Abstract
The retinal and cerebral microvasculatures share many morphological and physiological properties. Assessment of the cerebral microvasculature requires highly specialized and expensive techniques. The potential for using non-invasive clinical assessment of the retinal microvasculature as a marker of the state of the cerebrovasculature offers clear advantages, owing to the ease with which the retinal vasculature can be directly visualized in vivo and photographed due to its essential two-dimensional nature. The use of retinal digital image analysis is becoming increasingly common, and offers new techniques to analyse different aspects of retinal vascular topography, including retinal vascular widths, geometrical attributes at vessel bifurcations and vessel tracking. Being predominantly automated and objective, these techniques offer an exciting opportunity to study the potential to identify retinal microvascular abnormalities as markers of cerebrovascular pathology. In this review, we describe the anatomical and physiological homology between the retinal and cerebral microvasculatures. We review the evidence that retinal microvascular changes occur in cerebrovascular disease and review current retinal image analysis tools that may allow us to use different aspects of the retinal microvasculature as potential markers for the state of the cerebral microvasculature.
Keywords: anatomical and physiological homology, cerebral microvasculature, cerebrovascular disease, digital image analysis, retinal microvasculature
Introduction
The retina and the brain are highly metabolically active tissues with large demands on metabolic substrates via specialized vascular networks. Embryologically, the retina is an extension of the diencephalon, and both organs share a similar pattern of vascularization during development (Risau, 1997; Hughes et al. 2000; Dorrell et al. 2002; Lutty et al. 2002). There is a close anatomical correlation between both the macrovascular and the microvascular blood supply to the brain and the retina, and both vascular networks share similar vascular regulatory processes (Lassen, 1964; Hardy et al. 1997; Delaey & Van de Voorde, 2000b).
Assessment of the cerebral vasculature is important in determining an individual's risk of particular cerebrovascular diseases, such as vascular dementia (Mielke & Heiss, 1998; Varma et al. 2002; Yoshikawa et al. 2003) and stroke (Sadek & Hammeke, 2002; Powers & Zazulia, 2003). Investigative techniques currently used include transcranial Doppler ultrasound, positron emission tomography (PET), single-photon tomography (SPECT) and functional neuroimaging using magnetic resonance imaging (fMRI). fMRI techniques utilize either blood oxygen level-dependent (BOLD) contrast (Ogawa et al. 1992), dynamic contrast-enhanced imaging (Calamante et al. 1999) or arterial spin labelling (ASL) (Detre et al. 1992) and have become increasingly prevalent over the past decade. These techniques have provided invaluable tools in advancing our understanding of cerebrovascular pathophysiology (Kessler, 2003). However, these techniques are often expensive and available only in specialized centres, and therefore are not suitable candidates for more widespread screening of patients at risk of cerebrovascular disease. A simpler, more accessible technique is required.
Retinal digital image analysis may indirectly provide such a technique. Owing to the homology between the retinal and cerebral microvasculatures, changes in the retinal vasculature may reflect similar changes in the cerebral vasculature. The potential for using the retinal vasculature as a marker of the state of the cerebrovasculature offers clear advantages, owing to the ease with which the retinal vasculature can be directly visualized in vivo, and also photographed because of its essential two-dimensional nature. The use of retinal digital image analysis has become increasingly common over the past decade, and offers increasingly sophisticated techniques to analyse different aspects of retinal vascular topography, such as the widths of retinal microvessels. It has long been known that vascular topography, including the angles at which blood vessels bifurcate and the relationship between the widths of parent to daughter blood vessels at vascular junctions, is not necessarily random, but is optimized in order to minimize physical properties such as shear stress across any vascular network (Murray, 1926a, b; Zamir, 1976; Zamir et al. 1979; Sherman, 1981; Zamir & Medeiros, 1982). Changes in this optimal geometrical topography are known to occur in certain vascular conditions (Stanton et al. 1995b; Chapman et al. 2002). Analysis of these vascular properties through digital image analysis may offer an opportunity to study the potential for retinal microvascular abnormalities to act as markers of cerebrovascular pathology.
In this review, we outline the anatomical and physiological homology between the retinal and cerebral microvasculatures. We review the evidence that retinal microvascular changes occur in cerebrovascular disease and review recent advances in retinal image analysis tools that may allow us to consider retinal digital image analysis as a potential screening tool for cerebrovascular disease.
Comparative microvascular anatomy
Cerebral capillaries create a rich anastomotic vasculature throughout the brain, the density of which varies according to the activity and metabolic demand of the particular brain region, e.g. microvascular density in the grey matter of the brain is three times greater than that observed in the white matter, and sensory centres are more richly supplied than motor centres (Gjedde & Diemer, 1985; Klein et al. 1986). Retinal capillary density is greater in the central retina, but decreases towards the retinal periphery. The extreme retinal periphery is avascular. Specific regions of the retina identified as dominating the oxygen requirements of the retina include the inner segments of the photoreceptors (Linsenmeier, 1986) and the inner and outer plexiform layers (Yu & Cringle, 2001; Cringle et al. 2002).
The retinal circulation is a relatively low-flow (Alm & Bill, 1973) and high-oxygen-extraction system (Tornquist & Alm, 1979). The retinal capillary microvasculature has two distinct beds: the superficial capillary layer in the nerve fibre/ganglion cell layer, and the deeper capillary layer extending into the inner nuclear and outer plexiform layers (Toussaint et al. 1961). These capillaries have a diameter of approximately 5–6 µm (smaller than the cerebral capillaries) (Leber, 1903; Cogan & Kuwabara, 1984). Unlike the cerebral microvasculature, which has more abundant collateral channels, retinal blood vessels are end arteries without anastomotic connections, and therefore occlusion of these vessels leads to destruction of the inner layers of the retina (Yanoff & Fine, 1989). Unlike cerebral arterioles, retinal arterioles often show 90° vascular branching patterns (Cogan & Kuwabara, 1984).
Both the inner retinal and the cerebral circulation are ‘barrier’ circulations
The inner retinal and cerebral microcirculations share anatomical and physiological properties owing to their similar functions acting as ‘barrier’ endothelia. This barrier function serves to maintain the neuronal milieu from exogenous toxins, to buffer variations in blood composition and to restrict the transfer of small hydrophilic and large molecules and haematogenous cells in the brain and retina (Lightman et al. 1987; Bradbury & Lightman, 1990; Tornquist et al. 1990; Pardridge, 1995). This barrier consists of both mechanical and metabolic components (Fig. 1). The mechanical barrier is primarily attributed to the presence of tight junctional intercellular complexes between the endothelial cells of both the cerebral and the retinal vasculature on their luminal aspect. Barrier endothelia are no longer considered as static lipid membrane barriers, but as dynamic interfaces, with specific and selective membrane transport acting as a metabolic barrier (Cornford, 1985). The presence of specific carrier-mediated transport proteins is a feature of both the blood–brain barrier and the inner blood–retinal barrier (Betz et al. 1983; Bradbury, 1985; Tornquist & Alm, 1986; Neuwelt, 1989) (see below).
Fig. 1.
Schematic diagram of the mechanical and metabolic components of the blood–brain and blood–retinal barriers and the influence of glial cells on these barriers. The mechanical component consists of the presence of apical (luminal) tight junctions composed of proteins such as occludin, claudins and junctional adhesion molecules (JAMs), often in conjunction with submembranous tight-junction-associated proteins, such as zonula occludens. The metabolic component consists of transport proteins, including GLUT-1, P-glycoprotein and transferritin.
Constituents of the cerebral and retinal microvasculatures
Endothelial cells
The endothelial cells of the cerebral and retinal capillaries form a single layer around the capillary lumen. They are non-fenestrated and possess tight junctional intercellular complexes between the endothelial cells of both the cerebral and the retinal vasculature on their luminal aspect (Brightman, 1989; Pardridge, 1993; Wolburg & Lippoldt, 2002; Vorbrodt & Dobrogowska, 2003). Tight junctional complexes are composed of several proteins, including occludin (Morcos et al. 2001; Wolburg & Lippoldt, 2002), junctional adhesion molecules (JAM) (Martin-Padura et al. 1998) and claudins (Tsukita & Furuse, 1999; Morcos et al. 2001) (Fig. 1). Transmembrane proteins are often found in conjunction with submembranous tight junction-associated proteins [zonula occludens (ZO-1, ZO-2, ZO-3)] (Stevenson et al. 1986; Watson et al. 1991; Wolburg & Lippoldt, 2002). Tight junctions form the mechanical component of the blood–brain and inner blood–retinal barriers.
Endothelial cells lack fenestrations and have a paucity of pinocytotic vesicles (Coomber & Stewart, 1986; Bertossi et al. 1997; Farkas & Luiten, 2001). They are rich in mitochondria and the presence of specific carrier-mediated transport proteins is a feature of both vasculatures (Betz et al. 1983; Bradbury, 1985; Tornquist & Alm, 1986; Neuwelt, 1989) (Fig. 1). These transport mechanisms form an important role in the metabolic component of the blood–brain and blood–retina barriers and include GLUT1 and GLUT3 (for glucose transportation) (Maxwell et al. 1989; Takata et al. 1992; Cunha-Vaz, 1997; Badr et al. 1999; Mann et al. 2003), and specific amino acid protein transport systems (Tornquist & Alm, 1986; Tornquist et al. 1990; Mann et al. 2003). These metabolic markers of barrier endothelia provide specific carrier-mediated transport of nutrients such as glucose and amino acids across the tight junctions, as well as enzymatic degradation of molecules crossing the blood–brain and blood–retinal barriers. In addition, it has been postulated that the asymmetric distribution of plasma membrane proteins on the endothelia (luminal vs. abluminal) creates a polarized endothelium, which helps to create an electrical resistance to permeability (Crone & Olesen, 1982).
Pericytes
The pericyte surrounds the capillary endothelial cell. Pericytes are embedded within a common basement membrane with the endothelial cell, provide structural support to the microvasculature and are required for the establishment of the blood–brain and blood–retina barriers (LeBeux & Willemot, 1980; Farrell et al. 1987; Frey et al. 1991; Risau et al. 1992; Healey & Wilk, 1993; Song et al. 1993; Martin et al. 2000; Ramsauer et al. 2002). Retinal pericytes are known to cover more of the retinal endothelial network than their cerebral counterparts (Cogan & Kuwabara, 1984; Frank et al. 1987b) (Frank et al. 1990) (Fig. 2). Pericytes are the capillary counterparts of vascular smooth muscle cells, containing α-smooth muscle actin and having contractile properties (LeBeux & Willemot, 1980; Herman & D'Amore, 1985; Joyce et al. 1985a, b). Cerebral pericytes also have a phagocytic role, which may operate as a ‘second line of defence’ at the boundary between blood and brain (Jordan & Thomas, 1988; Tagami et al. 1990; Thomas, 1999; Rucker et al. 2000).
Fig. 2.
Schematic diagram of (a) the retinal and (b) the cerebral microvessel (not drawn to scale). Note the greater pericyte coverage on the retinal endothelium, and the smaller calibre of the retinal vessel. OBF, ocular blood flow; CBF, cerebral blood flow.
Basement membranes
As well as providing structural support to the microvasculature, other functions of the basement membranes include influencing endothelial function, filtration of macromolecules and cell adhesion (Perlmutter & Chui, 1990). Cerebral basement membrane is the site of deposition of β-amyloid peptide in Alzheimer's disease (Perlmutter, 1994; Farkas & Luiten, 2001), and is also thickened in Parkinson's disease and experimentally in spontaneously hypertensive rats (Tagami et al. 1990).
Pathological thickening of the retinal microvascular basement membrane occurs notably in diabetic retinopathy (Cai & Boulton, 2002; Tsilibary, 2003), as well as in the rare genetic small vessel disease, cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) (Haritoglou et al. 2004a).
Surrounding glial cells
Both the cerebral and the retinal microvasculatures are surrounded by numerous astrocytic processes (known as perivascular end feet) (Abbott et al. 1992; Holash & Stewart, 1993). In vitro studies show that as well as providing structural support, these astrocytic processes play an important role in the development of endothelial zonulae occludens (ZO-1 expression), and the production by cerebral endothelial cells of specific blood–brain barrier proteins (Arthur et al. 1987; Janzer & Raff, 1987; Maxwell et al. 1987; Maxwell et al. 1989; Meyer et al. 1991; Rauh et al. 1992; Hurwitz et al. 1993; Janzer, 1993; Sun et al. 1995; Joo, 1996; Hayashi et al. 1997). Retinal astrocytes (and Mueller cells) have also demonstrated induction of barrier properties in vascular endothelial cells (Janzer & Raff, 1987; Gardner et al. 1997; Wolburg & Lippoldt, 2002), via the release of humoral factors [such as glial cell line-derived neurotrophic factor (GDNF), bFGF, TGFbeta] and direct contact (Tao-Cheng et al. 1987; Tontsch & Bauer, 1991; Dehouck et al. 1994; Igarashi et al. 1999; Ramsauer et al. 2002). Both cerebral and retinal astrocytes may also play a role in angiogenesis, inducing endothelial cell and pericyte differentiation. In response to hypoxia, retinal astrocytes (which predominate in the nerve fibre layer) stimulate the release of vascular endothelial growth factor (VEGF), which in turn stimulates the growth of retinal blood vessels across the retinal surface, using the astrocytic processes as a template for angiogenesis (Zhang & Stone, 1997). Mueller cells, which extend radially from the inner limiting membrane of the retina to the external limiting membrane, serve as templates for retinal vascular growth inwards to the inner nuclear layer.
Perivascular microglia are a distinct subset of microglia within the central nervous system (Graeber et al. 1989; Stoll & Jander, 1999). The origin of the perivascular microglia has been shown to be from blood-derived monocytic precursor cells, from which they are regularly replaced (Hickey et al. 1992; Lassman et al. 1993). Both cerebral and retinal microglia have phagocytic properties, phagocytosing cerebral and retinal neurons after injury (Schnitzer, 1989; Thanos, 1991; Mato et al. 1996; Schuetz & Thanos, 2004).
Although the inner retinal and cerebral circulations are morphologically very similar, they can exhibit significantly different responses to various insults, and these differences may explain some of the variation between the two vasculatures in certain pathological processes (Lorenzi et al. 1986; Bradbury et al. 1989; Lightman & Yuen, 1989; Kern & Engerman, 1996; Grammas & Riden, 2003).
Regulation of cerebral and retinal circulation
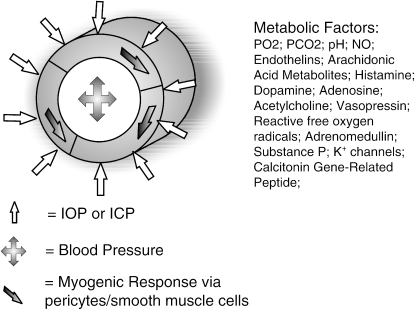
Both the brain and the retina have control mechanisms in place to allow a constant blood flow and hence delivery of nutrients in the face of a broad range of external factors, such as systemic blood pressure (Robinson et al. 1986). This local process of control (autoregulation) is a property of both the inner retinal and the cerebral circulation (Fig. 3).
Fig. 3.
Schematic diagram of the myogenic (via pericytes/vascular smooth muscle) and metabolic components of vascular autoregulation of the retinal and cerebral microvasculature. IOP, intraocular pressure; ICP, intracranial pressure; NO, nitric oxide.
The perfusion pressure of the cerebral circulation is related to systemic blood pressure and intracranial pressure by the following relationship:
The perfusion pressure of the ocular circulation is related to systemic blood pressure and intraocular pressure by a similar relationship:
In order for the retinal and cerebral circulations to maintain cerebral blood flow over a range of systemic blood pressures, their vascular resistance has to be altered accordingly. This is mediated by the vascular smooth muscle of the cerebral and retinal arterioles and pericytes (Alm & Bill, 1972; Riva et al. 1981; Riva et al. 1986; Delaey & Van de Voorde, 2000b; Vavilala et al. 2002) (predominantly via changes in vessel diameters). Cerebral blood flow is maintained between approximately 50 and 160 mmHg mean arterial blood pressure (MABP) (Wagner & Traystman, 1985; Paulson et al. 1990). In the case of increased intraocular pressure (IOP), the upper limit for autoregulation is approximately within 40–45 mmHg of the MABP (Grunwald et al. 1988). With increases of systemic blood pressure of approximately 40%, autoregulation is overcome, and retinal blood flow will increase (Robinson et al. 1986). The degree of autoregulation within the retinal vascular circulation has been shown to vary, with the region supplied by the superficial capillary bed being better regulated than the deeper capillary bed (Yu et al. 1994), and this may explain the frequent involvement of the deeper capillaries in retinal vascular disease (Yanoff & Fine, 1989).
Autoregulation consists of two components (Fig. 3).
(a) Myogenic
This is defined as the capacity of both vascular smooth muscle cells and pericytes of the retinal and cerebral microvasculature to contract in response to an increase in transmural pressure (Ursino, 1991), and has been directly visualized in isolated retinal and cerebral blood vessels, producing increased vascular tone and decreased luminal diameter (Wallis et al. 1996; Delaey & Van de Voorde, 2000a; Yu et al. 2003).
(b) Metabolic
Cerebral and retinal blood flow is related to local metabolic demand, which depends on regional neuronal activity. Hence, cerebral blood flow is coupled to the regional utilization of glucose (an indicator of neuronal activity) and cerebral oxygen metabolic rate (Hoge et al. 1999; Leenders et al. 1990). In the retina, blood flow has been found to be greater in the temporal retina (containing the metabolically active macula) than in the nasal retina (Riva et al. 1985; Rassam et al. 1996) and increases under conditions of light exposure (Bill & Sperber, 1990a, b; Kondo et al. 1997). Similarly, cerebral regulation of blood flow is driven by metabolic activity (Fox et al. 1988), and can vary regionally within the brain at any one time. As well as the local accumulation of metabolites, local parenchymal and endothelial substances have major impacts on vessel tone, such as nitric oxide (NO) (Palmer et al. 1987; Kondo et al. 1997; Harris et al. 1998), endothelins (Nyborg et al. 1991; Meyer et al. 1993; Dallinger et al. 2000; Polak et al. 2001), arachidonic acid metabolites (Nielsen & Nyborg, 1989; 1990; Hoste & Andries, 1991; Yu et al. 2001) and others (Benedito et al. 1991; Ferrari-Dileo et al. 1991; Ma et al. 1996; Krejcy et al. 1997; Abbott, 2000; Yu et al. 2003).
