Abstract
African elephants (Loxodonta africana) use their tusks for digging, carrying and behavioural display. Their healing ability following traumatic injury is enormous. Pain experience caused by dentin or pulp damage of tusks seems to be negligible in elephants. In this study we examined the pulp tissue and the nerve distribution using histology, electron microscopy and immunhistochemistry. The results demonstrate that the pulp comprises two differently structured regions. Randomly orientated collagen fibres characterize a cone-like part lying rostral to the foramen apicis dentis. Numerous nerve fibres and Ruffini endings are found within this cone. Rostral to the cone, delicate collagen fibres and large vessels are orientated longitudinally. The rostral two-thirds of the pulp are highly vascularized, whereas nerve fibres are sparse. Vessel and nerve fibre distribution and the structure of connective tissue possibly play important roles in healing and in the obviously limited pain experience after tusk injuries and pulp alteration. The presence of Ruffini endings is most likely related to the use of tusks as tools.
Keywords: dental pain, mechanoreceptors, pulpal nerves, Ruffini endings
Introduction
Tusks of African elephants (Loxodonta africana) are continuously growing permanent incisors of the upper jaw (Miller, 1890; Raubenheimer, 2000). Regarding the tusks, the elephant is a diphyodont species (Raubenheimer, 2000). The hard tissue of the tusk consists of dentin and a thin outer layer of cementum (Raubenheimer et al. 1998). Tusks are not involved in gnawing or mastication but are used for digging, carrying objects and behavioural display (Allen et al. 1984; Forstenpointner et al. 2001). Owing to their exposed position and shape they can easily be injured in both free-ranging and captive elephants (Fagan et al. 2001; Steiner et al. 2003; Weissengruber et al. 2003). With or without therapeutic intervention, the tusk pulp of the elephant shows remarkable healing ability and production of reparative dentin, which leads in most cases to the closing of the pulp cavity and recovery of the animal (Miller, 1891; Fagan et al. 2001; Forstenpointner et al. 2001). In elephants, the minor effects of tusk pulpitis and the remarkable restorative processes subsequent to pulpitis stand in striking contrast to the poor healing capacity of the pulp tissue especially in human teeth (Heine et al. 1989; Steenkamp, 2003). Because of the good clinical condition of animals after tusk injuries or inflammations it is often assumed by zoo veterinarians that elephants do not experience pain caused by tusk pulp alteration (Fagan et al. 1999). By contrast, injuries affecting the intra-alveolar root of the tusk or the alveolar walls tend to be associated with severe pain and discomfort (Fagan et al. 2001). There is considerable information concerning the structure of dental hard tissues (Raubenheimer et al. 1998) or pathological alterations of the dentin (Galippe, 1891; Von Solodkoff, 1988). However, not least owing to the scarcity of available specimens, our knowledge of the morphology of the tusk pulp is sparse and, furthermore, findings are controversial (Weatherford, 1940; Fagan et al. 1999; Boy & Steenkamp, 2004). The main aims of our study were to provide a detailed description of the tusk pulp structure and to detect morphological peculiarities related to function, pain perception and healing ability.
Materials and methods
Tusks of two female African elephants, aged 40 and 27 years, were cut longitudinally as well as transversally and tissue samples of the pulp were immediately extracted from the pulp cavity and fixed in buffered formalin. In addition, samples were taken from four different areas of the inner dentinal surface, which was covered by odontoblasts. Both animals were kept in the Vienna zoo. The older animal was put down by zoo veterinarians because of severe disorders affecting its locomotor system. The tusks were extracted during post-mortem examination. The second animal shed one tusk after a mechanical trauma with the tusk pulp showing no pathological alterations. This animal recovered rapidly and completely. In all specimens, pulp tissue samples (size 2 × 1 cm) were taken from five different positions moving from the foramen apicis dentis to the tip (rostral) of the pulp both from the pulpal surface and from the centre of the pulp.
