Abstract
The aim of the present study was to describe the ultrastructure of particular cells observed in the microvascular bed of the healthy human choroid, in close relation to the wall of the microvessels and resembling the periadventitial cells of other vascular areas of the human body. Serial sections of 12 fresh human eyes were studied by transmission electron microscopy. In all the eyes, the sections were obtained by cutting from the same zones (inner and outer choroid at the posterior pole of the eye). Standard techniques were used for transmission electron microscopy. Round cell bodies were found in the inner choroid at the posterior pole of the eye, mainly located in the intercapillary connective tissue. The cells were composed of an electron-transparent cytoplasm containing a few small mitochondria, and a dilated smooth surface of endoplasmic reticulum, at some points continuous with the nuclear membrane. These cells showed processes forming contact with the capillary wall. Some of these processes extended to the elastic layer of Bruch's membrane, but none had contact with the retinal pigment epithelium. A thin basement membrane surrounded both the cell bodies and processes. We believe that these cells are special cells resembling some type of periendothelial cells also localized in other microvascular districts of the human body. The close topographic correlation with the endothelial cells seems to indicate that these special cells play a role in the intrinsic control of proper endothelial functions.
Keywords: choriocapillary, endothelium, eye, humans, inner choroid, periadventitial cells, pericytes, TEM
Introduction
The vascular anatomy of the choroid was first described by Wibar (1954) in relation to human eye diseases. Many years later the cells and supporting structures of the human choroid, including its microvessels, were studied by means of electron microscopy (Wolter, 1956; Feeney & Hogan, 1961). Choroidal vascularization of rabbit eye was also investigated by Ruskell (1961). The choroid is the vascular tissue of the eye: it provides nourishment for the external retinal tissue and regulates ocular temperature (Alm & Bill, 1987). Microvessels of the choroid are similar to the microcapillaries of other vascular districts of the human body and have similar ultrastructure and similar organization (Feher & Artico, 1998). It is well known that in the anterior choroid (ciliary processes) the capillaries are large and fenestrated, as in the choriocapillaries, whereas in other zones of the choroid the capillaries are formed by a single layer of unfenestrated endothelium. In both cases the endothelium lies on a basement membrane and is surrounded by few pericytes and/or some periendothelial cells. The pericytes of the uveal capillaries were studied by Sakamoto et al. (1995a,b) and by Ishibashi et al. (1995) while, more recently, a functional role for the perivascular cells of the eye, including the pericytes, has been demonstrated (Witmer et al. 2003; Haritoglou et al. 2004). Recently, our group demonstrated the presence of special cells in the inner choroids that might fulfil intrinsic properties. These cells were interpreted as neural-like cells (Feher et al. 2002). In the present paper these preliminary observations were extended and the ultrastructural features of these special cells, localized in the inner choroid and closely related to the wall of the microvessels, were examined; they are interpreted as pericyte-like cells.
Materials and methods
Twelve human eyes were used in this study (seven female and five male, donors aged 36–87 years, mean age 68 years). The eyes were surgically removed owing to malignant tumour, absolute painful glaucoma or severe ocular trauma. For this study, only samples collected from the operating theatre were immediately fixed, to avoid post mortem phenomena that might disturb or severely modify the morphology of the structures under examination. The study was approved by the Ethics Committee of our University, and patients gave their informed written consent. The investigations were performed according to the guidelines of the declaration of Helsinki. Immediately after enucleation, the eye-bulb was dissected with a razor blade and cut into small samples. Choroidal and retinal samples of the equatorial and posterior pole regions were harvested for our morphological and ultrastructural studies.
Cross-sections of the retina and choroid were prepared for light and electron microscopy. Samples were fixed in 2% buffered glutaraldehyde for 2 h at 4 °C and post-fixed in 2% osmium tetroxide for another 2 h. The tissue samples were dehydrated and embedded in Araldite. Thick sections (5 µm) were cut with glass knives and stained with toluidine blue. Thin sections, for transmission electron microscopy (TEM), were cut with a diamond knife on an LKB ultramicrotome, counterstained with lead citrate and uranyl acetate, mounted on collodion-covered nickel grids and analysed using a Zeiss 109 electron microscope (Zeiss, Oberkochern, Germany). Sections for light microscopy served only as reference points for the ultrastructural observations. A large number of ultramicroscopic fields were observed and photographed.
