Abstract
The sensory innervation of the lacrimal gland (LG) in the cynomolgous monkey was studied using the retrograde wheat germ agglutinin/horsereadish peroxidase (WGA/HRP) tracer technique. A small solidified piece of WGA/HRP was implanted in the LG. Labelled sensory first-order neurons were found in the ipsilateral trigeminal ganglion (TG) and in the ipsilateral mesencephalic trigeminal nucleus (MTN). The distribution of labelled TG neurons was restricted to ophthalmic and maxillary ganglionic parts. Sensory innervation of LG by primary afferents is not only restricted to TG; an MTN involvement has also been found. This may imply that there is a central sensory role in the production and release of tears.
Keywords: lacrimal gland, mesencephalic, monkey, neuronal tracing, sensory innervation, trigeminal ganglion, trigeminal nucleus
Introduction
The general concept for autonomic lacrimal gland (LG) innervation is by parasympathetic nerve fibres originating in the pterygopalatine ganglion (PPG) and sympathetically by nerve fibres from the superior cervical ganglion (SCG).
Recently, Gordon Ruskell re-investigated the pathways of nerve fibres between PPG and the LG in human dissection room cadavers (Ruskell, 2004). The reason for this study was an attempt to resolve the uncertainty in the distribution of fine autonomic nerve fibres towards LG. In the late 1960s and early 1970s, Ruskell (among others) investigated the post-ganglionic autonomic innervation of LG, by nerve sectioning and by examining the degeneration of these nerves electron microscopically (Ruskell, 1967, 1969, 1970a,b, 1971, 1974, 1975). Although he performed unilateral nerve lesions, and also ophthalmic, maxillary or mandibular neurotomy or complete ablation of the trigeminal ganglion (TG), to study the Wallerian degeneration of nerve fibres in orbital and related structures, he did not investigate the sensory innervation of the LG. Instead he studied in monkeys and humans the sensory innervation of other orbital structures such as the cavernous sinus plexus, the cerebral arteries, the nasociliary nerve fibres towards the eyeball, the anterior eye segment, the trabeculum, the scleral spur, the palpebral conjunctiva, the conjunctival lymph follicles and the extraocular muscles (Ruskell, 1973, 1974, 1976, 1978, 1982, 1984a,b, 1985, 1988, 1993, 1994, 1999; Ruskell & Wilson, 1983; Ruskell & Simons, 1987, 1992; VanderWerf et al. 1996a,b; Ruskell & VanderWerf, 1997).
Other investigators have indicated that the TG has been involved in the afferent innervation of the nasal epithelium of the airway, and the orbital structures, such as the cornea, extraocular muscles, eyelids and LG (Corbin & Harrison, 1940; Lucier & Egizii, 1986; Porter, 1986; Marfurt & Echtenkamp, 1988; Buisseret-Delmas & Buisseret, 1990; Porter & Donaldson, 1991; VanderWerf et al. 1993, 1996a,b, 1997; Ndiaye et al. 2000, 2002). Anatomical studies have shown that sensory nerve fibres of the lacrimal branch of the ophthalmic division of TG supplies (among other things) the LG (Salvatore et al. 1999), the inferior conjunctiva (Oduntan & Ruskell, 1992), the lateral portion of the upper eyelid (Burton, 1992) and the skin (Shankland, 2001).
Sensory innervation of the LG has been studied in the monkey (VanderWerf et al. 1996a), the cat (Cheng et al. 2001), the rabbit (Salvatore et al. 1999) and the rat (Ten Tusscher et al. 1990; Tóth et al. 1999) using neuronal tracers. When the tracer was applied to LG, labelled neurons were found in PPG, in SCG, in the ciliary ganglion (CG) and in the TG (VanderWerf et al. 1996a; Tóth et al. 1999; Cheng et al. 2001). The distribution pattern of labelled neurons in TG was mainly restricted in the monkey to the ophthalmic and maxillary ganglionic parts. Double-labelling fluorescent cholera toxin B subunit tracing and nitric oxide synthase studies in the cat LG revealed double labelling in parasympathetic, sympathetic and sensory ganglia by neurons of TG, the superior vagal ganglion and superior glossopharyngeal ganglion (Cheng et al. 2001). Physiologically, a unilateral TG ablation in the rabbit resulted in disturbed protein production in LG (Meneray et al. 1998), but changes on tear IgA levels after TG ablation were not found (Sullivan et al. 1990).