The tissue samples were post-fixed for 24 h in 4% buffered formalin and embedded in paraffin (Tissue Tek, VIP3000, Miles Scientific, Sakura Finetek, Zoeterwoude, The Netherlands). Four-micrometre sections were cut and alternately stained with haematoxylin and eosin (H&E), van Gieson's connective tissue stain, Weigert's resorcin fuchsin for elastic fibres and Alcian blue (pH 4.0) with and without previous hyaluronidase digestion, according to the methods described in Romeis (1989).
For immunohistochemistry (IHC), sections were mounted on poly-l-lysine-coated SuperFrost slides, deparaffinized and rehydrated. Peroxidase activity was removed by addition of 0.6% H2O2 (Merck, Darmstadt, Germany) in methanol, then sections were washed in tap water, mounted with PBS (pH = 7.4) in Coverplates™ and put into a Sequenza® immunostaining centre (Thermo Shandon Int., Pittsburgh, PA, USA). Sections were treated with normal 1.5% goat serum for 30 min at room temperature and then incubated with the primary antibody overnight at 4 °C. Subsequently, sections were washed in PBS and incubated with secondary antibody (anti-rabbit or anti-mouse DAKO EnVision™ Poly HRP, DAKO, Vienna, Austria, or FITC-conjugated anti-goat or anti-mouse, Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. Sections were washed with PBS and peroxidase activity was detected with diaminobenzidine (DAB) for 10 min at room temperature. Then sections were rinsed, counterstained with haematoxylin, dehydrated and mounted with DPX (Fluka, Buchs, Switzerland). The following antibodies were used: anti-S-100 protein (DAKO, 1 : 1200), anti-glial fibrillary acidic protein (GFAP, DAKO, 1 : 500), anti-neuron-specific enolase (NSE, DAKO, 1 : 1000), anti-protein gene peptide 9.5 (PGP 9.5, UltraClone, UK, 1 : 6000), anti-substance P (SP, Zymed, CA, USA, 1 : 75), anti-calcitonin gene-related peptide (CGRP, Quartett, Berlin, Germany, 1 : 1000), anti-von Willebrand factor (vWF, DAKO, 1 : 1200), anti-α-sm-actin (DAKO, 1 : 400), anti-collagen type I (Southern Biotechnology, AL, USA, 1 : 30) and anti-collagen type III (Quartett, Berlin, Germany, 1 : 50). Pretreatment with protease (1 mg per 1 mL PBS) for 20 min at room temperature was required to stain for vWF. Collagen staining needed pepsin digestion (1 mg per 1 mL 0.5 m acetic acid) for 2 h at 37 °C. For negative controls, the primary antibody was omitted and unspecific mouse and rabbit IgG was used at the same protein concentration as the primary antibody. Sections from horse and elephant foot pads containing blood vessels and nerve fibres were stained accordingly as positive controls.
Formalin-fixed samples from the caudal part of the pulp were taken for electron microscopy (EM) studies of the neural tissue. Samples were washed in phosphate buffer and post-fixed for 2 h at room temperature in 1% phosphate-buffered osmium tetroxide (Plane W. Plannet GmbH, Wetzlar, Germany) and then rinsed in phosphate buffer. Dehydration was performed in a series of graded ethanol solutions. Infiltration with propylene oxide (Merck, Darmstadt, Germany) was followed by increasing the ratio of epoxy resin (Servy, Heidelberg, Germany) to propylene oxide (1 : 1, 3 : 1) and finally pure resin. After an additional change, the resin was polymerized at 60 °C. Ultrathin sections were stained with alkaline lead citrate and methanolic uranyl acetate, and viewed with a transmission electron microscope (EM 900, Zeiss, Oberkochen, Germany).
Anatomical terms are in accordance with the 4th edition of the Nomina Anatomica Veterinaria (NAV, 1994). Structures lying towards the apex cuspidis of the tusk are designated as rostral.
