Abstract
We investigated the development of cartilage canals to clarify their function in the process of bone formation. Cartilage canals are tubes containing vessels that are found in the hyaline cartilage prior to the formation of a secondary ossification centre (SOC). Their exact role is still controversial and it is unclear whether they contribute to endochondral bone formation when an SOC appears. We examined the cartilage canals of the chicken femur in different developmental stages (E20, D2, 5, 7, 8, 10 and 13). To obtain a detailed picture of the cellular and molecular events within and around the canals the femur was investigated by means of three-dimensional reconstruction, light microscopy, electron microscopy, histochemistry and immunohistochemistry [vascular endothelial growth factor (VEGF), type I and II collagen]. An SOC was visible for the first time on the last embryonic day (E20). Cartilage canals were an extension of the vascularized perichondrium and its mesenchymal stem cell layers into the hyaline cartilage. The canals formed a complex network within the epiphysis and some of them penetrated into the SOC were they ended blind. The growth of the canals into the SOC was promoted by VEGF. As the development progressed the SOC increased in size and adjacent canals were incorporated into it. The canals contained chondroclasts, which opened the lacunae of hypertrophic chondrocytes, and this was followed by invasion of mesenchymal cells into the empty lacunae and formation of an osteoid layer. In older stages this layer mineralized and increased in thickness by addition of further cells. Outside the SOC cartilage canals are surrounded by osteoid, which is formed by the process of perichondral bone formation. We conclude that cartilage canals contribute to both perichondral and endochondral bone formation and that osteoblasts have the same origin in both processes.
Keywords: bone formation, cartilage canals, chicken, electron microscopy, immunohistochemistry
Introduction
Cartilage canals are tubes of vascularized mesenchyme that are present in bones of vertebrates. Several studies have shown that they are formed during embryonic development in mammals (Burkus et al. 1993; Ganey et al. 1995; Rivas & Shapiro, 2002; Doschak et al. 2003). They contain a central arteriole that divides into several capillaries at the end from which a single venule passes back to the perichondrium (Wilsman & Van Sickle, 1972). According to Haines (1933) there is no doubt that they have a role in the nutrition of the cartilage and the elimination of waste products. In addition, several authors (Wilsman & Van Sickle, 1970; Claassen et al. 1996; Roach et al. 1998) have claimed that calcification of the cartilage matrix starts near the ends of cartilage canals, but this was questioned by Kimpel et al. (1999) on the basis of thyroid cartilage of Munich minipigs where no relationship between these canals and the beginning of mineralization was found. However, many authors have considered that in mammals cartilage canals provided stem cells that might contribute to the osteogenesis in the secondary ossification centre (SOC) (Wilsman & Van Sickle, 1970, Wilsman & Van Sickle, 1972; Kugler et al. 1979; Agrawal et al. 1984, 1986; Burkus et al. 1993; Ganey et al. 1995; Shapiro, 1998; Fritsch et al. 2001; Rivas & Shapiro, 2002) although clear evidence was never provided. Surprisingly, in the human distal phalangeal and proximal metacarpal epiphyses, cartilage canals are present, although an ossification centre is lacking (Soames, 1995). Other studies have suggested that cartilage canals may be involved in perichondral (intramembranous) bone formation (Gray & Gardner, 1969; Chandraraj & Briggs, 1988) and recently Ytrehus et al. (2004a, b) have shown that cartilage canals regress with age in the distal femur of pigs. All these studies are controversial and the exact role of cartilage canals is insufficiently understood so far (Roach et al. 1998).
In chicken several investigations (Lutfi 1970a,b; Doménech-Ratto et al. 1999, Blumer et al. 2004a, b) have indicated that the epiphysis of the femur and the tibia is well supplied with vascularized cartilage canals and hence the situation in avian is comparable with that in mammals. In chicken long bones the formation of the canals starts in early embryonic development and Doménech-Ratto et al. (1999) claimed that the only SOC in the avian skeleton occurs in the proximal epiphysis of the tibia at the 56th day after hatching. They assumed that the presence of cartilage canals is not necessarily associated with an SOC because canals also appear in the distal epiphysis of the femur, but here no ossification centre is found. However, these results are questionable when compared with those of mammals, where in most cases an SOC is present after cartilage canals have been formed (Wilsman & Van Sickle, 1970; Kugler et al. 1979; Cole & Cole, 1989; Visco et al. 1990; Burkus et al. 1993; Ganey et al. 1995; Shapiro, 1998; Rivas & Shapiro, 2002).
In the embryonic chicken femur it has also been shown that several so-called communicating cartilage canals pass through the proliferative zone of the epiphysis deeply into the hypertrophic zone of the diaphysis (Blumer et al. 2004a, b). At embryonic day 20 (E20) the lower segments of these canals bear no vessels and are not connected with processes of the bone marrow at E20 (Blumer et al. 2004b). They concluded that communicating canals contribute to bone formation within the primary ossification centre (POC).
In the present study we examined whether an SOC occurs in the distal epiphysis of the femur and we wished to clarify the function of cartilage canals when such a centre appears. Furthermore, we examined the fate of the communicating canals within the POC. We used different techniques [light microscopy, electron microscopy, histochemistry, immunohistochemistry and three-dimensional (3D) reconstruction] and compared our data with those for mammals.
Materials and methods
We studied the distal limbs of chicken (Gallus gallus) femurs. Embryonic chicken (E20) (Hamburger Hamilton Stage 45) and developmental stages after hatching (D2, 5, 7, 8, 10 and 13) were used. Chickens were killed with CO2, and subsequently legs were amputated and sectioned longitudinally. Fourteen individuals were used in this study.
Light and transmission electron microscopy (TEM)
The bones were fixed in 2.5% glutaraldehyde, 2% paraformaldehyde buffered in sodium cacodylate (0.1 m), pH 7.4, for 4 h at room temperature and rinsed in the same buffer. They were post-fixed in 0.5% osmium tetroxide, 1% potassium hexacyanoferrat III in distilled water overnight at 4 °C, rinsed in distilled water and decalcified in 3% ascorbic acid in sodium chloride (0.15 m) for 12 h at 4 °C. This was followed by dehydration in graded ethanol series and embedding in Spurr's epoxy resin. Ribbons of semithin sections (2 µm) were made on a Reichert Ultracut S microtome (Leica Microsystem, Wetzlar, Germany) with a histo-jumbo-diamond knife (Diatome, Biel, Switzerland) (Blumer et al. 2002) and stained with toluidine blue. Complete series of ultrathin sections (80 nm) were cut on a Reichert Utracut S microtome with an ultra-diamond knife, mounted on dioxan-formvar-coated copper slot-grids and stained with an aqueous solution of uranyl acetate (1%) for 30 min at 20 °C followed by lead citrate for 3 min at 20 °C. All ultrathin sections were examined with an electron microscope (10A, Zeiss, Oberkochen, Germany).