Neurogenic control of retinal and cerebral blood flow
The retinal vasculature is devoid of autononomic innervation beyond the level of the lamina cribrosa (Ehringer, 1966; Laties, 1967; Ferrari-Dileo et al. 1989; Hoste et al. 1990; Ye et al. 1990), and therefore the regulatory control mechanisms in the retinal circulation are not under neurogenic control and it relies predominantly on local vascular control mechanisms. However, the choroidal circulation is under neurogenic control, and vasoconstriction via sympathetic stimulation (including noradrenergic and neuropeptide-Y fibres) to the choroidal circulation as well as the extraocular circulation may assist myogenic and metabolic retinal autoregulatory mechanisms (Bill, 1962a, b; Stone et al. 1986). Likewise, there is no autonomic innervation to the cerebral vasculature beyond the pial vessels (Farkas & Luiten, 2001). However, there is evidence of a cholinergic pathway from the basal forebrain to the frontoparietal cortical microvasculature, capable of increasing regional cortical blood flow by vasodilatation (Biesold et al. 1989; Luiten et al. 1996; Barbelieven et al. 1999; Sato et al. 2001; Hamel, 2004), mediated via NO (Adachi et al. 1992; Zhang et al. 1995; Tong & Hamel, 2000). The sympathetic innervation to the large cerebral arteries is derived from the superior cervical ganglion, and includes the neuropeptides norepinephrine and neuropeptide-Y (Uddman & Edvinsson, 1989; Edvinsson et al. 1990). Monoaminergic brainstem centres such as the dorsal raphe nucleus, locus coeruleus or nucleus reticularis pontis oralis also influence vessel tone. The autonomic control of the larger cerebral vessels may exert a degree of regulation of cerebral blood flow, but the finer control on cerebral blood flow is exerted via myogenic and metabolic mechanisms of the cerebral microvasculature.
Evidence of retinal microvascular changes reflecting the cerebral microvasculature in aging and disease
Aging
Both the retinal and the cerebral microvasculatures undergo similar changes with aging. A reduction in cerebral blood flow (Tachibana et al. 1984; T et al. 1986; Marchal et al. 1992; Kawamura et al. 1993; Schultz et al. 1999; Slosman et al. 2001), decreased glucose and oxygen metabolism (Kuhl et al. 1982; 1984; Alavi, 1989; Eberling et al. 1995; Moeller et al. 1996), and impairment of the structural integrity of the anatomy of the microvasculature are all features of the aging brain. Similarly, retinal blood flow decreases incrementally with age (Rimmer et al. 1989; Costello et al. 1992; Grunwald et al. 1993; Williamson et al. 1995; Groh et al. 1996; Embleton et al. 2002), and exhibits decreased metabolic demand (Gramer & Dirmeyer, 1998). A recent study has shown evidence of an age-related decrease in retinal vascular autoregulation, related to increasing systemic blood pressure (Jeppesen et al. 2004). In contrast, another small study observed no such age-related change in retinal vascular diameter response to flicker light (Nagel et al. 2004). The cerebral vasculature undergoes morphological changes with age, including basement membrane thickening, and a decrease in endothelial and pericyte cell populations (Stewart et al. 1987; Mooradian, 1988; de Jong et al. 1991; Mooradian et al. 1991; Luiten et al. 1994; Farkas et al. 2000; Keuker et al. 2000; Farkas & Luiten, 2001). Retinal vascular age-related morphological changes have not been extensively studied. In one study using electron microscopy (Lee et al. 1987), features of aging-related vascular changes in two middle-aged eyes (52 years and 60 years) revealed extensive multilayering of the basement membrane and deposition of collagen. Density of the cerebral microvasculature decreases with age (Abernathy et al. 1993). Between the 6th and 7th decades, normal aged subjects demonstrate increased capillary diameter, volume and total length (Hunziker et al. 1979; Bell & Ball, 1981; Mann et al. 1986).
Retinal vascular changes in cognitive decline and dementia
Vascular dementia accounts for approximately 20% of all causes of dementia (Geldmacher & Whitehouse, 1996; Roman, 2003). Causes of vascular dementia include large cortical–subcortical infarcts and multiple infarcts, subcortical ‘small-vessel’ disease, and single infarcts in a strategic location critical to mental function (Roman, 2003). It is now recognized that cerebrovascular small-vessel disease with white-matter lesions and lacunar infarcts is an important cause of cognitive impairment of cerebrovascular origin (Erkinjuntti et al. 2000; Inzitari et al. 2000; Jellinger, 2002; Mok et al. 2004). Cerebral white-matter lesions are seen as hyperintensities on T2-weighted MRI scans, typically located in the periventricular areas and the anterior limb of the internal capsule. The severity of white-matter lesions is directly proportional to the degree of stenosis of the medullary arterioles due to arteriosclerosis (van Swieten et al. 1991). Lacunes result from occlusion of the lenticulostriate, thalamo-perforating and long medullary arterioles (Fisher, 1982). Lacunar infarcts are typically located in the thalamus, caudate nucleus, globus pallidus, internal capsule and frontal white matter (Lammie, 2002; Roman et al. 2002). A study in spontaneously hypertensive rats suggests retinal arterial changes may be predictive of cerebral arterial changes in hypertension (Tomassoni et al. 2002). Pathological studies have shown characteristic cerebral arteriolar changes (attenuation, increased tortuosity, increased capillary microaneurysms) associated with dementia (Miyakawa et al. 1988; Fischer et al. 1990; Kalaria, 1992; Buee et al. 1994; Moody et al. 1997).
Other dementias such as Alzheimer's disease are known to have a vascular component, with small-vessel disease and microinfarction (Ravona-Springer et al. 2003; Thal et al. 2003) and an increasingly recognized cause of dementia in the elderly is mixed Alzheimer's disease/vascular dementia (MRC/CFAS, 2001). As well as being an important cause of dementia in the elderly, cerebrovascular disease is also increasingly recognized as an important factor in development of mild cognitive impairment.
Few studies to date have explored retinal microvascular changes in cognitive impairment (Kwa et al. 2002; Wong et al. 2002b, c). Kwa et al. (2002) found that retinal arteriolar abnormalities, including narrowing, arteriolosclerosis and presence of retinal exudates, correlated with MRI signs of cerebral white-matter lesions. In addition, presence of lacunar infarction correlated with retinal exudation. The authors also found a substantial number of patients with retinal microvascular abnormalities who did not have evidence of cerebral white-matter lesions, and suggest that this may reflect a period of time required before the small vessel changes lead to the development of white-matter lesions. However, their study highlights the problem of using subjective observer-driven techniques to assess retinal microvascular abnormalities, as interobserver agreement was modest. To overcome this, only patients in which consensus was agreed between the two ophthalmologists were included in their study, but this may have an element of bias. However, their study supports the concept of retinal vascular imaging as a useful approach in screening patients at risk of cerebral small-vessel disease, and potentially as an indicator of those patients who may be at increased risk of developing cognitive impairment in later life.
The Atherosclerosis Risk in Communities Study (ARIC) was a large, population-based, cross-sectional study of 15 792 participants, ranging in age from 45 to 64 years. The ARIC study explored the relationship between retinal microvascular abnormalities and cognitive impairment (tested using the delayed word recall test, digit symbol subtest and word fluency test) in this middle-aged population (Wong et al. 2002c) and found that the presence of retinal microvascular abnormalities (presence of any retinopathy, micoraneurysms, retinal haemorrhages and exudates) was independently associated with a small decrease in cognitive function (two standard deviations lower than the mean score). The ARIC study lends further evidence that vascular permeability may be an important element in cerebral vascular changes leading to cognitive decline, as the retinal anomalies most consistently associated with cognitive impairment were micoraneurysms (odds ratio 1.62–3.00) and retinal haemorrhages (odds ratio 1.99–4.10), rather than arteriolar narrowing. Indeed, generalized arteriolar narrowing was not found to be correlated with cognitive function. Microaneurysms and retinal haemorrhages are indicative of more severe microvascular disease (Wong et al. 2002c), and, in conjunction with the finding that the same retinal features are most strongly associated with incident stroke, suggest that blood–brain barrier breakdown may be an important pathological feature in both cognitive impairment and stroke. An important drawback of the ARIC study is that the cognitive function tests were not done contemporaneously with the retinal photography, but were performed either 3 years previously or afterwards, and that the absence of visual acuity measurements may have had an effect on the outcomes, if those who could not optimally perform the cognitive function tests had visual impairment.
In addition, the ARIC study published evidence of retinal microvascular changes occurring independently in association with both MRI-defined white-matter lesions (Wong et al. 2002a) and sulcal widening and ventricular enlargement on MRI in this middle-aged population (Wong et al. 2003c). Again, the more severe signs of microvascular damage (microaneurysms, haemorrhages and exudates) were most strongly associated with MRI-defined white-matter lesions and cerebral atrophy. This study also showed that MRI-defined white-matter lesions were independently related to risk of clinical stroke. In the presence of retinopathy, those with MRI-defined white-matter lesions were 18.1 times more likely to develop stroke than those without either white-matter lesions or retinopathy.
The ARIC study also provided indirect evidence of retinal vascular abnormalities being related to impaired cognitive performance. They found a weak association between early age-related maculopathy (ARM) and cognitive function (word fluency test) in a middle-aged population (Wong et al. 2002b). Both ARM and cognitive function may share common vascular risk factors (Klein et al. 1993, 1997; Vingerling et al. 1995; Ott et al. 1998; Knopman et al. 2001). Indeed, the Blue Mountains Eye Study found a weak association between retinal microvascular changes (focal arteriolar narrowing and arteriovenous nicking) and ARM progression (Klein et al. 1993; Wang et al. 2004). The Rotterdam Study also found a weak association between late ARM and incidence of Alzheimer's disease in a population over 75 years of age (Klaver et al. 1999).
Retinal morphological changes are known to occur in Alzheimer's disease, including depletion of optic nerve ganglion cells, loss of nerve fibre layer and abnormal pattern ERG responses (reduced implicit time and amplitude) (Hinton et al. 1986; Katz et al. 1989; Trick et al. 1989; Blanks et al. 1996a, b; Parisi et al. 2001). Beta-amyloid and amyloid-associated proteins related to the pathogenesis of Alzheimer's disease have been isolated in retinal ganglion cells and nerve fibres (Loffler et al. 1995). There may be a common vascular component to the neurodegenerative process of both Alzheimer's disease and the resultant neuronal loss both at the cerebral and the retinal level.
There have been some studies looking at the association of diabetic retinopathy as a marker of microangiopathy, and its association with cognitive function. Some of these studies have been confounded by the presence of co-morbidity, such as hypertension (Dejgaard et al. 1991; Yousen et al. 1991; Lunetta et al. 1994). One study found an association between presence of microangiopathy and the presence of leukoaraiosis (Dejgaard et al. 1991), whereas another study found no evidence of cerebral MRI abnormalities in a cohort of patients who had had laser treatment for proliferative diabetic retinopathy (Yousen et al. 1991). More recently, Ferguson et al. (2003) reported on a cross-sectional study of young people with type 1 diabetes and found an association between the presence of diabetic retinopathy and small focal white-matter hyperintensities in the basal ganglia. In addition, presence of background diabetic retinopathy was associated with different domains of cognition, including fluid intelligence, information processing and attention ability. This association persisted when age, gender, premorbid IQ and duration of diabetes were accounted for. A possible explanation for the association may be a difference in visual function affecting the cognitive tests in the patients with background diabetic retinopathy. However, visual function processing, as assessed by the visual evoked potential P100 latency, is unaffected by background retinopathy, suggesting that the most likely explanation is that the retinal diabetic microangiopathy acts as a marker of suboptimal diabetic control, with chronic hyperglycaemia (Parisi et al. 1994). Long-duration type 1 diabetes complicated with retinopathy is known to be associated with impaired cerebrovascular responsiveness (Fulesdi et al. 1997).
Retinal and cerebral microcirculatory changes in hypertension and diabetes
Two of the most important cardiovascular risk factors for stroke are hypertension and diabetes. Both the cerebral and the retinal microcirculations exhibit morphological changes in these two conditions. However, whereas in hypertension the pathological changes are very similar, diabetes demonstrates how the microvasculatures may differ in response to certain conditions.
(a) Hypertension
Both cerebral and retinal microvasculatures undergo morphological changes with increasing blood pressure (Hill, 1970; Goto et al. 1975; Tso & Jampol, 1982). Hypertension causes generalized retinal arteriolar narrowing. In vessels with arteriolosclerosis, focal narrowing and dilatation may occur. The sclerotic phase is associated with tunica media hyperplasia and hyaline degeneration of the arteriolar wall, and in addition to vessel attenuation, may be associated with arteriovenous nicking and arteriolar tortuosity. Continued high blood pressure may lead to exudative changes and blood–retinal barrier breakdown, with fibrinoid necrosis, luminal narrowing and ischaemia, leading to retinal haemorrhages, micoraneurysms, exudates and nerve fibre layer ischaemia (cotton wool spots). Retinal microvascular changes appear not only to reflect current blood pressures, but also past blood pressures (Sharett et al. 1999), in particular generalized arteriolar narrowing and arteriovenous nicking.
Similar cerebral microvascular changes occur in hypertension, including hyaline arteriosclerosis (Lammie, 2002), leading to luminal narrowing (which correlates with systemic blood pressure) (Furuta et al. 1991). Pathologically, tunica media and the internal elastic lamina degenerate and are replaced by fibrous tissue. This leads to increased vessel tortuosity, and increased vessel permeability (Fredriksson et al. 1988), owing to breakdown of the blood–brain barrier. The pathological hallmark of acute hypertensive brain damage is fibrinoid necrosis (Gustafsson, 1997).
(b) Diabetes
The retinal microvascular changes occurring in diabetes mellitus are well documented (Cai & Boulton, 2002), including loss of retinal pericytes, basement membrane thickening, capillary microaneurysm formation, increased vascular permeability leading to exudation and tissue oedema, and capillary occlusion causing ischaemia, which may lead to retinal neovascularization. The cerebral circulation shows some significant differences and is much less affected in diabetes (Kern & Engerman, 1996). Cerebral vascular lesions reported to occur in diabetes are predominantly capillary basement membrane thickening (Mukai et al. 1980; Johnson et al. 1982; Frank et al. 1987a) or alterations in number or tortuosity of capillaries (Mukai et al. 1980; Jaboksen et al. 1987). Studies have found no evidence of pericyte loss or damage in histological cross-sections of cerebral cortex from patients said to have diabetic retinopathy (de Oliveira, 1966; Addison et al. 1970), and concluded that pericyte loss developed preferentially in the retina. Kern & Engerman (1996) found that microaneurysms, acellular capillaries and pericyte ‘ghosts’ developed in retinas of dogs with diabetes or galactosaemia, but did not develop in cerebral cortical vessels from the same animals. As stated earlier, pericytes cover more of the capillary circumference in retina than in brain (Frank et al. 1987b, 1990). Greater loss of pericytes from the retina compared with the cerebral cortex in diabetes thus might lead to differences in blood flow regulation between the two tissues. Consistent with this, Kern & Engerman (1996) found that capillary diameter tended to be increased in retinal capillaries from animals with diabetes, but cerebral capillaries were not. There are significant differences in the rate of glucose uptake between retinal and cerebral endothelial cells (Badr et al. 2000; Tang et al. 2000; Rajah et al. 2001), although the results are somewhat contradictory. Whereas Rajah et al. (2001) found an inability of retinal endothelial cells to down-regulate glucose uptake in the presence of high glucose levels, suggesting that this could make retinal microvessels more sensitive to the deleterious effects of hyperglycaemia, Badr et al. (2000) did find a down-regulation of the specific carrier-mediated transport protein GLUT-1 in retinal endothelial cells, but that no such down-regulation occurred in the cerebral microvasculature. Tang et al. (2000) reported a decrease in GLUT-1 expression in both tissues (55 vs. 36%, respectively). In addition, they report an increase in retinal endothelial glucose concentration in diabetes, with no such increase noted in cerebral tissue. Further studies are needed to clarify exactly the differences in glucose uptake between these two microvasculatures in hyperglycaemia. Other biochemical disparities include differences in gamma-glutamyl transpeptidase (Kowluru et al. 1994), protein kinase C (Shiba et al. 1993) and rate of glucose oxidation (Kennedy et al. 1983). One of the effects of diabetes on the retinal circulation is a reduction and redistribution of occludin in retinal endothelial cells with resultant increased vascular permeability (Antonetti et al. 1998; Barber et al. 2000). ZO-1 expression is greatly increased in brain-derived endothelial cells under hyperglycaemia, whereas retinal endothelial cells are unaffected (Grammas & Riden, 2003). Thus the cerebrum shows little or no permeability defect in diabetes (Mooradian, 1997). There is evidence to suggest that the retina has a reduced response to oxidative stress compared with the cerebral circulation with relatively lower levels of glutathione peroxidase and superoxide dismutase (Grammas & Riden, 2003).
Evidence of retinal microvascular changes in stroke
There is evidence that retinal microvascular anomalies reflect cerebrovascular changes relating to stroke (Aoki, 1975; Okada et al. 1976; Svardsudd et al. 1978; Tanaka et al. 1985; Sano et al. 1994; Kobayashi et al. 1997). After adjusting for other risk factors (age, sex, race, blood pressure, diabetes), the ARIC study found retinal microvascular anomalies were predictive of incident stroke (including ischaemic stroke) as well as MRI-detected subclinical stroke (Wong et al. 2001a). The relationship between retinal microvascular changes and stroke was strongest for micoraneurysms and soft exudates (adjusted relative risk 3.11 and 3.08, respectively).
The Cardiovascular Health Study similarly found a relationship between retinal microvascular changes and stroke, after controlling for blood pressure and other risk factors (Wong et al. 2003b). Participants with retinopathy were twice as likely to have a stroke as those without retinopathy (odds ratio 2.0, 95% CI 1.1–3.6).
