Abstract
Palisade endings form a cuff of nerve terminals around the tip of muscle fibres. They are found only in extraocular muscles, but no definite evidence for their role in eye movements has been established. Palisade endings have been reported in all species so far investigated except the rat. In this study we demonstrate that antibodies against SNAP-25, the synaptosomal associated protein of 25 kDa, reliably visualize the complete motor, sensory and autonomic innervation of the extraocular muscles in human, monkey and rat. The SNAP-25 antibody can be combined with other immunofluorescence procedures, and is used here to study properties of palisade endings. With SNAP-25 immunolabelling putative palisade endings are identified in the rat for the first time. They are not well branched, but fulfil several criteria of palisade endings, being associated with non-twitch fibres as shown by double labelling with ‘myosin heavy chain slow-twitch’ antibodies. The putative palisade endings of the rat lack α-bungarotoxin binding, which implies that these synapses are sensory. If palisade endings are sensory then they could function as an eye muscle proprioceptor. They seem to be a general feature of all vertebrate eye muscles, unlike the other two extraocular proprioceptors, muscle spindles and Golgi tendon organs, the presence of which varies widely between species.
Keywords: human, monkey, myotendinous cylinders, oculomotor proprioception, rat
Introduction
Dogiel was one of the first scientists to describe palisade endings. He found them in the extraocular muscles of human, monkey, horse, oxen, dogs and cats, and referred to three previous reports of similar structures in rabbit, camel and cow (Dogiel, 1906). They were subsequently found in the eye muscles of many other species but, thus far, have not been reported in the rat (Dogiel, 1906; Ruskell, 1999). Palisade endings consist of a cuff, or ‘palisade’, of fine nerve terminals at the myotendinous junction. They arise from myelinated nerve fibres that enter the muscle at the central nerve entry zone, run to the distal or proximal tip of the muscle and into the tendon, then turning back 180° to terminate around the tip of a muscle fibre. An alternative name for this type of terminal, plus its collagen capsule, is an ‘innervated myotendinous cylinder’ (Ruskell, 1978).
Palisade endings are thought to be confined to eye muscles, and they have not been found in any other vertebrate muscle up to now (Ruskell, 1999). Extraocular muscles have a particularly complex structure (reviewed by Spencer & Porter, 1988). They can be divided into an inner global and an outer orbital layer (Kato, 1938). The global layer is continuous from the annulus of Zinn to the tendinous insertion on the sclera of the globe, whereas the orbital layer terminates posterior to the scleral insertion on the fibroelastic capsule of Tenon (Porter et al. 1996; Demer, 2002; Miller et al. 2003). In contrast to skeletal muscles, which contain two main muscle fibre types, six fibre types can be distinguished in extraocular muscles. Spencer & Porter (1988) have reviewed their properties. Four of these fibre types are categorized as twitch muscle fibres (i.e. responding with an all-or-nothing response to stimulation), with a single ‘en plaque’ region of innervation similar to motor endplates found in skeletal muscles. The remaining two types are multiply innervated, one type lying in the orbital layer, the other in the global layer. The multiply innervated muscle fibres respond to stimulation with a slow tonic contraction, and are referred to here as non-twitch muscle fibres. The multiply innervated fibres of the orbital layer have mixed twitch and non-twitch properties (Pachter, 1984), whereas those of the global layer are ‘pure’ non-twitch fibres. Palisade endings innervate exclusively one specific type of extraocular muscle fibre, the multiply innervated non-twitch muscle fibres of the global layer.
The function of palisade endings has always been a highly controversial subject; and it is still unclear whether they have a sensory function, a motor function or perhaps a mixed function (Lukas et al. 2000). As early as 1910, Tozer and Sherrington showed that palisade endings in the monkey eye muscle did not degenerate when the sensory trigeminal nerve was sectioned (Tozer & Sherrington, 1910), but they did degenerate when the oculomotor nerves were cut. This result was confirmed by Sas & Schwab (1952), with similar methods. These findings imply that either the palisade endings do not have a sensory function and are therefore likely to have a motor function, or that the sensory afferents of palisade endings take a highly unusual route into the brain, for example via the oculomotor nerves.
The question of extraocular proprioception is also contentious, independent of the function of palisade endings. Both muscle spindles and Golgi tendon organs, the classical muscle proprioceptors, were only found in the extraocular muscles of cloven-hoofed species such as sheep (Harker, 1972), camel (Abuel-Atta et al. 1997), pig (Blumer et al. 2001a) and cow (Maier et al. 1974; Blumer et al. 2003). In contrast, some species, including humans, possessed only muscle spindles, but no Golgi tendon organs; by contrast, the eye muscles of numerous other species contained neither muscle spindles nor Golgi tendon organs (Cooper & Daniel, 1949; Lukas et al. 1994). No correlation could be established between the occurrence of proprioceptors and any other factor.
