerve fibres, for instance in the gastrointestinal tract and the bronchus (Pasternak, 1998; Fischer et al. 1998; Yazdani et al. 1999) as well as in immune cells (Mousa et al. 2002; Seale et al. 2004).
The aim of this study was to investigate the opioid peptidergic innervation of the anterior eye segment and to compare it with the innervation pattern of SP in order to clarify the putative functional relationship of these peptides.
Materials and methods
The eyes of 20 healthy adult Sprague–Dawley rats were investigated. The eyes were obtained from the animal laboratory of the University of Essen. The animals were killed in conjunction with other non-ocular protocols. None of the eyes showed pathological changes, either in the anterior or the posterior eye segments. The studies conformed with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation
Immediately after enucleation, all eyes were cut equatorially behind the ora serrata; the lens was removed and the anterior eye segments were dissected into quadrants. All quadrants were immersed either in Zamboni's fixative for 4–12 h or in paraformaldehyde (PFA, 4%) for 3–4 h for fixation. The posterior eye segments were not further processed.
After fixation, each quadrant was dissected into 2-mm-wide specimens. The specimens were washed for 24 h in phosphate-buffered saline (PBS) containing 20% sucrose for cryoprotection. In three or four specimens of each quadrant, serial tangential (flat) cryostat sections (12–16 µm) of the anterior eye segments were cut in a plane parallel to the inner wall of Schlemm's canal. In some cases sagittal cryostat sections were cut.
Tangential (flat) sections are particularly suitable to study the anatomy of nerves and nerval plexus (Selbach et al. 2000, 2004) because the course of the nerves can easily be investigated on such sections, whereas on sagittal sections the nerves are mostly visible in cross-section only, i.e. as a more or less punctate staining. However, it is difficult to obtain flat sections of good quality.
Serving as tissue for the positive control, brain and parts of the thoracic and lumbar spinal cord were carefully prepared and afterwards immersed in the same fixatives as mentioned above. Cross-sections (spinal cord) and frontal sections (brain) were cut on a cryostat.
Immunohistochemistry
Innervation was studied immunohistochemically. The sections were stained with antibodies against SP, nociceptin, nocistatin, endomorphin 1 and 2, and leu- and met-enkephalin (see also Table 1).
Table 1.
Antibodies used for immunohistochemistry
| Primary antibody | Source | Type | Host | Dilution |
|---|---|---|---|---|
| Substance P (SP) | Biotrend | po | Rabbit | 1 : 400 |
| (Cologne, Germany) | mo | Mouse | ||
| Leu-enkephalin | Chemicon (Temecula, CA) | po | Rabbit | 1 : 500 |
| Met-enkephalin | Chemicon | po | Rabbit | 1 : 500 |
| Endomorphin 1 | Chemicon | po | Rabbit | 1 : 50 |
| Endomorphin 2 | Chemicon | po | Rabbit | 1 : 200 |
| Nociceptin | Phoenix | po | Rabbit | 1 : 800 |
| (Orphanin FQ) | (Mountain View, CA) | |||
| Nocistatin | Phoenix | po | Rabbit | 1 : 800 |
Sections were placed on poly-l-lysine-coated slides and initially incubated with Blotto's dry milk solution at room temperature for 30 min to reduce non-specific background staining. Incubation with the primary antibodies (Table 1) was performed in a moist chamber for 12–36 h at room temperature. Afterwards the sections were rinsed in Tris-buffered saline (TBS) (3 × 10 min) and then incubated for 1 h with biotinylated secondary antibodies (Dako, Hamburg, Germany). Finally the antibodies were visualized either with streptavidin-Cy2 (Dianova, Hamburg, Germany), streptavidin-Cy3 (Dianova) and, after rinsing in TBS, mounted in Kaiser's glycerine jelly. To investigate possible co-localization of SP and enkephalin, a mouse anti-SP antibody was used (see Table 1) and direct immunofluorescence with Cy2 and Cy3 labelling was performed.
Control experiments were performed using either PBS or non-immune rabbit or mouse serum substituted for the primary antibody.
