Abstract
The aim of the study was to evaluate the role of lipid layer thickness and corneal sensation in the development of blinking in neonates. The study group comprised sixty-four neonates and infants (mean age 27.5 ± 15 (sd) weeks, range 3.4–52) whose mothers were attending a general practice healthy baby clinic. Spontaneous eye-blink activity was determined from digital videographic recordings; tear film lipid layer morphology wasexamined using interference patterns produced by the Keeler Tearscope™ Plus over a five-point grading scale (higher grades are associated with thick and stable lipid films); corneal sensation threshold was assessed with the Non-Contact Corneal Aesthesiometer (NCCA), using the eye-blink response as an objective indication that the cooling stimulus had been felt; palpebral aperture dimensions were measured using calibrated digital still images of the eye in the primary position. The overall mean spontaneous blink-rate was found to be 3.6 (± 0.3) blinks min−1, and the mean interblink time was 21.6 (± 2.8) s. The lowest blink-rates were observed in the 0–17-week age group (average 2 blinks min−1). The blink-rate showed a highly significant correlation with age (r = 0.46, P < 0.01). The overall mean lipid layer grading was 3.6 (± 0.2 SE) arbitrary units. Higher grades were found in the newborn and the mean grading score reduced with age (P < 0.01). The mean sensation threshold to blink (TTB) was 0.69 (0.04 SE) mbar, which did not differ from a control group of older subjects (P > 0.05). There was a rapid increase in palpebral aperture length and width from birth to 1 year old, with surface area increasing by 50% over the same period. We concluded that the low rate of spontaneous eye blink activity in neonates is associated with a thick stable lipid layer that may be a function of a small palpebral aperture. Furthermore, neonates appear to have the capacity to detect ocular surface cooling, which is a major trigger for spontaneous blinking.
Keywords: corneal sensation, lipid layer, neonates, tear film
Introduction
The preocular tear film is inherently unstable, and frequent blinking is necessary to prevent drying of the ocular surface. It is proposed that a possible trigger for normal spontaneous blinking is a localized evaporation-mediated reduction in ocular surface temperature (Mori et al. 1997). As the tear film evaporates, heat energy is drawn away from the epithelium and the resulting reduction in ocular surface temperature is detected by cold-sensitive receptors. By detecting the imminent tear break-up, a blink can be made to reform the tear film before break-up actually occurs. The superficial lipid layer acts as a barrier to retard tear evaporation and resists significant short-term evaporation-mediated tear film thinning, thereby reducing the requirement for frequent blinking.
Although reflex blinking to potentially noxious stimuli is well developed at birth, spontaneous blinking is infrequent in the newborn and in young infants. This is surprising given the importance of the blink process for the formation of the preocular tear film. The tear film of neonates and infants is associated with optical interference patterns commensurate with a thick surface lipid layer. It is proposed that this feature adapts the infant tear film to resist evaporation, preventing tear break-up and reducing the need for frequent blinking. An alternative explanation for the low spontaneous blink-rate could be that the afferent pathway, which detects a localized temperature change, may still be relatively immature.
The aim of this study was to address both of these possibilities, by investigating the incidence of lipid interference patterns associated with stable and unstable tear films using a Tearscope Plus™, and to assess the afferent limb of the blink response. The sensitivity of the cornea to a cooling stimulus was measured using the Non-Contact Corneal Aesthesiometer (NCCA), which delivers a fine air-stream of short duration that produces a localized reduction in corneal surface temperature by evaporative cooling (Murphy et al. 1996, 1999). This instrument was chosen because its stimulus closely mimics the cooling of the ocular surface that occurs during tear evaporation.
Materials and methods
Sixty-four neonates and infants with a mean gestational age of 27.5 (sd 15) weeks (range 3.4–52) were studied. All subjects were born at full term (post-conception age 38 weeks or greater) and were products of uncomplicated pregnancies. All subjects were patients attending a general practice healthy baby clinic. The City University, and the Kingston and Richmond Ethics Committees approved the study. Informed consent was obtained from parents or legal guardians of the participating subjects.