Results
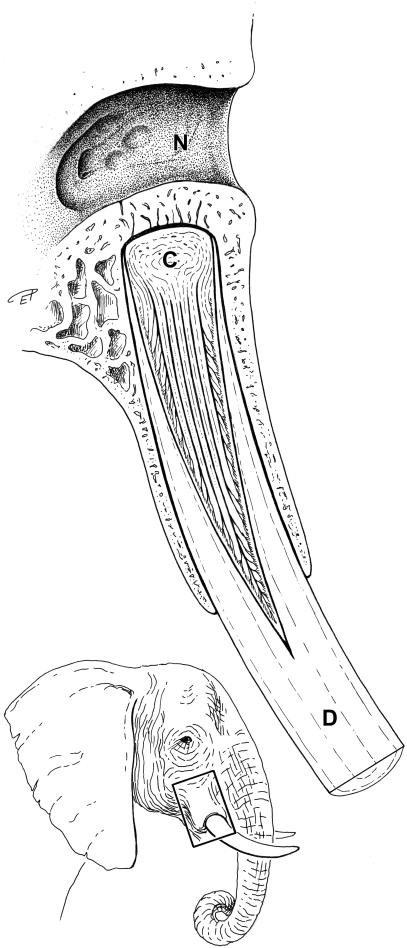
The dental pulp extended over approximately one-third of the total tusk length. The tip of the cone-shaped pulp lies a few centimetres outside the rim of the osseous alveolus and therefore the main part of the pulp is situated within the root (Fig. 1). In the 40-year-old animal, the foramen apicis dentis of the tusk was situated next to the osseous base of the alveolus (Fig. 1).
Fig. 1.
Longitudinal section through the right tusk and corresponding alveolus of an African elephant. N, nasal cavity; C, cone-like structure within the pulp rostral to the foramen apicis dentis; D, dentin (ivory).
Even macroscopically, two differently structured areas could be discerned in the pulp. A short blunt-ending cone lies rostral to the wide foramen apicis dentis (Fig. 1). The length of this cone (from the foramen apicis dentis to the ‘tip’ of the cone) takes up about one-sixth of the whole length of the pulp. The cone contains numerous densely packed randomly orientated collagen fibres. Rostral to this cone, the collagen fibres are delicate, loosely arranged and orientated parallel to the axis. These different arrangements of collagen bundles were demonstrated by van Gieson's connective tissue staining (Fig. 2a,b) and by immunohistochemical detection of collagen type I (Fig. 2c,d). Staining for collagen type III revealed a network of fibres surrounding the fibroblasts. Elastic fibres were not identified within the pulp tissue. The acidic extracellular matrix stained well with Alcian blue at pH 4.0 in all tissue samples of the pulp. Alcianophilia was abolished when sections were pretreated with hyaluronidase, revealing hyaluronan as its main constituent.
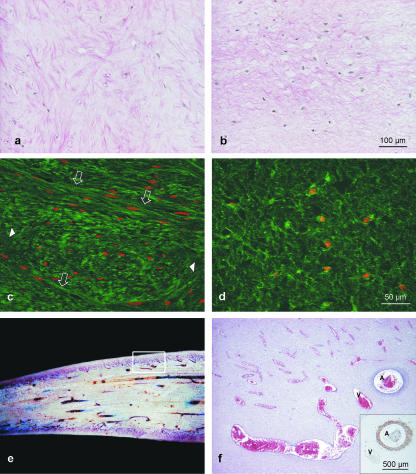
Fig. 2.