For histology, bones were fixed with 4% paraformaldehyde in PBS (0.1 m), rinsed in the same buffer and decalcified as described before. Subsequently, the bones were dehydrated in graded isopropanol and xylene series and embedded in paraffin. Serial sections (6 µm) were made on an HM 355S microtome (Microm, Walldorf, Germany) and stained with Trichrome. For histochemistry, Mayer's haematoxylin/Alcian blue/Sirius red (after Lison, 1954) was used as a nuclear stain and to distinguish bone matrix (red) from cartilage matrix (blue).
Immunohistochemistry
Immunohistochemistry (type I collagen) was carried out on paraffin sections and resin-embedded semithin sections (2 µm). Immunodetection of type II collagen and vascular endothelial growth factor (VEGF) was only performed on paraffin sections. Bones were fixed with 4% paraformaldehyde in PBS (0.1 m), rinsed in the same buffer, decalcified, dehydrated and embedded in paraffin or Spurr's epoxy resin as described above. Immunohistochemistry on paraffin sections as well as on resin sections was performed at 37 °C on a Ventana, Nexes IHC platform. Histological sections were deparaffinized and rinsed in PBS. Resin sections were pretreated with 3% NaOH in 100% ethanol for 2 min to dissolve the resin and were then rinsed in PBS. Rabbit anti-human VEGF (1 : 300) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-human collagen type I (1 : 100) (LF-67 from Prof. L. Fisher) and mouse anti-chicken type II collagen ready-to-use antibody (Biocarta, San Diego, CA, USA) were used. The Nexes detection protocol comprised a proteolytic digestion step using protease 1 (Ventana, Strasbourg, France), saturation of unspecific sites with 10% normal goat serum (NGS), primary antibody incubation, biotinylated secondary antibody incubation, application of streptavidin–horseradish peroxidase (SA-HRP) and chromogenic detection using the diaminobenzidine (DAB) detection kit. Sections were counterstained with haematoxylin.
Double labelling
Double labelling was carried out on paraffin sections with antibodies against type I and type II collagen. For type I collagen we used the same protocol as described above but without counterstaining. After washing, sections were incubated with mouse anti-chicken type II collagen, rinsed and the biotinylated secondary antibody was applied to the slides. Visualization of the antigen–antibody complex was obtained by incubation with Streptavidin Alkaline Phosphatase and Fast Red. Sections were counterstained with haematoxylin.
All control incubations without application of the primary antibody yielded no labelling.
Three-dimensional reconstruction
The distal limb of the femur (D2) was cut longitudinally and only the portion with the SOC was reconstructed. Ribbons of semithin cross-sections were made as described above and stained with toluidine blue. Every tenth semithin section was photographed as a colour image using a Zeiss AxioCam HR (Zeiss) running on a Pentium 4 (Intel Inc., Santa Cruz, CA, USA) with WindowsXP (Microsoft Inc., Redmond, WA, USA). The software package Axio-Vision 4.1 served to modulate image acquisition. Three-dimensional reconstruction was via an Apple Macintosh iBook (Apple, Cupertino, CA, USA). The files were converted into PICT format with GraphicConverter 4.9 (Lemke Software, Peine, Germany) and imported into SurfDriver 3.5 (Surfdriver, Kailua, Hawaii, USA). The covering structures were set quasi-transparent to improve the visibility of the cartilage canals. Post-processing of the 3D reconstruction created was via Adobe PhotoShop 6.0 (Adobe Inc., San Jose, CA, USA).
Results
Light microscopy and TEM
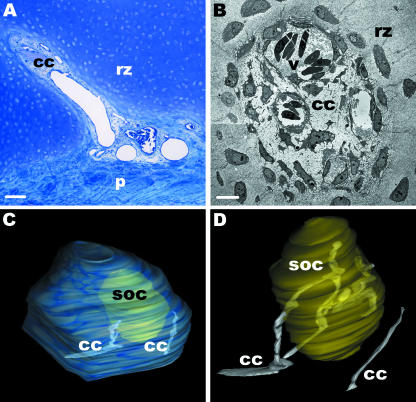
In late embryonic development (E20) and early postnatal phases (D2, 5, 7, 8, 10 and 13), cartilage canals formed a complex network within the reserve zone of the epiphysis. These canals were invaginations from the perichondrium (Fig. 1A). They were branched and contained vessels that were surrounded by numerous mesenchymal cells. Ultrastructural findings showed that the matrix of the canals was electron translucent and could be clearly differentiated from the remaining electron-dense cartilage matrix. No epithelium was elaborated around the canals (Fig. 1B). An SOC was present in the epiphysis and some canals passed into the hypertrophic zone of this centre, whereas the majority of the canals surrounded it or passed down into the hypertrophic zone of the diaphysis. Three-dimensional reconstructions based on complete series of semithin cross-sections through the whole epiphysis showed that canals within the SOC were connected with each other (Fig. 1C,D).
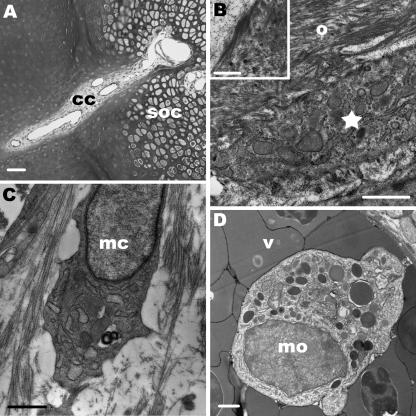
Fig. 1.
(A)Light microscopy. Semithin section through the epiphysis of the femur (D2). Cartilage canals originate from the perichondrium (p) and penetrate into the reserve zone (rz) of the cartilage matrix. Scale bar, 50 µm. (B) TEM. Cross-section through a cartilage canal (cc) of the reserve zone (rz). The canal contains mesenchymal cells and several vessels (v) (D2). The canal matrix is electron translucent whereas the cartilage matrix is electron dense. Note that no epithelium is formed around the canal. Scale bar, 5 µm. (C,D) The panels show three-dimensional images of the course of cartilage canals within the epiphysis at D2. We have reconstructed cartilage canals which enter the secondary ossification centre (SOC) and one canal which ends blind within the epiphysis. (C)An overview is shown.(D)The course of cartilage canals is shown in detail. The two cartilage canals which enter the SOC originate from one canal. Within the SOC the canals are connected with each other and additionally further ramification is detectable.
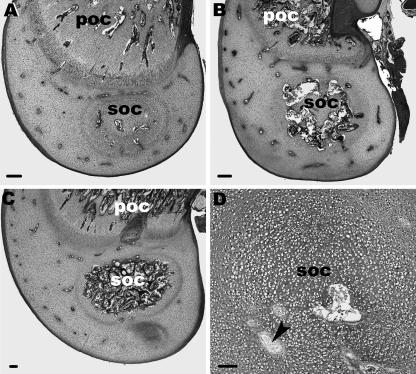
Dring postnatal development the SOC enlarged, and cartilage canals formed a large cavity within the SOC (Fig. 2A–C). The future SOC was visible in the last embryonic stage E20 as a nidus of hypertrophic cells with only one cartilage canal incorporated within it (Fig. 2D). At this embryonic stage the matrix around the canals showed no indication of calcification. Note that in two specimens (E20 and D5) no SOC was present although the epiphysis was rich in cartilage canals.