The Beaver Dam Eye Study found an association with the presence of retinal microaneurysms, haemorrhages, and retinal arteriolar narrowing and 10 years risk of stroke and coronary heart disease mortality (Wong et al. 2003a).
In a prospective study, the Blue Mountains Study found an increased relative risk for all forms of retinopathy (Wong, 2004), and unlike previous studies, the association with generalized arteriolar narrowing was as strong as the association with focal microaneurysms and haemorrhages [relative risk 3.0 (95% CI 1.1–8.2) vs. 3.0 (95% CI 1.9–5.2), respectively].
Other investigators have found a relationship between lacunar infarcts and retinal microvascular abnormalities (Korber et al. 1986; Schneider et al. 1993), and autopsy studies have shown a correlation between retinal and cerebral vasculature changes in patients who had died of stroke (Goto et al. 1975). Studies have also shown retinal arteriolar changes in the spontaneously hypertensive rat (Hamada, 1993). In addition, Kappelle et al. (1988) found in a prospective study that 78% of patients with lacunar stroke had retinal microvascular abnormalities. In another study, there was no difference in the prevalence of retinal lesions in patients with either lacunar infarcts or cortical strokes (Luijckx et al. 1998). Interestingly, Hiroki et al. (2003) reported on the association between central retinal artery Doppler parameters and cerebral small-vessel disease. They found that end-diastolic and mean velocities was related to the severity of cerebral small-vessel disease, independently of aging.
Hereditary small-vessel disease
This forms a small but important group of conditions that serve to illustrate the close relationship between the two barrier microcirculations of the brain and the retina, and their similar morphological response to disease. They include the conditions CADASIL, cerebrovascular retinopathy (CRV), hereditary endotheliopathy with retinopathy, nephropathy and stroke (HERNS) and hereditary vascular retinopathy (HVR).
CADASIL
CADASIL is a rare autosomal dominant microangiopathy, resulting from defects in the NOTCH 3 gene of 19q13.1 (Joutel et al. 1996), encoding for a receptor expressed on vascular smooth muscle cells and pericytes (Joutel et al. 2000). The clinical characteristics of those affected include recurrent transient ischaemic attacks (TIAs), early onset stroke, dementia and migraine with auras (Nishio et al. 1997; Dichgans et al. 1998; Haritoglou et al. 2004a, b). Patients invariably have diffuse white-matter hyperintensities and lacunar infarcts on neuroimaging (Dichgans et al. 1998). Retinal vascular findings are well documented in CADASIL, and have been found to resemble closely those of the cerebral microcirculation. Retinal features described include retinal arteriolar sheathing and attenuation (Robinson et al. 2001; Haritoglou et al. 2004b), arteriovenous nicking and remnants of branch retinal vein occlusions (Haritoglou et al. 2004b). Histopathological examinations of cerebral medullary arteries have shown loss of vascular smooth muscle cells, with adventitial fibrosis, but no evidence of vascular occlusion (Ruchoux et al. 1995; Ruchoux & Maurage, 1997; Okeda et al. 2002). Histopathological examination of retinal arterioles also shows loss of vascular smooth muscle cells, perivascular fibrosis, with thickened basement membrane and granular osmiophilic material in arterial walls, but with no evidence of arteriolar occlusion (Haritoglou et al. 2004a). Interestingly, no evidence of any choroidal vascular abnormalities was detected. The pathological hallmark of CADASIL (deposition of granular osmiophilic material within the basement membrane of smooth muscle cells) can be detected in other tissues outside of the central nervous system, and indeed skin or muscle biopsy is the normal mode of diagnosis of CADASIL. However, the clinical manifestations seem to be restricted to the cerebral and retinal microcirculations, possibly reflecting their specialized barrier function.
Cerebrovascular retinopathy
CVR is a rare genetic condition characterized by abnormal vasculature in the brain and retina (Grand et al. 1988; Gutmann et al. 1989). Unlike CADASIL, CRV is characterized by retinal capillary occlusion, with fluorescein angiography showing capillary closure, and the presence of shunt vessels. Histopathological examination of brain lesions (which often form intracerebral masses mimicking a brain tumour on neuroimaging: Weil et al. 1999; Niedermayer et al. 2000a, b) reveal arterial wall thickening, perivascular fibrosis, thrombosis and occlusion of small vessels, with fibrinoid necrosis and adjacent marked oedema and astrogliosis.
HERNS
HERNS was first described by Jen et al. (1997), affecting 11 members of a Chinese American family over three generations in an autosomal dominant inheritance pattern. Retinal changes described include retinal capillary occlusion, perifoveal telangiectasias and macular oedema. Neurological features included migraine, dysarthria, hemiparesis and apraxia. Neuroimaging revealed multiple subcortical lesions with surrounding oedema. In addition to brain and retinal involvement, several family members had renal involvement with proteinuria and haematuria. Brain biopsy from one of the patients revealed occlusion of small blood vessels, with intense subcortical white matter astrocytic gliosis. HERNS may be part of the same spectrum as CRV, in which systemic involvement has not been documented.
Hereditary vascular retinopathy
HRV is another autosomal dominant condition characterized by retinal occlusive microangiopathy (Storimans et al. 1991). As well as retinal features, cerebral symptoms, including migraine, mild cognitive decline and depression, have been described. White-matter abnormalities have also been reported.
HVR, CRV and HERNS have all been mapped via linkage analysis to a single locus on chromosome 3p21.1–p21.3 (Ophoff et al. 2001). Recently, a new hereditary small-vessel disease of the retina and brain has been described (Dichgans, 2003; Vahedi et al. 2003), characterized by retinal arterial tortuosity and retinal haemorrhages. Cerebral manifestations include infantile hemiparesis and migraine with aura. Neuroimaging revealed diffuse leucoencephalopathy with dilated perivascular spaces. Genetic analysis revealed no linkage to the 3p21 locus. This may be related to the condition hereditary retinal arterial tortuosity with retinal haemorrhages (Goldberg et al. 1972; Clearkin et al. 1986), which was believed only to affect the retinal arteries, although there is one report of a case with this condition suffering migraine, and having multiple small areas of midbrain ischaemia on neuroimaging (Sears et al. 1998).
These rare inherited small-vessel diseases typically involve both the inner retinal and the cerebral circulations. They illustrate how the pathological event occurring in the cerebral circulation seems to be mirrored by the retinal circulation. In any patient presenting with evidence of small-vessel disease, ophthalmoscopy is recommended to help in making the diagnosis (Robinson et al. 2001; Dichgans, 2003), particularly in young patients (Vahedi et al. 2003).
Table 1 summarizes the main associations found between retinal microvascular changes and stroke, cognitive impairment, cerebral white-matter lesions and cerebral atrophy.
Table 1.
Associations (odds ratios +95% confidence intervals) between cerebrovascular diseases and retinal microvascular changes
| Generalized arteriolar narrowing | Focal arteriolar narrowing | Focal retinopathy (microaneurysms, hamorrhages, exudates, cottonwool spots) | |
|---|---|---|---|
| Cognitive impairment (ARIC) | 1.1 (0.8–1.5) | 0.6 (0.4–0.9) | 2.6 (1.7–4.0) |
| Stroke | |||
| (a) CHS | 1.1 (0.7–1.8) | 1.2 (0.6–2.4) | 2.0 (1.1–3.6) |
| (b) ARIC† | 1.2 (0.7–2.3) | 1.2 (0.7–1.9) | 2.6 (1.6–4.2) |
| (c) BMES | 3.0 (1.1–8.2) | 2.6 (1.5–4.4) | 3.0 (1.9–5.2) |
| Cerebral white-matter lesions | |||
| (a) ARIC | 1.2 (0.8–1.9) | 2.1 (1.4–3.1) | 2.5 (1.5–4.0) |
| (b) Kwa et al. | 2.3 (1.1–4.6)* | Not assessed | 3.4 (1.5–8.1) |
| Cerebral atrophy (ARIC) | 1.0 (0.7–1.4) | 1.1 (0.8–1.6) | 1.9 (1.2–3.0) |
ARIC, Atherosclerosis Risk in Communities Study; CHS = Cardiovascular Health Study; BMES = Blue Mountains Eye Study.
Generalized narrowing subjectively assessed by ophthalmologist.
Prospective study; relative risk data.
Retinal vascular image analysis and its potential role in predicting cerebrovascular changes
Subjective evaluation of the retinal vasculature has been found to be unreliable (Kagan et al. 1966; Aoki, 1975; Dimmitt et al. 1989). More objective methods to assess the retinal microvascular topography are now possible, owing to the development of digital retinal image processing techniques. Digitalized image analysis techniques have been shown to be more reliable than previous micrometric techniques (Delori et al. 1988; Newsom et al. 1992; Sherry et al. 2002).
Image acquisition
Photography is the most common technique used to acquire retinal vascular images. This has usually been conventional film photography, using a fundus camera and requiring separate film processing. Processed photographic images can then be digitalized before being subjected to image analysis. However, recent improvements in the resolution of direct digital cameras has allowed direct digital capture of images using a charged coupled device (CCD) directly attached to a fundus camera. This has the advantage of immediate image acquisition, without the need for film processing. It also enables one to assess instantly the quality of the image, and repeat the process if necessary, in order to optimize the quality of the image. Any poor-quality images can be instantly discarded.
As well as conventional and digital photography, other newer techniques have been developed for retinal image acquisition. The retinal vessel analyser (RVA®; Imedos, Weimar, Germany) consists of a retinal fundus camera, a CCD video camera, a real-time monitor for electronic online image acquisition, and a PC for overall system control, image analysis and result archiving (Seifert & Vilser, 2002; Vilser et al. 2002). It allows real-time assessment of retinal vascular diameters at a maximum frequency of 50 Hz (allowing 25 vessel diameter readings per second) and has demonstrated reproducible results (Polak et al. 2000; Pache et al. 2002). Adaptive algorithms allow for measurement of retinal vessel widths, utilizing the absorbing properties of haemoglobin in each blood vessel. The system is able to correct automatically for slight adjustments in luminance that may occur due to slight eye movement, and thus vessel diameter can be recorded as a function of time as well as position along the vessel. A major limitation of the RVA® is that it assumes that the eye under measurement has no refractive error (emmetropia) and uses standardized units to measure vessel diameters. Therefore, the RVA® is unable to give actual measurements of vessel wall widths if a significant number of subjects may not conform to the assumptions of emmetropia. However, attempts at finding a value for the diameter of the central retinal artery in vivo using the RVA® have been performed, utilizing the diameters of all retinal veins entering the optic disc and laser Doppler velocimetry as a measure of the total retinal blood flow, and combining this with the velocity of blood flow in the central retinal artery (Dorner et al. 2002).
The scanning laser ophthalmoscope (SLO) (Nagel et al. 1992) provides a high-quality image of the fundus using less than 1 : 1000 of the light necessary to illuminate the fundus with conventional light ophthalmoscopy. During image acquisition, only one point on the fundus is illuminated at any one time. The laser sweeps across the fundus in a raster-like fashion so that a piece-by-piece image of the fundus is built up on the monitor. In addition, because the SLO only illuminates a small area of the fundus at any one time, only a small amount of the patient's pupil is used for illumination. This means that pupil dilation is not usually necessary when acquiring fundal images with the SLO. However, the optical resolution of the SLO is currently only 10–20 µm per pixel, and therefore is currently insufficient to be able to produce accurate measurements of retinal vessels. A non-mydriatic wide-field SLO is now commercially available.
The SLO can be combined with laser Doppler flowmetry to obtain measurements of retinal blood vessel diameter. A tracking stripe provided by a green 543-nm HeNe laser orientated perpendicular to the target vessel is used to measure the retinal vessel. The diameter of the vessel is determined automatically by computer analysis of the signal produced by the image of the vessel on the CCD sensor using the half height method described below. In order to correct for the refractive status of the subject, the operator has to input the axial length of the eye, and the Laser Doppler Flowmeter automatically measures the refractive error of the eye. A distinct advantage of the laser Doppler flowmeter in measuring individual vessels is that it takes numerous measurements over a 2-s time period, and therefore can be averaged to account for the different stages of the cardiac cycle. However, a significant drawback of the technique is that the resolution is limited to vessels greater than approximately 60 µm in diameter.
As well as measuring retinal blood vessel diameter, the laser Doppler flowmeter is able to calculate retinal blood flow, volume and velocity. Light reflected or scattered at moving objects is frequency shifted due to the optical Doppler effect. It interferes with unchanged light reflected at surrounding tissue. The interference causes characteristic temporal intensity variations of the measurable reflected light intensity. This frequency shift can then be used to obtain information on retinal blood flow, volume and velocity. However, the measurements obtained from laser Doppler flowmetry shows variable reproducibility (Guan et al. 2003; Yoshida et al. 2003; Jonescu-Cuypers et al. 2004).
Image analysis
Measurement of retinal vessel widths
One of the most important topographical features of retinal vessels amenable to objective measurement is the retinal vessel wall widths. Attempts at quantifying retinal arteriolar calibres were first considered by Wagener et al. (1947). During the 1960s and 1970s, the introduction of retinal photography allowed semi-objective methods of performing measurements on retinal vasculature using enlarged projected images (micrometric methods) and calipers (Burgess, 1967; Hodge et al. 1969; Parr & Spears, 1974a, b; Cunha-Vaz & Lima, 1978; Bracher et al. 1979; Arzabe et al. 1990; Hubbard et al. 1992). Since the mid-1980s, the introduction of digital image analysis has been used to provide more objective measurements of retinal vascular parameters, including measurements of vessel calibre (Brinchmann-Hansen, 1986; Delori et al. 1988; Eaton & Hatchell, 1988; Newsom et al. 1992; Penn & Gay, 1992; Rassam et al. 1994; Stromland et al. 1995; Wu et al. 1995; Gao et al. 2000).
These techniques commonly rely on intensity profiles of a greyscale image of the fundus (densitometry). A standard greyscale image is produced by eliminating the hue and saturation in the digitized colour fundus photograph while retaining the luminance. The subsequent intensity values will have a range from 0 (black) to 255 (white). A greyscale image consists of many elements, or pixels. The location of each pixel can be identified with spatial coordinates and each has a defined intensity, known also as its grey value.
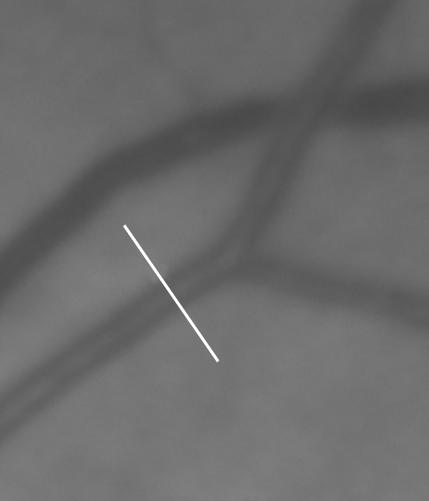
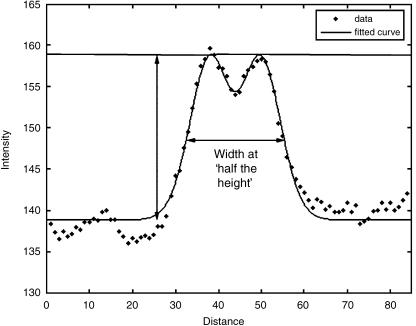
An intensity profile of a line crossing perpendicular to the blood vessel will tend to produce a distinct double-Gaussian curve against the background intensity of the surrounding retina (Figs 4 and 5). The single Gaussian model is given by the equation:
Fig. 4.
A greyscale image of a region of the retina, with a line drawn perpendicular to a retinal arteriole. Note the central light reflex from the arteriole.
Fig. 5.
A typical intensity profile of a cross-section of a retinal arteriole. A curve of best fit has been superimposed on the actual intensity data, showing a double-Gaussian configuration. The height of the intensity profile is calculated by subtracting the background intensity from the peak intensity measured across the vessel. The width of the blood vessel is then measured at the ‘half-height’.
where a1 is the the amplitude of the peak of the profile, a2 is the the position of the peak, a3 is a specific variable of the Gaussian function that controls the width of profile and a4 is the the background retinal intensity.
A smaller central Gaussian function (representing the central light reflex from retinal arterioles) is subtracted from this model, to produce the double-Gaussian curve. This central reflex is often absent in older eyes. The double-Gaussian curve can then be analysed using image processing software, and an estimate of the width of the blood vessel can then be obtained. The most common technique for acquiring the vessel width is to estimate the width of the vessel at half the height of the peak of the intensity profile of the double-Gaussian curve (half-height method). This strategy minimizes any effect of defocusing at the point of image acquisition, which otherwise may have an effect on the vessel width measurement.
Other techniques of automated vessel width measurement have included the use of edge detection masks (Gonzalez & Woods, 1992) and sliding linear regression filters (Chapman et al. 2001; Gang et al. 2002). Digitalized image analysis techniques have been shown to be more reliable than previous micrometric techniques (Delori et al. 1988; Newsom et al. 1992; Sherry et al. 2002).
However, all images captured from the retina are subject to image magnification, depending on the distance from the camera to the eye, and also the refractive error of the eye (Pach et al. 1989; Arnold et al. 1993). Therefore, the measurements recorded from any particular individual cannot be directly compared with another individual. Hence, the use of dimensionless measurements has been used to overcome this problem, so that different images from different subjects can be compared. The most commonly performed dimensionless measurement that has been used as a measure of the width of the retinal vessels is known as the arteriolar–venular ratio (AVR) (Hubbard et al. 1992, 1999; Stanton et al. 1995a). Hubbard et al. (1992) extended a previously published method for evaluating an index of generalized arteriolar width [central retinal artery equivalent (CRAE)] developed by Parr & Spears (1974a). The extended technique (known as the Parr–Hubbard formula) produced a method to evaluate an index of generalized venular width (central retinal vein equivalent, CRVE), and then combined this with the central retinal artery equivalent to obtain a generalized AVR. The AVR has other advantages, in that it takes into account the wide variety of vessel calibre within the normal population and the fact that people with narrow arterioles tend to have narrow venules (Wong et al. 2001b). Another dimensionless measure, which is predominantly affected by changes in vascular calibre, is the length–diameter ratio (L:D) (King et al. 1996). This is calculated as the length from the midpoint of a particular vascular bifurcation to the midpoint of the preceding bifurcation, expressed as a ratio to the diameter of the parent vessel at the bifurcation. Changes in the L:D should reflect changes in the widths of the vessels, rather than the length. The L:D has been found to be increased in hypertension (King et al. 1996). No relationship was observed between L:D and presence of peripheral vascular disease (Chapman et al. 2002). No studies have explored a relationship between L:D and cognitive function or stroke.