Cryostat sections of the spinal cord or brain were used as positive controls to show that the antibodies were working in the material (Lai et al. 1997; Martin-Schild et al. 1999; Okuda-Ashitaka & Ito, 2000; Pierce & Wessendorf, 2000).
Serial sections were viewed either by conventional fluorescence or confocal laser scanning microscopy (Bio-Rad MRC 1000; Bio-Rad Microscience Ltd, Hemel Hempstead, UK).
Results
Substance P
A dense innervation with SP-immunoreactive (SP-ir) nerve fibres could be found in the conjunctiva and especially in the subconjunctival tissue, both in free nerve endings and vessel-associated nerve fibres (data not shown). SP-ir varicose nerves were distributed abundantly throughout the corneal stroma, epithelium and limbus (Fig. 1a). Prominent nerve trunks entered the limbus where they branched into smaller fascicles. These gave rise to a dense superficial circular limbal nerve plexus. Most of the fibres of this plexus were vessel-associated (Fig. 1a). After giving off branches to the limbus, the main nerve trunks continued radially and entered the peripheral cornea where they branched repeatedly, forming a dense fibre plexus that was particularly dense in the subepithelial layer and the upper stroma. These SP-ir fibres continued in the epithelium. Only a few fibres were found in the deep stroma.
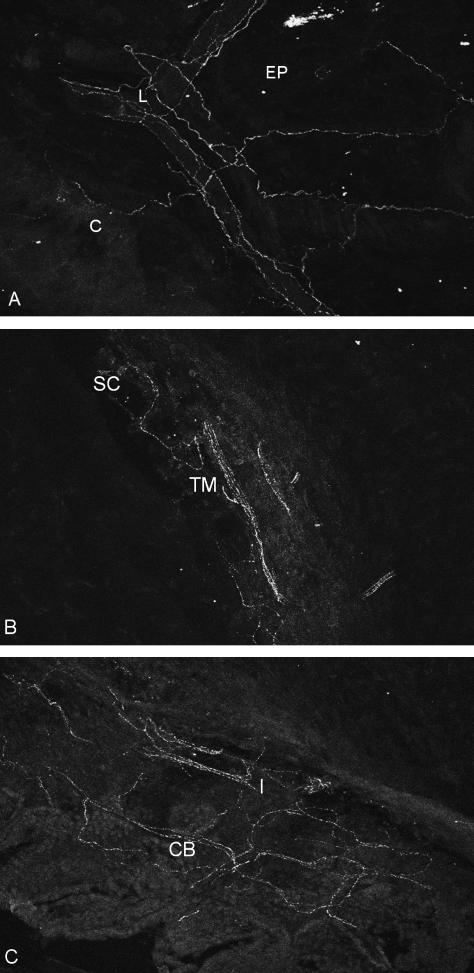
Fig. 1.
Tangential sections through a rat eye. (a) Cornea (C), corneal limbus (L) (perivascular nerve fibres) and episclera (EP); (b) trabecular meshwork (TM) and Schlemm's canal (SC); (c) iris root (I) and anteriormost ciliary body (CB) showing a plexus of SP-ir nerve fibres (×380).
Before entering the limbus the main nerve trunks run along the episclera. Here, branches were found closely associated with the episcleral vessels, both arterioles and veins (Fig. 1a).
Numerous SP-positive varicose nerve fibres were also seen in the layers of the trabecular meshwork (Fig. 1b). Larger fascicles entered the posterior part of the trabecular meshwork and branched immediately at approximately 90°. Most of these now circumferentially orientated SP-ir fibres were found in the outer portion of the meshwork close to the equivalent of Schlemm's canal.
Abundant nerve fibres staining for SP were also found in the iris, the iris root and the anteriormost portion of the ciliary body (Fig. 1c), forming a stromal and subepithelial plexus. Only a minority of these fibres were perivascular.