The results of physiological studies do not clarify the position. First, eye muscles do not show a stretch-reflex as skeletal muscles do, and the need for a sensory input from the eye muscles was often doubted, or considered not necessary, because the visual system exerts a constant feedback control over eye movements (Keller & Robinson, 1972). However, neural responses to stretching eye muscles have been reported in the superior colliculus, the cerebellum and brainstem (Donaldson & Long, 1980; Ruskell, 1999; Donaldson, 2000). In addition, a variety of oculomotor deficits were found when sensory afferents from extraocular muscles were destroyed, manipulated or inactivated (Fiorentini & Maffei, 1977; Maffei & Fiorentini, 1976; Pettorossi et al. 1995). The problems of eye muscle proprioception were pushed aside in the 1970s and became unfashionable for several years, until the early 1980s when investigations of scientists such as Gordon Ruskell generated new interest in the complicated issue (reviewed by Ruskell, 1999).
We consider that palisade endings may provide a proprioceptive signal from eye muscles in the absence of muscle spindles and Golgi tendon organs (Büttner-Ennever et al. 2003). To support this hypothesis, the sensory nature of the palisade endings must be established. Any such study of palisade endings must depend on a staining technique that visualizes the axon, the branches of the palisade endings and, most crucial of all, the terminal boutons of the putative receptors. Classical silver stains (Richmond et al. 1984) may fulfil some of these criteria, but they cannot be combined with modern immunohistochemical techniques to determine the functional properties of the palisade terminals. For example, positive stains for synaptophysin at a presynaptic terminal indicate the presence of synaptic vesicles and thereby a functional synapse (Wiedenmann & Franke, 1985).
In this paper we describe a staining technique that uses antibodies against SNAP-25 to visualize motor axons and endplates, as well as sensory axons and their endings. It is suitable for the complete staining of palisade ending axons, their branches and terminals. The procedure can be simply combined with other immunohistochemical techniques to establish the functional properties of these endings. The synaptosomal associated protein SNAP-25 is a t-SNARE (i.e. a target receptor associated with the presynaptic plasma membrane), involved in synaptic vesicle exocytosis (McMahon & Sudhof, 1995). Several studies showed that SNAP-25 is not only concentrated at synapses and in transport vesicles but also in the axonal membrane (Garcia et al. 1995; Tao-Cheng et al. 2000). Using SNAP-25 antibodies, our study shows for the first time that even rats have putative palisade endings associated with their global layer ‘non-twitch’ muscle fibres, implying that palisade endings may be present universally in mammalian eye muscles.
Methods
All experimental procedures conformed to the state and university regulations on Laboratory Animal Care, including the Principles of Laboratory Animal Care (NIH Publication 85-23, Revised 1985), and were approved by their Animal Care Officers and Institutional Animal Care and Use Committees.
Paraformaldehyde-fixed eye muscles were obtained from macaque monkey and three rats. The animals were killed with an overdose of nembutal (80 mg kg−1 body weight) and transcardially perfused with 0.9% saline (35 °C) followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB; pH 7.4) and 10% sucrose in 0.1 m PB (pH 7.4). Then, the eyes were removed from the orbits and the eye muscles were carefully dissected and equilibrated in 20 and 30% sucrose in 0.1 m PB for 3 days. Additional unfixed eye muscles were obtained from sheep. Human muscle specimens were removed during optical surgery, post-fixed in 4% PB-buffered paraformaldehyde, and equilibrated in 10, 20 and 30% sucrose. Fixed monkey and unfixed sheep eye muscles were shock frozen in isopentane (−60 °C) and kept at −20 °C until cutting. Rat and human eye muscles were directly frozen in the cryostat microtome (MICROM HM 560). All eye muscles were cut longitudinally at 20 µm and thaw-mounted onto glass slides (Superfrost Plus).
Prior to immunohistochemistry, sheep eye muscles were fixed for 5 min in 4% paraformaldehyde in 0.1 m PB. SNAP-25 immunoreactivity was revealed with the monoclonal mouse antibody SMI81 (Sternberger Monoclonals Inc.).
Single peroxidase staining of SNAP-25
All sections were pretreated with 3% H2O2/10% methanol in 0.1 m PB, pH 7.4, for 15 min to suppress endogenous peroxidase activity and then thoroughly washed. For the detection of SNAP-25 immunoreactivity, sections were blocked with 5% horse serum in 0.1 m PB, pH 7.4, containing 0.3% Triton X-100 for 1 h and subsequently processed with mouse anti-SNAP-25 antibodies (1 : 5000) overnight at room temperature. After several buffer washes the sections were treated with biotinylated horse antimouse antibody (1 : 200; Alexis) for 1 h at room temperature, washed and incubated in extravidin–horseradish peroxidase (1 : 1000; Sigma) for 1 h. Diaminobenzidine served as chromogen for the detection of SNAP-25 immunoreactivity. Some sections were counterstained with hemalaun (0.1%).
Double and triple fluorescence labelling
In order to verify that SNAP-25 is present in both nerve fibres and terminals, double immunofluorescence staining was performed on monkey and rat eye muscles using SNAP-25 antibodies combined with either anti-synaptophysin or anti-neurofilament-M (NF-M).