(a–d) Micrographs of the connective tissue arrangement in two different areas of the pulp, van Gieson's staining (a,b) and collagen type I staining (FITC labelling, nuclei are counterstained with propidium iodide, red, c,d). Densely packed irregular collagen fibres with random orientation of the fibre bundles (longitudinal fibres – empty arrows, cross-sectioned fibres – arrowheads) are present within the cone-like area rostral to the Foramen apicis dentis (a,c). Rostral to this cone (towards the tip of the pulp cavity) the thin collagen fibre bundles are loosely arranged (b,d). The vascular bed of the tusk pulp shows longitudinally aligned larger vessels giving branches to an extensive subodontoblastic plexus. (e) Macroscopic picture: longitudinal section through the central part of the pulp (diameter on right approximately 6 cm); inset, a representative area is demonstrated in (f), H&E staining. Arteries (A) and veins (V) were easily distinguished by α-sm-actin labelling (see inset in f).
Irregularly distributed blood vessels course within the cone described above. The central and major part of the pulp tissue rostral to the cone was characterized by large vessels (arteries and veins) running parallel to the longitudinal axis of the tusk (Fig. 2e). Numerous preterminal vessels and capillaries formed a dense network (Fig. 2f) towards the periphery beneath the pseudostratified layer of odontoblasts and at the tip of the pulp. This microvascular bed was clearly shown by IHC staining for vWF, and the smooth muscle cells of the vascular wall by actin labelling. Whereas arteries were provided with thin muscular walls, even large veins nearly lacked smooth muscle cells within the wall (Fig. 2f, inset). Lymphatic vessels were discerned from blood vessels as their endothelial cells do not stain with vWF. They were sparsely distributed in the dental pulp.
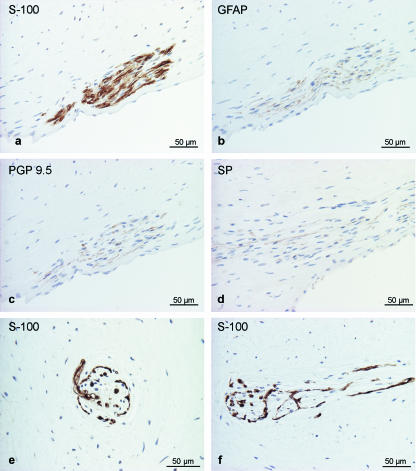
Nerve fibres were identified by staining for general markers of nervous tissue (S-100, GFAP, PGP 9.5 and NSE) as well as CGRP and SP specific for sensory nerve fibres. Nerve fibres were revealed best with anti-S-100, but were also positive for GFAP, PGP 9.5 (Fig. 3a–c) and weakly reactive for NSE (data not shown). Within nerve bundles, single axons reacted for SP (Fig. 3d) and CGRP (data not shown). Within the first third of the dental pulp, on average 7.9 nerve fibre profiles cm−2 were found. Several nerve fibre bundles entered into the pulp through the foramen apicis dentis. In particular within the cone of densely packed connective tissue, several bundles of myelinated and non-myelinated nerve fibres were seen (differentiation by EM), which are often accompanied by blood vessels but also course independently through the pulp. Longitudinal sections and cross-sections of especially structured nerve fibre terminals resembled Ruffini endings. These endings and myelinated nerve fibres were found exclusively within the cone-like structure rostral to the foramen apicis dentis (Fig. 3e,f). The Ruffini endings consist of an S-100-positive capsule enclosing a gelatinous core with a few fibroblasts and collagen fibers. At least one myelinated fibre, losing its myelin sheath on entering the capsule, gives off lateral branches that form an intricate terminal arborization among the collagen bundles. On average, we found 22 profiles of Ruffini endings per cm2 within the dense connective tissue of the cone. In the middle and final (rostral) third of the pulp, nerve fibres were rarely seen (average 1.3 nerve fibre profiles cm−2). Single axons were confined to deeper (axial) layers of the pulp, where these nerves mainly run independent of blood vessels. Only a few axons could be detected forming a perivascular plexus in the adventitia of larger arteries. No axons or neural plexus were identified in superficial layers of the pulp, in between odontoblasts or within the dentinal tubules.
Fig. 3.