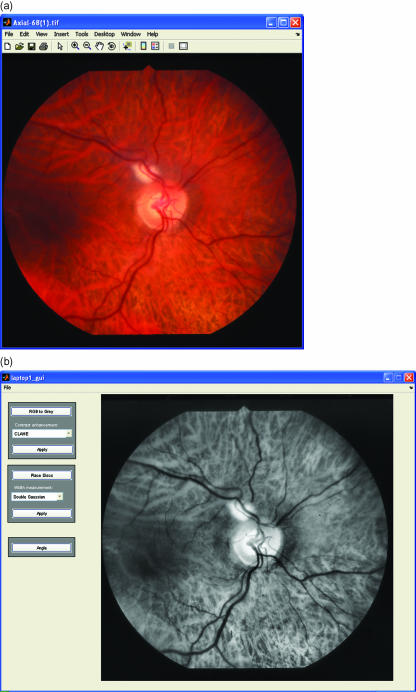
In addition to image magnification, any defocusing of the image will also have an effect on the measurements obtained from the final image (Heier & Brinchmann-Hansen, 1989). Although using the half-height method to measure vessel widths will minimize any effect of defocusing, it is important to obtain as sharp an image as possible. This is not always possible when photographing patients with medial opacities such as cataracts. Poor-quality captured images can be enhanced using grey-level transformation functions to modify the intensities (Gonzalez & Woods, 2002). This will improve the contrast of the retinal vessels, making the analysis easier. The simplest enhancement technique is a linear contrast stretch that linearly maps values in the input intensity image to new values in the output image to increase contrast. A slightly more complicated approach is histogram equalization, which involves transforming the intensity values of the input image so that the histogram of the output image approximately matches a specified histogram. The resulting output image contains intensity values that are nearly uniformly distributed throughout the range from 0 to 255. Alternatively, contrast-limited adaptive histogram equalization (CLAHE) can be used (Fig. 6a,b). This method operates on small regions in the image by enhancing the contrast so that the histogram of each output region approximately matches a specified histogram. The overall effect is to highlight subtle detail that would have been lost under normal histogram equalization. Fluorescein angiography can be used to enhance vessel contrast, but has the limitation of being an invasive procedure associated with occasional severe reactions.
Fig. 6.
(a) An unenhanced RGB image showing moderate delineation of the blood vessels. (b) Image in (a) after greyscale conversion and CLAHE enhancement. The retinal vessels are clearly more prominent.
Reliability of retinal vessel width measurements
Because of the scale and size of retinal blood vessels, vessel measurements (often approximately 10–20 pixels in diameter) need to be accurate and reproducible. Newsom et al. (1992) compared the retinal vessel width measurement techniques of observer-driven micrometric techniques (making manual measurements from a projected image) and objective computer-driven microdensitometry, based on a vessel's profile ‘grey-level’ intensity level, using the previously described ‘half-height’ technique, which has been shown to be the most accurate in the presence of focusing errors (Brinchmann-Hansen, 1986). The coefficient of variation for computer-driven microdensitometry was calculated as 1.5–7.5%, compared with 6–34% for the observer-driven technique. In addition, there was a tendency of the observer-driven technique to underestimate the size of small vessels. Other studies have shown a coefficient of variation of 1.5% using microdensitometry (Brinchmann-Hansen, 1986) Delori et al. (1988) also found a greater variability for micrometric than microdensitometric techniques.
In the ARIC study (Hubbard et al. 1999), correlation analysis (R) was used to evaluate interobserver agreement (R = 0.74, 0.77 and 0.79, for CRAE, CRVE and AVR, respectively, n = 151 eyes). For intraobserver agreement, R = 0.69, 0.89 and 0.84 for CRAE, CRVE and AVR, respectively.
Sherry et al. (2002) assessed the intra- and interobserver (two observers) reliability (using weighted kappa and correlation statistics) of computer-assisted retinal vessel measurement in the Blue Mountains Eye Study, using a similar system to that used in the ARIC study. They report intraobserver reliability kappa values ranging of 0.8 (for trunk AVR ratios) to 0.93 (for CRVE measurements). R2 correlation analysis showed agreement ranging from R2 = 0.79 to 0.92. For interobserver reliability, kappa ranged from 0.71 (for branch AVR measurements) to 0.9 (for CRVE measurements), and correlation statistics showed R2 ranging from 0.78 to 0.9. Although it is unclear as to why they chose a kappa statistic normally reserved for the analysis of agreement for categorical data, and correlation analysis is clearly vulnerable to high values in the presence of systematic bias (Ludbrook, 2002), these values suggest a reasonable level of agreement using computer-assisted vessel measurement. They also show better agreement for larger vessels (CRVE) and better intraobserver than interobserver agreement. For focal retinopathy (e.g. micoraneurysms and haemorrhages), kappa values range from 0.8 to 0.99 (Couper et al. 2002; Sherry et al. 2002; Wong et al. 2003a).
Other potential problems with image analysis include the fact that retinal vessel widths vary according to the cardiac cycle (Chen et al. 1994; Dumskyj et al. 1996; Knudtson et al. 2004), degree of systemic autonomic nerve stimulation (Lanigan et al. 1988) and degree of fundus pigmentation (Hubbard et al. 1992). Because retinal arterioles are small (approximately 50–200 µm in width), very high-resolution digital images must be obtained to perform accurate measurements from vessels that may be as small as 15–20 pixels in width.
In any study attempting to quantify changes in retinal vascular calibres, other factors that may affect the retinal microcirculation such as age, sex (Wellman et al. 1996; Sharett et al. 1999), hypertension (Sharett et al. 1999; Nagel et al. 2004; Wong et al. 2004a, b), cigarette smoking, diabetes (Klein et al. 2004), intraocular pressure (Nagel et al. 2000), and use of systemic medications and drugs (Tamaki et al. 1996; Okuno et al. 2002; Huemer et al. 2003; Polak et al. 2003) may need to be controlled.
Geometrical measurements at retinal vascular branching points
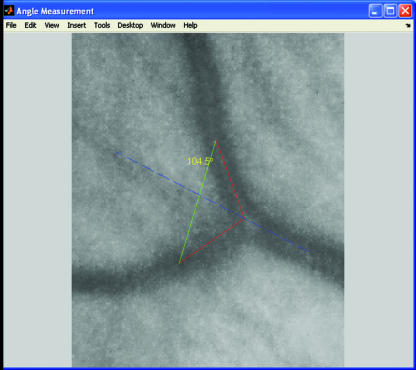
The geometric angle subtended between two branching offspring blood vessels at a bifurcation junction is an important aspect of arterial network topography (Murray, 1926b). Studies using digital image analysis techniques (Fig. 7) have shown that this angle between two offspring retinal arterioles is reduced in hypertension (Stanton et al. 1995) and in men with low birth weight (Chapman et al. 1997), but another study found no association between the angle at vascular bifurcations and peripheral vascular disease (Chapman et al. 2002). No studies to date have explored the relationship between retinal vascular bifurcation angles and cognitive function or stroke.
Fig. 7.
A region of interest within an enhanced greyscale fundal image has been selected to include a bifurcation of a parent vessel into two daughter vessels. Image analysis programming allows the angle between the two daughter vessels to be calculated.
Junctional exponents at retinal vascular junctions
Both theoretical and experimental studies have suggested that arterial diameters at any bifurcation should conform to a relationship that minimizes shear stress throughout any vascular network (Murray, 1926a, b; Zamir, 1976; Sherman, 1981; Zamir & Medeiros, 1982; Woldenberg, 1986). This relationship has been described by the following mathematical relationship:
where D0 is the diameter of the parent vessel, D1 and D2 are the diameters of the offspring vessels, X is a junctional exponent.
The junctional exponent has been calculated to be approximately equal to X = 3 when vascular network costs are minimized. Human and animal studies have shown values very close to this ideal value for the retinal circulation (Zamir et al. 1979; Zamir & Medeiros, 1982), as well as the coronary circulation (Hutchins et al. 1976). However, studies have shown that retinal junctional exponents deviate from optimal values with advancing age (Stanton et al. 1995), and in association with peripheral vascular disease (Chapman et al. 2002).
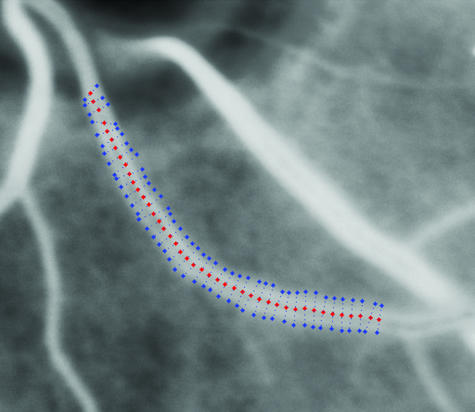
Automated vessel tracking techniques
Vessel tracking describes the process of automated vessel centre localization over a cross-section of a vessel's longitudinal axis, having been given a starting and ending position from which to search (Fig. 8) (Munch et al. 1995; Sinthanayothin et al. 1999). Typically, this takes the form of a Gaussian elongated filter, which is rotated through a number of angles and convolved with the image (Chaudhuri et al. 1989; Thackray & Nelson, 1993; Hoover et al. 2000). By providing information over different sections of the vessel's length, it potentially will give information regarding the variation of vessel widths across its length. This may allow an automated computer-driven objective index of vessel features such as venous beading (Gregson et al. 1995). Vessel tracking may also provide an index of venous tortuosity, by tracking the circuitous route of the blood vessel, and expressing it as a ratio to the shortest length between a nominated starting and end point of the vessel. In addition, automatic identification of retinal structures such as blood vessels (a process known as segmentation) may allow exploration of the fractal properties of the retinal vasculature. A study by Mainster (1990) found both retinal arterioles and venules exhibited fractal properties, and suggests that fractal geometry may offer a more accurate description of retinal vascular topography than conventional geometry.
Fig. 8.
Greyscale image showing a retinal vessel tracking procedure using a Gaussian elongated filter, having been given starting and ending points.
Whilst retinal digital image analysis may offer objective, semi-automated techniques to evaluate topographical features such as retinal vessel calibre, geometry at vessel bifurcations, fractal properties of the vascular network and indices of venous tortuosity, often clinical subjective evaluation of the images is still required to detect focal microvascular abnormalities such as presence of focal arteriolar attenuation, microvascular exudates and retinal haemorrhages. The reliability of grading of focal arteriolar changes has been shown to be largely dependent on the grading system used (Boehm et al. 2002). With the rapid advancement in digital image analysis, it may be possible in the future to enable image processing to detect and quantify these focal abnormalities, using techniques such as segmentation.
Conclusions
In this review, we have outlined the homology that exists between the retinal and the cerebral microcirculations. We have reviewed current evidence that retinal microvascular changes reflect cerebral microvascular changes in aging, as well as in diseases such as vascular dementia and stroke. This forms the basis for investigating retinal vascular topographical features as a potential marker of the state of the cerebral microvasculature in cerebrovascular disease. There are limitations to the sole use of traditional risk factors such as hypertension when assessing an individual's risk for cerebrovascular disease (Prospective Studies Collaboration, 1995), as a substantial proportion of cerebrovascular morbidity is not explained by these risk factors (Khaw et al. 1984; Curb et al. 1996; Menotti et al. 1996). Rather than obtaining a ‘snapshot’ of current blood pressure at any one particular time, retinal microvascular changes may reflect cumulative microcirculatory changes (Wong et al. 2001b). Current evidence suggests that retinal microvascular changes are independently related to past blood pressure and risk of stroke (Wong et al. 2001b). However, in the case of cognitive impairment, stroke, presence of cerebral white-matter lesions and cerebral atrophy, the strongest retinal microvascular associations (micoraneurysms and retinal haemorrhages) are of relatively low prevalence in the general population (approximately 2–15%; Yu et al. 1998; Wong et al. 2003a, b), and more commonly found lesions (such as generalized arteriolar narrowing) are only substantial in populations with hypertension or diabetes. A recent study found the prevalence of retinal vascular changes in never-treated hypertensives to be much greater than that of other quantitative markers of target organ damage, such as left ventricular hypertrophy, carotid abnormalities and microalbimunria (Cuspidi et al. 2004) and thus retinal vascular changes (based on observer-driven evaluation) cannot be used in amending the cardiovascular risk stratification in this particular population. Thus it is not clear how much more useful information regarding future risk of these clinical entities, over and above current standardized methods, will be obtained by retinal vascular analysis, and future studies need to address this question. As Wong (2004) has pointed out, a consistent demonstration that detection of retinal microvascular changes have independent predictive value and that they substantially improve traditional screening methods for cerebrovascular risk prediction has not been conclusively proved.
The ability to assess the retinal circulation in vivo offers potential advantages over other cerebral imaging techniques, which tend to be expensive and not necessarily widely available. Digital retinal image analysis is currently largely a research tool, as it is time-consuming and still undergoing development and refinement. However, digital image analysis of the retinal microvasculature is becoming increasingly available and easier to perform. With improving technology leading to a greater degree of automation (and hence less observer input and less time consumption) and the development of real-time image analysis systems, it may be possible in the future to analyse a digital image of the retinal vasculature quickly, and obtain readily accessible information regarding an individual's potential risk of cerebrovascular disease. Thus it may be possible for retinal vascular analysis to be translated into clinical practice. With the demographic increase in the aged population, the need for earlier identification of patients at risk of cognitive decline and stroke will only become more important, with the potential for earlier therapeutic intervention. Further studies assessing retinal vascular topography changes in cerebrovascular conditions and the continuing rapid advancement in retinal digital image analysis systems may ultimately offer retinal screening as a useful tool in risk identification for cerebrovascular disease.