Two candidate sensory neuronal structures could be considered for direct LG input: TG and the mesencephalic trigeminal nucleus (MTN), which is located in the mesencephalic part of the brainstem. MTN extends in rostrocaudal direction from the superior colliculus up to the trigeminal motor nucleus and is situated ventrolaterally on both sides of the fourth ventricle. The nucleus has a rod shape and consists of pseudo-unipolar small and large neurons (> 60 µm) and has in general a proprioceptive function for masticatory muscles (Corbin & Harrison, 1940; Luo & Dessem, 1996; Dessem & Luo, 1999; Luo et al. 2001) and extraocular muscles (Porter & Donaldson, 1991). In the rat, guinea-pig and cat MTN, it has been established that MTN projects to the masticatory muscles (Varathan et al. 2001; Zhang & Luo, 2002), periodontal ligaments (Zhang et al. 1992; Honma et al. 2001), nasal receptors (Lucier & Egizii, 1986) and extraocular muscles (Buisseret-Delmas & Buisseret, 1990; Ndiaye et al. 2000). The centrally placed ganglionic MTN neurons receive projections from the hypothalamus, parvocellular reticular formation and dorsal raphe nucleus (Nagy et al. 1986; Copray et al. 1991; Minkels et al. 1991). MTN itself projects, among others, to the motor nucleus of V, the sensory trigeminal complex (STC), the supratrigeminal area, the hypoglossal nucleus, reticular formation and the cerebellum (Buisseret-Delmas et al. 1997; Widmer et al. 1998; Zhang et al. 2001; Lazarov, 2002).
As the mechanism of tear regulation, the complex mechanisms mediating LG activities and the regulating neuronal network are still not clear, in the current study the sensory innervation of LG was studied in the monkey using the retrograde tracing technique with special attention given to the localization of sensory first-order neurons.
Materials and methods
Five adult cynomolgous monkeys (Macaca fascicularis) of both sexes weighing between 3.2 and 8.0 kg served as experimental subjects.
Each animal was anaesthetized with a mixture of ketamine, xylazine (Rompun) and atropine in a proportion 10 : 1 : 0.1 mg kg−1, respectively.
In all monkeys a solidified piece of wheat germ agglutinin/horsereadish peroxidase (WGA/HRP) (Sigma) in Willospon (Willpharma), prepared by soaking a small piece of Willospon (1.0 mm) in a 10% WGA/HRP phosphate buffer solution, was implanted by surgery in the orbital LG of the right orbit. The animals were anaesthetized with ketamine (0.4 mL kg−1) 48 or 72 h after surgery followed by 5000 units of thromboliquine kg−1. The animals were then killed by an overdose of pentobarbital and perfused directly through the internal carotid artery with 2 L phosphate-buffered saline, pH 7.4, at 37 °C. Subsequently, the animals were perfused with 2–3 L fixation fluid, containing 0.1 m phosphate-buffered 2% paraformaldehyde and 2.5% glutaraldehyde, pH 7.4, at 4 °C and finally perfused with 0.1 m phosphate buffer containing 10% sucrose at 4 °C. Immediately after perfusion, the orbital LG and its surrounding tissues of the right orbits, the brainstem and the right and left TG were dissected out. Transverse 50-µg sections of the brainstem were cut with a cryostat microtome from the level of the anterior part of the superior colliculus up to the spinal cord. In addition, 15–25-µm serial cryostat microtome sections of the TG of both sides were mounted directly on chrome–alum–gelatin-coated slides. The sections on the slides were then tested for the presence of WGA/HRP activity using either the tetramethylbenzidine (TMB) procedure (Mesulam, 1978) or the DAB gold-substituted silver peroxidase (GSSP) procedure (Van den Pol & Gorcs, 1986; Záborsky & Heimer, 1989). All samples were examined by bright- and dark-field microscopy for the presence of WGA/HRP-labelled neurons. Tissues directly surrounding the LG were dissected out and processed in the same way as the brainstems and the TGs in order to check eventual leakage of the neuronal tracer.