Results
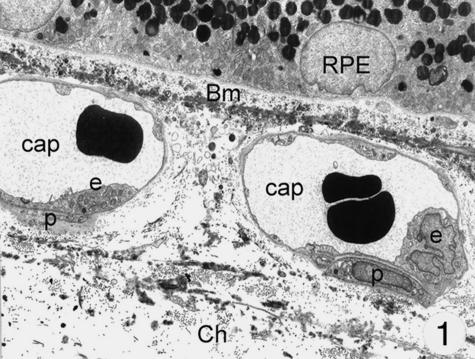
At the ultrastructural level, different cell types were identified in the wall of the choriocapillaris: endothelial cells showed an intense fenestration mainly towards Bruch's membrane, but also towards the outer surface (Fig. 1). They lined the inner surface of the vessels and were surrounded by a continuous basal membrane. A second type of cells showed features resembling pericytes. The choriocapillaris was only sparsely surrounded by pericytes, mainly at the outer and lateral surface of the capillaries at some distance from the fenestrations.
Fig. 1.
Electron microscopy of the choriocapillaris: a thin layer of fenestrated endothelial cells lie on a thin basement membrane. Only very rarely pericytes can be seen. Magnification ×8000. (Eye removed due to neovascular glaucoma.) Abbreviations for this and other figures: Bm, Bruch's membrane; RPE, retinal pigment epithelium; Ch, choroid; m, mitochondria; Gc, special cell; cap, capillary; e, endothelial cell; er, ergastoplasmatic reticulum; p, pericyte; d, cellular process.
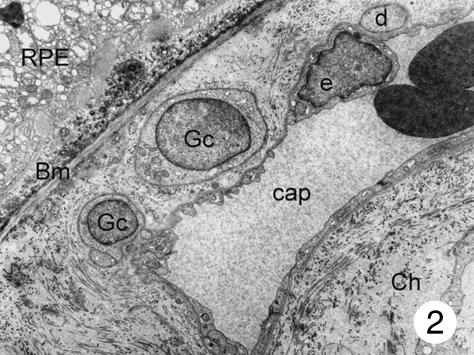
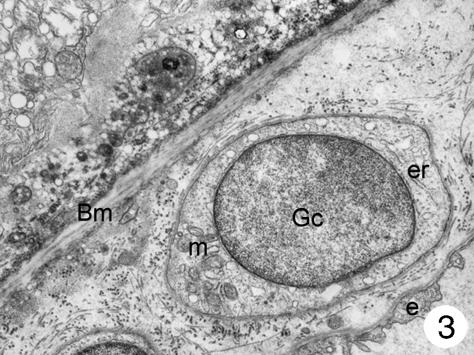
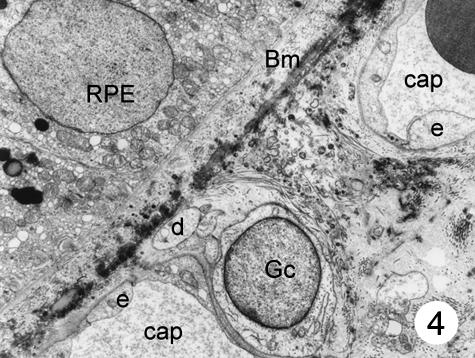
In addition to these cells of the capillary wall, a third type of cell was found in the inner choroids. These cells showed a round-shaped body and were mainly found in the posterior pole of the eye. Most of these cells were located in the intercapillary connective tissue, but they were also found in the outer connective tissue of the choriocapillaris and close to the Bruch's membrane (in small numbers only). The latter were located between the basal membrane of the endothelial cells and the elastic layer (Fig. 2). These cells had poorly electron-dense cytoplasm, which contained a few small mitochondria and several dilated the smooth surface of the endoplasmic reticulum, some continuous with the nuclear membrane. Most processes of these cells formed close contact with the capillary wall, separated from the endothelial cells by a thin basement membrane. At higher magnification, it was possible to see that the body of these cells contained a round nucleus surrounded by nuclear membrane that, in some places, was continuous with the dilated rough surface endoplasmic reticulum. Few mitochondria were observed. The plasmatic membrane showed focal electron densities (possibly caveols) (Fig. 3). Although some processes of these cells extended to the elastic layer of Bruch's membrane, none had contact with the retinal pigment epithelium. In places, the cells seemed to be surrounded by an incomplete sheath of a second cell layer; within the cytoplasm small groups of vesicles could be detected (Figs 2–4). A thin basement membrane surrounded both the cell bodies and their processes (Fig. 5).
Fig. 2.
Two cell bodies of special peri-adventitial cells can be seen in the widened outer collagen layer of Bruch's membrane. Some cellular processes from the smaller cell contact the capillary wall and only a thin basement membrane is interposed between these processes and the endothelial cells. No pericytes can be seen. Magnification ×8000. (Eye removed due to intraocular melanoma.) For abbreviations, see legend to Fig. 1.
Fig. 3.
Higher magnification of part of Fig. 2: the cell body contains a round nucleus surrounded by a nuclear membrane that, in some places, is continuous with the dilated rough surface endoplasmic reticulum. A few mitochondria can also be seen. The plasmatic membrane shows focal electron densities (caveols?) and is surrounded by a thin basement membrane. Magnification ×22 000. (Eye removed due to intraocular melanoma.) For abbreviations, see legend to Fig. 1.
