Abstract
The aim of the present study was to evaluate the regional differences in microvessel density (MVD) of the human medullary tegmentum in adults and newborns/infants. Transverse serial sections of formalin-fixed, paraffin-embedded brainstems, taken from 16 adult and eight newborn/infant subjects, were stained with anti-von Willebrand factor (vWF) polyclonal antibodies. The boundaries of the area postrema (AP), dorsal motor vagal nucleus (DMVN), solitary tract nucleus (STN), solitary tract (ST) and hypoglossal nucleus (XII) were defined, all vessels were counted and the values were divided by the areas. In adult cases, statistically significant heterogeneity in MVD was found among the nuclei studied (P < 0.001). DMVN and AP showed higher MVD with respect to XII and ST (P < 0.001). The MVD of STN was lower with respect to DMVN (P < 0.001) and higher with respect to XII and ST (P < 0.05). The MVD and capillary density of the AP of newborns/infants were not significantly different with respect to adults. In sections of the medulla of adult subjects stained with anti-vWF, all vessels showed an intense reaction of endothelial cells whereas in the DMVN, XII, STN and ST of newborns/infants, only rare, isolated vessels showed anti-vWF reactivity and in the AP, 41 ± 21% of vessels expressed vWF. Differences in MVD among the nuclei may be related to their different functions and metabolic demands. Light and heterogeneous expression of vWF in endothelial cells of newborns/infants indicates that differentiation of microvasculature in the human medullary tegmentum extends beyond fetal stages.
Keywords: anti-von Willebrand factor, dorsal motor vagal nucleus, human, microvessel density, solitary tract nucleus
Introduction
The regulatory centres of cardiovascular and respiratory functions are located in the medullary tegmentum, i.e. the area postrema (AP), solitary tract nucleus (STN) and dorsal motor vagal nucleus (DMNV). The STN receives multiple visceral afferents through the solitary tract (ST), with viscerotopical distribution. The cardiovascular and respiratory afferents impinge on the dorsomedial and ventrolateral parts, respectively, of the intermediate portion (Andresen & Kunze, 1994). The hypoglossal nucleus (XII), which is located in the tegmentum near the DMVN, has also been proposed to be involved in defining patterns of respiration (Friedland et al. 1995; Macchi et al. 2002).
The microvasculature of the medullary tegmentum plays a crucial role in the functioning of these nuclei, as many peptides acting on the AP, such as endothelin, angiotensin, endorphin and adrenomedullin, are brought to the nuclei by the blood (Szilagyi & Ferrario, 1981; Sander et al. 1989; Allen et al. 1997; Nakayama et al. 1997). Their actions are determined in part by the physiological featurers of the microcirculation, which is characterized by elevated vascular volume and slow blood flow, prolonging the time of interaction with local specific receptors (Gross, 1991). This kind of vascular bed, characterized by the absence of a blood–brain barrier and with elevated vascular volume, also favours ionic and osmotic monitoring of blood (McKinley et al. 2003). The microvasculature of the AP region also plays a role in connecting AP and STN. Short communicating vessels have been identified between these two centres, and a portal system, by means of which postremal venous drainage conveys neuroactive factors to STN, has been proposed (Roth & Yamamoto, 1968; Gross et al. 1991).
Analytical morphometric and functional data concerning the microvasculature of the medullary tegmentum have mainly been reported in experimental studies on rats (Roth & Yamamoto, 1968; Brizee & Klara, 1984; Gross et al. 1986, 1990, 1991; Shaver et al. 1990, 1991; Gross, 1991). The aim of the present report is to provide information about the microvessel densities (MVD) of the nuclei of the human medullary tegmentum. Differences in microvessel phenotypes and densities between adult and newborn/infant subjects are also presented.