First the eye muscle sections were blocked with 5% normal donkey serum in 0.1 m PB, pH 7.4, containing 0.3% Triton X-100 for 1 h. Then, the sections were processed with a mixture of mouse anti-SNAP-25 (1 : 1000) and either rabbit anti-synaptophysin (1 : 100; Synaptic Systems) or rabbit anti-NF-M (1 : 1000; Chemicon) overnight. For visualization of the applied antibodies, the sections were then reacted with a mixture of fluorochrome-tagged secondary antibodies from donkey, namely Alexa 488-anti-rabbit (1 : 200; Molecular Probes) and Cy3-anti-mouse (1 : 200; Dianova) for 2 h.
For the identification of putative palisade endings in rat extraocular muscles, longitudinal sections of 17 eye muscles were processed for the detection of SNAP-25 combined with α-bungarotoxin binding, and staining for synaptophysin or myosin heavy chain slow twitch. After blocking with 5% normal donkey serum in 0.1 m PB, pH 7.4, containing 0.3% Triton X-100 for 1 h, the sections were first treated with mouse anti-SNAP-25 (1 : 1000) overnight, followed by a 2-h incubation of Alexa 488-tagged donkey anti-mouse (1 : 200; Molecular Probes) secondary antibody. After extensive rinsing in 0.1 m PB, pH 7.4, the sections were then processed with mouse anti-myosin heavy chain slow-twitch (1 : 100; Novocastra) or rabbit anti-synaptophysin (1 : 100) overnight, followed by a 2-h incubation of Cy5-tagged donkey anti-mouse or donkey anti-rabbit (1 : 150; Dianova) secondary antibody. After washing in 0.1 m PB, the sections were then treated with Cy3-tagged α-bungarotoxin (1 : 200, Molecular Probes) for 30 min at room temperature and rinsed in 0.1 m PB.
All fluorochrome-stained sections were coverslipped with GEL/MOUNT permanent aqueous mounting medium (Biomeda) and stored in the dark at 4 °C.
Analysis
Images of bright-field photographs were digitalized by using the 3-CCD videocamera (Hamamatsu; C5810) mounted on a Leica DMRB microscope. The images were captured on a computer with Adobe Photoshop 5 software. Sharpness, contrast and brightness were adjusted to reflect the appearance of the labelling seen through the microscope.
Confocal microscopy and image processing
Fluorescence-stained eye muscle sections were imaged with a Leica TCS NT and a Leica TCS SP2 laser-scanning confocal fluorescence microscope (Leica, Heidelberg, Germany). Images were taken with a 40× oil objective, NA 1.4, at a resolution of approximately 200 nm per pixel. Dual or triple channel imaging of Cy2/Alexa 488, Cy3 and Cy5 fluorescence were sequentially recorded at 488 nm excitation/525–550 nm emission, 543 nm excitation/555–620 nm emission, and 633 nm excitation/650–750 nm emission, respectively. Z-series were collected from 0.5–1 µm optical sections taken through the specimen. Image stacks were processed using Metamorph 6.1 (Universal Imaging Inc., USA). Brightness and contrast were enhanced as required, and maximum intensity projections were generated for visualization.
All pictures were arranged and labelled with drawing software (CorelDraw 8 and 11; Corel).
Results
SNAP-25 immunolabelling is used to stain neural structures in the extraocular muscles of monkey, human, sheep and rat. It is shown here to stain motor nerves and endplates, sensory fibres and terminals, autonomic innervation of blood vessels, and palisade endings at the myotendinous junction.
Motor innervation
The motor nerves are completely visualized with the SNAP-25 antibody stain, from their entry into the extraocular muscle up to the fine ramifications where they yield the nerve terminals of ‘en plaque’ endings on the twitch muscle fibres (Fig. 1A). This was verified with double labelling of the ‘en plaque’ endings with synaptophysin antibodies (Fig. 1E). In all of these endings SNAP-25 and synaptophysin immunoreactivity were always co-localized (Fig. 1D-F). In contrast to SNAP-25 (red fluorescence), which clearly visualizes both nerves and terminals, NF-M (green fluorescence) is only present in the nerve branches (Fig. 1B,C).
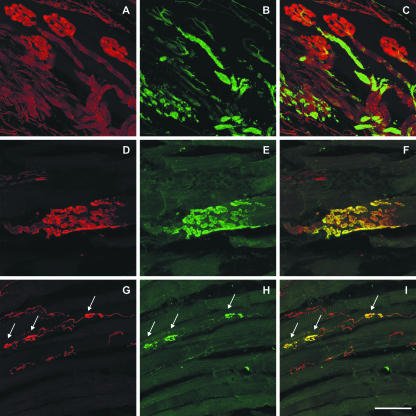
Fig. 1.