Opioid peptides
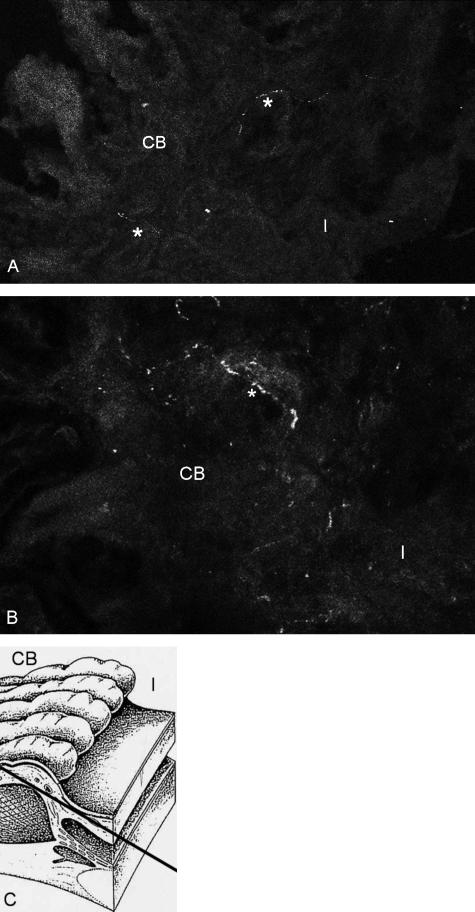
Few nerve fibres staining for met- and leu-enkephalin were found in the connective tissue between the iris root and the anterior basal part of the ciliary processes (Fig. 2a–c) in every quadrant of the circumference. They were not clearly vessel-associated. No fibres were found in association with the ciliary epithelium or the ciliary or pupillary muscles. However, staining was weak and only single fibres were found in three out of four investigated rat eyes. There was no plexus comparable with those with SP-ir nerve fibres. A co-localization with SP could was observed. This needs to be interpreted with some care because of the weak enkephalin staining in contrast to the intense SP staining. No met- or leu-enkephalin-immunoreactive nerve fibres were found in the conjunctiva, cornea, episclera or trabecular meshwork.
Fig. 2.
(a,b) Tangential section through a rat eye showing single nerve fibres (*) staining for met-enkephalin in the anteriormost ciliary body (CB) close to the iris root (I) (×680). (c) Schematic drawing to illustrate the section plane.
Nerve fibres immunoreactive for nociceptin, nocistatin, endomorphin 1 or 2 could not be detected in any part of the anterior segment of the rat eye, either in tangential or in sagittal sections.
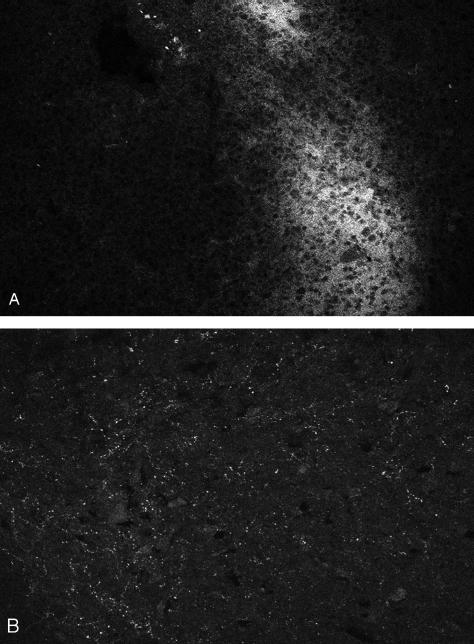
For these peptides, however, immunoreactivity could be identified in the dorsal horn of the spinal cord (nociceptin, nocistatin) and in parts of the brain (especially in the region of the hypothalamus, endomorphin 1 and 2), serving as positive controls (Fig. 3a,b).
Fig. 3.
Sections through (a) rat brain (hypothalamus) and (b) rat dorsal horn of the lumbar spinal cord showing immunoreactivity for (a) endomorphin 1 (×480) (diffuse staining) and (b) nociceptin (punctate staining, ×960).
Conclusions
The eye, as an example for peripheral SP innervation, has been extensively studied. In general, our results concerning innervation with SP-positive nerve fibres confirm those described by previous authors. The innervation pattern of the cornea is very similar to that described by Jones & Marfurt (1998). The findings regarding the innervation of the conjunctiva, episclera and iris also confirm those of other investigations (Luhtala & Uusitalo, 1991; Beckers et al. 1993; Selbach et al. 1998).