References
- Abbott NJ, Revest PA, Romero IA. Astrocyte–endothelial interaction: physiology and pathology. Neuropathol. Appl. Neurobiol. 1992;18:424–433. doi: 10.1111/j.1365-2990.1992.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Abbott NJ. Inflammatory mediators and modulation of blood–brain barrier permeability. Cell Mol. Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abernathy WB, Bell MA, Morris M, Moody DM. Microvascular density of the human paraventricular nucleus decreases with aging but not hypertension. Exp. Neurol. 1993;121:270–274. doi: 10.1006/exnr.1993.1095. [DOI] [PubMed] [Google Scholar]
- Adachi T, Inanami O, Sato A. Nitric Oxide (NO) is involved in increased cerebral cortical blood flow following stimulation of the nucleus basalis of Meynert in anesthetized rats. Neurosci. Lett. 1992;139:201–204. doi: 10.1016/0304-3940(92)90552-i. [DOI] [PubMed] [Google Scholar]
- Addison D, Garner A, Ashton N. Degeneration of intramural pericytes in diabetic retinopathy. Br. Med. J. 1970;1:264–266. doi: 10.1136/bmj.1.5691.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi A. The aging brain. J. Neuropsychiatry Clin. Neurosci. 1989;1:S51–S55. [PubMed] [Google Scholar]
- Alm A, Bill A. The oxygen supply to the retina, I, Effects of changes in intraocular and arterial blood pressures and in arterial Po2 and Pco2 on the oxygen tension in the vitreous body of the cat. Acta Physiol. Scand. 1972;84:261. doi: 10.1111/j.1748-1716.1972.tb05177.x. [DOI] [PubMed] [Google Scholar]
- Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp. Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- Aoki N. Epidemiological evaluation of fundoscopic findings in cerebrovascular diseases, II. a multivariate analysis of fundoscopic findings. Jpn Circ. J. 1975;39:271–282. doi: 10.1253/jcj.39.271. [DOI] [PubMed] [Google Scholar]
- Arnold JV, Gates JWC, Taylor KM. Possible errors in the measurement of retinal lesions. Invest. Ophthalmol. Vis. Sci. 1993;34:2576–2580. [PubMed] [Google Scholar]
- Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Arzabe C, Jalkh A, Fariza E, Akiba J, Quiroz M. A simple device to standardize measurements of retinal structures in fundus photographs and retinal angiograms. Am. J. Ophthalmol. 1990;109:107–108. doi: 10.1016/s0002-9394(14)75601-6. [DOI] [PubMed] [Google Scholar]
- Badr GA, Zhang J-Z, Tang JF, Kern TS, Ismail-Beigi F. Glut1 and Glut3 expression, but not capillary density, is increased by cobalt chloride in rat cerebrum and retina. Mol. Brain Res. 1999;64:24–33. doi: 10.1016/s0169-328x(98)00301-5. [DOI] [PubMed] [Google Scholar]
- Badr GA, Tang J, Ismail-Beigi F, Kern TS. Diabetes down-regulates Glut1 expression in retina and its microvessels, but not in cerebral cortex or its microvessels. Diabetes. 2000;49:1016–1021. doi: 10.2337/diabetes.49.6.1016. [DOI] [PubMed] [Google Scholar]
- Barbelieven A, Bertrand N, Besret L, Beley A, MacKenzie ET, Dauphin F. Neurochemical stimulation of the rat substantia innominata increases cerebral blood flow (but not glucose use) through the parallel activation of cholinergic and non-cholinergic pathways. Brain Res. 1999;840:115–124. doi: 10.1016/s0006-8993(99)01736-9. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Invest. Ophthalmol. Vis. Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- Bell MA, Ball MJ. Morphometric comparison of hippocampal microvasculature in ageing and demented people: diameters and densities. Acta Neuropathol. 1981;53:299–318. doi: 10.1007/BF00690372. [DOI] [PubMed] [Google Scholar]
- Benedito S, Prieto D, Nielsen PJ, Nyborg NCB. Histamine induces endothelium-dependent relaxation of bovine retinal arteries. Invest. Ophthalmol. Vis. Sci. 1991;32:32–28. [PubMed] [Google Scholar]
- Bertossi M, Virgintino D, Maiorano E, Occhiogrosso RL. Ultrastructural and morphometric investigation of human brain capillaries in normal and peritumoral tissues. Ultrastruct. Pathol. 1997;21:41–49. doi: 10.3109/01913129709023246. [DOI] [PubMed] [Google Scholar]
- Betz AL, Bowman PD, Goldstine GW. Hexose transport in microvascular endothelial cells cultured from bovine retina. Exp. Eye Res. 1983;36:269–277. doi: 10.1016/0014-4835(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci. Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- Bill A. Aspects of physiological and pharmacological regulation of uveal blood flow. Acta Soc. Med. Upsalien. 1962a;67:122. [Google Scholar]
- Bill A. Autonomic nervous control of uveal blood flow. Acta Physiol. Scand. 1962b;56:70. doi: 10.1111/j.1748-1716.1962.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Bill A, Sperber GO. Aspects of oxygen and glucose consumption in the retina: effects of high intraocular pressure and light. Graefe's Arch. Clin. Exp. Ophthalmol. 1990a;228:124–127. doi: 10.1007/BF00935720. [DOI] [PubMed] [Google Scholar]
- Bill A, Sperber GO. Control of retinal and choroidal blood flow. Eye. 1990b;4:319–325. doi: 10.1038/eye.1990.43. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Schmidt SY, Torigoe Y, Hinton DR. Retinal pathology in Alzheimer's disease. II. Regional neuron loss and glial changes in GCL. Neurobiol. Aging. 1996a;1996:385–395. doi: 10.1016/0197-4580(96)00009-7. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer's disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol. Aging. 1996b;17:377–384. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- Boehm AG, Bowd C, Vasile C, El-Beltagi TAMB, Zangwill LM, Weinreb RN. Comparison of two grading methods to evaluate focal narrowing of retinal arterioles in glaucoma. Graefe's Arch. Clin. Exp. Ophthalmol. 2002;240:810–815. doi: 10.1007/s00417-002-0533-4. [DOI] [PubMed] [Google Scholar]
- Bracher D, Dozzi M, Lotmar W. Measurement of vessel width on fundus photographs. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1979;211:35–48. doi: 10.1007/BF00414652. [DOI] [PubMed] [Google Scholar]
- Bradbury MWB. The blood–brain barrier: transport across the cerebral endothelium. Circ. Res. 1985;57:213–222. doi: 10.1161/01.res.57.2.213. [DOI] [PubMed] [Google Scholar]
- Bradbury MWB, Lightman SL, Pinter GG. Microvascular permeability in experimental diabetes in the anaesthetized rat; brain, optic nerve, and sciatic nerve. J. Physiol. 1989;417:48P. [Google Scholar]
- Bradbury MWB, Lightman SL. The blood–brain interface. Eye. 1990;4:249–254. doi: 10.1038/eye.1990.36. [DOI] [PubMed] [Google Scholar]
- Brightman W. The anatomic basis of the blood–brain barrier. In: Neuwelt E, editor. Implications of the Blood-Brain Barrier, its Manipulation. New York: Plenum; 1989. pp. 53–83. [Google Scholar]
- Brinchmann-Hansen O. The light reflex on retinal arteries and veins. A theoretical study and a new technique for measuring width and intensity profiles across retinal vessels. Acta Ophthalmol. 1986;179(Suppl):1–53. [PubMed] [Google Scholar]
- Buee L, Hof PR, Bouras C., et al Pathological alterations of the cerebral microvasculature in Alzheimer's disease and related dementing disorders. Acta Neuropathol. (Berl.) 1994;87:469–480. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- Burgess A. Objective measurements of the retinal vessels. Ann. Intern. Med. 1967;67:1346–1347. doi: 10.7326/0003-4819-67-6-1346. [DOI] [PubMed] [Google Scholar]
- Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye. 2002;16:242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J. Cereb. Blood Flow Metab. 1999;19:701–735. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Chapman N, Mohamudally A, Cerutti A, et al. Retinal vascular network architecture in low birth weight males. J. Hypertens. 1997;15:1449–1453. doi: 10.1097/00004872-199715120-00012. [DOI] [PubMed] [Google Scholar]
- Chapman N, Witt N, Gao X, et al. Computer algorithms for the automated measurement of retinal arteriolar diameters. Br. J. Ophthalmol. 2001;85:74–79. doi: 10.1136/bjo.85.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman N, Dell'omo G, Sartini MS, et al. Peripheral vascular disease is associated with abnormal arteriolar diameter relationships at bifurcations in the human retina. Clin. Sci. 2002;103:111–116. doi: 10.1042/cs1030111. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S, Chatterjee S, Katz N, Nelson M, Goldbaum M. Detection of retinal blood vessels in retinal images using two-dimensional matched filters. IEEE Trans. Med. Imaging. 1989;8:263–369. doi: 10.1109/42.34715. [DOI] [PubMed] [Google Scholar]
- Chen HC, Patel V, Wiek J, Rosson SM, Kohner EM. Vessel diameter changes during the cardiac cycle. Eye. 1994;8:97–103. doi: 10.1038/eye.1994.19. [DOI] [PubMed] [Google Scholar]
- Clearkin LG, Rose H, Patterson A, Mody CH. Development of retinal arteriolar tortuosity in previously unaffected family members. Trans. Ophthalmol. Soc. UK. 1986;105:568–574. [PubMed] [Google Scholar]
- Cogan DG, Kuwabara T. Comparison of retinal and cerebral vasculature in trypsin digest preparations. Br. J. Ophthalmol. 1984;68:10–12. doi: 10.1136/bjo.68.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber BL, Stewart PA. Three-dimensional reconstructural of vesicles in endothelium of blood–brain barrier versus highly permeable microvessels. Anat. Rec. 1986;215:256–261. doi: 10.1002/ar.1092150308. [DOI] [PubMed] [Google Scholar]
- Cornford EM. The blood–brain barrier, a dynamic regulatory interface. Mol. Physiol. 1985;7:219–260. [Google Scholar]
- Costello J, Salmenson BD, Sinclair SH. Age-related changes in macular capillary circulation as demonstrated with the blue field entoptic phenomenon. Invest. Ophthalmol. Vis. Sci. 1992;33(Suppl):810. [Google Scholar]
- Couper D, Klein R, Hubbard L, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am. J. Ophthalmol. 2002;133:78–88. doi: 10.1016/s0002-9394(01)01315-0. [DOI] [PubMed] [Google Scholar]
- Cringle SJ, Yu D-Y, Yu PK, Su EN. Intraretinal oxygen consumption in the rat in vivo. Invest. Ophthalmol. Vis. Sci. 2002;41:864–869. [PubMed] [Google Scholar]
- Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241:49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG, Lima JJ. Studies on retinal blood flow. I. Estimation of human retinal blood flow by slit-lamp fluorophotometry. Arch. Ophthalmol. 1978;96:893–897. doi: 10.1001/archopht.1978.03910050495022. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG. The blood–ocular barriers: past, present and future. Doc. Ophthalmol. 1997;93:149–157. doi: 10.1007/BF02569055. [DOI] [PubMed] [Google Scholar]
- Curb J, Abott R, MacLean C, et al. Age-related changes in stroke risk in men with hypertension and normal blood pressure. Stroke. 1996;27:819–824. doi: 10.1161/01.str.27.5.819. [DOI] [PubMed] [Google Scholar]
- Cuspidi C, Salerno M, Salerno D, et al. High prevalence of retinal vascular changes in never-treated essential hypertensives: an inter- and intra-observer reproducibility study with non-mydriatic retinography. Blood Press. 2004;13:25–30. doi: 10.1080/08037050310025753. [DOI] [PubMed] [Google Scholar]
- Dallinger S, Dorner GT, Wenzel R, et al. Endothelin-1 contributes to hypoxia-induced vasoconstriction in the human retina. Invest. Ophthalmol. Vis. Sci. 2000;41:864–869. [PubMed] [Google Scholar]
- Dehouck B, Dehouck MP, Fruchart JC, Cecchelli R. Upregulation of the low-density lipoprotein receptor at the blood–brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J. Cell Biol. 1994;126:465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejgaard A, Gade A, Larsson H, Balle V, Parving A. Evidence for diabetic encephalopathy. Diabet Med. 1991;8:162–167. doi: 10.1111/j.1464-5491.1991.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Delaey C, Van de Voorde J. Pressure-induced myogenic responses in isolated bovine retinal arterioles. Invest. Ophthalmol. Vis. Sci. 2000a;14:1871–1875. [PubMed] [Google Scholar]
- Delaey C, Van de Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000b;32:249–256. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- Delori FK, Fitch KA, Feke GT, Deupree DM, Weiter JJ. Evaluation of micrometric and microdensitometric methods for measuring the width of retinal vessel images on fundus photographs. Graefe's Arch. Clin. Exp. Ophthalmol. 1988;226:393–399. doi: 10.1007/BF02172974. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn. Reson. Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Dichgans M, Mayer M, Uttner I, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann. Neurol. 1998;44:731–739. doi: 10.1002/ana.410440506. [DOI] [PubMed] [Google Scholar]
- Dichgans M. A new cause of hereditary small vessel disease. Neurology. 2003;60:8–9. doi: 10.1212/wnl.60.1.8. [DOI] [PubMed] [Google Scholar]
- Dimmitt SB, West JN, Eames SM, Gibson JM, Gosling P, Littler WA. Usefulness of ophthalmoscopy in mild to moderate hypertension. Lancet. 1989;1:1103–1106. doi: 10.1016/s0140-6736(89)92384-2. [DOI] [PubMed] [Google Scholar]
- Dorner GT, Polska E, Garhoefer G, Zawinka C, Frank B, Schmetterer L. Calculation of the diameter of the central retinal artery from noninvasive measurements in humans. Curr. Eye Res. 2002;25:341–345. doi: 10.1076/ceyr.25.6.341.14231. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest. Ophthalmol. Vis. Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- Dumskyj MJ, Ishii N, Nishihara Y, Horie A. The accurate assessment of changes in retinal vessel diameter using multiple frame electrocardiograph synchronised fundus photography. Curr. Eye Res. 1996;15:625–632. doi: 10.3109/02713689609008902. [DOI] [PubMed] [Google Scholar]
- Eaton AM, Hatchell DL. Measurement of retinal blood veseel width using computerized image analysis. Invest. Ophthalmol. Vis. Sci. 1988;29:1258–1264. [PubMed] [Google Scholar]
- Eberling JL, Nordahl TE, Kusbov N, Reed BR, Budinger TF, Jagust WJ. Reduced temporal lobe glucose metabolism in aging. J. Neuroimaging. 1995;5:178–182. doi: 10.1111/jon199553178. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Uddman R, Juul R. Peptidergic innervation of the cerebral circulation. Role in subarachnoid hemorrhage in man. Neurosurg. Rev. 1990;13:265–272. doi: 10.1007/BF00346363. [DOI] [PubMed] [Google Scholar]
- Ehringer B. Adrenergic nerves to the eye and to related structures in man and in the cynomolgus monkey (Macca irus) Invest. Ophthalmol. Vis. Sci. 1966;5:42–52. [Google Scholar]
- Embleton SJ, Hosking SL, Roff Hilton EJ, Cunliffe IA. Effect of senescence on ocular blood flow in the retina, neuroretinal rim and lamina cribrosa, using scanning laser Doppler flowmetry. Eye. 2002;16:156–162. doi: 10.1038/sj.eye.6700100. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Inzitari D, Pantoni L. Research criteria for subcortical ischemic vascular dementia in clinical trials. J. Neural Transm. Suppl. 2000;59:23–30. doi: 10.1007/978-3-7091-6781-6_4. [DOI] [PubMed] [Google Scholar]
- Farkas E, de Jong GI, Apro E, Keuker JIH, Luiten PGM. Calcium antagonists decrease capillary wall damage in aging hypertensive rat brain. Neurobiol. Aging. 2000;100:395–402. doi: 10.1016/s0197-4580(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PGM. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Farrell CR, Steward PA, Farrell CL, Del Maestro RF. Pericytes in human cerebral microvasculature. Anat. Rec. 1987;218:466–469. doi: 10.1002/ar.1092180416. [DOI] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes. Relation to microangiopathy and preceeding severe hypoglycaemia. Diabetes. 2003;52:149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- Ferrari-Dileo G, Davis EB, Anderson DR. Biochemical evidence for cholinergic activity in retinal blood vessels. Invest. Ophthalmol. Vis. Sci. 1989;30:473–477. [PubMed] [Google Scholar]
- Ferrari-Dileo G, Davis EB, Anderson DR. Angiotensin II binding receptors in retinal and optic nerve head blood vessels. Invest. Ophthalmol. Vis. Sci. 1991;32:21–26. [PubMed] [Google Scholar]
- Fischer VW, Siddiqi A, Yusufaly Y. Altered angioarchitecture in selected areas of brains with Alzheimer's disease. Acta Neuropathol. (Berl.) 1990;79:672–679. doi: 10.1007/BF00294246. [DOI] [PubMed] [Google Scholar]
- Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32:871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- Fox P, Raichle M, Mintun M, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frank R, Dutta S, Frank S. Cerebral cortical capillary basement membrane thickening in galactosemic rats. Diabetologia. 1987a;30:739–744. doi: 10.1007/BF00296999. [DOI] [PubMed] [Google Scholar]
- Frank RN, Dutta S, Mancini MA. Pericyte coverage is greater in the retinal than in the cerebral capillaries. Invest. Ophthalmol. Vis. Sci. 1987b;28:1086–1091. [PubMed] [Google Scholar]
- Frank R, Turczyn TJ, Das A. Pericyte coverage of retinal and cerebral capillaries. Invest. Ophthalmol. Vis. Sci. 1990;31:999–1007. [PubMed] [Google Scholar]
- Fredriksson K, Nordbood C, Kalimo H, Olsson Y, Johansson BB. Cerebral microangiopathy in stroke-prone spontaneously hypertensive rats: an immunohistochemical and ultrastructural study. Acta Neuropathol. 1988;75:241–252. doi: 10.1007/BF00690532. [DOI] [PubMed] [Google Scholar]
- Frey A, Meckelein B, Weiler-Guettler H, Moeckel B, Flach R, Gassen HG. Pericytes of the brain microvasculature express [gamma]-glutamyl transpeptidase. Eur. J. Biochem. 1991;202:421–429. doi: 10.1111/j.1432-1033.1991.tb16391.x. [DOI] [PubMed] [Google Scholar]
- Fulesdi B, Limburg M, Bereczki D, et al. Impairment of cerebrovascular reactivity in long-term type 1 diabetes. Diabetes. 1997;46:1840–1845. doi: 10.2337/diab.46.11.1840. [DOI] [PubMed] [Google Scholar]
- Furuta A, Nobuyoshi N, Nishihara Y, Honie A. Medullary arteries in aging and dementia. Stroke. 1991;22:442–446. doi: 10.1161/01.str.22.4.442. [DOI] [PubMed] [Google Scholar]
- Gang L, Chutatape O, Krishnan SM. Detection and measurement of retinal vessels in fundus images using amplitude modified second-order Gaussian filter. IEEE Trans. Biomed. Eng. 2002;49:168–172. doi: 10.1109/10.979356. [DOI] [PubMed] [Google Scholar]