Fig. 2.
Light microscopy. (A–D) Longitudinal sections through the femur of different developmental stages show that numerous cartilage canals are located within the epiphysis. Additionally, the POC and the SOC are seen. (A) At D2 the ends of cartilage canals within the SOC are slightly enlarged.(B) At D7 this enlargement has increased. (C) At D10 the SOC has markedly increased and cartilage canals form a large cavity. Scale bars, 200 µm. (D) At E20 the SOC is very small and contains only one cartilage canal. The arrowhead points to a cartilage canal located outside the SOC. Scale bar, 100 µm.
Cartilage canals that penetrate into the SOC (D2, 7, 8, 10 and 13)
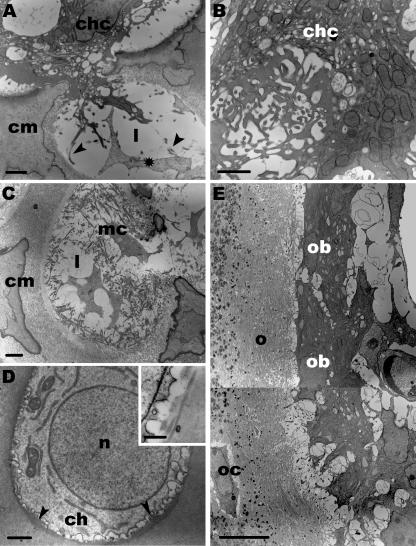
These canals were composed of two segments, which were distinguishable by their ultrastructural characteristics. The segments inside the SOC ended blind with capillaries forming glomerulus-like structures. The cartilage matrix adjacent to the canals was calcified, but further away from the canals no sign of mineralization was detectable at D2. Monocytes and multinucleated chondroclasts were embedded in the canal matrix. The latter cells opened the lacunae of hypertrophic chondrocytes and remnants of these cells were found within the lacunae (Fig. 3A). Only a few chondroclasts were present at D2. They contained clear vesicles, lysosomes, numerous mitochondria and their cell surface was elaborated to several cytoplasmic processes (Fig. 3B). Furthermore, we found mesenchymal cells that invaded the opened lacunae (Fig. 3C). A striking feature was that hypertrophic chondrocytes adjacent to the cartilage canals showed an amorphous layer inside their intact lacunae. This layer contained type I collagen fibrils (Fig. 3D). It should be emphasized that in the lacunae of hypertrophic chondrocytes further away from the canals no type I collagen was seen and the cells themselves appeared to degenerate. At D2 parts of the canals were already lined by newly formed osteoid, which was rich in collagen I and showed mineralization foci. Osteocytes were embedded in this extracellular matrix and numerous mesenchymal cells of the canals bordered on it like a string of pearls. The latter cells were interpreted as osteoblasts. They were arranged close to each other, were irregularly shaped and were characterized by a large amount of endoplasmic reticulum (Fig. 3E).
Fig. 3.
TEM. Segments of cartilage canals within the SOC are shown at D2. (A) A chondroclast (chc) opens the lacuna (l) of a hypertrophic chondrocyte. Within the lacuna type I collagen fibrils (arrowheads) and the remnant of a hypertrophic chondrocyte (asterisk) is visible. The surrounding cartilage matrix (cm) is calcified. Scale bar, 1 µm. (B) The margin of a chondroclast (chc) is differentiated into numerous cytoplasmic processes. In addition, the cytoplasm contains numerous mitochondria and clear vesicles. Scale bar, 1 µm. (C) A mesenchymal cell (mc) which derives from the cartilage canal enters the empty lacunae (l) in which type I collagen fibrils are detectable. Scale bar, 1 µm. (D) Hypertrophic chondrocyte (ch) adjacent to a cartilage canal. The nucleus (n) is spherical. At the margin of the lacuna an electron-dense layer is seen (arrowheads). Scale bar, 1 µm. The inset shows a higher magnification of this layer. Type I collagen fibrils with their typical banding pattern are present. Scale bar, 0.5 µm. (E)At the edge of a cartilage canal numerous densely arranged osteoblasts (ob) are found. Adjacent to these cells the osteoid layer (o) is mineralized and osteocytes (oc) are embedded within this matrix. Scale bar, 5 µm.
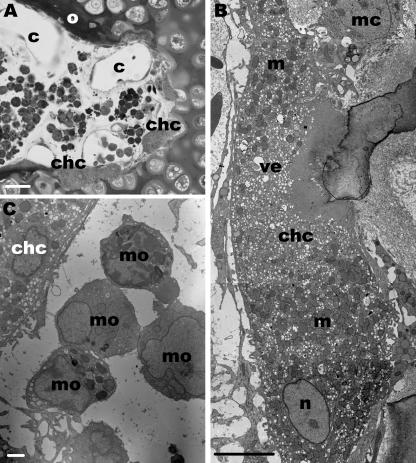
At D7, 8, 10 and 13 the structural events of these segments did not change with the exception that multinucleated chondroclasts were more numerous (Fig. 4A,B). These cells were resorbing the calcified cartilage matrix and hence widening the ends of the canals. Additionally monocytes – occasionally densely arranged – were abundant within the canals (Fig. 4C). These cells were spherical and contained numerous lysosomes and mitochondria. The layer of osteoid lining the cartilage canals was more pronounced in these developmental stages. Hypertrophic chondrocytes adjacent to cartilage canals showed the same ultrastructural features as described before. At D7 the cartilage matrix within the SOC was completely mineralized.
Fig. 4.
Segments of cartilage canals within the SOC are shown at D7 and 10. (A) Light microscopy. Cross-section through the terminal part of a cartilage canal which contains several capillaries (c). At the interface between the canal and the calcified cartilage matrix chondroclasts (chc) open the lacunae of hypertrophic chondrocytes. Parts of the canal are surrounded by newly formed osteoid (o). Scale bar, 20 µm. (B)TEM. A chondroclast (chc) with numerous mitochondria (m) and clear vesicles (ve) is located adjacent to the calcified cartilage matrix and a mesenchymal cell (mc). The chondroclast contains numerous nuclei (n) but in this section only one is present. Scale bar, 5 µm. (C) TEM. Monocytes (mo) which form a cluster are embedded in the canal matrix. These cells are located close to a chondroclast (chc). Scale bar, 1 µm.
The segments outside the SOC were surrounded by flat cells and by a dense layer of type I collagen fibrils (osteoid). At D13 the flat cells were completely trapped within this osteoid layer (Fig. 5A,B). The cartilage matrix around the canals showed no signs of calcification in all stages. Vessels, mesenchymal cells and bundles of type I collagen were present within the canals. Endoplasmic reticulum was abundant in the mesenchymal cells and their cell surface was irregularly shaped (Fig. 5C). In addition to blood cells, vessels contained monocytes that had the same ultrastructural features as described above (Fig. 5D). The segments were successively incorporated into the SOC during proceeding postnatal development during which the SOC enlarged.