For electron microscopy, 50-µm cryostat microtome sections of the brainstem were rinsed in Tris-buffered saline. Thereafter, free-floating sections were incubated with DAB. The reaction product was intensified using the GSSP technique.The sections were post-fixed for 15 min in 1% OsO4 and rinsed in 0.1 m sodium cacodylate buffer, pH 7.4, containing 1% potassium ferricyanide. Subsequently, sections were dehydrated in a series of ethanol/acetone mixtures and flat-embedded in Epon. Areas of sections containing WGA/HRP-labelled neurons were selected light microscopically, cut out and mounted on prepolymerized Epon blocks. Ultrathin sections were cut and stained with uranyl acetate and lead citrate, after which they were examined with a Philips EM 201 electron microscope.
Results
Histologically, the tracer implant was restricted to LG and the tissues directly surrounding LG were devoid of WGA/HRP. No visible leakage of the neuronal tracer was detected in the surrounding tissues of the examined sections. Some ectopic WGA/HRP-labelled neurons were observed in the walls of the ductal system of LG. Labelled nerve fibres were present in the stroma, the ductal system and the acini of LG.
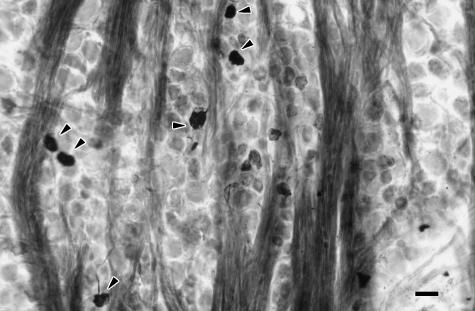
In all cynomolgous monkey experiments, pseudo-unipolar WGA/HRP-labelled somata of neurons were found in the ophthalmic and maxillary ganglionic parts of the ipsilateral TG (Fig. 1). The number of labelled TG neurons ranged between 1300 and 1500 in the ophthalmic ganglionic part (approximately 20%) and between 600 and 650 in the maxillary ganglionic part (approximately 5%). Labelled neurons were not observed in the mandibular ganglionic part. Labelled TG neurons varied in size; small (< 40 µm) as well as large somata (40–60 µm) were found.
Fig. 1.
Bright-field light micrograph of WGA/HRP-labelled somata in the ophthalmic ganglionic part of the trigeminal ganglion. Bar, 50 µm.
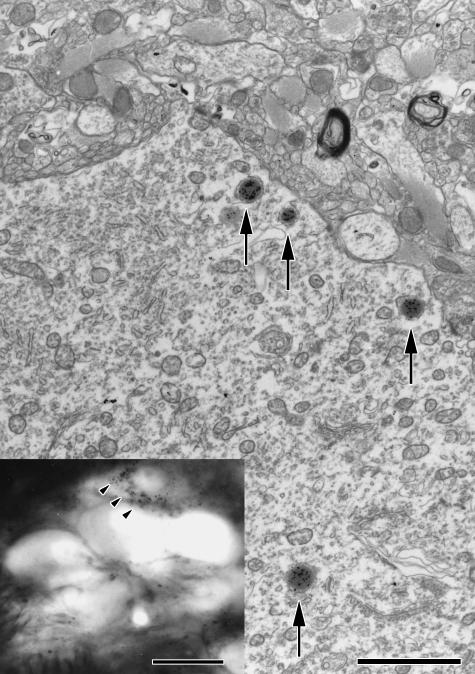
In the brainstem, WGA/HRP-labelled neurons were detected mostly in the caudal half of the ipsilateral MTN (Fig. 2). The number of labelled neurons in each of the five animals was 45, 34, ten, seven and five. MTN consisted of labelled neurons of small as well as large type. Electron microscopic examination of these neurons revealed WGA/HRP labelling in the lysosome-like bodies in the cytoplasm of the perikarya (Fig. 3).
Fig. 2.
Part of a bright-field light micrograph containing a labelled mesencephalic trigeminal nucleus (MTN) neuron (arrows) surrounded by unlabelled MTN neurons. Bar, 50 µm.
Fig. 3.
Electron micrograph of the inserted labelled mesencephalic trigeminal nucleus neuron. The WGA/HRP-reaction product is mostly located in lysosome-like bodies of the cytoplasm. Bar, 0.5 µm.
Discussion
After WGA/HRP-tracer application to LG, many primary sensory neurons were labelled in the ophthalmic ganglionic part (approximately 20%) and a substantial number were observed in the maxillary ganglionic part (approximately 5%) of the ipsilateral TG. Labelled neurons were also found in the ipsilateral MTN.