Laser scanning photomicrographs of longitudinal sections of extraocular eye muscles. (A–C) Double immunofluorescence labelling of a rat inferior rectus muscle with antibodies for SNAP-25 (red) and NF-M (green). A motor nerve is entering the eye muscle and branches into several ‘en plaque’ endings. SNAP-25 antibodies visualize both the nerve fibres and the ‘en plaque’ endings (A) whereas NF-M immunoreactivity is present only in the majority of the nerve fibres (B). This is clearly shown in the overlay of both markers in C. (D–I) Double immunofluorescence labelling of a monkey inferior rectus muscle with antibodies for SNAP-25 (red) and synaptophysin (green). Detailed view of an ‘en plaque’ ending on a twitch muscle fibre (D–F) and ‘en grappe’ endings (arrows) on a multiply innervated muscle fibre (G–I). In both types of endings, SNAP-25 and synaptophysin immunoreactivity clearly outline the morphology of these endings and are co-localized in all terminals, as indicated in the overlays (F,I). Note that the motor nerve giving rise to the ‘en grappe’ endings lacks synaptophysin and is only visualized with SNAP-25 antibodies (H,I). Scale bar, 50 μ.
The motor nerve and the small ‘en grappe’ motor endplates along the length of multiply innervated muscle fibres were strongly labelled with SNAP-25 antibodies (Fig. 1G,I). All ‘en grappe’ endplates were double labelled for synaptophysin and SNAP-25 (Fig. 1H,I).
Sensory innervation
In order to test the ability of SNAP-25 to label sensory nerves and their terminals, sheep eye muscles, which contain muscle spindles, were stained with SNAP-25 antibody. Muscle spindles were easily identified in van Giesson stains by the collagen fibre capsule. The sensory annulospiral endings on the equatorial region of the intrafusal fibre of muscle spindles were fully labelled with SNAP-25 antibodies (Fig. 2A).
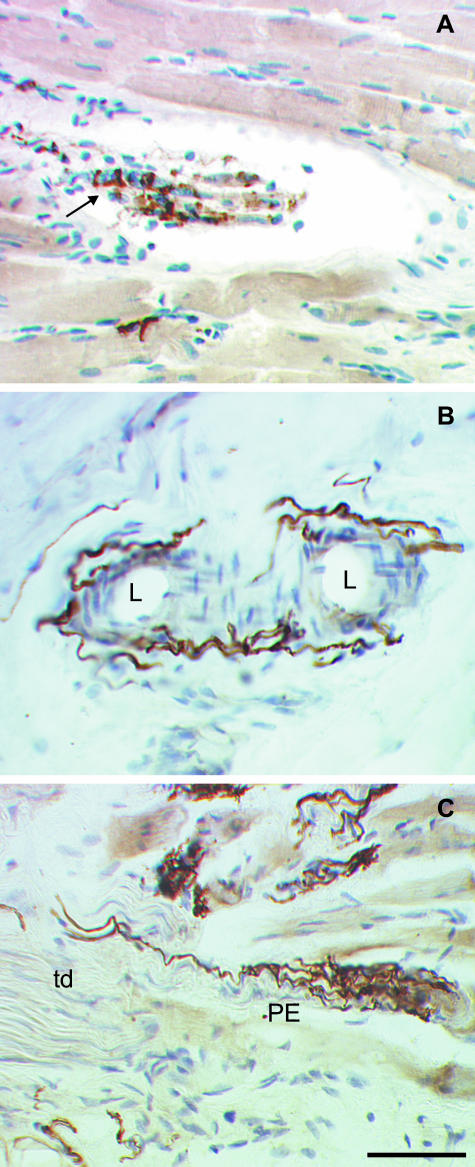
Fig. 2.
Photomicrographs of neural structures in longitudinal sections of sheep and human extraocular eye muscles labelled with SNAP-25 antibodies and counterstained with hemalaun. (A) Identified muscle spindle in a sheep inferior rectus muscle. With SNAP-25 immunolabelling, a nerve fibre which enters the collagenous capsule is visualized. On the intrafusal muscle fibres an annulospiral ending which winds around the fibres is clearly SNAP-25 positive (arrow). (B) Blood vessels in the tendon of a human extraocular muscle. Using SNAP-25 antibodies, a fine network of varicose nerve fibres surrounding the lumen (L) of the vessels could be identified. (C) A palisade ending at the myotendinous junction of a human extraocular eye muscle. SNAP-25 antibodies completely label the palisade ending (PE), the axon which enters from the tendon (td) as well as the bunch of branches and small terminals which terminate on a muscle fibre. Scale bar, 50 μm.
Autonomic innervation
Blood vessels within the extraocular muscle are innervated by a network of fine varicose nerves which are clearly SNAP-25 positive (Fig. 2B).
Palisade endings
Only palisade endings in which the supplying axon could be seen to enter the muscle fibre from the distal tendon were analysed in this study. Because staining for SNAP-25 labels both nerve fibres and terminals of motor and sensory axons, it could be used for the complete visualization of palisade endings at the myotendinous junction. In Fig. 2(C), the palisade ending in a human eye muscle is visualized to its full extent with SNAP-25 antibodies. The reconstruction of the double labelled monkey palisade ending shows the red fluorescence of SNAP-25 positive afferent axon and cuff of terminals around the tip of a muscle fibre (Fig. 3A). In contrast, NF-M immunoreactivity (green fluorescence) is present only in the axonal branches and not in the terminals (Fig. 3B,C).