Nerve fibres in the tusk pulp detected by immunohistochemical staining for general neural markers. (a) S-100, (b) GFAP, (c) PGP 9.5, (d) SP-positive axons within the nerve bundle. Special nerve fibre terminals resembling Ruffini endings were found within the pulp (e, cross-section; f, longitudinal section) stained for S-100 protein.
Discussion
We describe here the morphology of the pulp tissue of tusks in African elephants. The results indicate that the pulpal structure of tusks reveals some differences to that of other tooth types. Although the pulpal tissues of only two female elephants were examined and differences between the sexes in circumference and total length of the tusk have been identified previously (Pilgram & Western, 1986), it is unlikely that tusk pulps of other individuals, and males in particular, reveal a markedly different structure or innervation. Because age-related changes in the pulp structure of teeth of humans and other animals are well documented, it is likely that similar changes occur in elephants with advancing age (more than 40 years).
The diameter of the pulp is wide in the apical region as is the case in hypsodont teeth of other species (Hildebrand et al. 1995; Steenkamp, 2003). In brachydont teeth, the foramina at the apex are small and the pulp is a low-compliance tissue (Matthews & Andrew, 1995). Owing to the rigid encasement of the pulp and the small foramina, inflammation leads to an increase in both interstitial fluid volume and tissue pressure, eventually causing so-called self-strangulation of the pulp (Matthews & Andrew, 1995; Heyeraas & Berggreen, 1999). Thus, pressure-induced vascular occlusion and infarction is not likely in the tusk pulp as the pressure resulting from the inflammatory exudate is released through the large apical foramen.
In humans and other mammals, dental pulp tissue is characterized by a three-dimensional meshwork of fibroblasts formed by elongated cell processes and by a large amount of matrix between the cells (Pischinger & Stockinger, 1968; Müller & Städtler, 1987; Heine et al. 1989). Considering the densely packed collagen fibres, the absence of the mentioned meshwork and the presence of large, longitudinally orientated vessels, the structure of the tusk pulp is completely different to that found in other mammalian pulps. It is likely that the apparently good vascularization plays an important role in mediating an immunological response, forming granulation tissue, producing (secondary) dentin and in supporting other mechanisms of defence and repair. In the human tooth a subodontoblastic zone that is free of cell nuclei (Weil's basal zone) occurs within the pulp of the tooth crown (Heine et al. 1989). According to our findings, such a zone is absent in the tusk pulp; this omission has also been described for rats (Müller & Städtler, 1987). In our specimens of African elephants a pseudostratified layer of odontoblasts is present. A similar arrangement is visible in one figure in Raubenheimer et al. (1998), indicating that it may be the normal pattern in African elephants.
In accordance with the findings of Boy & Steenkamp (2004) and those on other continuously growing teeth such as incisors of rodents (Bishop, 1981; Hildebrand et al. 1995), the tusk pulps examined for the present study contain rather few axons. As in other mammalian teeth (Pischinger & Stockinger, 1968), unmyelinated fibres were detected more towards the periphery of the tusks, whereas the myelinated nerve fibres were only present in the first part of the tusk pulp supplying the mechanoreceptors. In the specimens examined here, axons show no structural association with odontoblasts, and a subodontoblastic plexus of Raschkow is lacking. The sensory nerve fibres of the tusk pulp express a number of neuropeptides such as SP and CGRP, which play a role in vascular regulations and possess immunoregulatory capacities (Fried, 1992; Okiji et al. 1997; Hargreaves et al. 2003). It is a common finding that dental pulp vessels are richly innervated. Pulp vessels of the continuously erupting rat incisor are accompanied by numerous nerve fibres and the terminal arterioles receive dense nerve supply (Zhang et al. 1998). In comparison, the nerve supply of the vessels in the tusk pulp is sparse. In the human tooth (Ramieri et al. 1990) and also in the rat incisor (Zhang et al. 1998) penetration of nerve fibres into the dentin has been shown. Axons within the dentinal tubules of tusks were neither observed in African (present study) nor Asian (Weatherford, 1940) elephants. Considering the increase of neural density with caries progression and the dynamic changes of SP expression within the inflamed pulp in the human tooth (Rodd & Boissonnade, 2000), it remains unclear whether similar mechanisms occur in elephant tusks during or after injury or inflammation. As age-related changes such as a decrease in CGRP- and SP-immunoreactivity of nerve fibres and loss of axons are commonly observed in the tooth pulp (Fried, 1992), similar phenomena could also be found in the hypsodont tusk. These changes might be responsible for failing to detect nerves within the tusk pulp as mentioned by Fagan et al. (1999).