Materials and methods
The study was performed on 24 brainstems sampled during autopsy from 16 adult subjects (13 male, three female; age range 33–84 years, mean 38 years) and eight newborn/infant subjects (five male, three female; age range 4–120 days, mean 54 days). Autopsy was performed within 48 h of death. The brain was cut after fixation in 10% formalin for 15 days. In all cases, examination revealed the absence of acute, chronic, localized or diffuse brain lesions. Histological examination was performed on 5-µm-thick transverse serial sections of the paraffin-embedded medulla. Sections were stained with haematoxylin-eosin, Nissl, Kluver-Barrera and azan-Mallory. In each case, 12 sections were collected, at 100 µm from each other, at the level of the AP. They were stained with anti-von Willebrand factor (vWF) for detecting small blood vessels.
For immunohistochemical study, sections were hydrated gradually through decreasing concentrations of ethanol and then washed in deionized H2O. Antigen unmasking was performed through trypsin treatment for 10 min. Sections were then incubated in 0.3% hydrogen peroxide in deionized H2O to remove endogenous peroxidase activity and enhance antibody penetration into the tissue, and then washed in 0.01 m phosphate-buffered saline (PBS). They were incubated for 30 min in blocking serum to eliminate non-specific binding. The blocking serum was made with 0.2% bovine serum albumin (BSA; Sigma, USA) and normal goat serum (DAKO, Italy) at a dilution of 1 : 200 in PBS. Sections were then incubated at room temperature for 45 min, with polyclonal antibody raised in rabbit against human vWF (DAKO, code A 0082) at a dilution of 1 : 8000 in PBS. After primary antibody incubation, sections were washed in PBS three times for 5 min, incubated at room temperature for 30 min with anti-mouse and anti-rabbit serum (Universal Immuno-Peroxidase Polymer, Histofine, Japan), placed in 3,3′-diaminobenzidine (DAB; Sigma, Italy) containing H2O2, and finally counterstained with haematoxylin. Sections incubated without the primary antibody showed no immunoreactivity.
For each section stained by immunohistochemistry, all the vascular structures in the context of the AP, STN, ST, DMVN and XII were counted. The boundaries of these centres were defined according to Paxinos & Huang (1995) and McRitchie et al. (1990) (Fig. 1). Data regarding the interstitial subnucleus of STN, which is located within the context of ST, were counted with STN. For DMVN, dorsal and medial fringes were not considered. Vascular structures were considered those with positive reaction and a visible lumen or well-defined linear vessel shape. The MVD of the above-mentioned nuclei were calculated in each section by dividing the number of vessels by the area, obtained with the help of image analysis software (Qwin Leica Imaging System, Cambridge, UK). The mean MVD, with standard deviations, were calculated for each case and for the entire sample. In order to evaluate differences in MVD among the nuclei, a one-way anova and Tukey's multiple comparison test were performed. P < 0.05 was considered to be statistically significant. For each nucleus, statistical analysis of the linear correlation between MVD and age was also performed (P < 0.05 was considered to be statistically significant).
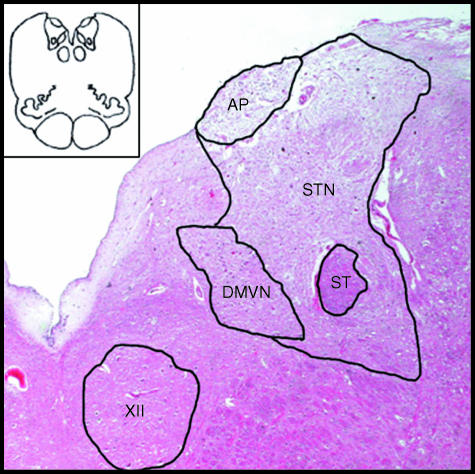
Fig. 1.
Schematic drawing of a transverse section of human medulla and a histological image of medullary tegmentum, showing boundaries of districts examined: area postrema (AP), solitary tract nucleus (STN), solitary tract (ST), dorsal motor vagal nucleus (DMVN) and hypoglossal nucleus (XII) (haematoxylin-eosin, ×4).
As regards AP, capillary density was also calculated. For each section, vessels with luminal diameter of less than 7 µm were separately counted and the value was then divided by the area. The capillary density of AP was also calculated for each case and for the entire sample.