Spontaneous eye-blink activity was determined non-invasively from digital videographic recordings, in alert neonates and infants, of at least 3-min duration. Recordings were made in the same clinical environment (examination room in General Practice Clinic) under identical testing conditions. The mean interblink time, i.e. the time from the end of one blink to the start of the next, and the mean blink-rate was determined from each recording.
The precorneal tear film was examined in specular reflection using a hand-held Keeler Tearscope™ Plus (Keeler Ltd, Windsor, UK) with a focusing lens attachment. The lipid layer interference patterns were classified using a five-point grading scale (modified from Guillon, 1998), where Grade 1 represents the thinnest lipid layer and Grade 5 the thickest (Table 1).
Table 1.
Tear lipid grading scale (modified from Guillon, 1998)
| Tear grading | Description |
|---|---|
| 1 | Open meshwork pattern: very thin lipid layer with no detail visible |
| 2 | Amorphous pattern: even well-mixed lipid layer with minimal detail visible |
| 3 | Wave pattern: grey streaks visible with flow pattern during the blink process |
| 4 | Colour fringe pattern: interference colours (1st order) visible in part of the field |
| 5 | Colour fringe pattern: interference colours (2nd order) covering the majority of the field |
Sensation thresholds were determined with the NCCA, using the eye-blink response as an objective indication that the cooling stimulus had been felt. The use of the eye-blink for threshold determination in corneal aesthesiometry has been used successfully in human and animal studies (Millodot, 1973). The stimulus air-nozzle of the NCCA was positioned 1 cm away from the central cornea, and the air-stream duration was set to 0.9 s, and the threshold to blink (TTB) was measured using ascending and descending stimulus presentations as previously described (Murphy et al. 1996). As a control population, TTB was measured in a group of older subjects [n = 27, mean age 29 years (range 5.8–73)].
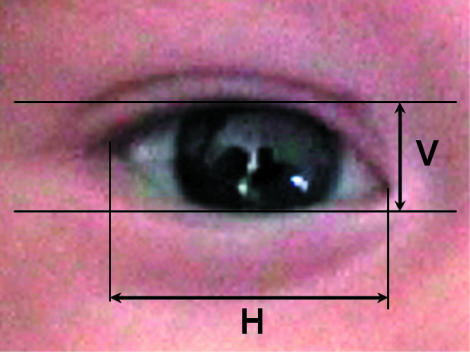
Calibrated still images of the eye in the primary position were grabbed from digital video recordings. Measurements of palpebral aperture dimensions were taken using Scion Image (V4.02) (Scion Corp., Frederick, MD, USA) image analysis software. The horizontal palpebral aperture length was measured from canthus to canthus and the vertical width was taken at the widest point between the lids (Fig. 1). The mean of three measurements was taken for each image. For exposed ocular surface area measurements, the area between the lids was outlined with the cursor (including the caruncle and lacus lacrimalis). This planar value was converted into the curved ocular surface by applying the formula described by Zaman et al. (1998). This entailed multiplying the planar area measurement by 1.23.
Fig. 1.
Measurement of horizontal and vertical palpebral aperture dimensions.