The results of the current study are in agreement with studies in the monkey, cat and rat, which showed that LG is innervated ipsilaterally by sympathetic, parasympathetic and sensory nerves (VanderWerf et al. 1996a; Tóth et al. 1999; Cheng et al. 2001). The peripheral sensory innervation is not restricted to the ipsilateral TG; in addition, the superior vagal and superior glossopharyngeal ganglia project to LG directly (Cheng et al. 2001). Centrally, ipsilateral second-order neurons of the oral subnucleus (5o) of the STC were labelled after tracer injections into LG (Tóth et al. 1999). In the current study, first-order neurons were found in the ipsilateral MTN.
Although the number of MTN neurons was relatively low compared with the number of labelled neurons in TG (less than 3%), their number was consistent in every experiment and similar to the number of labelled MNT neurons observed in the study of Lucier & Egizii (1986).
The technique used, application by microsurgery of a small solidified piece of Willospon (1.0 mm), soaked in the retrograde tracer WGA/HRP, minimized spread of the tracer in adjacent tissues. The advantage of our technique compared with pressure tracer injections is that tracer uptake occurs gradually through the nerve fibres involved, whereas pressure injections are less accurate and spread the tracer over a larger area through the tissues. Surrounding tissues should be protected against leakage. Inadvertent uptake of the tracer by cutaneous branches of the lacrimal nerve could not be excluded, but is unlikely to have occurred because no visible leakage of the neuronal tracer into the skin area was seen. Interestingly, in other studies in which retrograde WGA/HRP tracing and fluorescent tracing experiments were performed in 18 monkeys under the same conditions and using the same technique, labelled neurons were never observed in MTN (VanderWerf et al. 1993, 1996a,b, 1997, 1998a; VanderWerf, 1998).
Owing to the survival time (48–72 h after WGA/HRP application), labelled nerve terminals were almost absent in STC.
Functionally, the sensory innervation participates in the secretory process of proteins in LG and may control, as a feedback system, the amount of tear production in LG. An immunohistochemical study on the innervation of LG in rat and guinea-pig showed nerve fibres positive for substance P around the lacrimal duct and nerve fibres positive for VIP were mainly concentrated around the acini (Nikkinen et al. 1984). These substance P nerve fibres originate most probably from PPG and TG, as PPG and TG somata contain substance P (for a review see Lazarov, 2002). Immunohistochemical experiments in the rat and monkey TG showed substance P in the somata of primary sensory neurons (Lee et al. 1985; Prins et al. 1993) and in nerve terminals projecting towards MTN neurons (VanderWerf, 1993). The LG innervation pathway is not restricted to the peripheral TG–PPG–LG loop, which is known in trigeminal-parasympathetic reflexes (Drummond, 1995), but centrally sensory second-order neurons could also participate in tear reflexes. It is known that 5o projects directly to the ipsilateral superior salivatory nucleus, which projects to PPG.
Moreover, 5o also projects ipsilaterally towards the thoracic spinal cord, which projects towards the SCG (Tóth et al. 1999). Both pathways innervate LG efferently and activate LG for tear production.
Minkels et al. (1991) showed that MTN projects towards the reticular formation (RF). RF projects also towards PPG or SCG. Physiologically, a unilateral TG ablation in the rabbit resulted in an increase of protein by the denervated LG in response to carbachol or isoproterenol (Meneray et al. 1998). Based on the effects of sensory denervation, these authors suggested a direct sensory input for LG secretion control. This is supported by investigations regarding neurothrophin-4 (NT-4) and their receptors in LG. NT-4, a member of the nerve growth factor family, and its receptors have recently been found in the rat LG (Ghinelli et al. 2003). These authors suggested that NT-4 in LG might represent a key point in the network created by the ocular surface, the nerves, the brainstem and LG. Reflex activation of efferent nerves in LG may cause a release of NTs in this gland. In another rat study the NT-4 protein was found in MTN neurons and neurons of dorsal root ganglia (Ritsuko et al. 2003). Moreover, NT-4 elongates primary sensory TG neurons in culture studies (Ulupinar et al. 2004). The results of this study provide morphological evidence that primary sensory neurons of TG and MTN are involved in the feedback mechanism during tear production and its release.
Addendum
The enthusiasm of Gordon Ruskell for the neuroanatomical research elucidated the nature of many unknown nerve pathways towards orbital structures. By joining our research programme with his expertise in the field of electron microscopy, a fruitful co-operation was born. This resulted in some publications concerning sensory innervation of orbital structures. He would undoubtedly have welcomed the outcome of this study.