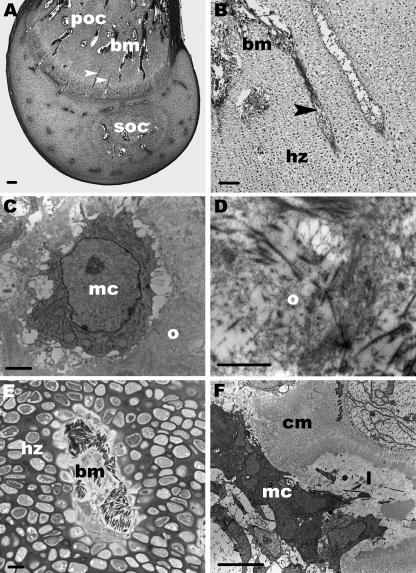
Fig. 5.
(A)Light microscopy. Semithin longitudinal section through a cartilage canal (cc) which transports mesenchymal stem cells into the SOC. Scale bar, 50 µm. The following micrographs show segments of the canals which are located outside the SOC.(B) TEM. Longitudinal section through the edge of a canal. At D13 these segments are surrounded by a dense layer of type I collagen fibrils or osteoid (o) and by flat cells (asterisk). Scale bar, 1 µm. Inset: higher magnification of the type I collagen fibrils. Scale bar, 0.5 µm. (C)TEM. Bundles of type I collagen are also present within these segments (D7). Scale bar, 1 µm. (D)TEM. Oblique section through a vessel (v) which contains a monocyte (mo) (D2). Scale bar, 1 µm.
Cartilage canals that did not penetrate into the SOC (D2, 7, 8, 10 and 13)
These canals had the same ultrastructural features as described above and several of them terminated blind either within the reserve zone or within the proliverative zone of the epiphysis without having any contact with the SOC. Other canals passed from epiphysis down into the hypertrophic zone of the diaphysis where they were connected with processes of the bone marrow (Fig. 6A,B). These communicating canals were not branched and were orientated in parallel to the longitudinal axis of the bone. The lower segment of the communicating canals contained no vessels and was exclusively composed of mesenchymal cells trapped in a type I collagen-rich matrix (Fig. 6C,D).
Fig. 6.
(A,B) Light microscopy. Longitudinal section through the femur (D2).(A) Arrowheads point to communicating cartilage canals which pass down from the epiphysis into the hypertrophic zone of the POC. Bone marrow processes (bm) penetrate up into the proliferative zone. Scale bar, 200 µm. (B) The hypertrophic zone (hz) is shown. A communicating canal (arrowhead) is connected with a bone marrow process (bm). Scale bar, 100 µm. (C,D) TEM. Cross-sections through the lower segment of a communicating canal (D2 and 7). No vessels are present in this part and the canal is composed of mesenchymal cells (mc) and osteoid (o) which is rich in type I collagen. Scale bar, 1 µm. (E) Light microscopy. Semithin cross-section through the hypertrophic zone (hz) and a bone marrow process (bm). At the border of this process the lacunae of hypertrophic cells are opened. Scale bar, 20 µm. (F) TEM. The margin of a bone marrow process is shown and mesenchymal cells (mc) entering an empty lacuna (l). The neighbouring cartilage matrix (cm) is calcified. Scale bar, 5 µm.
Processes of the bone marrow (D2, 7, 8, 10 and 13)
At D2 the growth plate of the POC was very wide, with numerous cell layers of hypertrophic chondrocytes. The flattened chondrocytes of the proliferative zone were clearly arranged in columns and finger-shaped processes of the bone marrow extended up into this zone (Fig. 6A). Within the hypertrophic zone the cartilage matrix was mineralized adjacent to the bone marrow processes. At D7, 8, 10 and 13 the pattern of the marrow processes was more regular and the cell layers of the hypertrophic zone decreased. Processes of the bone marrow were composed of mesenchymal cells, monocytes, multinucleated chondroclasts and blood vessels. In all stages the cellular components were densely arranged in the processes. Furthermore, our findings indicated that the chondroclasts opened the lacunae of hypertrophic chondrocytes. Mesenchymal cells invaded these lacunae in which type I collagen was already present (Fig. 6E,F).
Histochemistry
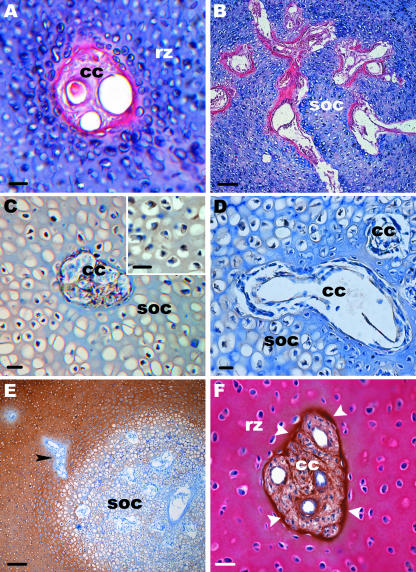
Staining with Sirius red was used to distinguish bone matrix (red) from cartilage matrix (blue). The staining indicated that a layer of bone-like matrix was elaborated around cartilage canals within the epiphysis (Fig. 7A). This layer was thicker in the SOC and around the processes of the bone marrow (Fig. 7B). In addition, the perichondrium and the lower ends of the communicating canals stained red. This was found in all stages.
Fig. 7.
Light microscopy. Histological sections through the epiphysis of different developmental stages are shown. (A) Cross-section through a cartilage canal (cc) within the reserve zone (rz). The section is stained with Sirius red. The canal is surrounded by a matrix which contains bone matrix components (D7). Scale bar, 20 µm. (B) Section through the SOC stained with Sirius red. Here the bone matrix around the canals is much thicker (D2). Scale bar, 100 µm. (C,D) Sections through the SOC. (C) At E20 cartilage canals (cc) and hypertrophic chondrocytes label for VEGF. Inset: a higher magnification of VEGF-positive hypertrophic chondrocytes. (D) At D2 and later postnatal stages no labelling was detectable. Scale bar, 20 µm. (E) An overview. Only weak labelling for type II collagen is visible in the SOC whereas the remaining cartilage matrix shows strong labelling. Cartilage canals are type II collagen negative. Arrowhead points to a canal which enters the SOC. Note that a distinct layer around this canal is also negative for type II collagen. Scale bar, 100 µm. (F) Immunostaining for type I and II collagen. Cross-section through a cartilage canal (cc) within the reserve zone (rz). The matrix of the canal (cc) and a distinct layer around the canal (arrowheads) labels for type I collagen (brown). This corresponds with E where this layer does not label for type II collagen. The cartilage matrix of the reserve zone (rz) labels strongly against type II collagen (red) (D7). Scale bar, 20 µm.