Results
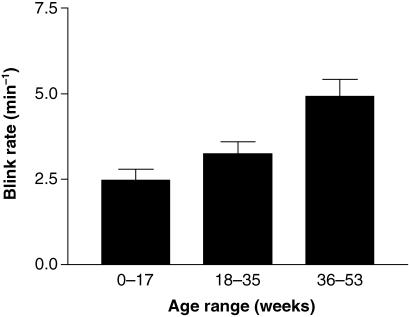
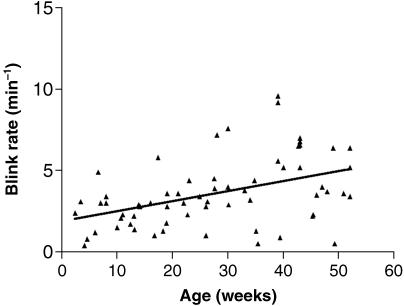
The overall mean spontaneous blink-rate in the experimental group was 3.6 (SE 0.3) blinks min−1 and the mean interblink time was 21.6 (SE 2.8) (range 6.8–82) s. The lowest blink-rates were observed in the 0–17-week age group (Fig. 2) with a mean blink-rate of approximately 2 blinks min−1. The blink-rate thereafter increased progressively, with a mean rate in the older age group (36–53 weeks) of approximately 5 blinks min−1. This difference was statistically significant (Kruskal–Wallis one-way anova with Dunn's post test, P < 0.001). The older age groups also showed a greater variability in blink-rate. Overall, the blink-rate showed a highly significant positive correlation with age (Fig. 3) (Spearman rank correlation, r = 0.4561, P = 0.0002).
Fig. 2.
Variation in blink-rate with age (means ± SE).
Fig. 3.
Scatter plot showing correlation between blink-rate and age over the first year of life.
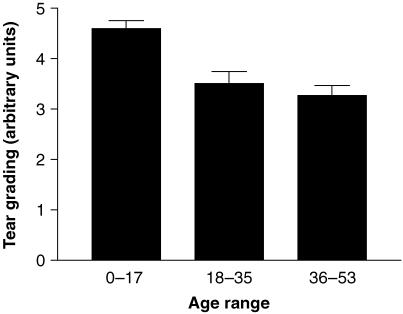
The overall mean lipid layer grading for the study population was 3.6 (SE 0.2) arbitrary units. The mean grading score progressively reduced with age, reflecting a thinning of the lipid layer (Fig. 4). In the 0–17-week age group, the lipid layer typically showed a colour fringe pattern in specular reflection. Three-quarters of this group were classified as Grade 5 with interference colours covering the whole of the cornea. By contrast in the 36–53-week age group, only 10% were classified as Grade 5. The differences between the 0–17-week group and the 18–35- and 36–53-week groups were statistically significant (Kruskal–Wallis one-way anova with Dunn's post test, P < 0.01 and < 0.001, respectively). Moreover, the tear grading score showed a highly significant negative correlation with age (Spearman rank correlation, r = −0.5081, P < 0.0001).
Fig. 4.
Variation in tear grading with age (means ± SE).
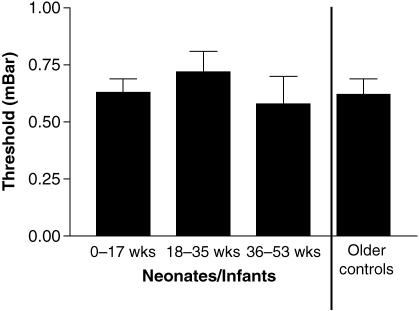
The mean TTB for neonatal and infant subjects was 0.69 (SE 0.04) mbar, which did not differ significantly from the control group of older children and adults (Mann–Whitney test, P > 0.05). Furthermore, the sensation threshold showed no significant variation with age over the first year of life (Fig. 5).
Fig. 5.
Variation in corneal sensation threshold with age.