References
- Buisseret-Delmas C, Buisseret P. Central projections of extraocular muscle afferents in cat. Neurosci. Lett. 1990;109:48–53. doi: 10.1016/0304-3940(90)90536-i. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Pinganaud G, Compoint C, Buisseret P. Projection from trigeminal nuclei toneurons of the mesencephalic trigeminal nucleus in rat. Neurosci. Lett. 1997;229:189–192. doi: 10.1016/s0304-3940(97)00452-7. [DOI] [PubMed] [Google Scholar]
- Burton H. Somatic sensations from the eye. In: Hart WM, editor. Adler's Physiology of the Eye. St Louis: Mosby; 1992. pp. 71–100. [Google Scholar]
- Cheng SB, Kuchiiwa S, Kuchiiwa T, Nonaka S, Nakagawa S. Presence of neuronal nitric oxide synthase in autonomic and sensory ganglion neurons innervating the lacrimal gland of the cat: an immunofluorescent and retrograde tracer double-labeling study. J. Chem. Neuroanat. 2001;22:147–155. doi: 10.1016/s0891-0618(01)00125-9. [DOI] [PubMed] [Google Scholar]
- Copray JCVM, Liem RSB, Ter Horst GJ, Van Willigen JD. Origin, distribution and morphology of serotonergic afferents to the mesencephalic trigeminal nucleus of the rat. Neurosci. Lett. 1991;121:97–101. doi: 10.1016/0304-3940(91)90658-g. [DOI] [PubMed] [Google Scholar]
- Corbin KB, Harrison F. Function of mesencephalic root of fifth cranial nerve. J. Neurophysiol. 1940;3:432–435. [Google Scholar]
- Dessem D, Luo P. Jaw-muscle spindle afferent feedback to the cervical spinal cord in the rat. Exp. Brain Res. 1999;128:451–459. doi: 10.1007/s002210050868. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Lacrimation and cutaneous vasodilatation in the face induced by painful stimulation of the nasal ala and upper lip. J. Autonomic Nervous System. 1995;51:109–116. doi: 10.1016/0165-1838(94)00121-y. [DOI] [PubMed] [Google Scholar]
- Ghinelli E, Johansson J, et al. Presence and localization of neurotrophins and neurotrophin receptors in rat lacrimal gland. Invest. Ophthalmol. Visual Sci. 2003;44:3352–3357. doi: 10.1167/iovs.03-0037. [DOI] [PubMed] [Google Scholar]
- Honma S, Moritani M, Zhang LF, et al. Quantitative ultrastructure of synapses on functionally identified primary afferent neurones in the cat trigeminal mesencephalic nucleus. Exp. Brain Res. 2001;137:150–162. doi: 10.1007/s002210000632. [DOI] [PubMed] [Google Scholar]
- Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog. Neurobiol. 2002;66:19–59. doi: 10.1016/s0301-0082(01)00021-1. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kawai Y, Shiosaka S, et al. Coexistence of calcitonoin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat. Brain Res. 1985;330:194–196. doi: 10.1016/0006-8993(85)90027-7. [DOI] [PubMed] [Google Scholar]
- Lucier GE, Egizii R. Central projections of the ethmoidal nerve of the cat as determined by the horseradish peroxidase tracer technique. J.Comparative Neurol. 1986;247:123–132. doi: 10.1002/cne.902470108. [DOI] [PubMed] [Google Scholar]
- Luo P, Dessem D. Morphological evidence for recurrent jaw-muscle spindle afferent feedback within the mesencephalic trigeminal nucleus. Brain Res. 1996;710:260–264. doi: 10.1016/0006-8993(95)01439-x. [DOI] [PubMed] [Google Scholar]
- Luo P, Moritani M, Dessem D. Jaw-muscle spindle afferent pathways to the trigeminal motor nucleus in the rat. J.Comparative Neurol. 2001;435:341–353. doi: 10.1002/cne.1034. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Echtenkamp SF. Central projections and trigeminal ganglion location of corneal afferent neurons in the monkey, Macaca fascicularis. J. Comparative Neurol. 1988;272:370–382. doi: 10.1002/cne.902720307. [DOI] [PubMed] [Google Scholar]
- Meneray MA, Bennett DJ, Nguyen DH, Beuermann RW. Effect of sensory denervation on the structure and physiologic responsiveness of rabbit lacrimal gland. Cornea. 1998;17:99–107. doi: 10.1097/00003226-199801000-00015. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic bleu reaction-product with superior sensitivity for visualizating neural afferents and efferents. J. Histochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Minkels RF, Jüch PJW, Ter Horst GJ, Van Willigen JD. Projections of the parvocellular reticular formation to the contralateral mesencephalic trigeminal nucleus in the rat. Brain Res. 1991;547:13–21. doi: 10.1016/0006-8993(91)90569-h. [DOI] [PubMed] [Google Scholar]