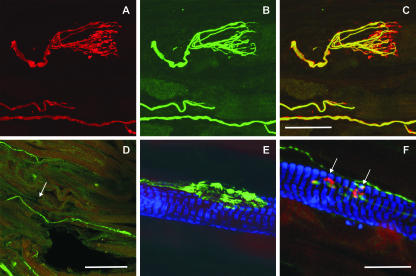
Fig. 3.
Laser scanning photomicrographs of longitudinal sections of extraocular eye muscles. (A–C) Double immunofluorescence labelling of a palisade ending at the myotendinous junction of a monkey inferior rectus muscle with antibodies for SNAP-25 (red) and NF-M (green). The afferent axon and the bunch of axonal branches are both visible in the SNAP-25 (A) stain and the NF-M (B) stain. In contrast, the cuff of terminals lacks NF-M immunoreactivity and can only be identified with SNAP-25 antibodies (B), which is clearly demonstrated in the overlay of both markers in C. (D–F) Double (D) or triple (E,F) immunofluorescence labelling of a rat inferior rectus muscle with antibodies for SNAP-25 (green), myosin heavy chain slow-twitch (MHCslow-twitch; blue) and additional applied α-bungarotoxin (red). Panels D–F show overlays of the applied markers. (D) At the myotendinous junction (tendon left, muscle right), a putative palisade ending is identified with SNAP-25 antibodies. A nerve fibre enters from the distal tendon (arrow) and forms a clump of terminals on a muscle fibre. At higher magnification, a similar clump of terminals of the putative palisade ending in a different section can be clearly identified as several individual terminals, which form neuromuscular contacts on an MHC slow-twitch positive multiply innervated muscle fibre (E). In the same section, two α-bungarotoxin and SNAP-25 positive ‘en grappe’ endings (arrows) on an MHC slow-twitch-positive multiply innervated muscle fibre are clearly visible (F). Note that in contrast to these ‘en grappe’ endings, the terminals of the putative palisade endings in D and E do not show red any α-bungarotoxin binding. Scale bars: 50 µm (A–C), 200 µm (D), 20 m (E,F).
The study of 17 rat extraocular muscles stained with SNAP-25 antibodies showed a more widespread network of neural structures at the distal tip of the muscle and the adjacent tendon compared with in monkey or human. Some of these could be identified as ‘en grappe’ endplates on muscle fibres, whereas others were associated with blood vessels and connective tissue. In addition, axons could be traced from the distal tendon, and were found to terminate in close proximity to the muscle fibre tips, where the global layer inserts into the sclera (Blumer et al. 2001b; Miller et al. 2003). These axons were of a larger calibre than the fine nerves innervating blood vessels. In spite of the difficulty in obtaining intact myotendinous junctions in rat eye muscles, 27 such structures were found and they varied in their morphology from a fine network to a compact clump of terminals (Fig. 3D,E). A review of the spectrum of terminals is beyond the scope of this paper. Here, one such terminal is selected as an example, and used to illustrate some of the histochemical properties of structures at the myotendinous junction of the rat eye muscles.
Nineteen of the presumed palisade endings were tested for α-bungarotoxin binding, and all but one were completely negative, implying the absence of motor endplates. In contrast, a control labelling of ‘en grappe’ endplates on a multiply innervated muscle fibre in the same section shows clear α-bungarotoxin binding (Fig. 3F). Additional staining with antibodies for synaptophysin on ten of the bungarotoxin-negative terminals verified the presence of synapses. In the few terminals in which α-bungarotoxin binding is present, it was assumed to be due to the presence of ‘en grappe’ endplates from a distally projecting motor nerve adjacent to the presumed palisade ending.
Palisade endings are always associated with multiply innervated muscle fibres of the global layer (Alvarado-Mallart & Pincon Raymond, 1979). In order to support our assumption that the terminals at the myotendinous junction of the rat eye muscles share the same properties of palisade endings we combined the SNAP-25 staining with immunolabelling for the myosin heavy chain slow-twitch isoform, which is only expressed in the multiply innervated muscle fibres according to several authors (Kranjc et al. 2001; Rubinstein & Hoh, 2001; Kjellgren et al. 2003; D. Porter, personal communication). We found that the putative palisade endings in rat were always attached to a myosin heavy chain slow-twitch-positive, multiply innervated muscle fibre (Fig. 3E,F).
The putative palisade endings terminate in close proximity (about 200 µm) to the distal tip of the muscle fibre, but do not show the typical palisade-like branching seen in monkeys (Fig. 3). No neurotendinous terminals were found in the rat experiments. Accordingly, in this light microscopic study, all terminals appear to contact the muscle fibres.