Immunohistochemistry
Immunodetection for VEGF was performed on paraffin sections (E20, D2, 7, 8, 10 and 13). The growth factor was observed in cartilage canals and in hypertrophic chondrocytes of the POC and SOC (Fig. 7C). This was found at E20 but not in the following developmental stages where cartilage canals as well as hypertrophic chondrocytes did not label for VEGF (Fig. 7D). Chondrocytes of the reserve zone and the proliferative zone were VEGF-negative in all stages.
Immunodetection for type II collagen was carried out on paraffin sections. Type II collagen was visible in the cartilage matrix of the reserve zone and the proliferative zone but only weak staining was detectable in the hypertrophic zone of both ossification centres. In addition, the matrix of the cartilage canals and a distinct layer around the canals was type II collagen-negative (Fig. 7E). Double labelling against type I (brown) and II collagen (now in red) clearly showed the distribution of these two types of collagen. Type I collagen was visible within the canals and in addition a distinct layer was present around the canals. In contrast, type II collagen was only present in the cartilage matrix (Fig. 7F). These results were found in all stages.
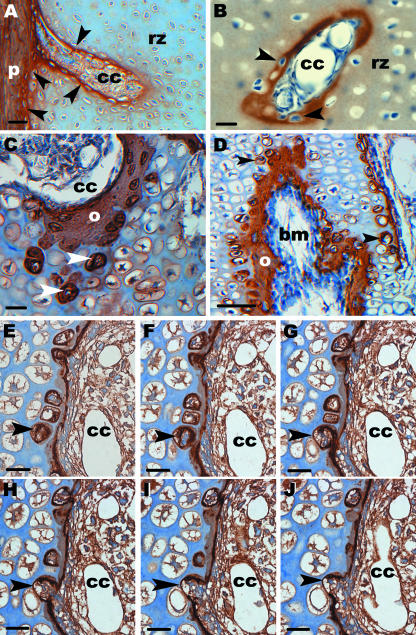
Immunohistochemistry for type I collagen was carried out on both resin and paraffin sections and all stages were examined. Resin sections were made with a histo-jumbo-diamond knife and had several advantages over paraffin sections made with a steal knife: the quality of sections improved, the sections had a thickness of only 2 µm and consequently could analysed in greater detail. The labelling pattern for cartilage canals was the same as described above and the type I collagen layer around the canals increased with development (Fig. 8A,B). It was always thicker inside the SOC than outside the SOC (Fig. 8C). Furthermore, a pronounced layer of type I collagen was detectable around bone marrow processes. (Fig. 8D). In addition, type I collagen was found in the lacunae of hypertrophic chondrocytes adjacent to the canals in the SOC (only in postnatal stages) and to the bone marrow processes in the POC (all stages). Series of consecutive semithin resin sections through the SOC showed that type I collagen-positive lacunae were opened from cells that derived from the canal (Fig. 8E–J). Type I collagen was not detectable in hypertrophic chondrocytes further from cartilage canals and bone marrow processes. Furthermore, the perichondrium, the periosteum and perichondral bone labelled positively for type I collagen.
Fig. 8.
Immunohistochemistry for type I collagen which is a major component of bone matrix is shown on both paraffin sections (B–D) and resin sections (A, E–J). (A) Longitudinal section through a cartilage canal (cc) within the reserve zone (rz) of the epiphysis (D2). The canal derives from the perichondrium (p). Note that the outer layer of the canal labels strongly for type I collagen and is composed of flat cells. This layer is a continuation of the inner layer of the perichondrium (arrowheads). Scale bar, 20 µm. (B) Cross-section through a cartilage canal (cc) within the reserve zone (rz). At D13 the thickness of the type I collagen layer around the canal has increased and cells (arrows) are embedded within this matrix. Scale bar, 20 µm. (C) Section through a cartilage canal (cc) within the SOC. A thick layer of early bone matrix (o) is elaborated. In the lacunae of hypertrophic chondrocytes bone matrix is also visible (arrowheads). Scale bar, 20 µm. (D) Longitudinal section through a bone marrow process (bm) within the POC (D2). Bone matrix is found around these processes and in addition in the lacunae of adjacent hypertrophic chondrocytes (arrowheads). In chondrocytes further from the bone marrow processes no labelling for type I collagen is seen. Scale bar, 50 µm. (E–J). Consecutive semithin sections through a cartilage canal (cc) within the SOC (D8). The sections show the interface between the canal and the hypertrophic zone. A layer of osteoid is already present around the canal. An arrowhead points to one hypertrophic chondrocyte. Type I collagen is already present in the lacuna (E–G) before it is opened, presumably by a multinucleated giant cell (H–J). Scale bar, 20 µm.
Table 1 shows the major events of femur bone development for embryonic stage E20 until D13 after hatching.
Table 1.
Summary of the major events in the development of the chicken femur
| E20 | Cartilage canals form a complex network in the distal epiphysis of the femur. Appearance of future SOC with one cartilage canal incorporated into it. Hypertrophic chondrocytes were VEGF positive. |
| D2 | Penetration of more canals into SOC. Bone formation initiated within SOC; canals lined by newly formed osteoid which has started to mineralize. |
| Type I collagen present in hypertrophic chondrocytes adjacent to the canals. Communicating canals which pass down into the hypertrophic zone of POC are connected with bone marrow processes. | |
| D7 | Cartilage matrix within SOC completely calcified. |
| E20–D13 | Osteoid layer around cartilage canals outside the SOC increases in thickness but without mineralization. |
Discussion
General observations
The present study focuses on the role of cartilage canals in bone formation in the developing chicken femur (E20, D2, 5, 7, 8, 10 and 13). The canals appear at E9, their number increases during embryonic development and at E20 a complex network is formed in the distal part of the femur (Doménech-Ratto et al. 1999; Blumer et al. 2004a). A SOC appears long after the formation of these canals. This was also found in the chicken tibia (Lutfi 1970a; Hunt et al. 1979) and in long bones of mammals (dogs, rabbits, pigs and humans) (Wilsman & Van Sickle, 1970; Kugler et al. 1979; Cole & Cole, 1989; Visco et al. 1990; Burkus et al. 1993; Ganey et al. 1995; Shapiro, 1998; Rivas & Shapiro, 2002; Doschak et al. 2003). Similarly, in the short bone without epiphysis, such as the human talus (Agrawal et al. 1984) and calcaneus (Agrawal et al. 1986), cartilage canals appear long before the endochondral ossification nucleus is formed. Only in rats do these chronological events diverge and cartilage canals occur shortly before the formation of A SOC (Kugler et al. 1979; Delgado-Baeza et al. 1991). Our findings contradict the view of Doménech-Ratto et al. 1999) who postulated that A SOC is only present in the avian tibia. We assume that there is variation in the time of appearance of such a centre because we found no SOC in the femur of two individuals (E20, D5). This was also detectable in the long bones of swine (Visco et al. 1990). Our data provide evidence that the cartilage matrix of the SOC starts to calcify around cartilage canals and confirm what has been shown in the humeral head of dogs (Wilsman & Van Sickle, 1970) and in long bones of swine (Visco et al. 1990). In chicken the calcification proceeds successively and at D7 the whole matrix of the SOC is mineralized.