- Nagy Ji, Buss M, Dadona PE. On the innervation of trigeminal mesencephalic primary afferent neurons by adenosine deaminase-containing projections from the hypothalamus in the rat. Neuroscience. 1986;17:141–156. doi: 10.1016/0306-4522(86)90232-0. [DOI] [PubMed] [Google Scholar]
- Ndiaye A, Pinganaud G, VanderWerf F, Buisseret-Delmas C, Buisseret P. Connections between the trigeminal mesencephalic nucleus and the superior colliculus in the rat. Neurosci. Lett. 2000;294:17–20. doi: 10.1016/s0304-3940(00)01519-6. [DOI] [PubMed] [Google Scholar]
- Ndiaye A, Pinganaud G, Buisseret-Delmas C, Buisseret P, VanderWerf F. Organization of trigeminocollicular connections and their relations to the sensory innervation of the eyelids in the rat. J.Comparative Neurol. 2002;448:373–387. doi: 10.1002/cne.10269. [DOI] [PubMed] [Google Scholar]
- Nikkinen A, Lehtosalo JI, Uusitalo H, Palkema A, Panula P. The lacrimal glands of the rat and guinea pig are innervated by nerve fibres containing immunoreactivities for substance P and vasoactive intestinal peptide. Histochemistry. 1984;81:23–27. doi: 10.1007/BF00495396. [DOI] [PubMed] [Google Scholar]
- Oduntan O, Ruskell GL. The source of sensory fibres of the inferior conjunctiva of monkeys. Von Graefes Arch. Clin. Exp. Ophthalmol. 1992;230:258–263. doi: 10.1007/BF00176301. [DOI] [PubMed] [Google Scholar]
- Porter JD. Brainstem terminations of extraocular muscle primary afferent neurones in the monkey. J. Comparative Neurol. 1986;247:133–143. doi: 10.1002/cne.902470202. [DOI] [PubMed] [Google Scholar]
- Porter JD, Donaldson IML. The anatomical substrate for cat extraocular muscle proprioception. Neuroscience. 1991;43:473–481. doi: 10.1016/0306-4522(91)90309-c. [DOI] [PubMed] [Google Scholar]
- Prins M, VanderWerf F, Baljet B, Otto AJ. Calcitonin gene-related peptide and substance P immunoreactivity in the monkey trigeminal ganglion, an electron microscopic study. Brain Res. 1993;629:315–318. doi: 10.1016/0006-8993(93)91337-r. [DOI] [PubMed] [Google Scholar]
- Ritsuko K, Satoshi I, Yoshio H, et al. Nt-4 protein is localized in neuronal cells in the brain stem as well as the dorsal root ganglion of embryonic and adult rats. J. Neurochem. 2003;86:660–668. doi: 10.1046/j.1471-4159.2003.01874.x. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Vasomotor axons of the lacrimal glands of monkeys and the ultrastructural identification of sympathetic terminals. Z.Zellforschung. 1967;83:321–333. doi: 10.1007/BF00336861. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Changes in nerve terminals and Acini of the lacrimal gland and changes in secretion induced by autonomic denervation. Z. Zellforschung. 1969;94:261–281. doi: 10.1007/BF00339361. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. An ocular parasympathetic nerve pathway of facial nerve origin and its influence on intraocular pressure. Exp. Eye Res. 1970a;10:319–330. doi: 10.1016/s0014-4835(70)80044-6. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. The orbital branches of the pterygopalatine ganglion and their relationship with internal carotid nerve branches in primates. J. Anat. 1970b;106:323–339. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. The distribution of autonomic post-ganglionic nerve fibres to the lacrimal gland in monkeys. J. Anat. 1971;109:229–242. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. Sympathetic innervation of the ciliary muscle in monkeys. Exp. Eye Res. 1973;16:183–190. doi: 10.1016/0014-4835(73)90212-1. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Ocular fibres of the maxillary nerve in monkeys. J. Anat. 1974;118:195–203. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. Nerve terminals and epithelial cell variety in the human lacrimanl gland. Cell Tissue Res. 1975;158:121–136. doi: 10.1007/BF00219955. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. The source of nerve fibres of the trabeculae and adjacent structures in monkey eyes. Exp. Eye Res. 1976;23:449–459. doi: 10.1016/0014-4835(76)90174-3. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J. Neurocytol. 1978;7:693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Innervation of the anterior segment of the eye. In: Lütjen-Drecoll E, editor. Basic Aspects of Glaucoma Research. Stuttgart: Schattauer Verlag; 1982. pp. 49–66. [Google Scholar]
- Ruskell GL, Wilson J. Spiral nerve endings and dapple motor end plates in monkey extraocular muscles. J. Anat. 1983;136:85–95. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. Sheathing of muscle fibres at neuromuscular junctions and at extra-junctional loci in human extra-ocular muscles. J. Anat. 1984a;138:33–44. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. Spiral nerve endings in human extraocular muscles terminate in motor end plates. J. Anat. 1984b;139:33–43. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. Innervation of the conjunctiva. Trans. Ophthalmol. Soc. UK. 1985;104:390–395. [PubMed] [Google Scholar]
- Ruskell GL, Simons T. Trigeminal nerve pathways to the cerebral arteries in monkeys. J. Anat. 1987;155:23–37. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. The tentorial nerve in monkeys is a branch of the cavernous plexus. J. Anat. 1988;157:67–77. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL, Simons T. The internal carotid artery has a sleeve of increased innervation density within the cavernous sinus in monkeys. Brain Res. 1992;595:116–120. doi: 10.1016/0006-8993(92)91459-r. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Distribution of otic postganglioninc and recurrent mandibular nerve fibres to the cavernous sinus plexus in monkeys. J. Anat. 1993;182:187–195. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. Trigeminal innervation of the scleral spur in cynomolgus monkeys. J. Anat. 1994;184:511–518. [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL, VanderWerf F. Sensory innervation of conjunctival lymph follicles in cynomolgus monkeys. Invest. Opthalmol. Visual Sci. 1997;38:884–892. [PubMed] [Google Scholar]
- Ruskell GL. Extraocular muscle proprioceptors and proprioception. Prog. Retinal Eye Res. 1999;18:269–291. doi: 10.1016/s1350-9462(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Distribution of pterygopalatine efferents to the lacrimal gland in man. Exp. Eye Res. 2004;78:329–335. doi: 10.1016/j.exer.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Pedroza L, Beuermann RW. Denervation of rabbit lacrimal gland increases levels of transferrin and unidentified tear proteins of 44 and 36 kDa. Current Eye Res. 1999;18:455–466. doi: 10.1076/ceyr.18.6.455.5270. [DOI] [PubMed] [Google Scholar]
- Shankland WE. The trigeminal nerve. Part II; The ophthalmic division. J. Craniomandibular Prac. 2001;19:8–12. doi: 10.1080/08869634.2001.11746145. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Hann LE, Soo CH, Yee L, Edwards JA, Allansmith MR. Neural-immune relationship: effect of topic, sympathetic, temporofacial, or sensory denervation on the secretory immune system of the lacrimal gland. Regulatory Immunol. 1990;3:204–212. [PubMed] [Google Scholar]
- Ten Tusscher MPM, Klooster J, Baljet B, VanderWerf F, Vrensen GFJM. Pre- and post-ganglionic nerve fibres of the pterygopalatine ganglion and their allocation to the eyeball in rats. Brain Res. 1990;517:315–323. doi: 10.1016/0006-8993(90)91043-g. [DOI] [PubMed] [Google Scholar]