- Gao XW, Bharath A, Stanton A, Hughes A, Chapman N, Thom S. Quantification and characterization of arteries in retinal images. Comput. Meth. Programs Biomed. 2000;63:133–134. doi: 10.1016/s0169-2607(00)00082-1. [DOI] [PubMed] [Google Scholar]
- Gardner TWLE, Khin SA, Barber AJ, et al. Astrocytes increase barrier properties and ZO-1 expression in retinal vascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 1997;38:2423–2427. [PubMed] [Google Scholar]
- Geldmacher DS, Whitehouse PJ. Evaluation of dementia. N. Engl. J. Med. 1996;335:330–336. doi: 10.1056/NEJM199608013350507. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Diemer NH. Double-tracer study of the fine regional blood–brain barrier glucose transfer in the rat by computer-assisted autoradiography. J. Cereb. Blood Flow Metab. 1985;5:282–289. doi: 10.1038/jcbfm.1985.36. [DOI] [PubMed] [Google Scholar]
- Goldberg MF, Pollack IP, Green WR. Familial retinal arteriolar tortuosity with retinal hemorrhages. Am. J. Ophthalmol. 1972;73:183–191. [Google Scholar]
- Gonzalez RC, Woods RE. Digital Image Processing. Reading, MA: Addison-Wesley; 1992. pp. 418–420. ch. 10. [Google Scholar]
- Gonzalez RC, Woods RE. Digital Image Processing. Reading, MA: Addison-Wesley; 2002. pp. 85–94. Ch. 3. [Google Scholar]
- Goto I, Kimoto K, Katsuki S, Mimatsu T, Ikui H. Pathological studies on the intracerebral and retinal arteries in cerebrovascular and noncerebrovascular diseases. Stroke. 1975;6:263–269. doi: 10.1161/01.str.6.3.263. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Kreutzberg GW. Identity of ED2-positive perivascular cells in rat brain. J. Neurosci. Res. 1989;22:103–106. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- Gramer E, Dirmeyer M. Optical coherence tomography (OCT) to measure nerve fiber layer thickness in normal eyes. Invest. Ophthalmol. Vis. Sci. 1998;39:S933. (ARVO abstract, 4296) [Google Scholar]
- Grammas P, Riden M. Retinal endothelial cells are more susceptible to oxidative stress and increased permeability than brain-derived endothelial cells. Microvasc. Res. 2003;65:18–23. doi: 10.1016/s0026-2862(02)00016-x. [DOI] [PubMed] [Google Scholar]
- Grand MG, Kaine J, Fulling K, et al. Cerebroretinal vasculopathy. A new hereditary syndrome. Ophthalmology. 1988;95:649–659. doi: 10.1016/s0161-6420(88)33131-3. [DOI] [PubMed] [Google Scholar]
- Gregson PH, Shen Z, Scott RC, Kozousek V. Automated grading of venous beading. Comput. Biomed. Res. 1995;28:291–304. doi: 10.1006/cbmr.1995.1020. [DOI] [PubMed] [Google Scholar]
- Groh MJ, Michelson G, Langhans MJ, Harazny J. Influence of age on retinal and optic nerve head blood circulation. Ophthalmology. 1996;103:529–534. doi: 10.1016/s0161-6420(96)30662-3. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Kozart DM. Retinal circulation during a spontaneous rise of intraocular pressure. Br. J. Ophthalmol. 1988;72:754–752. doi: 10.1136/bjo.72.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Piltz J, Patel N, Bose S, Riva CE. Effect of aging on retinal macular microcirculation: a blue field simulation study. Invest. Ophthalmol. Vis. Sci. 1993;34:3609–3613. [PubMed] [Google Scholar]
- Guan K, Hudson C, Flanagan JG. Variability and repeatability of retinal blood flow measurements using the Canon Laser Blood flowmeter. Microvasc. Res. 2003;65:145–151. doi: 10.1016/s0026-2862(03)00007-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson F. Hypertensive arteriolar necrosis revisited. Blood Press. 1997;6:71–77. doi: 10.3109/08037059709061802. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, Fischbeck KH, Sergott RC. Hereditary retinal vasculopathy with cerebral white matter lesions. Am. J. Med. Genet. 1989;34:217–220. doi: 10.1002/ajmg.1320340217. [DOI] [PubMed] [Google Scholar]
- Hamada Y. Ophthalmological study of the M-strain stroke-prone spontaneously hypertensive rats (2). Retinal arteriolar changes in fluorescein angiogram. Nippon Ganka Gakkai Zasshi. 1993;97:690–697. [PubMed] [Google Scholar]
- Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog. Brain Res. 2004;145:171–178. doi: 10.1016/S0079-6123(03)45012-7. [DOI] [PubMed] [Google Scholar]
- Hardy P, Varma DR, Chemtoc S. Control of cerebral and ocular blood flow autoregulation in neonates. Pediatric Clinics North Am. 1997;44:137–152. doi: 10.1016/s0031-3955(05)70467-3. [DOI] [PubMed] [Google Scholar]
- Haritoglou C, Hoops JP, Stefani FH, Mehraein P, Kampik A, Dichgans M. Histopathological abnormalities in ocular blood vessels of CADASIL patients. Am. J. Ophthalmol. 2004a;138:302–305. doi: 10.1016/j.ajo.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Haritoglou C, Rudolph G, Hoops J, Opherk C, Kampik A, Dichgans M. Retinal vascular abnormalities in CADASIL. Neurology. 2004b;62:1202–1205. doi: 10.1212/01.wnl.0000118296.16326.e1. [DOI] [PubMed] [Google Scholar]
- Harris A, Ciulla TA, Chung HS, Martin B. Regulation of retinal and optic nerve blood flow. Arch. Ophthalmol. 1998;116:1491–1495. doi: 10.1001/archopht.116.11.1491. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood–brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19:13–26. [PubMed] [Google Scholar]
- Healey DP, Wilk S. Localization of immunoreactive glutamyl aminopeptidase in rat brain. II. Distribution and correlation with angiotensin II. Brain Res. 1993;606:295–303. doi: 10.1016/0006-8993(93)90997-2. [DOI] [PubMed] [Google Scholar]
- Heier H, Brinchmann-Hansen O. Reliable measurements fromm fundus photographs in the presence of focusing errors. Invest. Ophthalmol. Vis. Sci. 1989;30:674–677. [PubMed] [Google Scholar]
- Herman IM, D'Amore PA. Microvascular pericytes contain muscle and nonmuscle actins. J. Cell Biol. 1985;101:43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J. Neuropathol. Exp. Neurol. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Hill G. Studies on the pathogenesis of hypertesnive vascularb disease. Effects of high-pressure intra-arterial injections in rats. Circ. Res. 1970;27:657–668. doi: 10.1161/01.res.27.5.657. [DOI] [PubMed] [Google Scholar]
- Hinton DR, Sadun SA, Blanks IC, Miller CA. Optic-nerve degeneration in Alzheimer's disease. N. Engl. J. Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- Hiroki M, Miyashita K, Yoshida H, Hirai S, Fukuyama H. Central retinal artery Doppler flow parameters reflect the severity of cerebral small-vessel disease. Stroke. 2003;34:e92–e94. doi: 10.1161/01.STR.0000075768.91709.E4. [DOI] [PubMed] [Google Scholar]
- Hodge JV, Parr JC, Spears GF. Comparison of methods of measuring vessel widths on retinal photographs and the effect of fluorescein injection on apparent vessel calibers. Am. J. Ophthalmol. 1969;68:1060–1068. doi: 10.1016/0002-9394(69)93448-5. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc. Natl Acad. Sci. USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash JA, Stewart PA. The relationship of astrocyte-like cells to the vessels that contribute to the blood–ocular barriers. Brain Res. 1993;629:218–224. doi: 10.1016/0006-8993(93)91323-k. [DOI] [PubMed] [Google Scholar]
- Hoover A, Kouznetsova V, Goldbaum M. Locating blood vessels in retinal images by piecewise threshold probing of a matched filter response. IEEE Trans. Med. Imaging. 2000;19:203–210. doi: 10.1109/42.845178. [DOI] [PubMed] [Google Scholar]
- Hoste AM, Boels PJ, Andries LJ, Brutsaert DL, de Lacey JJ. Effects of beta-antagonists on contraction of bovine retinal microarteries in vitro. Invest. Ophthalmol. Vis. Sci. 1990;31:1231–1237. [PubMed] [Google Scholar]
- Hoste AM, Andries LJ. Contracted responses of isolated bovine retinal microarteries to acetylcholine. Invest. Ophthalmol. Vis. Sci. 1991;32:1996–2005. [PubMed] [Google Scholar]
- Hubbard LD, Ehrhardt B, Klein R. The association between generalized artreiolar narrowing and blood pressure. Invest. Ophthalmol. Vis. Sci. 1992;33(Suppl):804. [Google Scholar]
- Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associate with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- Huemer KH, Garhofer G, Zawinka C, et al. Effects of dopamine on human retinal vessel diameter and its modulation during flicker stimulation. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H358–H363. doi: 10.1152/ajpheart.00642.2002. [DOI] [PubMed] [Google Scholar]
- Hughes S, Yang H, Chan-Ling T. Vacsularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest. Ophthalmol. Vis. Sci. 2000;41:1217–1228. [PubMed] [Google Scholar]
- Hunziker O, Abdel'Al S, Schulz U. The aging human cerebral cortex: a stereological characterization of changes in the capillary net. J. Gerentol. 1979;34:345–350. doi: 10.1093/geronj/34.3.345. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Berman JW, Rashbaum WK, Lyman WD. Human fetal astrocytes induce the expression of blood–brain barrier specific proteins by autologous endothelial cells. Brain Res. 1993;625:238–243. doi: 10.1016/0006-8993(93)91064-y. [DOI] [PubMed] [Google Scholar]
- Hutchins G, Miner M, Boitnott J. Vessel calibre and branch angle of human coronary artery branch points. Circ. Res. 1976;38:572–576. doi: 10.1161/01.res.38.6.572. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Utsumi H, Chiba H, et al. Glial cell line-derived neurotrophic factor (GDNF) enhances barrier function of endothelial cells forming the blood–brain barrier. Biochem. Biophys. Res. Commun. 1999;261:108–112. doi: 10.1006/bbrc.1999.0992. [DOI] [PubMed] [Google Scholar]
- Inzitari D, Erkinjunnti T, Wallin A, del Ser T, Romanelli M, Pantoni L. Subcortical vascular dementia as a specific target for clinical trials. Ann. N. Y. Acad. Sci. 2000;903:510–521. doi: 10.1111/j.1749-6632.2000.tb06407.x. [DOI] [PubMed] [Google Scholar]
- Jaboksen J, Sidenius P, Gundersen H, Osterby R. Quantitative changes of cerebral neocortical structure in insulin-treated long-term streptozocin-induced diabetes in rats. Diabetes. 1987;36:597–601. doi: 10.2337/diab.36.5.597. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325:253–256. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Janzer RC. The blood–brain barrier: cellular basis. J. Inherit. Metab. Dis. 1993;16:639–647. doi: 10.1007/BF00711897. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Vascular-ischemic dementia: an update. J. Neural Transm. Suppl. 2002;62:1–23. doi: 10.1007/978-3-7091-6139-5_1. [DOI] [PubMed] [Google Scholar]
- Jen J, Cohen AH, Yue Q, et al. Hereditary endotheliaopathy with retinopathy, nephropathy, and stroke (HERNS) Neurology. 1997;49:1322–1330. doi: 10.1212/wnl.49.5.1322. [DOI] [PubMed] [Google Scholar]
- Jeppesen P, Gregersen PA, Bek T. The age-dependent decrease in the myogenic response of retinal arterioles as studies with the Retinal Vessel Analyzer. Graefe's Arch. Clin. Exp. Ophthalmol. 2004;242:914–919. doi: 10.1007/s00417-004-0945-4. [DOI] [PubMed] [Google Scholar]
- Johnson P, Brendel K, Meezan E. Thickened cerebral cortical capillary basement membranes in diabetes. Arch. Pathol Lab. Med. 1982;106:214–217. [PubMed] [Google Scholar]
- Jonescu-Cuypers CP, Harris A, Bartz-Schmidt KU, et al. Reproducibility of circadian retinal and optic nerve head blood flow measurements by Heidelberg retina flowmetry. Br. J. Ophthalmol. 2004;88:348–353. doi: 10.1136/bjo.2003.024885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong GI, Jansen ASP, Horvath E, Gispen WH, Luiten PGM. Nimodipine effects on cerebral microvessels and sciatic nerve in aging rats. Neurobiol. Aging. 1991;13:73–81. doi: 10.1016/0197-4580(92)90012-m. [DOI] [PubMed] [Google Scholar]
- Joo F. Endothelial cells of the brain and other organ systems: some similarities and differences. Prog. Neurobiol. 1996;48:255–273. doi: 10.1016/0301-0082(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Jordan FL, Thomas WE. Brain macrophages: questions of origin and inter relationship. Brain Res. 1988;472:165–178. doi: 10.1016/0165-0173(88)90019-7. [DOI] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, et al. NOTCH 3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- Joutel A, Andreux F, Gaulis S, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J. Clin. Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce NC, Haire MF, Palade GE. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J. Cell Biol. 1985a;100:1379–1385. doi: 10.1083/jcb.100.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce NC, Haire MF, Palade GE. Contractile proteins in pericytes. II. Immunocytochemical localization of tropomyosin. J. Cell Biol. 1985b;100:1387–1395. doi: 10.1083/jcb.100.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan A, Aurell E, Dobree J. A note of signs in the fundus oculi and arterial hypertension: conventional assessment and significance. Bull. WHO. 1966;34:955–960. [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN. The blood–brain barrier and cerebral microcirculation in Alzheimer disease. Cerebrovasc. Brain Metab. Rev. 1992;4:226–260. [PubMed] [Google Scholar]
- Kappelle LJ, Koudstaal PJ, van Gijn J, Ramos LM, Keunen JE. Carotid angiography in patients with lacunar infarction. A prospective study. Stroke. 1988;19:1093–1096. doi: 10.1161/01.str.19.9.1093. [DOI] [PubMed] [Google Scholar]
- Katz B, Rimmer S, Iragui V, Katzman R. Abnormal pattern electroretinogram in Alzheimer's disease: evidence for retinal ganglion cell degeneration? Ann. Neurol. 1989;26:221–225. doi: 10.1002/ana.410260207. [DOI] [PubMed] [Google Scholar]
- Kawamura J, Terayama Y, Takashima S, et al. Leukoaraiosis and cerebral perfusion in normal aging. Exp. Aging Res. 1993;19:225–240. doi: 10.1080/03610739308253935. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Frank R, Varma S. Aldose reductase activity in retinal and cerebral microvessels and cultured vascular cells. Invest. Ophthalmol. Vis. Sci. 1983;24:1250–1258. [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Capillary lesion develop in retina rather than in cerebral cortex in diabetes and experimental galactosemia. Arch. Ophthalmol. 1996;114:306–310. doi: 10.1001/archopht.1996.01100130302013. [DOI] [PubMed] [Google Scholar]
- Kessler RM. Imaging methods for evaluating brain function in man. Neurobiol. Aging. 2003;24:S21–S35. doi: 10.1016/s0197-4580(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Keuker JI, Luiten PG, Fuchs E. Capillary changes in hippocampal CA1 and CA3 areas of the aging rhesus monkey. Acta Neuropathol. (Berl.) 2000;100:665–672. doi: 10.1007/s004010000227. [DOI] [PubMed] [Google Scholar]
- Khaw K, Barrett-Connor E, Suarez L, Criqui M. Predictors of stroke-associated mortality in the elderly. Stroke. 1984;15:244–248. doi: 10.1161/01.str.15.2.244. [DOI] [PubMed] [Google Scholar]
- King LA, Stanton AV, Sever PS, Thom S, Hughes A. Arteriolar length: diameter (L: D) ratio: a geometric parameter of the retinal vasculature diagnostic of hypertension. J. Hum. Hypertens. 1996;10:417–418. [PubMed] [Google Scholar]
- Klaver CC, Ott A, Hofman A, Assink J, Breteler MM, de Jong P. Is age-related maculopathy associated with Alzheimer's disease? The Rotterdam study. Am. J. Epidemiol. 1999;150:963–968. doi: 10.1093/oxfordjournals.aje.a010105. [DOI] [PubMed] [Google Scholar]
- Klein B, Kuschinsky W, Schroeck H, Vetterlein F. Inter-dependency of local capillary density, blood-flow and metabolism in rat brains. Am. J. Physiol. 1986;251:H1333–H1340. doi: 10.1152/ajpheart.1986.251.6.H1333. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Franke T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy. The Beaver Dam Eye study. Ophthalmology. 1993;100:406–414. doi: 10.1016/s0161-6420(93)31634-9. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Jensen SC. The relation of cardiovascular disease and its risk factors to the 5-year incidence of age-related maculopathy. The Beaver Dam Eye study. Ophthalmology. 1997;104:1804–1812. doi: 10.1016/s0161-6420(97)30023-2. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, et al. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy. XIX: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch. Ophthalmol. 2004;122:76–83. doi: 10.1001/archopht.122.1.76. [DOI] [PubMed] [Google Scholar]
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- Knudtson MD, Klein BEK, Klein R, et al. Variation associated with measurement of retinal vessel diameters at different points in the pulse cycle. Br. J. Ophthalmol. 2004;88:57–61. doi: 10.1136/bjo.88.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Okada K, Koide H, Bokura H, Yamaguchi S. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke. 1997;28:1932–1939. doi: 10.1161/01.str.28.10.1932. [DOI] [PubMed] [Google Scholar]
- Kondo M, Wang L, Bill A. The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta. Ophthalmol. Scand. 1997;75:232–235. doi: 10.1111/j.1600-0420.1997.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Korber N, Schneider R, Brockmann M. Circulatory parameters of the retina in patients with lacunar stroke. J. Neurol. 1986;233:30–33. doi: 10.1007/BF00313988. [DOI] [PubMed] [Google Scholar]
- Kowluru R, Kern T, Engerman R. Abnormalities of retinal metabolism in diabetes or galactosemia, II: comparison of gamma-glutamyl transpeptidase in retina and cerebral cortex, and effects of antioxidant therapy. Curr. Eye Res. 1994;13:891–896. doi: 10.3109/02713689409015092. [DOI] [PubMed] [Google Scholar]
- Krejcy K, Woltz M, Kreuzer C, et al. Characterization of angiotensin II effects on cerebral and ocular circulation by noninvasive methods. Br. J. Clin. Pharmacol. 1997;43:501–508. doi: 10.1046/j.1365-2125.1997.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl DE, Metter EJ, Riege WH, Phelps ME. Effects of human aging on patterns of local cerebral glucose utilization determined by the [18F]-fluorodeoxyglucose method. J. Cereb. Blood Flow Metab. 1982;2:163–171. doi: 10.1038/jcbfm.1982.15. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Metter EJ, Riege WH, Hawkins RA. The effect of normal aging on patterns of local cerebral glucose utilization. Ann. Neurol. 1984;15:S133–S137. doi: 10.1002/ana.410150726. [DOI] [PubMed] [Google Scholar]
- Kwa VI, van der Sande JJ, Stam J, Tijmes N, Vrooland JL. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002;59:1536–1540. doi: 10.1212/01.wnl.0000033093.16450.5c. [DOI] [PubMed] [Google Scholar]
- Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 2002;12:358–370. doi: 10.1111/j.1750-3639.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanigan LP, Clark CV, Hill DW. Retinal circulation responses to systemic autonomic nerve stimulation. Eye. 1988;2:412–417. doi: 10.1038/eye.1988.75. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Autoregulation of cerebral blood flow. Circ. Res. 1964;14:201–206. [PubMed] [Google Scholar]
- Lassman H, Schmeid M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- Laties AM. Central retinal artery innervation. Arch. Ophthalmol. 1967;77:405–409. doi: 10.1001/archopht.1967.00980020407021. [DOI] [PubMed] [Google Scholar]
- Leber T. Circulations and Ernahrungsverhaltnisse des Auges. In: Graefe A, Saenusch T, editors. Handbuch der Gesamten Augenheilkunde. Leipzig: Springer-Verlag; 1903. [Google Scholar]
- LeBeux YVI, Willemot J. Actin- and myosin-like filaments in retinal pericytes and endothelial cells. Invest. Ophthalmol. Vis. Sci. 1980;19:1433–1441. [PubMed] [Google Scholar]
- Lee WR, Blass GE, Shaw DC. Age-related retinal vasculopathy. Eye. 1987;1:296–303. doi: 10.1038/eye.1987.48. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood Volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Palestine AG, Rapoport AG, Rechtand E. Quantitative assessment of the permeability of the rat blood–retinal barrier to small water-soluble non-electrolytes. J. Physiol. 1987;389:483–490. doi: 10.1113/jphysiol.1987.sp016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL, Yuen L. Blood–retinal barrier permability in the streptozocin diabetic rat. J. Physiol. 1989;417:49P. [Google Scholar]
- Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J. Gen. Physiol. 1986;88:521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler KU, Edward DP, Tso MO. Immunoreactivity against tau, amyloid precursor protein, and beta-amyoil in the human retina. Invest. Ophthalmol. Vis. Sci. 1995;36:24–31. [PubMed] [Google Scholar]
- Lorenzi M, Healy DP, Hawkins R, Printz JM, Printz MP. Studies on the permeability of the blood brain barrier in experimental diabetes. Diabetologia. 1986;29:58–62. doi: 10.1007/BF02427282. [DOI] [PubMed] [Google Scholar]
- Ludbrook J. Statistical tcehniques for comparing measurers and measurements: a critical review. Clin. Exp. Pharmacol. Physiol. 2002;29:527–536. doi: 10.1046/j.1440-1681.2002.03686.x. [DOI] [PubMed] [Google Scholar]
- Luijckx G-J, Boiten J, van Kroonenburgh M, et al. Systemic small-vessel disease is not exclusively related to lacunar stroke. A pilot study. J. Stroke Cerebrovasc. Dis. 1998;7:52–57. doi: 10.1016/s1052-3057(98)80021-9. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, de Jong GI, Schuurman T. Cerebrovascular, neuronal, and behavioural effects of long-term calcium channel blockade in aging normotensive and hypertensive rat strains. Ann. NY Acad. Sci. 1994;747:431–451. doi: 10.1111/j.1749-6632.1994.tb44427.x. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, de Jong GI, Van der Zee EA, van Dijken H. Ultrastructural localization of cholinergic muscarinic receptors in rat brain cortical capillaries. Brain Res. 1996;13:225–229. doi: 10.1016/0006-8993(96)00195-3. [DOI] [PubMed] [Google Scholar]
- Lunetta M, Damanti AR, Fabbri G, Lombardo M, Di Mauro M, Mughini L. Evidence by magnetic resonance imaging of cerebral alterations of atrophy type in young insulin-dependent diabetic patients. J. Endocrinol. Invest. 1994;17:241–245. doi: 10.1007/BF03348967. [DOI] [PubMed] [Google Scholar]
- Lutty GA, McLeod DS, Hughes S, Chu Y, Baxter L, Chan-Ling T. Astrocyte–endothelial interactions during human retinal vasculogenesis. Invest Ophthalmol. Vis Sci. 2002;43 doi: 10.1167/iovs.03-1169. (ARVO abstract #1934) [DOI] [PubMed] [Google Scholar]
- Mainster MA. The fractal properties of retinal vessels: embryological and clinical implications. Eye. 1990;4:235–241. doi: 10.1038/eye.1990.33. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Eaves NR, Marcyniuk B, Yates PO. Quantitative changes in cerebral cortical microvasculature in ageing and dementia. Neurobiol. Aging. 1986;7:321–330. doi: 10.1016/0197-4580(86)90158-2. [DOI] [PubMed] [Google Scholar]
- Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol. Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- Marchal G, Rioux P, Petit-Taboue M, et al. Regional cerebral oxygen consumption, blood flow, and blood Volume in healthy human aging. Arch. Neurol. 1992;49:1013–1020. doi: 10.1001/archneur.1992.00530340029014. [DOI] [PubMed] [Google Scholar]
- Martin AR, Bailie JR, Robson T, et al. Retinal pericytes control expression of nitric oxide synthase and endothelin-1 in microvascular endothelial cells. Microvasc. Res. 2000;59:131–139. doi: 10.1006/mvre.1999.2208. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998;87:736–745. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato M, Okawara S, Sakamoto A, et al. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc. Natl Acad. Sci. USA. 1996;93:3269–3274. doi: 10.1073/pnas.93.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Berliner JA, Cancilla PA. Induction of gamma glutamyltranspeptidase in cultured cerebral endothelial cells by a product released by astrocytes. Brain Res. 1987;410:309–314. doi: 10.1016/0006-8993(87)90329-5. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Berliner JA, Cancilla PA. Stimulation of glucose analogue uptake in cerebral microvessel endothelium by a product released by astrocytes. J. Neuropathol. Exp. Neurol. 1989;48:69–80. doi: 10.1097/00005072-198901000-00006. [DOI] [PubMed] [Google Scholar]
- Menotti A, Jacobs DJ, Blackburn H, et al. Twenty-five year prediction of stroke deaths in the seven countries study: the role of blood pressure and its changes. Stroke. 1996;27:381–387. doi: 10.1161/01.str.27.3.381. [DOI] [PubMed] [Google Scholar]
- Meyer J, Rauh J, Galla H-J. The susceptibility of cerebral endothelial cells to astroglial induction of blood–brain barrier enzymes depends on their proliferate state. J. Neurochem. 1991;57:1971–1977. doi: 10.1111/j.1471-4159.1991.tb06411.x. [DOI] [PubMed] [Google Scholar]
- Meyer P, Flammer J, Luscher TF. Endothelium-dependent regulation of the ophthalmic microcirculation in the perfused porcine eye: role of nitric oxide and endothelins. Invest. Ophthalmol. Vis. Sci. 1993;34:3614–3621. [PubMed] [Google Scholar]
- Mielke R, Heiss WD. Positron emission tomography for diagnosis of Alzheimer's disease and vascular dementia. J. Neural Transm. Suppl. 1998;53:237–250. doi: 10.1007/978-3-7091-6467-9_21. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Uehara Y, Desaki J, Kimura T, Kuramoto R. Morphological changes of microveseels in the brain with Alzheimer's disease. Jpn. J. Psychiatry Neurol. 1988;42:819–824. doi: 10.1111/j.1440-1819.1988.tb01171.x. [DOI] [PubMed] [Google Scholar]
- Moeller J, Ishikawa T, Dhawan V, et al. The metabolic topography of normal aging. J. Cereb. Blood Flow Metab. 1996;16:385–398. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- Mok V, Wong A, Lam W, et al. Cognitive impairment and functional outcome after stroke associated with small vessel disease. J. Neurol. Neurosurg. Psychiatry. 2004;75:560–566. doi: 10.1136/jnnp.2003.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DM, Brown WR, Challa VR, Ghazi-Birry HS, Reboussin DM. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer's disease. Ann. NY Acad. Sci. 1997;826:103–116. doi: 10.1111/j.1749-6632.1997.tb48464.x. [DOI] [PubMed] [Google Scholar]
- Mooradian AD. Effect of aging on the blood–brain barrier. Neurobiol. Aging. 1988;9:31–39. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morin AM, Cipp LJ, Haspel HC. Glucose transport is reduced in the blood–brain barrier of aged rats. Brain Res. 1991;551:145–149. doi: 10.1016/0006-8993(91)90926-m. [DOI] [PubMed] [Google Scholar]
- Mooradian A. Central nervous system complications of diabetes mellitus – a perspective from the blood–brain barrier. Brain Res. Rev. 1997;23:210–218. doi: 10.1016/s0165-0173(97)00003-9. [DOI] [PubMed] [Google Scholar]
- Morcos Y, Hosie MJ, Bauer HC, Chan-Ling T. Immunolocalization of occludin and claudin-1 to tight junctions in intact CNS vessels of mammalian retina. J. Neurocytol. 2001;30:107–123. doi: 10.1023/a:1011982906125. [DOI] [PubMed] [Google Scholar]
- MRC/CFAS. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Mukai N, Hori S, Pomeroy S. Cerebral lesions in rats with streptozotocin-inducced diabetes. Acta. Neuropathol. (Berl.) 1980;51:79–84. doi: 10.1007/BF00688853. [DOI] [PubMed] [Google Scholar]
- Munch K, Vilser W, Lindloh C, Klein S. Adaptive algorithm for automatic measurement of retinal vascular diameter. Biomed. Tech (Berl.) 1995;40:322–325. [PubMed] [Google Scholar]
- Murray C. The physiological principle of minimum work 1. The vascular system and the cost of blood volume. Proc. Natl. Acad. Sci. USA. 1926a;12:207–214. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. The physiological principle of minimum work applied to the angle of branching arteries. J. Gen. Physiol. 1926b;9:835–841. doi: 10.1085/jgp.9.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel E, Vilser W, Lindloh C, Klein S. Measuring retinal vascular diameter using the scanning laser ophthalmoscope and computer. Initial results. Ophthalmologe. 1992;89:432–436. [PubMed] [Google Scholar]
- Nagel E, Vilser W, Fuhrmann G, Vilser W, Lang GE. Dilatation of large retinal vessels after increased intraocular pressure. Ophthalmologe. 2000;97:742–747. doi: 10.1007/s003470070021. [DOI] [PubMed] [Google Scholar]
- Nagel E, Vilser W, Lanzl I. Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Invest. Ophthalmol. Vis. Sci. 2004;45:1486–1492. doi: 10.1167/iovs.03-0667. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. Implications of the Blood–Brain Barrier and its Manipulation. Vol. 1. New York: Plenum; 1989. [Google Scholar]
- Newsom R, Sullivan P, Rassam S, Jagoe R, Kohner E. Retinal vessel measurement: comparison between observer and computer driven methods. Graefe's Arch. Clin. Exp. Ophthalmol. 1992;230:221–225. doi: 10.1007/BF00176292. [DOI] [PubMed] [Google Scholar]
- Niedermayer I, Graf N, Schmidbauer J, Reiche W, Feiden W. Cerebroretinal vasculopathy mimicking a brain tumour. Neurology. 2000a;54:1878. doi: 10.1212/wnl.54.9.1878-a. [DOI] [PubMed] [Google Scholar]
- Niedermayer I, Reiche W, Graf N, Mestres P, Feiden W. Cerebroretinal vasculopathy and leukoencephalopathy mimicking a brain tumor. Report of two early-onset cases with Fanconi's anemia-like phenotypes suggesting an autosomal-recessive inheritance pattern. Clin. Neuropathol. 2000b;19:285–295. [PubMed] [Google Scholar]
- Nielsen PJ, Nyborg NCB. Calcium antagonist-induced relaxation of the prostaglandin-F2 response of isolated calf retinal resistance arteries. Exp. Eye Res. 1989;48:329–335. doi: 10.1016/s0014-4835(89)80002-8. [DOI] [PubMed] [Google Scholar]
- Nielsen PJ, Nyborg NCB. Contractile and relaxing effects of arachidonic acid derivatives on isolated bovine retinal resistance arteries. Exp. Eye Res. 1990;50:305–311. doi: 10.1016/0014-4835(90)90215-g. [DOI] [PubMed] [Google Scholar]
- Nishio T, Arima K, Eto K, Ogawa M, Sunohara N. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: report of an autopsied Japanese case. Clin. Neurol. 1997;37:910–916. [PubMed] [Google Scholar]
- Nyborg NCB, Prieto D, Benedito S, Nielsen PJ. Endothelin-1 induced contraction of bovine retinal small arteries is reversible and abolished by nitrendipine levels. Invest. Ophthalmol. Vis. Sci. 1991;32:27–31. [PubMed] [Google Scholar]
- Ogawa S, Tank D, Menon R, Ellermann J, Kim S, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl Acad. Sci. USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Horibe H, Yoshiyuki O, Hayakawa N, Aoki N. A prospective study of cerebrovascular disease in Japanese rural communities, Akabane and Asahi, part 1: evaluation of risk factors in the occurrence of cerebral hemorrhage and thrombosis. Stroke. 1976;7:599–607. doi: 10.1161/01.str.7.6.599. [DOI] [PubMed] [Google Scholar]
- Okeda R, Arima K, Kawai M. Arterial changes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in relation to pathogenesis of diffuse myelin loss of cerebral white matter: examination of cerebral meduallary arteries by reconstruction of serial sections of an autopsy case. Stroke. 2002;33:2565–2569. doi: 10.1161/01.str.0000032620.91848.1c. [DOI] [PubMed] [Google Scholar]
- Okuno T, Sugiyama T, Tominaga M, Kojima S, Ikeda T. Effects of caffeine on microcirculation of the human ocular fundus. Jpn. J. Ophthalmol. 2002;46:170–176. doi: 10.1016/s0021-5155(01)00498-1. [DOI] [PubMed] [Google Scholar]
- de Oliveira F. Pericytes in diabetic retinopathy. Br. J. Ophthalmol. 1966;50:134–143. doi: 10.1136/bjo.50.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophoff RA, DeYoung J, Service S, et al. Hereditary vascular retinopathy, cerebroretinal vasculopathy, and hereditary endotheliopathy with retinopathy, nephropathy, and stroke map to a single locus on chromosome 3p21.1-p21.3. Am. J. Med. Genet. 2001;69:447–453. doi: 10.1086/321975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Slooter AJ, Hofman A, et al. Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–1843. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- Pach J, Pennel DO, Romano PE. Optic disc photogrammetry: magnification factors for eye position, centration, and ametropias, refractive and axial; and their application in the diagnosis of optic nerve hyperplasia. Ann. Ophthalmol. 1989;21:454–462. [PubMed] [Google Scholar]
- Pache M, Nagel E, Flammer J. Reproducibility of measurements with the retinal vessel analyzer under optimal conditions. Klin. Monatsbl. Augenheilkd. 2002;219:523–527. doi: 10.1055/s-2002-33589. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. The Blood–Brain Barrier: Cellular and Molecular Biology. New York: Raven Press; 1993. [Google Scholar]
- Pardridge WM. Transport of small molecules through the blood–brain barrier: biology and methodology. Advanced Drug. Delivery Rev. 1995;15:5–36. [PubMed] [Google Scholar]
- Parisi V, Uccioli L, Montocone G, Parisi L, Menzinger G, Bucci MG. Visual evoked potentials after photostress in insulin-dependent diabetic patients with or without diabetic retinopathy. Graefe's Arch. Clin. Exp. Ophthalmol. 1994;232:193–198. doi: 10.1007/BF00184004. [DOI] [PubMed] [Google Scholar]
- Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer's disease patients. Clin. Neurophysiol. 2001;112:1860–1867. doi: 10.1016/s1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- Parr JC, Spears GF. General calibre of the retinal arteries expressed as the equivalent width of the central retinal artery. Am. J. Ophthalmol. 1974a;77:472–477. doi: 10.1016/0002-9394(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Parr JC, Spears GF. Mathematical relationships between the width of a retinal artery and the widths of its branches. Am. J. Ophthalmol. 1974b;77:478–483. doi: 10.1016/0002-9394(74)90458-9. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Penn JS, Gay CA. Computerized digital image analysis of retinal vessel intensity: application to normoxic and hyperoxic rearing of the newborn rat. Exp. Eye Res. 1992;54:329–336. doi: 10.1016/0014-4835(92)90045-t. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS, Chui HC. Microangiopathy, the vascular basement membrane and Alzheimer's disease. Brain Res. Bull. 1990;24:677–686. doi: 10.1016/0361-9230(90)90007-m. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS. Microvascular pathology and vascular basement membrane components in Alzheimer's disease. Mol. Neurobiol. 1994;9:33–40. doi: 10.1007/BF02816103. [DOI] [PubMed] [Google Scholar]
- Polak K, Dorner G, Kiss B, et al. Evaluation of the Zeiss retinal vessel analyser. Br. J. Ophthalmol. 2000;84:1285–1290. doi: 10.1136/bjo.84.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak K, Petternel V, Luksch A, et al. Effect of endothelin and BQ123 on ocular blood flow parameters in healthy subjects. Invest. Ophthalmol. Vis. Sci. 2001;42:2949–2956. [PubMed] [Google Scholar]
- Polak K, Wimpissinger B, Berisha F, Georgopoulos M, Schmetterer L. Effects of sildenafil on retinal blood flow and flicker-induced retinal vasodilatation in healthy subjects. Invest. Ophthalmol. Vis. Sci. 2003;44:4872–4876. doi: 10.1167/iovs.03-0177. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Zazulia AR. The use of positron emission tomography in cerebrovascular disease. Neuroimaging Clin. N. Am. 2003;13:741–758. doi: 10.1016/s1052-5149(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration. Prospective Studies Collaboration Cholesterol, diastolic blood pressure and stroke: 13 000 strokes in 450 000 people in 45 prospective cohorts. Lancet. 1995;346:1647–1653. [PubMed] [Google Scholar]
- Rajah TT, Olson AL, Grammas P. Differential glucose uptake in retina- and brain-derived endothelial cells. Microvasc. Res. 2001;62:236–242. doi: 10.1006/mvre.2001.2337. [DOI] [PubMed] [Google Scholar]
- Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood–brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002;16:1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- Rassam SMPV, Brinchmann-Hansen O, Engvold O, Kohner EM. Accurate vessel width measurement from fundus photographs: a new concept. Br. J. Ophthalmol. 1994;78:24–29. doi: 10.1136/bjo.78.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassam SMB, Patel V, Chen HC, Kohner EM. Regional retinal blood flow and vascular autoregulation. Eye. 1996;10:331–337. doi: 10.1038/eye.1996.69. [DOI] [PubMed] [Google Scholar]
- Rauh J, Meyer J, Beuckmann C, Galla HJ. Development of an in vitro cell culture system to mimic the blood–brain barrier. Prog. Brain Res. 1992;91:117–121. doi: 10.1016/s0079-6123(08)62325-0. [DOI] [PubMed] [Google Scholar]