Structure of cartilage canals
In the chicken femur cartilage canals contain arterioles, venules, capillaries and mesenchymal cells which are embedded in the canal matrix (Lutfi 1970a; Hunt et al. 1979; Doménech-Ratto et al. 1999; Blumer et al. 2004a, b). Additionally, our observations demonstrate that the matrix of the canals contains no type II collagen, whereas the surrounding cartilage matrix of the reserve and the proliferative zone is rich in this type of collagen. Thus, a demarcation between the canals and the surrounding cartilage matrix appears and ultrastructural data clearly show that no epithelium is elaborated around the canals. This is in contrast with previous findings in chicken (Lutfi 1970a, b; Doménech-Ratto et al. 1999) and rabbit (Ganey et al. 1995; Roach et al. 1998). In these studies it was stated that early and mature cartilage canals are surrounded by an epithelium but these results were only based on histological and immunohistochemical data, and not on ultrastructural observations. However, the present results and our previous data (Blumer et al. 2004a) demonstrate that no epithelium is visible around cartilage canals during bone development.
Shapiro (1998) postulated that the structural characteristics of cartilage canals are similar within vertebrates and that one function of the canals is to provide nutrients to the epiphyseal cartilage. This was also concluded by many other authors (Wilsman & Van Sickle, 1970, 1972; Lutfi 1970a; Hunt et al. 1979; Delgado-Baeza et al, 1991; Ganey et al. 1995; Shapiro, 1998; Fritsch et al. 2001; Rivas & Shapiro, 2002; Blumer et al. 2004a). Additionally, it was suggested that cartilage canals provide ossification centres (SOC in long bones and ossification nucleus in short bones) with mesenchymal cells (Wilsman & Van Sickle, 1970, 1972; Lutfi 1970a; Kugler et al. 1979; Agrawal et al. 1984, 1986; Visco et al. 1990; Burkus et al. 1993; Ganey et al. 1995; Fritsch et al. 2001) although this was never demonstrated.
The function of cartilage canals in bone formation
In studies on the relationship between cartilage canals and the SOC in mammals it was suggested that cartilage canals are a continuation of the perichondrium into the hyaline cartilage of the epiphysis (Wilsman & Van Sickle, 1970; Agrawal et al. 1984; Burkus et al. 1993; Ganey et al. 1995; Shapiro, 1998). This was also assumed in an investigation of the human thyroid cartilage (Claassen et al. 1996) and can be confirmed by the present data. Our data provide evidence that the perichondrium and the cartilage canals label positively for type I collagen and stain with Sirius red, indicating the presence of cells that are of the osteogenic cell line. This is in accordance with our ultrastructural data, which show bundles of type I collagen within and a dense matrix of type I collagen (osteoid) around the canals. Pechak et al. (1986a,b) demonstrated that in chicken the perichondrium is composed of an inner layer that has the characteristics of osteoprogenitor cells and an outer fibroblastic layer. Our data indicate that both layers can provide their mesenchymal stem cells, which migrate into and along the cartilage canals. Several cartilage canals penetrate into the SOC and multinucleated chondroclasts resorb the calcified cartilage matrix and hence open the lacunae of hypertrophic chondrocytes. We consider that the chondroclasts derive from monocytes which originate from blood vessels and are released into the canal matrix where consequently the multinucleated state is derived by cell fusion. The mesenchymal cells of the canals enter the free lacunae and form a layer of early bone which is deposited on the calcified cartilage around the canals. This is followed by mineralization of this layer, and the increase in bone girth in older postnatal stages is achieved by further addition of canal cells. During the radial growth of the SOC cartilage canals are successively incorporated into it. We conclude that cartilage canals are extensions of the perichondrium and that the cells of both layers can serve as a source of osteoblasts. Therefore, the present data demonstrate that the osteoblasts not only derive from the vessels of the canals as was suggested previously (Roach et al. 1998; Rivas & Shapiro, 2002). The cartilage canals deliver the latter cells into the SOC and it is clear that the canals are essential for its development and growth. We propose that the structural events found in chicken may also occur in the epiphysis of mammal long bones during the formation of an SOC.
The present findings also demonstrate that communicating canals pass down into the hypertrophic zone of the POC where they are connected with processes of the bone marrow. This connection is visible for the first time at D2 but could not be detected in late embryonic stages where communicating canals ended blind in the lower hypertrophic zone (E20) (Blumer et al. 2004b). The ultrastructure of the communicating canals was described in detail in our last study (Blumer et al. 2004b) and does not alter in postnatal stages. The lower segments of these canals are also composed of mesenchymal cells. which are trapped within a collagen I-rich matrix and thus the cells are interpreted as osteoblasts. These results strengthen our previous observations (Blumer et al. 2004b) that communicating cartilage canals contribute to bone formation within the POC.
In the perichondral bone formation of chicken, osteoblasts derive from the inner layer of the perichondrium and osteoid rich in type I collagen is laid down just outside the cartilage core. Later, the osteoid becomes mineralized and bone is formed (Pechak et al. 1986a,b). In the present study we show that osteoid is deposited on non-calcified cartilage around cartilage canals outside the SOC and that this extra cellular matrix is also delectable within the lower segments of communicating canals. It should be emphasized that chondroclasts are not present in these segments of the canals and hence the cartilage cannot be replaced by bone. Thus formation of the osteoid layer is analogous to the process of perichondral ossification. This was also suggested for the developing human humerus and vertebrae (Gray & Gardner, 1969; Chandraraj & Briggs, 1988) although the illustrations do not clearly show the bone matrix around the canals. Recently, it has been shown that in older developmental stages (several weeks) of the pig femur, cartilage canals undergo regression in which vessels, nerve fibres and mesenchymal stem cells within the canal are replaced by cartilage. This process is initiated at the distal ends of the canals (Ytrehus et al. 2004a, b). Consequently, these canals are not involved in bone formation. However, in the current study chondrification of cartilage canals was never observed and based on our data we conclude that cartilage canals contribute to both perichondral and endochondral bone formation depending on their position. Furthermore, our results provide evidence that the bone-forming cells originate from the perichondrium in both processes and thus their origin is identical.
We have also examined the ultrastructure and the immunohistochemical events of the bone marrow processes within the POC. These processes are composed of vessels, mesenchymal cells, monocytes and chondroclasts. In chicken it was clearly demonstrated that these vessels and the associated cells invade the cartilage core from the periosteum during embryonic development (Pechak et al. 1986a). The present study indicates that the calcified cartilage matrix is resorbed by chondroclasts and subsequently the empty lacunae of hypertrophic chondrocytes are occupied by mesenchymal cells. Resorption of calcified cartilage matrix by multinucleated giant cells was also shown by Roach & Shearer (1989) around bone marrow processes in the embryonic chicken tibia. Furthermore, some lacunae close to the bone marrow processes contain type I collagen. Thus, the morphological and immunohistochemical picture within the POC obviously does not differ from that as outlined for the SOC. Wilsman & Van Sickle (1970) and Fritsch et al. (2001) pointed out that the principal mechanisms of endochondral ossification are the same within the SOC and those observed at the metaphyseal side of the POC and this can be confirmed by the present study.