In newborn/infant specimens, preliminary evaluation of immunohistochemical staining showed positive vessels almost exclusively in the AP, so that quantitative evaluations were performed only for this district. For each section of the AP, vessels and capillaries with positive reaction were separately counted and divided, respectively, by the total number of visible vessels and capillaries to determine the percentage of vascular positivity and capillary positivity. The mean percentages of positivity were then calculated for each case and for the entire sample. An unpaired Student's t-test was performed to verify any differences between the percentages of vascular and capillary positivity. P < 0.05 was considered to be statistically significant.
MVD and capillary density, with standard deviations, were also calculated for each case and for the entire sample, following the same procedure described for adults, but taking into account all vascular structures, both positive and negative at immunohistochemistry, with a visible lumen or well-defined linear vessel shape. An unpaired Student's t-test was performed to verify any differences in MVD and capillary density in the AP between adults and newborns/infants. P < 0.05 was considered to be statistically significant.
Results
In sections of the medulla of adult subjects stained with anti-vWF, all vessels showed an intense reaction of endothelial cells. Thus, for detecting vascular structures, immunohistochemistry with anti-vWF proved as effective as staining with anti-CD31 (Porzionato et al. 2004).
The one-way anova revealed that the differences in MVD among the nuclei were statistically significant (P < 0.001). The DMVN showed the highest value of MVD, corresponding to 149 ± 84 mm−2. Tukey's test revealed significant differences when comparing this value to that for STN (97 ± 32 mm−2, P < 0.001), ST (59 ± 21 mm−2, P < 0.001) and XII (61 ± 25 mm−2, P < 0.001). The difference in MVD between DMVN and AP (123 ± 36 mm−2) was not statistically significant (P > 0.05).
The MVD in the AP was higher than in ST (P < 0.001) and XII (P < 0.001). The difference in MVD between AP and STN was not statistically significant (P > 0.05). Within the context of the AP, clusters of vessels grouped together were frequently found. The AP showed a capillary density of 48 ± 26 mm−2, corresponding to a percentage of capillaries of 45 ± 18%.
Differences in MVD between STN and ST (P < 0.05) and XII (P < 0.05) were statistically significant. However, there was no statistically significant difference in the MVD between ST and XII (P > 0.05) (Fig. 2).
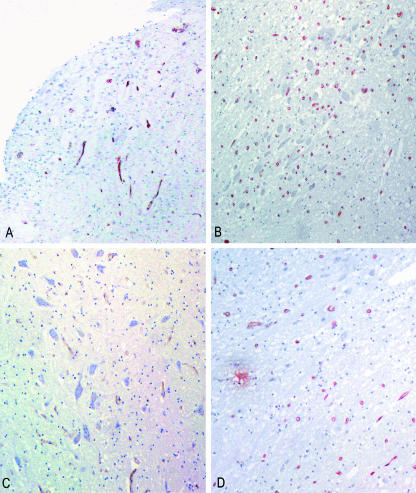
Fig. 2.
Sections of medullary tegmentum from an adult subject, stained immunohistochemically for von Willebrand factor, showing higher microvessel density in area postrema (A, ×16) and dorsal motor vagal nucleus (B, ×16) with respect to hypoglossal nucleus (C, ×16) and solitary tract nucleus (D, ×16).
No significant statistical correlation between MVD and age was found.
In sections of medulla of newborn/infant subjects, staining with anti-vWF was weaker and only a few vessels showed positive reaction. Rare, isolated vessels were stained in STN, ST, DMVN and XII. The AP showed the highest percentage of positivity, with 41 ± 21% of vessels stained at immunohistochemistry (Fig. 3). Immunopositivity in capillaries was significantly less (34 ± 19%) than that in non-capillaries (48 ± 25%).