Discussion
The staining properties of antibodies against the synaptosomal associated protein SNAP-25 enabled us to stain both motor and sensory nerve axons and terminals in extraocular muscles. In particular, palisade endings and their terminals could be reliably visualized in human and monkey, and for the first time putative palisade endings are described in rat. In many previous studies silver staining methods were used to identify palisade endings (Dogiel, 1906; Alvarado-Mallart & Pincon Raymond, 1979; Richmond et al. 1984). The visualization of palisade endings using SNAP-25 antibodies is at least as good as with the silver staining techniques, and it avoids their major disadvantages. (1) Silver stains cannot be combined with immunohistochemical stains, (2) the technique is complex and not easy to reproduce (3) as stated by some authors (Richmond et al. 1984), some structures may remain unstained. Looking for alternative stainings to SNAP-25 immunolabelling, there are at least two other antibodies which visualize both nerve fibres and terminals and can also be combined with other immunohistochemical techniques: neurocalcin and protein gene product 9.5 (PGP 9.5). Neurocalcin is a member of the EF-hand family of calcium-binding proteins and stains myelinated axons and motor nerve endings in extraocular muscles (Junttila et al. 1995). However, this antibody is not commercially available. PGP 9.5 is a cytoplasmatic neuronal protein identified as a ubiquitin carboxyl-terminal hydrolase (Wilkinson et al. 1989) and has also been used to stain palisade endings. But up to now, the PGP 9.5 stainings of palisade endings have not yielded the high-resolution morphology seen here with SNAP-25 immunolabelling (Lukas et al. 2000).
The morphology of rat palisade endings varies and appeared to be less elaborate in comparison with the highly branched terminals in monkey and human, but they always showed typical properties of palisade endings, in that they (1) are innervated by a nerve arising from the tendon (Fig. 3D), and (2) terminate on a multiply innervated muscle fibre at the myotendinous junction of the global layer (Fig. 3E) (Dogiel, 1906; Richmond et al. 1984; Ruskell, 1999). The structures in Fig. 3(D,E) represent only one type of ending with a rather compact clump of terminals. In spite of the fact that the appearance of this structure is so unlike a palisade, we assume it to be homologous to a palisade ending, because of its other attributes.
In addition, the rat putative palisade endings were shown to contain synaptophysin immunoreactivity, proving the presence of synapses at their terminals. The presence of rudimentary palisade endings in rats may not be surprising, because rats are not highly visual animals and would not be expected to have well-developed receptors in their oculomotor system.
The function of palisade endings is still controversial, and current studies have attributed both motor and sensory properties to them. We combined the staining for SNAP-25 in palisade endings with the classical proof for the presence of motor endplates, the binding of α-bungarotoxin (Anderson & Cohen, 1974), and found no evidence for motor endplates at the terminals of rat palisade endings. According to Alvarado-Mallart & Pincon Raymond (1979) and Lukas et al. (2000), a defining feature of palisade endings is that they form mainly neurotendinous contacts, whereas neuromuscular contacts were rare. An exception to this was found in the rabbit, where Blumer et al. (2001b) showed that palisade endings form exclusively myoneural contacts. In our analysis of the putative palisade endings of the rat we only found neuromuscular contacts, implying a similarity to the rabbit. Exclusive neuromuscular contacts combined with α-bungarotoxin positive terminals led Blumer et al. (2001b) to assume that rabbit palisade endings are motor and may act as effectors. However, the rat putative palisade endings never bind α-bungarotoxin, implicating a sensory function. It is hard to believe that these contradictions are due to species differences; and a possible explanation has been put forward by Lukas et al. (2000). They suggest that palisade endings are proprio-effectors with mixed sensory and motor properties.
The presence of palisade-like endings in rat opens up the possibility that they are a universal feature of all eye muscles, whereas the occurrence of muscle spindles and Golgi tendon organs is not. Several authors have suggested that palisade endings could be the source of sensory afferent signals (Ruskell, 1999; Donaldson, 2000; Steinbach, 2000; Weir et al. 2000; Büttner-Ennever et al. 2002). The ultrastructural morphology of palisade endings in cat, rhesus monkey and sheep has been shown to be typical of a sensory structure (Ruskell, 1978; Alvarado-Mallart & Pincon Raymond, 1979; Blumer et al. 1998). However, Blumer et al. (2001b), Lukas et al. (2000), and Konakci et al. (2005) show that in rabbit, and to a minor extent in human and in cat, palisade terminals exhibit motor-terminal-like properties. The problem is compounded by the conflicting evidence for the location of the cell soma of the palisade ending. If the palisade endings are sensory, their cell body should lie in the trigeminal ganglion or in the mesencephalic trigeminal nucleus; by contrast, if the endings are of a motor origin then they would have cell bodies associated with the motor nuclei of the extraocular muscles. Tozer & Sherrington (1910) as well as Sas & Scháb (1952) provided evidence for their location in the oculomotor nerve or nucleus, a result more compatible with a motor role for the palisade endings (Gentle & Ruskell, 1997; Ruskell, 1999), whereas the results of other studies point to the trigeminal ganglion as the location of palisade ending soma (Billig et al. 1997), and imply a sensory function.
The work of Gordon Ruskell has revived the interest in palisade endings, but their function still remains unclear. We have shown that the synaptosomal associated protein SNAP-25 is a useful marker for studying their properties. Finally, our demonstration that palisade-like endings occur in the extraocular muscles of the rat implies that palisade endings may be a general feature of mammalian eye muscles, unlike extraocular proprioceptors such as muscle spindles and Golgi tendon organs.
Acknowledgments
This study was sponosored by a grant from the Deutsche Forschungsgemeinschaft (Ho 1639/4-1; GRK 267). We also thank Christine Glombik for her technical assistance, Ahmed Messoudi for his expertise and Professor Lange for his continual support.