The function of hypertrophic cells
Hypertrophic chondrocytes produce VEGF and it was considered that synthesis of this growth factor stimulates the directional growth of vessels into the hypertrophic zone of the POC (Carlevaro et al. 2000; Petersen et al. 2002). Within the SOC hypertrophic chondrocytes and cartilage canals are VEGF positive at E20 but not in postnatal stages. Down-regulation in hypertrophic chondrocytes was also demonstrated by Carlevaro et al. (2000) in older embryonic stages of the mouse and chicken. At the late embryonic stage (E20) the future SOC is small and contains only one canal. At D2 the number of canals has increased and thus we conclude that the hypertrophic chondrocytes induce the invasion of further canals. This is consistent with the results from the femoral chondroepiphysis in rabbits where VEGF also plays an important role during vascular invasion and formation of the SOC (Doschak et al. 2003).
In bone-forming areas such as the SOC and the POC, type I collagen is present in the intact lacunae of numerous hypertrophic chondrocytes adjacent to vascularized cartilage canals and bone marrow processes. On the ultrastructural level these cells appear as viable cells surrounded by a layer of type I collagen. In previous studies on endochandral bone formation in avian and rabbit it was postulated that hypertrophic chondrocytes can differentiate into osteoblasts (Roach & Shearer, 1989; Galatto et al. 1994; Roach et al. 1995; Roach, 1997,Roach, 1999; Blumer et al. 2004b). This was found around the walls of marrow tunnels in the POC (Roach et al. 1995; Roach, 1997), near the marrow cavity in the SOC (Roach, 1999), beneath the vascularized periosteum (Galatto et al. 1994) and adjacent to communicating cartilage canals (Blumer et al. 2004b). We suggest that factors from the vessels or the osteoprogenitor cells diffuse to the neighbouring hypertrophic chondrocytes and induce the synthesis of type I collagen. Our observations show that the lacunae which contain type I collagen are opened by chondroclasts and we conclude that later the hypertrophic cells undergo apoptosis. Therefore, the osteoblast-like stage of hypertrophic chondrocytes is only transitory and we assume that most hypertrophic chondrocytes that are adjacent to vascularized areas share this fate. However, we cannot exclude the possibility that some of these hypertrophic chondrocytes switch into osteoblasts without degenerating and dying. Hypertrophic chondrocytes further from cartilage canals and bone marrow processes do not synthesize type I collagen and in addition show features of degeneration. It appears possible that these cells undergo an aberrant programmed cell death, which is called chondroptosis (Roach et al. 2004). These authors pointed out that the most crucial difference between apoptosis and chondroptosis is the elimination of cellular remnants, which occurs by self-destruction in the latter process and hence is not dependent on phagocytosis by other cells (macrophages or chondroclasts).
Conclusions
The present work demonstrates that (a) an SOC appears in the femur of chicken, (b) cartilage canals are an extension of the perichondrium and its layers of mesenchymal stem cells, (c) some canals penetrate into the SOC supplying stem cells for endochondral bone formation, (d) cartilage canals contribute to both endochondral and perichondral bone formation, and (e) the origin of osteoblasts is the same in both processes.
Acknowledgments
We thank Professor H. Dietrich for kindly providing the material for this study and Professor L. Huber for providing technical equipment. We gratefully acknowledge the provision of anti-collagen type I by Professor L. Fisher (Bethesda). We especially thank Professors K. Pfaller and T. Pérez for discussion and critically reading the manuscript.
References
- Agrawal P, Atre PR, Kulkarni DS. The role of cartilage canals in the ossification of the Talus. Acta Anat. 1984;199:238–240. doi: 10.1159/000145891. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Kulkarni DS, Atre PR. The participation of cartilage canals in the ossification of the human fetal calcaneum. J. Anat. 1986;147:135–142. [PMC free article] [PubMed] [Google Scholar]
- Blumer MJF, Gahleitner P, Narzt T, Handl C, Ruthensteiner B. Ribbons of semithin sections: an advanced method with a new type of diamond knife. J. Neurosci. Methods. 2002;120:11–16. doi: 10.1016/s0165-0270(02)00166-8. [DOI] [PubMed] [Google Scholar]
- Blumer MJF, Fritsch H, Pfaller K, Brenner E. Cartilage canals in the chicken embryo: ultrastructure and function. Anat. Embryol. 2004a;207:453–462. doi: 10.1007/s00429-003-0363-0. [DOI] [PubMed] [Google Scholar]
- Blumer MJF, Longato S, Fritsch H. Cartilage canals in the chicken embryo are involved in the process of endochondral bone formation within the epiphyseal growth plate. Anat. Rec. 2004b;279A:692–700. doi: 10.1002/ar.a.20058. [DOI] [PubMed] [Google Scholar]
- Burkus KJ, Ganey TM, Ogden JA. Development of the cartilage canals and the secondary center of ossification in the distal chondroepiphysis of the prenatal human femur. Yale J. Biol. Med. 1993;66:193–202. [PMC free article] [PubMed] [Google Scholar]
- Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J. Cell Sci. 2000;113:59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- Chandraraj S, Briggs ChA. Role of cartilage canals in the osteogenesis and growth of the vertebral centra. J. Anat. 1988;158:121–136. [PMC free article] [PubMed] [Google Scholar]
- Claassen H, Kirsch T, Simons G. Cartilage canals in human thyroid cartilage characterized by immunolocalization of collagen types I, II, pro-III, IV and X. Anat. Embryol. 1996;194:147–153. doi: 10.1007/BF00195008. [DOI] [PubMed] [Google Scholar]
- Cole AA, Cole MB. Are perivascular cells in the cartilage canals chondrocytes? J. Anat. 1989;165:1–8. [PMC free article] [PubMed] [Google Scholar]
- Delgado-Baeza Giménez-Ribotta M, Miralles-Flores Nieto-Chaguaceda A, Santos-Alvarez I. Morphogenesis of cartilage canals: experimental approach in the rat tibia. Acta Anat. 1991;142:132–137. doi: 10.1159/000147177. [DOI] [PubMed] [Google Scholar]
- Doménech-Ratto G, Fernández-Villacañas Marín M, Ballester-Moreno A, Doménech-Asensi P. Development and segments of cartilage canals in the chick embryo. A light microscope study. Eur. J. Anat. 1999;3:121–126. [Google Scholar]