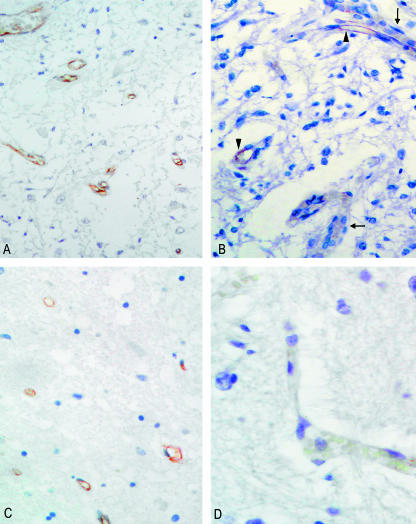
Fig. 3.
(A) Section of area postrema of an adult subject, stained with anti-von Willebrand factor, showing positive reaction of all vessels (×20). (B) Area postrema of an infant subject, showing coexistence of positive (arrowheads) and negative (arrows) vessels (×25). (C) Section of solitary tract nucleus of adult, with positive staining of all vascular structures (anti-von Willebrand factor, ×25). (D) Vessel in solitary tract nucleus of infant, showing negative staining at immunohistochemistry (anti-von Willebrand factor, ×40).
The MVD in the AP of newborn/infant subjects was 114 ± 50 mm−2. Moreover, the AP showed a capillary density of 55 ± 32 mm−2, corresponding to a percentage of capillaries of 50 ± 15%. There were no statistically significant differences between adult and newborn in either the MVD or the capillary density in the AP.
Discussion
Microvascular specializations and differences in capillary densities have been observed in the dorsal medulla of rats (Gross et al. 1990, 1991; Gross, 1991; Shaver et al. 1991). Shaver et al. (1991) found that the AP had higher capillary density than STN and DMVN, and also that the dorsal and medial zones of AP had higher values of capillary density than lateral or ventral zones. In the STN, higher capillary density was found in the medial subnuclei with respect to dorsal strip subnuclei and commissural subnucleus (Gross et al. 1991; Shaver et al. 1991). In addition, the microvessels of the medial complex of the STN showed fenestrated capillaries with wide Virchow–Robin spaces, suggesting specialized microvascular mechanisms increasing the blood-sensing properties of STN (Gross, 1991).
In our study, heterogeneity in MVD among nuclei of dorsal medulla was found, partially in analogy to findings in rats. DMVN and AP showed higher values of MVD with respect to XII and ST. MVD of the AP was in agreement with the value obtained in a previous study with anti-CD31 immunohistochemistry (114.5 mm−2) (Porzionato et al. 2004). STN showed lower MVD with respect to DMVN and higher MVD with respect both to ST and to XII.
This microvessel heterogeneity may be ascribed to the different metabolic demands of the nervous structures involved or to particular functions of microvasculature in blood–tissue exchanges. Although Gjedde & Diemer (1985) stated that brain capillaries are related to metabolic demands, in that they are basic units with the same characteristics in all parts of the nervous system, the differences in the microvasculatures of the nuclei of the dorsal medulla found in our study were also related to their differing functions. Thus the high MVD of AP and STN may be ascribed to the role of microvasculature in favouring the local action of neuropeptides and in blood monitoring. Instead, differences in MVD between DMVN and XII are ascribed to differing metabolic demands. In particular, the higher relative MVD of DMVN with respect to that in rat indicates the peculiarity of human medullary microvascularization.
Between adult and newborn/infant subjects, no statistically significant differences were found in MVD or percentage of capillaries. Developmental changes in the capillary densities in various brain structures have been studied in rat (Zeller et al. 1996). A significant increase was found in most grey matter districts between the 10th and 20th postnatal days, in relation to increased functional activity. The homogeneity of microvessel and capillary densities in the AP of newborn/infant and adult subjects found here is ascribed to its early initial development.