However, our study is the first to describe SP-ir nerve fibres in the trabecular meshwork of the rat eye. SP-ir nerve fibres have been found in the trabecular meshwork of humans and monkeys (Laties et al. 1981; Selbach et al. 2000).
The rat eye has become increasingly important as an animal model because, with a few differences, its anatomy shows great similarities to the human eye (van der Zypen, 1977). This is in marked contrast to other laboratory animals such as rabbits. In glaucoma research in particular, rat eyes are used for various models, e.g. coagulation of episcleral veins. van der Zypen (1977) investigated in detail the anatomy of the filtration angle of the rat eye. A trabecular meshwork, similar to the trabeculum cribriforme in primate eyes, lies directly below the inner wall of a circular, flattened canal, which corresponds in its topographical position and in its ultrastructure to the canal of Schlemm in humans and primates. The rat trabecular meshwork is, however, less extensive and less developed than that of humans and monkeys.
The innervation with SP-ir nerve fibres we found in the rat eye is very similar to that found in human and monkey eyes (Stone & Kuwayama, 1985; Selbach et al. 2004). It is generally presumed that SP-positive nerve fibres represent afferent trigeminal axons, i.e. that the innervation is sensory in nature (Elsas et al. 1994). It is tempting to speculate that the SP-positive varicose nerve fibres in the trabecular meshwork might be involved – in a yet unknown way – in the regulation of aqueous humor outflow (Ruskell, 1976, 1994; Selbach et al. 2000, 2004).
SP and CGRP (calcitonin gene-related peptide) as neurotransmitters of primary afferent neurons are, when released at the peripheral end of the axon, principal mediators of neurogenic inflammation. In the peripheral nervous system, especially in the airways, endogenous opioid peptides (especially endomorphin 1 and nociceptin) are believed to be inhibitors of neurogenic inflammation (Rouget et al. 2004). Nociceptin and endomorphin inhibit bronchoconstriction and plasma extravasation in the airways by inhibiting tachykinin release (especially SP) (Fischer et al. 1998; Rouget et al. 2004). This is important for the pathophysiology of asthma. In the stomach and small intestine nociceptin inhibits cholinergic neurotransmission (Yazdani et al. 1999).
SP- (and CGRP-) induced neurogenic inflammation is also known in the eye, where it leads to miosis, vasodilation and a breakdown of the blood–aqueous barrier. At least in monkey eyes, the anteriormost portion of the ciliary body close to the iris root is markedly involved in such changes as provoked by paracentesis or prostaglandin treatment (Okisaka, 1976a,b; Lütjen-Drecoll & Tamm, 1988; Tamm & Lütjen-Drecoll, 1996). In this region we found a dense plexus of SP-ir nerve fibres (Fig. 1c) and interestingly also a few opioid-peptidergic nerves (Fig. 2).
Enkephalin-positive nerve fibres in the iris have also been described by Björklund et al. (1984). Other reports of opioid-peptidergic innervation in tissues of the anterior segment are lacking.
Thus it seems that under physiological conditions there is only little opioid peptidergic innervation in the anterior eye segment of rat eyes. Therefore it is questionable whether peripheral endogenous opioids play a major role in modulation of ocular sensory neurotransmission, pain perception or normal aqueous humor outflow. However, it cannot be excluded that under certain (pathological) conditions (e.g. inflammation) peptide expression is up-regulated (Hökfelt et al. 2000; Mousa et al. 2002) and opioid peptides may also be involved in the modulation of (neurogenic) inflammation in the eye.
Acknowledgments
This work is dedicated to the memory of Gordon Ruskell. It was supported by grants from IFORES (University of Essen) (J.M.S., S.H.B.) and the Deutsche Ophthalmologische Gesellschaft (J.M.S.).