Fig. 4.
The cell body and some processes of a special cell can be seen in the intercapillary connective tissue, next to a choriocapillary. One of the processes reaches the elastic layer of Bruch's membrane. No pericytes can be seen. Magnification ×8000. (Eye removed due to intraocular melanoma.) For abbreviations, see legend to Fig. 1.
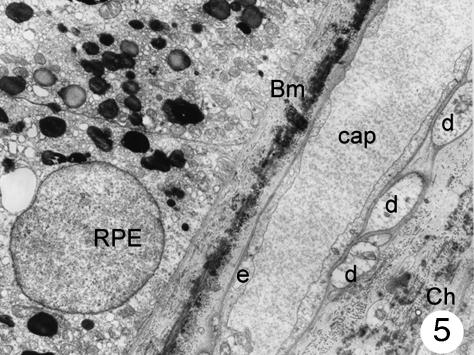
Fig. 5.
Several cellular processes in contact with the outer side of choriocapillaries. A very thin basement membrane is interposed between these processes and the endothelial cells. No pericytes can be seen. Magnification ×8000. (Eye removed due to intraocular melanoma). For abbreviations, see legend to Fig. 1.
Discussion
Evidence was found of a special population of cells around the microvessels of the human inner choroid. These cells may be interpreted as special cells, localized in pericapillary connective tissue and closely related to the endothelial cells. These cells are similar to the cells found in other vascular districts (skin, dental pulp, placenta, brain, etc.) The skin microvessels are organized into upper and lower horizontal plexi with dermal capillary loops of junction. The wall of the microvessels is made up of endothelial cells. These cells lie on a basal membrane. Periadventitial cells (pericytes and veil cells) are present around all microvessels. Their size and number appear to be correlated with the quantity of mural basement membrane material found in skin microvessels (Braverman, 1989). The presence of pericytes and transitional cells, in the microvessels of the human dental pulp, was studied by Carlile et al. (2000). Three types of periendothelial cells were identified: pericytes, transitional cells and fibroblasts. Pericytes were embedded within the capillary basement membrane. Transitional cells were partly surrounded by basement membrane, but separated from the endothelium by collagen fibrils; fibroblasts were located outside, but adjacent to the basement membrane and closely associated with collagen fibrils.
Pericytes were also studied in the human placenta (Challier et al. 1999). The placental microvessels examined by TEM showed many pericytes with foot processes included in the basement membrane. The origin and the potential functions of the pericytes (contractility, basement membrane secretion, angiogenesis and phagocytosis) in the placental vascular bed remain unknown.
In the human brain, pericytes may be involved in oedema. In fact, pericyte-like endothelial cells exhibited remarkable oedematous changes, increased vascular and vescicular transport, and formation of transient trans-pericytal channels and tubular structures, demonstrating pericyte/brain barrier dysfunction. Some pericytes displayed hypertrophic and necrotic changes. Phagocytic pericytes exhibited ingested erythrocytes. Hypertrophic pericytes inducing basement membrane splitting and degenerated pericytes with lacunar enlargement of endoplasmic reticulum were found; in addition were deposits of dense osmiophylic bodies and glycogen granules, vacuolization, hydropic changes of Golgi apparatus and pleomorphic mitochondria (Castejon, 1984).
Pericytes originate from mesenchymal cells. They form an incomplete envelope around the endothelial cells, and within the microvascular basement membrane of capillaries and postcapillary venules. Morphologically, they appear as long, slender, polymorphic cells, with an elongated cell body, from which longitudinal and circumferential branches arise. Cell bodies and cytoplasmic processes of pericytes, as well as the endothelial cells, are enveloped by the same basal lamina, except at the points where they are directly in contact with each other. Several general functions for the pericytes have been postulated: contractability; permeability regulator; integrity maintainer; endothelial cell growth modulator; and cell progenitor with considerable mesenchymal potential (Diaz-Flores et al. 1991).
The special cells described in this paper and presumed to be special, pericyte-like cells, may exert the same functions listed above. In fact, the close topographic correlation between these cells and the capillary endothelial cells suggests that they intrinsically control endothelial functions. In addition, the caveols described in the plasmatic membrane of these special cells, localized near the Bruch's membrane, can be assumed to be morphological age-related changes. Finally, because only a few pericytes were observed around the choriocapillaries, the pericytes themselves seem to play only a minor role in the regulation of choriocapillary blood flow. The presence of some new, special cells (called pericyte-like cells) near the choriocapillaries leads us to suggest that these cells have an intrinsic control on the proper functions of the endothelium of the choriocapillaries.