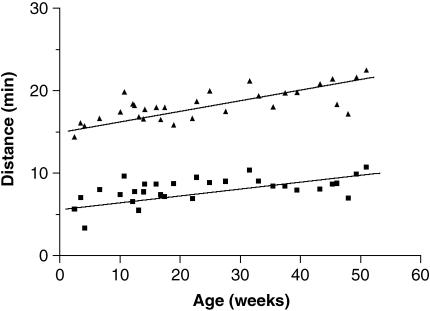
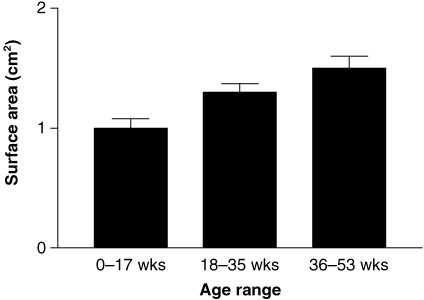
A rapid increase in palpebral aperture length and width occurred from birth to 1 year of age (Fig. 6). The greatest increase rate of change occurred for the horizontal dimension. The ratio between length and width was approximately 1 : 2 at birth, increasing to 1 : 2.5 by the end of the first year. Surface area showed an approximately 50% increase from the 0–17-week group to the 36–53-week group (Fig. 7) This difference was statistically significant (Kruskal–Wallis one-way anova with Dunn's post test, P < 0.01).
Fig. 6.
Variation in vertical width (▴) and horizontal length (▪) with age.
Fig. 7.
Variation in exposed ocular surface area with age.
Discussion
The factors involved in the control of spontaneous blinking are not well understood, although it is likely to involve central and peripheral triggers. Evidence that the central nervous system plays a role in the control of blinking comes from several sources. The blink-rate is highly dependent on attention and cognitive states (Karson, 1988) and blink activity is substantially influenced by experimental manipulation of dopaminergic circuits in the basal ganglia (Taylor et al. 1999). Significantly, altered blink-rates are also observed in several neuropsychiatric disorders that are known to affect dopaminergic neurotransmission (Karson, 1983). There is increasing evidence that sensory stimuli arising from the exposed ocular surface, and the environmental factors that affect them, are also major determinants of blink-rate (Tsubota, 1998). Significantly lower blink-rates are recorded during corneal anaesthesia (Collins et al. 1989), and higher rates occur during conditions that favour tear evaporation (Nakamori et al. 1997). Blinking plays an important role in maintaining a healthy ocular surface by inducing the formation of the preocular tear film. The tear film prevents drying and maintains a smooth and regular refractive surface of high optical quality. It is a dynamic structure that is inherently unstable. The tear film is re-formed during each blink, and if blinking is deliberately prevented, the tear film will soon thin and break up in a random manner (Tsubota, 1998). It has recently been suggested that evaporation-mediated cooling, which occurs during the process of tear break-up, may be detected by thermo-sensitive corneal afferents and provide the signal for a blink to reform the tear film (Mori et al. 1997). Blinking is also linked to the transfer of lipid from the ductal system of the meibomian glands onto the lid margins (Bron & Tiffany, 1998). Meibomian-derived lipid forms the anterior layer of the tear film, which provides a barrier to limit evaporation and therefore contributes to tear stability (Craig & Tomlinson, 1997). The lipid layer can be visualized in specular reflection and estimates of its thickness can be made based on the appearance of the resulting interference patterns (Guillon, 1998; Khamene et al. 2000). Using this technique it is possible to differentiate between lipid patterns associated with stable and unstable tear films (Craig & Tomlinson, 1997; Guillon, 1998). Given the importance of periodic blinking for tear film reformation, the low rate of spontaneous eye-blink activity in infants is surprising. One possible explanation is that the infant tear film is adapted to resist significant short-term evaporation-mediated tear film thinning. Alternatively, the afferent pathway, which detects an evaporation-mediated reduction in ocular surface temperature, may still be relatively immature.