With regard to hypo-expression of vWF by endothelial cells in newborn/infant subjects, Tonnesen et al. (1985) found that expression of vWF was scant in the blood vessels of fetal skin, bright but focal in newborns, and intense and almost confluent in adults. On the basis of these findings, vWF was considered to be a marker of endothelial cell differentiation. Expression of this protein has also been studied in neovessels of neoplastic tissues that are not completely differentiated. Sawada et al. (1988) and Unger et al. (2002) reported that vWF is poorly expressed in proliferating immature endothelial cells of brain tumours and ascribed this finding partly to the microenvironment of brain tumours. Zeller et al. (1996) reported developmental changes in endothelial cell gene expression in rat AP, with decreasing Glut1 staining at the 5th and 10th postnatal days. In our newborn/infant cases, the almost absent expression of vWF in the STN, DMVN and XII indicates that this protein is normally expressed in a later period. It may be hypothesized that the process of morphological and functional differentiation of microvasculature in the human central nervous system extends, at least as regards some characteristics, beyond fetal stages in the first months of life.
Besides varying in relation to vascular maturation, expression of vWF has also been shown to vary between differing blood vessels and vascular beds. For instance, it is highly expressed in venules with respect to arterioles and, conversely, is less expressed in hepatic and splenic sinusoids (Aird et al. 1997). In our newborn/infant cases, vWF was differently expressed in the AP, where 41 ± 21% of vessels stained positively, and in tegmental nuclei, where rare, isolated vessels stained. The capillary vascular bed also showed a percentage of positivity that was statistically insignificant with respect to non-capillaries. This finding supports heterogeneity in vWF gene regulation in endothelial cells of various regions and vascular beds. It has recently been proposed that tissue-specific endothelial cell gene expression is controlled by multiple signalling pathways arising from the microenvironment and interacting with intracellular transcriptional networks (Aird et al. 1997; Ribatti et al. 2002; Scavelli et al. 2004). Further studies should evaluate how the microenvironment of the AP, with its peculiarities among the districts of the dorsal medulla, may influence (besides being influenced by) the morpho-functional characteristics of the microvasculature.
Acknowledgments
We are grateful to Giuliano Carlesso for his skilful technical assistance.
References
- Aird WC, Edelberg JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J. Cell. Biol. 1997;138:1117–1124. doi: 10.1083/jcb.138.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MA, Smith PM, Ferguson AV. Adrenomedullin microinjection into the area postrema increases blood pressure. Am. J. Physiol. 1997;272:R1698–R1703. doi: 10.1152/ajpregu.1997.272.6.R1698. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius – gateway to neural circulatory control. Ann. Rev. Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Brizee KR, Klara PM. The structure of the mammalian area postrema. Fed. Proc. 1984;43:2944–2948. [PubMed] [Google Scholar]
- Friedland DR, Eden AR, Laitman JT. Naturally occurring motoneuron cell death in rat upper respiratory tract motor nuclei: a histological, fast DiI and immunocytochemical study in the hypoglossal nucleus. J. Neurobiol. 1995;27:520–534. doi: 10.1002/neu.480270407. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Diemer NH. Double-tracer study of the fine regional blood-brain glucose transfer in the rat by computer-assisted autoradiography. J. Cereb. Blood Flow Metab. 1985;5:282–289. doi: 10.1038/jcbfm.1985.36. [DOI] [PubMed] [Google Scholar]