References
- Ahmadi S, Muth-Selbach U, Lauterbach A, Lipfert P, Neuhuber WL, Zeilhofer HU. Facilitation of spinal NMDA receptor currents by spillover of synaptically released glycine. Science. 2003;300:2094–2097. doi: 10.1126/science.1083970. [DOI] [PubMed] [Google Scholar]
- Beckers HJM, Klooster J, Vrensen GFJM, Lamers WPMA. Substance P in rat corneal and iridial nerves: an ultrastructural immunohistochemical study. Ophthalmic Res. 1993;25:192–200. doi: 10.1159/000267291. [DOI] [PubMed] [Google Scholar]
- Björklund H, Hoffer B, Olson L, Palmer M, Seiger A. Enkephalin immunoreactivity in iris nerves: distribution in normal and grafted irides, persistance and enhanced fluorescence after denervations. Histochemistry. 1984;80:1–7. doi: 10.1007/BF00492763. [DOI] [PubMed] [Google Scholar]
- Elsas T, Edvinsson L, Sundler F, Uddman R. Neuronal pathways to the rat conjunctiva revealed by retrograde tracing and immunohistochemistry. Exp. Eye Res. 1994;58:117–126. doi: 10.1006/exer.1994.1201. [DOI] [PubMed] [Google Scholar]
- Fischer A, Forssmann WG, Undem BJ. Nociceptin-induced inhibition of tachykinergic neurotransmission in guinea-pig bronchus. J. Pharmacol. Exp. Ther. 1998;285:902–907. [PubMed] [Google Scholar]
- Hökfelt T, Broberger C, Xu ZQD, Sergeyev V, Ubink R, Diez M. Neuropeptides – an overview. Neuropharmacology. 2000;39:1337–1356. doi: 10.1016/s0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp. Eye Res. 1998;66:421–435. doi: 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- Kuwayama Y, Stone RA. Distinct substance P and calcitonin gene-related peptide immunoreactive nerves in the guinea pig eye. Invest. Ophthalmol. Vis. Sci. 1987;28:1947–1954. [PubMed] [Google Scholar]
- Lai CC, Wu SY, Dun SL, Dun NJ. Nociceptin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons. Neuroscience. 1997;81:887–891. doi: 10.1016/s0306-4522(97)00251-0. [DOI] [PubMed] [Google Scholar]
- Laties AM, Stone RA, Brecha NC. Substance P-like immunoreactive nerve fibers in the trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 1981;21:484–486. [PubMed] [Google Scholar]
- Luhtala J, Uusitalo H. The distribution and origin of substance P immunoreactive nerve fibres in the rat conjunctiva. Exp. Eye Res. 1991;53:641–646. doi: 10.1016/0014-4835(91)90224-3. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2α. Exp. Eye Res. 1988;47:761–769. doi: 10.1016/0014-4835(88)90043-7. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J. Comp. Neurol. 1999;405:450–471. [PubMed] [Google Scholar]
- Mousa SA, Machelska H, Schafer M, Stein C. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J. Neuroimmunol. 2002;126:5–15. doi: 10.1016/s0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- Muth-Selbach U, Dybek E, Kollosche K, et al. The spinal antinociceptive effect of nocistatin in neuropathic rats is blocked by d-serine. Anesthesiology. 2004;101:753–758. doi: 10.1097/00000542-200409000-00025. [DOI] [PubMed] [Google Scholar]
- Okisaka S. Effects of paracentesis on the blood–aqueous barrier: a light and electron microscopic study on cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 1976a;10:824–834. [PubMed] [Google Scholar]
- Okisaka S. The effects of prostaglandin E1 on the ciliary epithelium and the drainage angle of cynomolgus monkeys: a light and electron microscopic study. Eyp. Eye Res. 1976b;22:141–154. doi: 10.1016/0014-4835(76)90041-5. [DOI] [PubMed] [Google Scholar]