The basic neuronal circuitry for reflex blinking is well developed at birth. Reflex contraction of the orbicularis oculi in response to direct trigeminal stimulation has been demonstrated as early as 10–10.5 weeks of fetal life (Humphrey, 1964). Assessment of this primitive reflex, by tapping of the glabella, is part of a standard neurological assessment of the newborn (Anday et al. 1990). A recent study by Snir et al. (2002) using a mechanical stimulus found only a minority of newborns to have a tactile corneal reflex. However, this reflex rapidly developed for all newborns during the first 3 months after birth. Bell's phenomenon also develops quickly, although slightly more slowly than the corneal reflex, requiring up to 4 months for presentation in healthy babies (Snir et al. 1996). By contrast, the maturation of spontaneous blinking occurs much later in development. In their seminal study of the factors affecting human blinking, Ponder & Kennedy (1928) thought that ‘true’ blinks first appeared at 6 months and increased in frequency thereafter. However, more recent observers have recorded low rates of spontaneous blinking in very young infants under 2 months of age (Zametkin et al. 1979).
The present study found very low spontaneous eye-blink frequencies in neonates and young infants. The blink-rate was significantly correlated with age during the first year of life. The lowest blink-rates were found in newborns, which had blink-rates that were typically between 2 and 3 blinks min−1. Published values of blink-rates in adults lie in the range 12–20 blinks min−1 (Ponder & Kennedy, 1928; Collins et al. 1989; Tsubota, 1998). Thus throughout the neonatal period and early infancy the rate of periodic blinking is much lower than in adults. The relative importance of the sensory input from the ocular surface, tear stability and centrally mediated factors in the determination of spontaneous blinking in neonates and infants is not completely understood. The role of central dopamine activity and spontaneous eye-blink rates in the adult is well established (Karson, 1983). Although dopaminergic neurons appear early in human development, there are significant postnatal changes in the neuronal organization of the basal ganglia that are associated with altered levels of endogenous dopamine and changes in dopamine receptor subtype expression (Meng et al. 1999; Herlenius & Lagercrantz, 2001). The relationship between dopaminergic plasticity and spontaneous eye-blink frequency in the neonate is unclear. However, it is possible that the low rate of spontaneous blinking observed at birth is a function of a relatively immature dopaminergic system.
The aims of the present study were to investigate the role of peripheral factors: for example, the possibility that the sensory pathway that detects evaporative cooling in neonates is not fully developed, and/or that the neonatal tear film is adapted to obviate partly the requirement for frequent blinking. Thermo-selective ‘cold’ neurons conducting in the C-range have been identified in adult cats and rabbits (Belmonte et al. 1997), and cooling of the cornea in adult human subjects evokes a perceptible cold sensation (Beuerman & Tanelian, 1979). The present study utilized an aesthesiometer to produce localized and controlled cooling of the ocular surface (Murphy et al. 1999). The sensation threshold in neonates and infants was not significantly different from that found in older children and adults, suggesting that a thermosensitive pathway is well developed at birth, which could potentially be capable of detecting evaporative cooling or drying of the cornea. This leaves the possibility that the neonatal tear film is inherently stable. (Nakamori et al. 1997) have measured the longest period during which normal adult subjects can comfortably keep their eyes open without blinking. They found a mean value of approximately 11 s. The babies in our study showed a mean interblink time of approximately 23 s, and interblink times greater than 1 min were not unusual. Although the tear break-up time was not measured, neonates were shown to have a high incidence of lipid interference patterns typical of thick films. A thick lipid layer could potentially retard tear evaporation and thereby increase tear stability. It is significant that rabbits, which display an interblink time approximately 45 times longer than that of humans, also possess a thick and stable lipid layer (Korb et al. 1998). A recent study by Isenberg et al. (2003) also found a thicker lipid layer in neonates and infants, in the first 6 months after birth, which was associated with a longer tear break up time. They suggested that the thicker lipid layer may be related to a smaller palpebral aperture. Previous measurements of the palpebral aperture dimensions in neonates and infants have found a smaller aperture than in adults (Hymes, 1928; Jones et al. 1978; Mehes, 1980; Sivan et al. 1982; Isenberg et al. 1987). The results from this study show a similar gradual widening in the palpebral aperture. Indeed, there was a 50% increase in the mean surface area from 0–17 weeks to 36–53 weeks (Fig. 7), which would also increase the available area for tear evaporation. In addition to a thicker lipid layer, it is possible that infant tear lipids differ in their biophysical characteristics. A previous study has found that Meibomian lipids, collected from infants of 1.5 years, showed a greater ability to withstand repeated compression and expansion than equivalent samples taken from adults (Kaercher et al. 1994).