In the human injured tooth pulp, no correlation was found between pain experience and overall neural density (Rodd & Boissonnade, 2000). In elephants, the structure of the tusk pulp is completely different to that in the human tooth. Furthermore, peripheral mechanisms of pain are complex and not well understood (Byers & Narhi, 1999). It seems likely that the small number of nerve fibres within the rostral two-thirds of the tusk pulp is associated with a limited ability to perceive pain. Considering the exposed position of the tusk, its length and its use as tool, it is clear that this tooth can easily be injured. From an evolutionary standpoint, the enormous healing ability of the tusk and the obviously limited pain experience and discomfort after pulp alterations (Fagan et al. 1999; Forstenpointner et al. 2001) could be advantageous adaptations.
The nerve fibre terminals (Ruffini endings) found in the pulp resemble mechanoreceptors occurring in the skin, in joint structures (capsules, labra, menisci) and in the periodontal ligament of different tooth types and species (Truex & Carpenter, 1968; Berry et al. 1995; Kim & Azuma, 1995; Maeda et al. 1999; Jayawardena et al. 2002). They contain highly branched non-myelinated nerve endings that invade and ramify among bundles of collagen fibres in order to record changes in pressure and tension (Truex & Carpenter, 1968; Berry et al. 1995). Within periodontal ligaments, Ruffini endings are also frequently found in the dense collagenous tissue areas (Maeda et al. 1999). The Ruffini endings in tusk pulps may play a similar role in mechanoreception as those lying in the periodontium.
In conclusion, the present study provides histological evidence that the vascularization and structure of the pulp of the tusk may explain its good healing ability following injury and inflammation. The obviously limited dental pain experience after tusk injuries and pulp alterations may be due to a rather sparse innervation of the rostral parts of the pulp. The Ruffini corpuscles possibly play an important role when the tusks are used for mechanical work such as digging and carrying. Nonetheless, further research, particularly in injured tusk pulps, may lead to a greater insight into the complex mechanisms of healing and dental pain.
Acknowledgments
We wish to thank Mrs Magdalena Helmreich (Histology and Embryology, Department of Pathobiology, University of Veterinary Medicine Vienna) for technical support and Mag. med. vet. Eva Polsterer for graphical support. We appreciate the cooperation of the Vienna Zoo Schönbrunn.