- Gross PM, Sposito NM, Pettersen SE, Fenstermacher JD. Differences in function and structure of the capillary endothelium in gray matter, white matter and a circumventricular organ of rat brain. Blood Vessels. 1986;23:261–270. doi: 10.1159/000158652. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am. J. Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Gross PM. Morphology and physiology of capillary systems in subregions of the subfornical organ and area postrema. Can. J. Physiol. Pharmacol. 1991;69:1010–1025. doi: 10.1139/y91-152. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Wainman DS, Shaver SW. Subregional topography of capillaries in the dorsal vagal complex of rats. II. Physiological properties. J. Comp. Neurol. 1991;306:83–94. doi: 10.1002/cne.903060107. [DOI] [PubMed] [Google Scholar]
- Macchi V, Snenghi R, De Caro R, Parenti A. Monolateral hypoplasia of the motor vagal nuclei in a case of sudden infant death syndrome. J. Anat. 2002;200:195–198. doi: 10.1046/j.0021-8782.2001.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Davern P, et al. The sensory circumventricular organs of the mammalian brain. Adv. Anat. Embryol. Cell Biol. 2003;172:1–122. doi: 10.1007/978-3-642-55532-9. [DOI] [PubMed] [Google Scholar]
- McRitchie DA, Tork I, Rikard-Bell GC, Paxinos G. Autonomic regulatory centers in the medulla oblongata. In: Paxinos G, editor. The Human Nervous System. New York: Harcourt Brace. Jovanovich Publishers; 1990. pp. 221–259. [Google Scholar]
- Nakayama Y, Takano Y, Eguchi K, et al. Modulation of the arterial baroreceptor reflex by the vasopressin receptor in the area postrema of the hypertensive rats. Neurosci. Lett. 1997;226:179–182. doi: 10.1016/s0304-3940(97)00274-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF. Atlas of the Human Brainstem. San Diego: Academic Press; 1995. [Google Scholar]
- Porzionato P, Macchi V, Parenti A, De Caro R. The distribution of mast cells in the human area postrema. J. Anat. 2004;204:141–147. doi: 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A, Roncali L, Dammacco F. Endothelial cell heterogeneity and organ specificity. J. Hematother. Stem Cell Res. 2002;11:81–90. doi: 10.1089/152581602753448559. [DOI] [PubMed] [Google Scholar]
- Roth GI, Yamamoto WS. The microcirculation of the area postrema in the rat. J. Comp. Neurol. 1968;133:329–340. doi: 10.1002/cne.901330304. [DOI] [PubMed] [Google Scholar]
- Sander GE, Lowe RF, Given MB, Giles TD. Interaction between circulating peptides and the central nervous system in hemodynamic regulation. Am. J. Cardiol. 1989;64:44–50. doi: 10.1016/0002-9149(89)90683-8. [DOI] [PubMed] [Google Scholar]
- Sawada T, Nakamura M, Sakurai I. An immunohistochemical study of neovasculature in human brain tumors. Acta Pathol. Jpn. 1988;38:713–721. doi: 10.1111/j.1440-1827.1988.tb02343.x. [DOI] [PubMed] [Google Scholar]
- Scavelli C, Weber E, Aglianò M, et al. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J. Anat. 2004;204:433–449. doi: 10.1111/j.0021-8782.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver SW, Sposito NM, Gross PM. Quantitative fine structure of capillaries in subregions of the rat subfornical organ. J. Comp. Neurol. 1990;294:145–152. doi: 10.1002/cne.902940111. [DOI] [PubMed] [Google Scholar]
- Shaver SW, Pang JJ, Wall KM, Sposito NM, Gross PM. Subregional topography of capillaries in the dorsal vagal complex of rats. I. Morphometric properties. J. Comp. Neurol. 1991;306:73–82. doi: 10.1002/cne.903060106. [DOI] [PubMed] [Google Scholar]
- Szilagyi JE, Ferrario CM. Central opiate system modulation of the area postrema pressor pathway. Hypertension. 1981;3:313–317. doi: 10.1161/01.hyp.3.3.313. [DOI] [PubMed] [Google Scholar]
- Tonnesen MG, Jenkins D, Jr, Siegal SL, Lee LA, Huff JC, Clark RA. Expression of fibronectin, laminin, and factor VIII-related antigen during development of the human cutaneous microvasculature. J. Invest. Dermatol. 1985;85:564–568. doi: 10.1111/1523-1747.ep12277410. [DOI] [PubMed] [Google Scholar]
- Unger RE, Oltrogge JB, von Briesen H, et al. Isolation and molecular characterization of brain microvascular endothelial cells from human brain tumors. In Vitro Cell. Dev. Biol. Anim. 2002;38:273–281. doi: 10.1290/1071-2690(2002)038<0273:IAMCOB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zeller K, Vogel J, Kuschinsky W. Postnatal distribution of Glut1 glucose transporter and relative capillary density in blood–brain structures and circumventricular organs during development. Dev. Brain Res. 1996;91:200–208. doi: 10.1016/0165-3806(95)00177-8. [DOI] [PubMed] [Google Scholar]