In conclusion, neonates and infants have a low spontaneous blink-rate that leads to prolonged periods of eye opening. The finding of a high incidence of interference patterns associated with a thick and stable lipid layer in this age group may provide a mechanism to resist evaporation-mediated tear film thinning, and prevent drying of the ocular surface.
Acknowledgments
This study was funded by a grant from the British Contact Lens Association. We thank the staff and patients of Hampton Wick Surgery for their help with this study.
References
- Anday EK, Cohen ME, Hoffman HS. The blink reflex: maturation and modification in the neonate. Dev. Med. Child Neurol. 1990;32:142–150. doi: 10.1111/j.1469-8749.1990.tb16913.x. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Garcia-Hirschfeld J, Gallar J. Neurobiology of ocular pain. Prog. Ret. Eye Res. 1997;16:117–156. [Google Scholar]
- Beuerman RW, Tanelian DL. Corneal pain evoked by thermal stimulation. Pain. 1979;7:1–14. doi: 10.1016/0304-3959(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM. The meibomian glands and tear film lipids: structure, function and control. Adv. Exp. Med. Biol. 1998;438:281–295. doi: 10.1007/978-1-4615-5359-5_40. [DOI] [PubMed] [Google Scholar]
- Collins M, Seeto R, Campbell L, Ross M. Blinking and corneal sensitivity. Acta Ophthalmol. 1989;67:525–531. doi: 10.1111/j.1755-3768.1989.tb04103.x. [DOI] [PubMed] [Google Scholar]
- Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom. Vis. Sci. 1997;74:8–13. doi: 10.1097/00006324-199701000-00014. [DOI] [PubMed] [Google Scholar]
- Guillon JP. Non-invasive Tearscope Plus routine for contact lens fitting. Cont. Lens Ant. Eye. 1998;21(Suppl):31–40. doi: 10.1016/s1367-0484(98)80035-0. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early neural development. Early Hum. Dev. 2001;65:21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- Humphrey T. Some correlations between the appearance of human fetal reflexes and the development of the nervous system. Prog. Brain Res. 1964;4:93–112. [Google Scholar]
- Hymes C. The postnatal growth of the cornea and palpebral fissure, and the projection of the eyeball in early life. J. Comp. Neurol. 1928;48:415–440. [Google Scholar]
- Isenberg SJ, Ward McCarty J, Rich R. Growth of the conjunctival fornix and orbital margin in term and premature infants. Ophthalmology. 1987;92:1276–1280. doi: 10.1016/s0161-6420(87)80011-8. [DOI] [PubMed] [Google Scholar]
- Isenberg SJ, Del Signore M, Chen A, Wei J, Guillon JP. The lipid layer and stability of the preocular tear film in newborns and infants. Ophthalmology. 2003;110:1408–1411. doi: 10.1016/S0161-6420(03)00451-2. [DOI] [PubMed] [Google Scholar]
- Jones KL, Hanson JW, Smith DW. Palpebral fissure size in newborn infants. J. Pediatr. 1978;92:787–788. doi: 10.1016/s0022-3476(78)80156-5. [DOI] [PubMed] [Google Scholar]
- Kaercher T, Mobius D, Welt R. Biophysical behaviour of the infant lipid layer. Int. Ophthalmol. 1994;18:15–19. doi: 10.1007/BF00919408. [DOI] [PubMed] [Google Scholar]
- Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–653. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- Karson CN. Physiology of normal and abnormal blinking. Adv. Neurol. 1988;40:25–37. [PubMed] [Google Scholar]