In conclusion, our electron microscopic observations show the presence of special cells in the choriocapillaries. The few pericytes observed probably exert a modest role in the regulation of choriocapillary blood flow. Further research regarding the possible functions of the above-mentioned cells may be useful for new aetiopathogenetic interpretations of some ocular pathologies such as chronic simple glaucoma, diabetic retinopathy, vascular retinopathy and other macular pathologies.
Acknowledgments
The present study was supported in part by a grant from University of Rome ‘La Sapienza’, Ricerche di Ateneo, 03.05.016. We are greatly indebted to Dr C. A. May and A. Kovacs for their useful suggestions and criticisms. Medline and the informatic consulting services of Dr Mauro Cameroni, the technical assistance of Mr Dario Caporuscio, the secretarial work of Mrs Silvana Casamento, the photographical co-operation of Mr Giuseppe Leoncini, and the kind help of P. Patrick Roger in the revision of the English text are gratefully acknowledged.
References
- Alm A, Bill A. Ocular circulation. In: Moses RA, Hart WM Jr, editors. Adle's Physiology of the Eye: Clinical Application. St Louis: Mosby; 1987. pp. 183–203. [Google Scholar]
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J. Invest. Dermatol. 1989;93(Suppl. 2):2S–9S. doi: 10.1111/1523-1747.ep12580893. [DOI] [PubMed] [Google Scholar]
- Carlile MJ, Sturrock MG, Chisholm DM, Ogden GR, Schor AM. The presence of pericytes and transitional cells in the vasculature of the human dental pulp: an ultrastructural study. Histochem. J. 2000;32:239–245. doi: 10.1023/a:1004055118334. [DOI] [PubMed] [Google Scholar]
- Castejon OJ. Submicroscopic changes of cortical capillary pericytes in human perifocal brain edema. J. Submicrosc. Cytol. 1984;16:601–618. [PubMed] [Google Scholar]
- Challier JC, Galtier M, Kacemi A, Guillaumin D. Pericytes of term human foeto-placental microvessels: ultrastructure and visualization. Cell Mol. Biol. (Noisy-le-Grand) 1999;45:89–100. [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol. Histopathol. 1991;6:269–286. [PubMed] [Google Scholar]
- Feeney L, Hogan MJ. Electron microscopy of the human choroid. I. Cells and supporting structure. Am. J. Ophthalmol. 1961;51:1057–1072. doi: 10.1016/0002-9394(61)91795-0. [DOI] [PubMed] [Google Scholar]
- Feher J, Artico M. Anatomy of the anterior uvea. In: Feher J, editor. Pathophysiology of the Eye 4: Glaucoma. Budapest: Akademiai Kladò; 1998. pp. 51–78. [Google Scholar]
- Feher J, Papale A, Gualdi L, Balacco Gabrieli C. Intrinsic nervous plexus of the human choriocapillary. An ultrastructural study. Invest. Ophthalmol. Vis. Sci. 2002 ARVO Congress abstract 192. [Google Scholar]
- Haritoglou C, Hoops JP, Stefani FH, Mehraein P, Kampik A, Dichgans M. Histo-pathological abnormalities in ocular blood vessels of CADASIL patients. Am. J. Ophthalmol. 2004;138:302–305. doi: 10.1016/j.ajo.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Inomata H, Sakamoto T, Ryan SJ. Pericytes of newly formed vessels in experimental subretinal neovascularization. Arch. Ophthalmol. 1995;113:227–231. doi: 10.1001/archopht.1995.01100020111041. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Choroidal vascularization in the rabbit. Am. J. Ophthal. 1961;52:807–815. doi: 10.1016/0002-9394(61)90905-9. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakamoto H, Hinton DR, Spee C, Ishibashi T, Ryan SJ. In vitro studies of human choroidal endothelial cells. Curr. Eye Res. 1995a;14:621–627. doi: 10.3109/02713689508998488. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakamoto H, Murphy TL, et al. Vessel formation by choroidal endothelial cells in vitro is modulated by retinal pigment epithelial cells. Arch. Ophthalmol. 1995b;113:512–520. doi: 10.1001/archopht.1995.01100040134039. [DOI] [PubMed] [Google Scholar]
- Wibar KC. Vascular anatomy of the choroid in relation to selective localization of ocular disease. Br. J. Ophthalmol. 1954;38:513–517. doi: 10.1136/bjo.38.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, van Blijswijk BC, van Noorden CJ, Vrensen GF, Schlingemann RO. In vivo angiogenic phenotype of endothelial cells and pericytes indu-ced by vascular endothelial growth factor-A. J. Histochem. Cytochem. 2003;52:39–52. doi: 10.1177/002215540405200105. [DOI] [PubMed] [Google Scholar]
- Wolter JR. The pericytes of the choroid of the human eye. Am. J. Ophthalmol. 1956;41:990–995. doi: 10.1016/0002-9394(56)91048-0. [DOI] [PubMed] [Google Scholar]