References
- Abuel-Atta AA, DeSantis M, Wong A. Encapsulated sensory receptors within intraorbital skeletal muscles of a camel. Anat. Rec. 1997;247:189–198. doi: 10.1002/(SICI)1097-0185(199702)247:2<189::AID-AR5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Alvarado-Mallart RM, Pincon Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979;11:567–584. doi: 10.1016/0040-8166(79)90063-6. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Cohen MW. Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle. J. Physiol. 1974;237:385–400. doi: 10.1113/jphysiol.1974.sp010487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig I, Buisseret-Delmas C, Buisseret P. Identification of nerve endings in cat extraocular muscles. Anat. Rec. 1997;248:566–575. doi: 10.1002/(SICI)1097-0185(199708)248:4<566::AID-AR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Blumer R, Lukas JR, Wasicky R, Mayr R. Presence and structure of innervated myotendinous cylinders in sheep extraocular muscle. Neurosci. Lett. 1998;248:49–52. doi: 10.1016/s0304-3940(98)00331-0. [DOI] [PubMed] [Google Scholar]
- Blumer R, Wasicky R, Brugger PC, Hoetzenecker W, Wicke WL, Lukas JR. Number, distribution, and morphologic particularities of encapsulated proprioceptors in pig extraocular muscles. Invest. Ophthalmol. Vis. Sci. 2001a;42:3085–3094. [PubMed] [Google Scholar]
- Blumer R, Wasicky R, Hötzenecker W, Lukas JR. Presence and structure of innervated myotendinous cylinders in rabbit extraocular muscle. Exp. Eye Res. 2001b;73:787–796. doi: 10.1006/exer.2001.1085. [DOI] [PubMed] [Google Scholar]
- Blumer R, Konakci KZ, Brugger PC, et al. Muscle spindles and Golgi tendon organs in bovine calf extraocular muscle studied by means of double-fluorescent labeling, electron microscopy, and three-dimensional reconstruction. Exp. Eye Res. 2003;77:447–462. doi: 10.1016/s0014-4835(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AKE, Graf W, Ugolini G. Modern concepts of brainstem anatomy. Ann. NY Acad. Sci. 2002;956:75–84. doi: 10.1111/j.1749-6632.2002.tb02810.x. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Eberhorn A, Horn AKE. Motor and sensory innervation of extraocular eye muscles. Ann. NY Acad. Sci. 2003;1004:40–49. doi: 10.1111/j.1749-6632.2003.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Cooper S, Daniel PM. Muscle spindles in human extrinsic eye muscles. Brain. 1949;72:1–24. doi: 10.1093/brain/72.1.1. [DOI] [PubMed] [Google Scholar]
- Demer JL. The orbital pulley system: a revolution in concepts of orbital anatomy. Ann. NY Acad. Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- Dogiel AS. Die Endigungen der sensiblen Nerven in den Augenmuskeln und deren Sehnen beim Menschen und den Säugetieren. Arch. Mikrosk. Anat. 1906;68:501–526. [Google Scholar]
- Donaldson IML, Long AC. Interactions between extraocular proprioceptive and visual signals in the superior colliculus of the cat. J. Physiol. 1980;298:85–110. doi: 10.1113/jphysiol.1980.sp013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson IML. The functions of the proprioceptors of the eye muscles. Phil. Trans. R. Soc. Lond. [Biol.] 2000;355:1685–1754. doi: 10.1098/rstb.2000.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A, Maffei L. Instability of the eye in the dark and proprioception. Nature. 1977;269:330–331. doi: 10.1038/269330a0. [DOI] [PubMed] [Google Scholar]
- Garcia EP, McPherson PS, Chilocote TJ, Takei K, De Camilli P. rb-Sec1A and B colocalize with synataxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J. Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle A, Ruskell GL. Pathway of the primary afferent nerve fibres serving proprioception in monkey extaocular muscles. Ophthalmol. Physiol. Opt. 1997;17:225–231. [PubMed] [Google Scholar]
- Harker DW. The structure and innervation of sheep superior rectus and levator palpebrae extraocular eye muscles. II: Muscle spindles. Invest. Ophthalmol. Vis. Sci. 1972;11:970–979. [PubMed] [Google Scholar]
- Junttila T, Koistinaho J, Rechardt L, Hidaka H, Okazaki K, Pelto-Huikko M. Localization od neurocalcin-like immunoreactivity in rat cranial motoneurons and spinal cord interneurons. Neuro. sci. Lett. 1995;183:100–103. doi: 10.1016/0304-3940(94)11124-2. [DOI] [PubMed] [Google Scholar]
- Kato T. Über histologische Untersuchungen der Augenmuskeln von Menschen und Säugetieren. Okajimas Folia Anat. Jap. 1938;16:131–145. [Google Scholar]
- Keller EL, Robinson DA. Abducens unit behavior in the monkey during vergence movements. Vision Res. 1972;12:369–382. doi: 10.1016/0042-6989(72)90082-x. [DOI] [PubMed] [Google Scholar]