- Khamene A, Negahdaripour S, Tseng SCG. A spectral-discrimination method for tear-film lipid layer thickness estimated from fringe pattern images. IEE Trans. Biomed. Eng. 2000;47:249–258. doi: 10.1109/10.821773. [DOI] [PubMed] [Google Scholar]
- Korb DR, Griener JV, Glonek T, et al. Human and rabbit lipid layer and interference pattern observations. Adv. Exp. Med. Biol. 1998;438:305–308. doi: 10.1007/978-1-4615-5359-5_42. [DOI] [PubMed] [Google Scholar]
- Mehes K. Palpebral fissure length in newborn infants. Acta Pediatr. Acad. Sci. Hung. 1980;21:55–56. [PubMed] [Google Scholar]
- Meng SZ, Ozawa Y, Itoh M, Takashima S. Development and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain. 1999;843:136–144. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- Millodot M. Objective measurement of corneal sensitivity. Acta Ophthalmol. 1973;51:325–334. doi: 10.1111/j.1755-3768.1973.tb06010.x. [DOI] [PubMed] [Google Scholar]
- Mori A, Oguchi Y, Okusawa Y, Ono M, Fujishima H, Tsubota K. Application of high-speed high resolution thermography for evaluation of tear film layer. Am. J. Ophthalmol. 1997;124:729–735. doi: 10.1016/s0002-9394(14)71689-7. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Patel S, Marshall J. A new non-contact corneal aesthesiometer (NCCA) Ophthal. Physiol. Opt. 1996;16:101–107. [PubMed] [Google Scholar]
- Murphy PJ, Morgan PB, Patel S, Marshall J. Corneal surface temperature change as the mode of stimulation of the non-contact corneal aesthesiometer. Cornea. 1999;18:333–342. doi: 10.1097/00003226-199905000-00016. [DOI] [PubMed] [Google Scholar]
- Nakamori K, Odawara M, Nakajima T, Mizutani T, Tsubota K. Blinking is controlled primarily by ocular surface conditions. Am. J. Ophthalmol. 1997;124:24–30. doi: 10.1016/s0002-9394(14)71639-3. [DOI] [PubMed] [Google Scholar]
- Ponder E, Kennedy WP. On the act of blinking. Q. J. Exp. Physiol. 1928;18:89–110. [Google Scholar]
- Sivan Y, Merlob P, Reisner SH. Eye measurements in preterm and term newborn infants. J. Craniofac. Genet. Dev. Biol. 1982;2:239–242. [PubMed] [Google Scholar]
- Snir M, Kremer I, Kuperman A, Merlob P, Yassur Y. Bell's phenomenon in newborns and premature babies. Br. J. Ophthalmol. 1996;80:553–555. doi: 10.1136/bjo.80.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snir M, Axer-Siegel R, Bourla D, Kramer I, Benjamini Y, Weinberger D. Tactile corneal reflex development in full-term babies. Ophthalmology. 2002;109:526–529. doi: 10.1016/s0161-6420(01)00978-2. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Lawrence MS, Sladek JR, Roth RH, Redmond DE. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Exp. Neurol. 1999;158:214–220. doi: 10.1006/exnr.1999.7093. [DOI] [PubMed] [Google Scholar]
- Tsubota K. Tear dynamics and dry eye. Prog. Ret. Eye Res. 1998;17:565–596. doi: 10.1016/s1350-9462(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Zaman ML, Doughty MJ, Button NF. The exposed occular surface and it's relationship to spontaneous eyeblink rate in elderly caucasians. Exp. Eye. Res. 1998;67:681–686. doi: 10.1006/exer.1998.0571. [DOI] [PubMed] [Google Scholar]
- Zametkin AJ, Stevens JR, Pittman R. Ontogeny of spontaneous blinking and of habituation of the blink reflex. Ann. Neurol. 1979;5:453–457. doi: 10.1002/ana.410050509. [DOI] [PubMed] [Google Scholar]