- Okuda-Ashitaka E, Ito S. Nocistatin: a novel neuropeptide encoded by the gene for the nociceptin/orphanin FQ precursor. Peptides. 2000;21:1101–1109. doi: 10.1016/s0196-9781(00)00247-3. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. The central questions in pain perception may be peripheral. Proc. Natl. Acad. Sci. USA. 1998;95:10354–10355. doi: 10.1073/pnas.95.18.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce TL, Grahek MD, Wessendorf MW. Immunoreactivity for endomorphin-2 occurs in primary afferents in rats and monkey. Neuroreport. 1998;9:385–389. doi: 10.1097/00001756-199802160-00005. [DOI] [PubMed] [Google Scholar]
- Pierce TL, Wessendorf MW. Immunocytochemical mapping of endomorphin-2-immunoreactivity in rat brain. J. Chem. Neuroanat. 2000;18:181–207. doi: 10.1016/s0891-0618(00)00042-9. [DOI] [PubMed] [Google Scholar]
- Rouget C, Cui YY, D'Agostino B, et al. Nociceptin inhibits airway microvascular leakage induced by HCl intra-oesophageal installation. Br. J. Pharmacol. 2004;141:1077–1083. doi: 10.1038/sj.bjp.0705704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. The source of nerve fibres of the trabeculae and adjacent structures in monkey eyes. Exp. Eye Res. 1976;23:449–459. doi: 10.1016/0014-4835(76)90174-3. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Trigeminal innervation of the scleral spur in cynomolgus monkeys. J. Anat. 1994;184:511–518. [PMC free article] [PubMed] [Google Scholar]
- Sanderson Nydahl K, Skinner K, Julius D, Basbaum AI. Co-localization of endomorphin-2 and substance P in primary afferent nociceptors and effects of injury: a light and electron microscopic study in the rat. Eur. J. Neurosci. 2004;19:1789–1799. doi: 10.1111/j.1460-9568.2004.03284.x. [DOI] [PubMed] [Google Scholar]
- Seale JV, Jessop DS, Harbuz MS. Immunohistochemical staining of endomorphin 1 and 2 in the immune cells of the spleen. Peptides. 2004;25:91–94. doi: 10.1016/j.peptides.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Selbach JM, Schönfelder U, Funk RHW. Arteriovenous anastomoses of the episcleral vasculature in the rabbit and rat eye. J. Glaucoma. 1998;7:50–57. [PubMed] [Google Scholar]
- Selbach JM, Gottanka J, Wittmann M, Lütjen-Drecoll E. Efferent and afferent innervation of primate trabecular meshwork and scleral spur. Invest. Ophthalmol. Vis. Sci. 2000;41:2184–2191. [PubMed] [Google Scholar]
- Selbach JM, Rohen JW, Steuhl KP, Lütjen-Drecoll E. Angioarchitecture and innervation of the primate anterior episclera. Curr. Eye Res. 2005 doi: 10.1080/02713680590934076. in press. [DOI] [PubMed] [Google Scholar]
- Stone RA, Laties AM, Brecha NC. Substance P-like immunoreactive nerves in the anterior segment of the rabbit, cat and monkey eye. Neuroscience. 1982;7:2459–2468. doi: 10.1016/0306-4522(82)90207-x. [DOI] [PubMed] [Google Scholar]
- Stone RA, Kuwayama Y. Substance P-like immunoreactive nerves in the human eye. Arch. Ophthalmol. 1985;103:1207–1211. doi: 10.1001/archopht.1985.01050080119031. [DOI] [PubMed] [Google Scholar]
- Stone RA. Nervous system and intraocular pressure. In: Ritch E, Shields MB, Krupin, editors. The Glaucomas. St Louis, MO: CV Mosby; 1996. pp. 357–383. [Google Scholar]
- Tamm ER, Lütjen-Drecoll E. Ciliary body. Microsc. Res. Technique. 1996;33:390–439. doi: 10.1002/(SICI)1097-0029(19960401)33:5<390::AID-JEMT2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Yazdani A, Takahashi T, Bagnol D, Watson SJ, Owyang C. Functional significance of a newly discovered neuropeptide, Orphanin FQ, in rat gastrointestinal motility. Gastroenterology. 1999;116:108–117. doi: 10.1016/s0016-5085(99)70234-9. [DOI] [PubMed] [Google Scholar]
- van der Zypen E. Experimental morphological study on structure and function of the filtration angle of the rat eye. Ophthalmologica. 1977;174:285–298. doi: 10.1159/000308617. [DOI] [PubMed] [Google Scholar]