- Kjellgren D, Thornell L-E, Andersen J, Pedrosa-Domellöf F. Myosin heavy chain isoforms in human extraocular muscles. Invest. Ophthalmol. Vis. Sci. 2003;44:1419–1425. doi: 10.1167/iovs.02-0638. [DOI] [PubMed] [Google Scholar]
- Konakci KZ, Streicher J, Hoetzenecker W, Blumer MJF. Molecular characteristics suggest an effector function of palisade endings in extra-ocular muscles. Invest. Opthalmol. Vis. Sci. 2005;46:155–165. doi: 10.1167/iovs.04-1087. [DOI] [PubMed] [Google Scholar]
- Kranjc BS, Sketelj J, Albis AD, Ambroz M, Erzen I. Fibre types and myosin heavy chain expression in the ocular medial rectus muscle of the adult rat. J. Muscle Res. Cell Motil. 2001;21:753–761. doi: 10.1023/a:1010362926221. [DOI] [PubMed] [Google Scholar]
- Lukas JR, Aigner M, Blumer R, Heinzl H, Mayr R. Number and distribution of neuromuscular spindles in human extraocular muscles. Invest. Ophthalmol. Vis. Sci. 1994;35:4317–4327. [PubMed] [Google Scholar]
- Lukas JR, Blumer R, Denk M, Baumgartner I, Neuhuber W, Mayr R. Innervated myotendinous cylinders in human extraocular muscles. Invest. Ophthalmol. Vis. Sci. 2000;41:2422–2431. [PubMed] [Google Scholar]
- Maffei L, Fiorentini A. Asymmetry of motility of the eyes and change of binocular properties of cortical cells in adults cats. Brain Res. 1976;105:73–78. doi: 10.1016/0006-8993(76)90923-9. [DOI] [PubMed] [Google Scholar]
- Maier A, DeSantis M, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J. Morphol. 1974;143:397–408. doi: 10.1002/jmor.1051430404. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Sudhof TC. Synaptic core complex of synaptobrevin, synatxin, and SNAP-25 forms high affinity alpha-SNAP binding site. J. Biol. Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- Miller JM, Demer JL, Poukens V, Pavlovski DS, Nguyen HN, Rossi EA. Extraocular connective tissue architecture. J. Vis. 2003;3:240–251. doi: 10.1167/3.3.5. [DOI] [PubMed] [Google Scholar]
- Pachter BR. Rat extraocular muscle. 3. Histochemical variability along the length of multiply-innervated fibers of the orbital surface layer. Histochemistry. 1984;80:535–538. [PubMed] [Google Scholar]
- Pettorossi VE, Ferraresi A, Draicchio F, et al. Extraocular muscle proprioception and eye position. Acta Otolaryngol.(Stockholm) 1995;115:137–140. doi: 10.3109/00016489509139276. [DOI] [PubMed] [Google Scholar]
- Porter JD, Poukens V, Baker RS, Demer JL. Structure-function correlations in the human medial rectus extraocular muscle pulleys. Invest. Ophthalmol. Vis. Sci. 1996;37:468–472. [PubMed] [Google Scholar]
- Richmond FJR, Johnston WSW, Baker RS, Steinbach MJ. Palisade endings in human extraocular muscle. Invest. Ophthalmol. Vis. Sci. 1984;25:471–476. [PubMed] [Google Scholar]
- Rubinstein NA, Hoh JF. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest. Ophthalmol. Vis. Sci. 2001;41:3391–3398. [PubMed] [Google Scholar]
- Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles in rhesus monkey. J. Neurocytol. 1978;7:693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Extraocular muscle proprioceptors and proprioception. Prog. Retin Eye Res. 1999;18:269–291. doi: 10.1016/s1350-9462(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Sas J, Scháb R. Die sogenannten ‘Palisaden-Endigungen’ der Augenmuskeln. Acta Morph. Acad. Sci. (Hungary) 1952;2:259–266. [Google Scholar]
- Spencer RF. Structural organization of the extraocular muscles. In: Büttner-Ennever JA, editor. Neuroanatomy of the Oculomotor System. Amsterdam: Elsevier; 1988. pp. 33–79. [PubMed] [Google Scholar]
- Steinbach MJ. The palisade ending: an afferent source for eye position information in humans. In: Lennerstrand G, Ygge J, Laurent T, editors. Advances in Strabismus Research.Basic and Clinical Aspects. London: Portland Press; 2000. pp. 33–42. [Google Scholar]
- Tao-Cheng J-H, Du J, McBain CJ. Snap-25 is polarized to axons and abundant along the axolemma: an immunogold study of intact neurons. J. Neurocytol. 2000;29:67–77. doi: 10.1023/a:1007168231323. [DOI] [PubMed] [Google Scholar]
- Tozer FM, Sherrington CS. Receptors and afferents of the third, fourth and sixth cranial nerves. Proc. R. Soc. London Ser. 1910;82:451–457. [Google Scholar]
- Weir CR, Knox PC, Dutton GN. Does extraocular muscle proprioception influence oculomotor control? Br. J. Ophthalmol. 2000;84:1071–1074. doi: 10.1136/bjo.84.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38 000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is an ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]