- Doschak MR, Cooper DML, Huculak CN, et al. Angiogenesis in the distal femoral chondroepiphysis of the rabbit during the development of the secondary centre of ossification. J. Anat. 2003;203:223–233. doi: 10.1046/j.1469-7580.2003.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch H, Brenner E, Debbage P. Ossification in the human calcaneus: a model for spatial bone development and ossification. J. Anat. 2001;199:609–616. doi: 10.1046/j.1469-7580.2001.19950609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatto M, Campanile G, Robino G, Cancedda FD, Bianco P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in the developing chick embryo. J. Bone Min. Res. 1994;9:1239–1249. doi: 10.1002/jbmr.5650090814. [DOI] [PubMed] [Google Scholar]
- Ganey TM, Ogden JA, Sasse J, Neame PJ, Hilbelink DR. Basement membrane composition of cartilage canals during development and ossification of the epiphysis. Anat. Rec. 1995;241:425–437. doi: 10.1002/ar.1092410318. [DOI] [PubMed] [Google Scholar]
- Gray DJ, Gardner E. The prenatal development of the human humerus. Am. J. Anat. 1969;124:431–446. doi: 10.1002/aja.1001240403. [DOI] [PubMed] [Google Scholar]
- Haines RW. Cartilage canals. J. Anat. 1933;68:45–64. [PMC free article] [PubMed] [Google Scholar]
- Hunt CD, Ollerich DA, Nielsen FH. Morphology of the perforating cartilage canals in the proximal tibial growth plate of the chick. Anat. Rec. 1979;194:143–157. doi: 10.1002/ar.1091940110. [DOI] [PubMed] [Google Scholar]
- Kimpel M, Claassen H, Fleiner B, Tillmann B. Vascularization and cartilage mineralization of the thyroid cartilage of Munich minipigs and domestic pigs. Anat. Embryol. 1999;199:281–290. doi: 10.1007/s004290050228. [DOI] [PubMed] [Google Scholar]
- Kugler JH, Tomlinson A, Wagstaff A, Ward SM. The role of cartilage canals in the formation of secondary centres of ossification. J. Anat. 1979;129:493–506. [PMC free article] [PubMed] [Google Scholar]
- Lison L. Alcian blue 8G with Chlorantine fast red 5B. A technic for selective staining of mucopolysaccharides. Stain Technol. 1954;29:131–138. doi: 10.3109/10520295409115457. [DOI] [PubMed] [Google Scholar]
- Lutfi AM. Mode of growth, fate and functions of cartilage canals. J. Anat. 1970a;106:135–145. [PMC free article] [PubMed] [Google Scholar]
- Lutfi AM. Study of cell multiplication in the cartilaginous upper end of the tibia of the domestic fowl by tritiated thymidine autoradiography. Acta Anat. (Basel) 1970b;76:454–463. doi: 10.1159/000143507. [DOI] [PubMed] [Google Scholar]
- Pechak DG, Kujawa MJ, Caplan AI. Morphological and histochemical events during first bone formation in embryonic chick limbs. Bone. 1986a;7:441–458. doi: 10.1016/8756-3282(86)90004-9. [DOI] [PubMed] [Google Scholar]
- Pechak DG, Kujawa MJ, Caplan AI. Morphology of bone development and bone remodelling in embryonic chick limbs. Bone. 1986b;7:459–472. doi: 10.1016/8756-3282(86)90005-0. [DOI] [PubMed] [Google Scholar]
- Petersen W, Tsokos M, Pufe Th. Expression of VEGF121 and VEGF165 in hypertrophic chondrocytes of the human growth plate and epiphyseal cartilage. J. Anat. 2002;201:153–157. doi: 10.1046/j.1469-7580.2002.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas R, Shapiro F. Structural stages in the development of the long bones and epiphyses: a study in the New Zealand white rabbit. J. Bone Joint Surg. Am. 2002;84-A1:85–100. doi: 10.2106/00004623-200201000-00013. [DOI] [PubMed] [Google Scholar]
- Roach HI, Shearer JR. Cartilage resorption and endochondral bone formation during the developmet of long bones in chick embryos. Bone Miner. 1989;6:289–309. doi: 10.1016/0169-6009(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Roach HI, Erenpreisa J, Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J. Cell Biol. 1995;131:483–494. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach HI. New aspects of endochondral ossification in the chick: chondrocyte apoptosis, bone formation by former chondrocytes, and acid phosphatase activity in the endochondral bone matrix. J. Bone Miner. Res. 1997;12:795–805. doi: 10.1359/jbmr.1997.12.5.795. [DOI] [PubMed] [Google Scholar]
- Roach HI, Baker JE, Clarke NMP. Initiation of the bony epiphysis in long bones: chronology of interactions between the vascular system and chondrocytes. J. Bone Min. Res. 1998;13:950–961. doi: 10.1359/jbmr.1998.13.6.950. [DOI] [PubMed] [Google Scholar]
- Roach HI. Association of matrix acid and alkaline phosphatases with mineralization of cartilageand endochondral bone. Histochem. J. 1999;31:53–61. doi: 10.1023/a:1003519104980. [DOI] [PubMed] [Google Scholar]
- Roach HI, Aigner T, Kouri JB. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis. 2004;9:265–277. doi: 10.1023/b:appt.0000025803.17498.26. [DOI] [PubMed] [Google Scholar]
- Shapiro F. Epiphyseal and physeal cartilage vascularization: a light microscopic and tritiated thymidine autoradiographic study of cartilage canals in newborn and young postnatal rabbit bone. Anat. Rec. 1998;252:140–148. doi: 10.1002/(SICI)1097-0185(199809)252:1<140::AID-AR12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Soames RW. Gray's Anatomy. New York: Churchill Livingstone; 1995. Skeletal systems; pp. 425–736. [Google Scholar]
- Visco DM, Hill MA, Van Sickle DC, Kincaid SA. The development of centres of ossification of bones forming elbow joints in young swine. J. Anat. 1990;171:25–39. [PMC free article] [PubMed] [Google Scholar]
- Wilsman NJ, Van Sickle DC. The relationship of cartilage canals to the initial osteogenesis of secondary centers of ossification. Anat. Rec. 1970;168:381–392. doi: 10.1002/ar.1091680305. [DOI] [PubMed] [Google Scholar]
- Wilsman NJ, Van Sickle DC. Cartilage canals, their morphology and distribution. Anat. Rec. 1972;173:79–93. doi: 10.1002/ar.1091730107. [DOI] [PubMed] [Google Scholar]
- Ytrehus B, Carlson CS, Lundeheim N, et al. Vascularisation and osteochondrosis of the epiphyseal grpwth cartilage of the distal femur in pigs – development with age, growth rate, weight and joint shape. Bone. 2004a;34:454–465. doi: 10.1016/j.bone.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Ytrehus B, Ekman S, Carlson CS, Teige J, Reinholt FP. Focal changes in blood supply during normal epiphyseal growth are central in the pathogensis of osteochondrosis in pigs. Bone. 2004b;35:1294–1306. doi: 10.1016/j.bone.2004.08.016. [DOI] [PubMed] [Google Scholar]