References
- Allen JL, Welsch B, Jacobson ER, Turner TA, Tabeling H. Medical and surgical management of a fractured tusk in an African elephant. J. Am. Vet. Med. Assoc. 1984;185:1447–1449. [PubMed] [Google Scholar]
- Berry M, Bannister LH, Standring SM. Nervous system. In: Williams PL, editor. Gray's Anatomy. Edinborough: Churchill Livingstone; 1995. pp. 901–1367. [Google Scholar]
- Bishop MA. A fine-structural survey of the pulpal innervation in the rat mandibular incisor. Am. J. Anat. 1981;160:213–229. doi: 10.1002/aja.1001600207. [DOI] [PubMed] [Google Scholar]
- Boy SC, Steenkamp G. Neural innervation of the tusk pulp of the African elephant (Loxodonta africana) Vet. Rec. 2004;154:372–374. doi: 10.1136/vr.154.12.372. [DOI] [PubMed] [Google Scholar]
- Byers MR, Narhi MV. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit. Rev. Oral Biol. Med. 1999;10:4–39. doi: 10.1177/10454411990100010101. [DOI] [PubMed] [Google Scholar]
- Fagan DA, Benirschke K, Simon JHS, Roocroft A. Elephant dental pulp tissue: where are the nerves? J. Vet. Dent. 1999;16:169–172. doi: 10.1177/089875649901600403. [DOI] [PubMed] [Google Scholar]
- Fagan DA, Oosterhuis JE, Roocroft A. Captivity disorders in elephants impacted molars and broken tusks. Zool. Garten N. F. 2001;71:281–303. [Google Scholar]
- Forstenpointner G, Weissengruber GE, Kuebber-Heiss A, Burger H, Voracek T. Healing and repair in tusk, incisivum and maxilla of an African elephant. Proceedings of the Joint Conference of the AAZV, AAWV, ARAV, NAZWV; Orlando, FL: American Association of Zoo Veterinarians; 2001. p. 393. [Google Scholar]
- Fried K. Changes in pulpal nerves with aging. Proc. Finn. Dent. Soc. 1992;88(Suppl. 1):517–528. [PubMed] [Google Scholar]
- Galippe V. Recherches d'anatomie normale et pathologique sur l'appareil dentaire de l'elephant. J. Anat. Physiol. 1891;27:285–343. [Google Scholar]
- Hargreaves KM, Bowles WR, Jackson DL. Intrinsic regulation of CGRP release by dental pulp sympathetic fibers. J. Dent. Res. 2003;82:398–401. doi: 10.1177/154405910308200514. [DOI] [PubMed] [Google Scholar]
- Heine H, Schaeg G, Türk R. Funktionelle Morphologie des Pulpagewebes. Z. Mikrosk. Anat. Forsch. 1989;103:367–375. [PubMed] [Google Scholar]
- Heyeraas KJ, Berggreen E. Interstitial fluid pressure in normal and inflamed pulp. Crit. Rev. Oral Biol. Med. 1999;10:328–336. doi: 10.1177/10454411990100030501. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Fried K, Tuisku F, Johansson CS. Teeth and tooth nerves. Prog. Neurobiol. 1995;45:165–222. doi: 10.1016/0301-0082(94)00045-j. [DOI] [PubMed] [Google Scholar]
- Jayawardena CK, Takahashi N, Takano Y. A unique localization of mechanoreceptors in the periodontal tissue of Guinea pig teeth. Arch. Histol. Cytol. 2002;65:233–244. doi: 10.1679/aohc.65.233. [DOI] [PubMed] [Google Scholar]
- Kim YT, Azuma H. The nerve endings of the acetabular labrum. Clin. Orthopaedics Related Res. 1995;320:176–181. [PubMed] [Google Scholar]
- Maeda T, Ochi K, Nakakura-Oshima K, Youn SH, Wakisaka S. The Ruffini ending as the primary mechanoreceptor in the periodontal ligament: its morphology, cytochemical features, regeneration, and development. Crit. Rev. Oral Biol. Med. 1999;10:307–327. doi: 10.1177/10454411990100030401. [DOI] [PubMed] [Google Scholar]
- Matthews B, Andrew D. Microvascular architecture and exchange in teeth. Microcirculation. 1995;2:305–313. doi: 10.3109/10739689509148275. [DOI] [PubMed] [Google Scholar]