- Tóth IE, Boldogköi Z, Medveczky I, Palkovits M. Lacrimal preganglionic neurons form a subdivision of the superior salivatory nucleus of rat: transneuronal labeling by pseudorabies virus. J. Autonomic Nervous System. 1999;77:45–54. doi: 10.1016/s0165-1838(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Ulupinar E, Ünal N, Erzurumlu RS. Morphometric analysis of embryonic rat trigeminal neurons treated with different neurotrophins. Anat. Record Part A. 2004;277A:396–407. doi: 10.1002/ar.a.20029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Pol AN, Gorcs T. Synaptic relationships between neurons containing vasopressin, gastrin-relasing peptides, vasoactive intestinal polipeptides and glutamate decarboxylase immunoreactivity in the suprachiasmatic nucleus: dual ultrastructural immunohistochemistry with gold-substituted silver peroxidase. J. Comparative Neurol. 1986;252:507–521. doi: 10.1002/cne.902520407. [DOI] [PubMed] [Google Scholar]
- VanderWerf F. Amsterdam: 1993. Autonomic and sensory innervation of some orbital structures in the primate. Thesis. [Google Scholar]
- VanderWerf F, Baljet B, Prins M, Timmerman A, Otto AJ. Innervation of superior tarsal (Müller) muscle in the cynomolgus monkey: a retrograde tracing study. Invest. Ophthalmol. Visual Sci. 1993;34:2333–2340. [PubMed] [Google Scholar]
- VanderWerf F, Baljet B, Prins M, Otto AJ. Innervation of the lacrimal gland in the cynomolgus monkey: a retrograde tracing study. J. Anat. 1996a;188:591–601. [PMC free article] [PubMed] [Google Scholar]
- VanderWerf F, Baljet B, Prins M, Ruskell GL, Otto AJ. Innervation of the palpebral conjunctiva and the superior tarsal muscle in the cynomolgus monkey: a retrograde fluorescent tracing study. J. Anat. 1996b;189:285–292. [PMC free article] [PubMed] [Google Scholar]
- VanderWerf F, Aramideh M, Ongerboer de Visser BW, Baljet B, Speelman JD, Otto AJ. A retrograde double fluorescent tracing study of the levator palpebrae superioris muscle in the cynomolgus monkey. Exp. Brain Res. 1997;113:174–179. doi: 10.1007/BF02454155. [DOI] [PubMed] [Google Scholar]
- VanderWerf F. The structure of the facial nuclei related to blinking: a retrograde traing study in the monkey. In: Valls-Solé J, Tolosa E, editors. Brainstem Reflexes and Functions. Madrid: ENE Publicidad; 1998. pp. 7–25. [Google Scholar]
- VanderWerf F, Aramideh M, Otto AJ, Ongerboer de Visser BW. Retrograde tracing studies of subdivisions of the orbicularis oculi muscle in the rhesus monkey. Exp. Brain Res. 1998;121:433–441. doi: 10.1007/s002210050478. [DOI] [PubMed] [Google Scholar]
- Varathan V, Shigenaga Y, Takemura M. Nitric oxide synthase/Nicotinamide adenine Dinucleotide phosphate-diaphorase in the brainstem trigeminal nuclei after transection of the massetric nerve in rats. J. Neurosci. Res. 2001;66:428–438. doi: 10.1002/jnr.1235. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Morris-Wiman JA, Calhoun JC. Development of trigeminal mesencephalic and motor nuclei in relation to masseter muscle innervation in mice. Brain Res. Dev. Brain Research. 1998;108:1–11. doi: 10.1016/s0165-3806(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Záborsky L, Heimer L. Combinations of tracer techniques, especially HRP and PHA-L, with transmitter identification for correlated light and electron microscopic studies. In: Heimer L, Záborsky L, editors. In Neuroanatomical Tract-Tracing Methods 2, Recent Progress. New York: Plenum Press; 1989. pp. 49–77. [Google Scholar]
- Zhang JD, Wang L, Wang BR, Li JS. Mesencephalic trigeminal nucleus neurons with collaterals to both the masseter and the inferior alveolar nerve. A fluorescent double-labeling study in the rat. Neurosci. Lett. 1992;139:224–226. doi: 10.1016/0304-3940(92)90558-o. [DOI] [PubMed] [Google Scholar]
- Zhang J, Luo P, Pendlebury WW. Light and electron microscopic observations of a direct projection from mesencephalic trigeminal neurons to hypoglossal motoneurones in the rat. Brain Res. 2001;917:67–80. doi: 10.1016/s0006-8993(01)02911-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Luo P. Orexin B immunoreactive fibers and terminals innervate the sensory and motor neurones of jaw elevator muscles in the rat. Synapse. 2002;44:106–110. doi: 10.1002/syn.10050. [DOI] [PubMed] [Google Scholar]