- Miller WD. Studies on the anatomy and pathology of the tusks of the elephant. Dental Cosmos. 1890;32:337–348. [Google Scholar]
- Miller WD. Studies on the anatomy and pathology of the tusks of the elephant. The exposed pulp. Dental Cosmos. 1891;33:169–175. [Google Scholar]
- Müller S, Städtler R. Zellen und Bindegewebselemente der Zahnpulpa. Z. Mikrosk. Anat. Forsch. 1987;101:295–300. [PubMed] [Google Scholar]
- NAV. Nomina Anatomica Veterinaria. 4th. Zürich: World Ass. Vet. Anat; 1994. [Google Scholar]
- Okiji T, Jontell M, Belichenko P, Dahlgren U, Bergenholtz G, Dahlström A. Structural and functional association between substance P- and calcitonin gene-related peptide-immunoreactive nerves and accessory cells in the rat dental pulp. J. Dent. Res. 1997;76:1818–1824. doi: 10.1177/00220345970760120301. [DOI] [PubMed] [Google Scholar]
- Pilgram T, Western D. Inferring the sex and age of African elephants from tusk measurements. Biol. Conserv. 1986;36:39–52. [Google Scholar]
- Pischinger A, Stockinger L. Die Nerven der menschlichen Zahnpulpa. Z. Zellforsch. 1968;89:44–61. [PubMed] [Google Scholar]
- Ramieri G, Anselmetti GC, Baracchi F. The innervation of human teeth and gingival epithelium as revealed by means of an antiserum for protein gene product 9.5 (PGP 9.5) Am. J. Anat. 1990;189:146–154. doi: 10.1002/aja.1001890205. [DOI] [PubMed] [Google Scholar]
- Raubenheimer EJ, Bosman MC, Vorster R, Noffke CE. Histogenesis of the chequered pattern of ivory of the African elephant (Loxodonta africana) Arch. Oral Biol. 1998;43:969–977. doi: 10.1016/s0003-9969(98)00077-6. [DOI] [PubMed] [Google Scholar]
- Raubenheimer EJ. Early development of the tush and the tusk of the African elephant (Loxodonta africana) Arch. Oral Biol. 2000;45:983–986. doi: 10.1016/s0003-9969(00)00068-6. [DOI] [PubMed] [Google Scholar]
- Rodd HD, Boissonnade FM. Substance P expression in human tooth pulp in relation to caries and pain experience. Eur. J. Oral Sci. 2000;108:467–474. doi: 10.1034/j.1600-0722.2000.00924.x. [DOI] [PubMed] [Google Scholar]
- Romeis B. In: Mikroskopische Technik. Böck p., editor. München: Urban und Schwarzenberg; 1989. [Google Scholar]
- Steenkamp G. Oral biology and disorders of tusked mammals. Vet. Clin. Exot. Anim. 2003;6:689–725. doi: 10.1016/s1094-9194(03)00035-5. [DOI] [PubMed] [Google Scholar]
- Steiner M, Gould AR, Clark TJ, Burns R. Induced elephant (Loxodonta africana) tusk removal. J. Zoo Wildl. Med. 2003;34:93–95. doi: 10.1638/1042-7260(2003)34[0093:IELATR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Truex RC, Carpenter M. Human Neuroanatomy. 6. Baltimore: Williams & Wilkins; 1968. [Google Scholar]
- Von Solodkoff M. Two cases of bullet injuries in elephant tusks. Tieraerztl. Prax. 1988;16:201–203. [PubMed] [Google Scholar]
- Weatherford HL. Some observations on the tusk of an Indian elephant – the innervation of the pulp. Anat. Rec. 1940;76:81–93. [Google Scholar]
- Weissengruber GE, Egerbacher M, Forstenpointner G. Mechanisms of loss and repair in traumatically injured tusks of African elephants. Proc. Inst. Zoo Wildlife Res. Berlin. 2003;5:425. [Google Scholar]
- Zhang JQ, Nagata K, Iijima T. Scanning electron microscopy and immunohistochemical observations of the vascular nerve plexuses in the dental pulp of rat incisor. Anat. Rec. 1998;251:214–220. doi: 10.1002/(SICI)1097-0185(199806)251:2<214::AID-AR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]