- Ravona-Springer R, Davidson M, Noy S. The role of cardiovascular risk factors in Alzheimer's disease. CNS Spectr. 2003;8:824–833. doi: 10.1017/s109285290001926x. [DOI] [PubMed] [Google Scholar]
- Rimmer T, Scialdioni A, Kohner E. Reduced macular blood velocity with age. Invest. Ophthalmol. Vis. Sci. 1989;30(Suppl):153. [Google Scholar]
- Risau W, Dingler A, Albrecht U, Dehouck M-P, Cecchelli R. Blood–brain barrier pericytes are the main source of [gamma]-glutamyltranspeptidase activity in brain capillaries. J. Neurochem. 1992;58:667–672. doi: 10.1111/j.1471-4159.1992.tb09769.x. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Riva CE, Sinclair SH, Grunwald JE. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Invest. Ophthalmol. Vis. Sci. 1981;21:34. [PubMed] [Google Scholar]
- Riva CE, Grunwald JE, Sinclair SH, Petrig BL. Blood velocity and volume tric flow rate in human retinal vessels. Invest. Ophthalmol. Vis. Sci. 1985;26:1124–1132. [PubMed] [Google Scholar]
- Riva CE, Grunwald JE, Petrig BL. Autoregulation of human retinal blood flow; an investigation with laser Doppler velocimetry. Invest. Ophthalmol. Vis. Sci. 1986;27:1706. [PubMed] [Google Scholar]
- Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest. Ophthalmol. Vis. Sci. 1986;27:722. [PubMed] [Google Scholar]
- Robinson W, Galetta SL, McClusky L, Forman MS, Balcer LJ. Retinal findings in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) Surv. Ophthalmol. 2001;45:445–448. doi: 10.1016/s0039-6257(00)00206-x. [DOI] [PubMed] [Google Scholar]
- Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Roman GC. Vascular dementia: distinguishing characteristics, treatment and prevention. J. Am. Geriatr. Soc. 2003;51:S296–S304. doi: 10.1046/j.1532-5415.5155.x. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo J-P, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol. 1995;89:500–512. doi: 10.1007/BF00571504. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Maurage CA. CADASIL: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J. Neuropathol. Exp. Neurol. 1997;56:947–964. [PubMed] [Google Scholar]
- Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res. Bull. 2000;51:363–369. doi: 10.1016/s0361-9230(99)00260-9. [DOI] [PubMed] [Google Scholar]
- Sadek JR, Hammeke TA. Functional neuroimaging in neurology and psychiatry. CNS. Spectr. 2002;7:286–290. doi: 10.1017/s1092852900017703. [DOI] [PubMed] [Google Scholar]
- Sano T, Arai H, Ogawa Y. [Relationship of fundus oculi changes to declines in mental and physical health conditions among elderly living in a rural community.] Nippon Koshu Eisei Zasshi. 1994;41:219–229. [PubMed] [Google Scholar]
- Sato A, Sato Y, Uchida S. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Int. J. Dev. Neurosci. 2001;19:327–337. doi: 10.1016/s0736-5748(01)00017-x. [DOI] [PubMed] [Google Scholar]
- Schneider R, Rademacher M, Wolf S. Lacunar infarcts and white matter attenuation: ophthalmologic and microcirculatory aspects of the pathophysiology. Stroke. 1993;24:1874–1879. doi: 10.1161/01.str.24.12.1874. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Enzyme-histochemical demonstration of microglial cells in the adult and postnatal rabbit retina. J. Comp. Neurol. 1989;282:249–263. doi: 10.1002/cne.902820207. [DOI] [PubMed] [Google Scholar]
- Schuetz E, Thanos S. Neuro–glial interactions in the adult rat retina after reaxotomy of ganglion cells: examination of neuron survival and phagocytic microglia using fluorescent tracers. Brain Res. Bull. 2004;62:391–396. doi: 10.1016/j.brainresbull.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Schultz SK, O'Leary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to mid-life adults. Neuroreport. 1999;10:2493–2496. doi: 10.1097/00001756-199908200-00011. [DOI] [PubMed] [Google Scholar]
- Sears J, Gilman J, Sternberg P. Inherited retinal arteriolar tortuosity with retinal hemorrhages. Arch. Ophthalmol. 1998;116:1185–1188. doi: 10.1001/archopht.116.9.1185. [DOI] [PubMed] [Google Scholar]
- Seifert BU, Vilser W. Retinal Vessel Analyzer (RVA): design and function. Biomed Tech.(Berl.) 2002;47(Suppl. 1):678–681. doi: 10.1515/bmte.2002.47.s1b.678. [DOI] [PubMed] [Google Scholar]
- Sharett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- Sherman T. On connecting large vessels to small: the meaning of Murray's law. J. Gen. Physiol. 1981;78:431–453. doi: 10.1085/jgp.78.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry LM, Wang JJ, Rochtchina E, et al. Reliability of computer-assisted retinal vessel measurement in a population. Clin. Exp. Ophthalmol. 2002;30:179–182. doi: 10.1046/j.1442-9071.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- Shiba T, Inoguchi T, Sportsman J, Heath W, Bursell S, King G. Correlation of diacylglycerol and protein kinase C in rat retina to retinal circulation. Am. J. Physiol. 1993;265:783–793. doi: 10.1152/ajpendo.1993.265.5.E783. [DOI] [PubMed] [Google Scholar]
- Sinthanayothin C, Boyce JF, Cook HL, Williamson TH. Automated localisation of the optic disc, fovea, and retinal blood vessels from digital colour fundus images. Br. J. Ophthalmol. 1999;83:902–910. doi: 10.1136/bjo.83.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosman DO, Chicherio C, Ludwig C, et al. (133) Xe SPECT cerebral blood flow study in a healthy population: determination of T-scores. J. Nucl. Med. 2001;42:864–870. [PubMed] [Google Scholar]
- Song L, Wilk E, Wilk S, Healy DP. Localization of immunoreactive glutamyl aminopeptidase in rat brain. I. Association with cerebral microvessels. Brain Res. 1993;606:286–294. doi: 10.1016/0006-8993(93)90996-z. [DOI] [PubMed] [Google Scholar]
- Stanton AV, Mullaney P, Mee, O'Brien ET, O'Malley K. A method for quantifying retinal microvascular alterations associated with blood pressure and age. J. Hypertens. 1995a;13:41–48. [PubMed] [Google Scholar]
- Stanton AV, Wasan B, Cerutti A, et al. Vascular network changes in the retina with age and hypertension. J. Hypertens. 1995b;13:1724–1728. [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;1986:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA, Maglicco M, Hayakawa K, et al. A quantitative analysis of blood–brain barrier ultrastructure in the aging human. Microvasc. Res. 1987;33:270–282. doi: 10.1016/0026-2862(87)90022-7. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog. Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Stone RA, Laties AM, Emson PC. Neuropeptide Y and the ocular innervation of the rat, guinea pig, cat and monkey. Neuroscience. 1986;17:1207. doi: 10.1016/0306-4522(86)90088-6. [DOI] [PubMed] [Google Scholar]
- Storimans CWJM, Van Schooneveld MJ, Oosterhuis JA, Bos PJ. A new autosomal dominant vascular retinopathy syndrome. Eur. J. Ophthalmol. 1991;1:73–78. doi: 10.1177/112067219100100204. [DOI] [PubMed] [Google Scholar]
- Stromland K, Hellstrom A, Gustavsson T. Morphometry of the optic nerve head and retinal vessels in children by computer-assisted analysis of fundus photographs. Graefe's Arch. Clin. Exp. Ophthalmol. 1995;233:150–153. doi: 10.1007/BF00166607. [DOI] [PubMed] [Google Scholar]
- Sun D, Lytle C, O'Donnell ME. Astroglial cell-induced expression of Na-K-Cl cotransporter in brain microvascular endothelial cells. Am. J. Physiol. 1995;269:C1506–C1512. doi: 10.1152/ajpcell.1995.269.6.C1506. [DOI] [PubMed] [Google Scholar]
- Svardsudd K, Wedel H, Aurell E, Tibber G. Hypertensive eye ground changes: prevalence relation to blood pressure and prognostic importance. Acta. Med. Scand. 1978;204:159–167. [PubMed] [Google Scholar]
- van Swieten JC, van Den Hout JW, van Ketel BA, Hijdra A, Wokke JHJ, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly: a morphometric correlation with arteriosclerosis and dilated perivascular spaces. Brain. 1991;114:761–774. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- T Y, Kanno I, Uemura K, et al. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke. 1986;17:1220–1228. doi: 10.1161/01.str.17.6.1220. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Meyer JS, Okayasu H, Kandula P. Changing topographic patterns of human cerebral blood flow with age measured by xenon CT. Am. J. Roentgenol. 1984;142:1027–1034. doi: 10.2214/ajr.142.5.1027. [DOI] [PubMed] [Google Scholar]
- Tagami M, Nara Y, Kubota A, Fujino H, Yamori Y. Ultrastructural changes in cerebral pericytes and astrocytes of stroke-prone spontaneously hypertensive rats. Stroke. 1990;21:1064–1071. doi: 10.1161/01.str.21.7.1064. [DOI] [PubMed] [Google Scholar]
- Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Ultracytochemical localisation of the erthyrocyte/HepG2-type glucose transporter (GLUT1) in cells of the blood–retinal barrier in the rat. Invest. Ophthalmol. Vis. Sci. 1992;33:377–383. [PubMed] [Google Scholar]
- Tamaki Y, Araie M, Tomita K, Tomidokoro A. Time-course of changes in nicardipine effects on microcirculation in retina and optic nerve head in living rabbit eyes. Jpn. J. Ophthalmol. 1996;40:202–211. [PubMed] [Google Scholar]
- Tanaka H, Hayashi M, Date C, et al. Epidemiologic studies of stroke in Shibata, a provincial Japanese city: preliminary report on risk factors for cerebral infarction. Stroke. 1985;16:773–780. doi: 10.1161/01.str.16.5.773. [DOI] [PubMed] [Google Scholar]
- Tang J, Zhu XW, Lust WD, Kern TS. Retina accumulates more glucose than does the embryologically similar cerebral cortex in diabetic rats. Diabetologia. 2000;43:1417–1423. doi: 10.1007/s001250051548. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J. Neurosci. 1987;7:3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray BD, Nelson AC. Semi-automatic segmentation of vascular network images using a rotating structuring element (rose) with matthematical morphology and dual feature thresholding. Pattern Recognition. 1993;3:385–392. doi: 10.1109/42.241865. [DOI] [PubMed] [Google Scholar]
- Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J. Neuropathol. Exp. Neurol. 2003;62:1287–1301. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- Thanos S. The relationship of microglial cells to dying neurons during natural neuronal cell death and axotomy-induced degeneration of the rat retina. Eur. J. Neurosci. 1991;3:1189–1207. doi: 10.1111/j.1460-9568.1991.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res. Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Tomassoni D, Mancinelli G, Mignini F, Sabbatini M, Amenta F. Quantitative image analysis of choroid and retinal vasculature in SHR: a model of cerebrovascular hypertensive changes? Clin. Exp. Hypertens. 2002;24:741–752. doi: 10.1081/ceh-120015349. [DOI] [PubMed] [Google Scholar]
- Tong XK, Hamel E. Basal forebrain nitric oxide synthase (NOS)-containing neurons project to microvessels and NOS neurons in the rat neocortex: cellular basis for cortical blood flow regulation. Eur. J. Neurosci. 2000;12:2769–2780. doi: 10.1046/j.1460-9568.2000.00158.x. [DOI] [PubMed] [Google Scholar]
- Tontsch U, Bauer HC. Glial cells and neurons induce blood brain barrier related enzymes in cultured cerebral ECs. Brain Res. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- Tornquist P, Alm A. Retinal and choroidal contribution to retinal metabolism in vivo: a study in pigs. Acta. Physiol. Scand. 1979;106:351–357. doi: 10.1111/j.1748-1716.1979.tb06409.x. [DOI] [PubMed] [Google Scholar]
- Tornquist P, Alm A. Carrier-mediated transport of amino acids through the blood-retinal and blood–brain barriers. Graefe's Arch. Clin. Exp. Ophthalmol. 1986;224:21–25. doi: 10.1007/BF02144127. [DOI] [PubMed] [Google Scholar]
- Tornquist P, Alm A, Bill A. Permeability of ocular vessels and transport across the blood–retinal barrier. Eye. 1990;4:303–309. doi: 10.1038/eye.1990.41. [DOI] [PubMed] [Google Scholar]
- Toussaint D, Kuwabara T, Cogan DG. Retinal vascular patterns. Arch. Ophthalmol. 1961;65:575. doi: 10.1001/archopht.1961.01840020577022. [DOI] [PubMed] [Google Scholar]
- Trick GL, Barris MC, Bickel-Blut M. Abnormal pattern electroretinogram in patients with senile dementia of the Alzheimer type. Ann. Neurol. 1989;26:226–231. doi: 10.1002/ana.410260208. [DOI] [PubMed] [Google Scholar]
- Tsilibary EC. Microvascular basement membranes in diabetes mellitus. J. Pathol. 2003;200:537–546. doi: 10.1002/path.1439. [DOI] [PubMed] [Google Scholar]
- Tso M, Jampol L. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132–1145. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L. Neuropeptides in the cerebral circulation. Cerebrovasc. Brain Metab. Rev. 1989;1:230–252. [PubMed] [Google Scholar]
- Ursino M. Mechanisms of cerebral blood flow regulation. Crit. Rev. Biomed. Eng. 1991;18:255–288. [PubMed] [Google Scholar]
- Vahedi K, Massin P, Guichard J, et al. Hereditary infantile hemiparesis, retinal arteriolar tortuosity, and leukoencephalopathy. Neurology. 2003;60:57–63. doi: 10.1212/wnl.60.1.57. [DOI] [PubMed] [Google Scholar]
- Varma AR, Adams W, Lloyd JJ, et al. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer's disease, frontotemporal dementia and vascular dementia. Acta. Neurol. Scand. 2002;105:261–269. doi: 10.1034/j.1600-0404.2002.1o148.x. [DOI] [PubMed] [Google Scholar]
- Vavilala MS, Lee LA, Lam AM. Cerebral blood flow and vascular physiology. Anesthesiol. Clin. North. Am. 2002;20:247–264. doi: 10.1016/s0889-8537(01)00012-8. [DOI] [PubMed] [Google Scholar]
- Vilser W, Nagel E, Lanzl L. Retinal Vessel Analysis – new possibilities. Biomed. Tech. (Berl.) 2002;47(Suppl. 1):682–685. doi: 10.1515/bmte.2002.47.s1b.682. [DOI] [PubMed] [Google Scholar]
- Vingerling JR, Klaver CC, Hofman A, de Jong PT. Epidemiology of age-related macular degeneration. Epidemiol. Rev. 1995;17:347–360. doi: 10.1093/oxfordjournals.epirev.a036198. [DOI] [PubMed] [Google Scholar]
- Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist's view. Brain Res. Rev. 2003;42:221–242. doi: 10.1016/s0165-0173(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Wagener HP, Clay GE, Gipner JF. Classification of retinal lesions in the presence of vascular hypertension. Trans. Am. Ophthalmol. Soc. 1947;45:57–73. [PMC free article] [PubMed] [Google Scholar]
- Wagner EM, Traystman RJ. Cerebrovascular transmural pressure and autoregulation. Ann. Biomed Eng. 1985;13:311–320. doi: 10.1007/BF02584249. [DOI] [PubMed] [Google Scholar]
- Wallis SJ, Firth J, Dunn WR. Pressure-induced myogenic responses in human isolated cerebral resistance vessels. Stroke. 1996;27:2287–2290. doi: 10.1161/01.str.27.12.2287. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Mitchell P, Rochtchina E, Tan AG, Wong TY, Klein R. Retinal vessel wall signs and the 5 year incidence of age related maculopathy: the Blue Mountains Eye Study. Br. J. Ophthalmol. 2004;88:104–109. doi: 10.1136/bjo.88.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PM, Anderson JM, Van Itallie CM, Doctrow SR. The tight-junction-specific protein ZO-1 is a component of the human and rat blood–brain barriers. Neurosci. Lett. 1991;129:6–10. doi: 10.1016/0304-3940(91)90708-2. [DOI] [PubMed] [Google Scholar]
- Weil S, Reifenberger G, Dudel C, Yousry TA, Schriever S, Noachtar S. Cerebroretinal vasculpathy mimicking a brain tumour: a case of a rare hereditary syndrome. Neurology. 1999;53:629–631. doi: 10.1212/wnl.53.3.629. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide and Ca(2+)-dependent K+ channels. Circ. Res. 1996;79:1024–1030. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- Williamson TH, Lowe GD, Baxter GM. Influence of age, systemic blood pressure, smoking, and blood viscosity on orbital blood velocities. Br. J. Ophthalmol. 1995;79:17–22. doi: 10.1136/bjo.79.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier: development, composition, and regulation. Vasc. Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Woldenberg MJ. Relation of branching angles to optimality for four cost principles. J. Theor. Biol. 1986;122:187–204. doi: 10.1016/s0022-5193(86)80081-9. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001a;358:1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Klein BEK, Tielsch JM, Hubbard LD, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular diseases and mortality. Surv. Ophthalmol. 2001b;46:59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett A, et al. Cerebral white matter lesion, retinopathy and risk of clinical stroke: the Atherosclerosis Risk in Communities Study. JAMA. 2002a;288:67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Nieto FJ, et al. Is early age-related maculopathy related to cognitive function? The atherosclerosis risk in communities study. Am. J. Ophthalmol. 2002b;134:828–835. doi: 10.1016/s0002-9394(02)01672-0. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons. The Atherosclerosis Risk Communities Study. Stroke. 2002c;33:1487–1492. doi: 10.1161/01.str.0000016789.56668.43. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Nieto F, et al. Retinal microvascular abnormalities and ten-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003a;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett A, et al. The prevalence and risk factors of microvascular abnormalities in older people: the Cardiovascular Health Study. Ophthalmology. 2003b;110:658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- Wong TY, Mosley T, Jr, Klein R, et al. Retinal microvascular changes and MRI signs of cerebral atrophy in healthy, middle-aged people. Neurology. 2003c;61:806–811. doi: 10.1212/01.wnl.0000086372.05488.8d. [DOI] [PubMed] [Google Scholar]
- Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol. 2004;3:179–183. doi: 10.1016/s1474-4422(04)00682-9. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann. Intern. Med. 2004a;17:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. Br. Med. J. 2004b;329:79. doi: 10.1136/bmj.38124.682523.55. Epub 2004 June 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Schwartz B, Schwoerer J, Banwatt R. Retinal blood vessel width measured on colour fundus photographs by image analysis. Acta. Ophthalmol. Scand. 1995;215(Suppl):33–40. doi: 10.1111/j.1600-0420.1995.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Yanoff M, Fine BS. Ocular Pathology: a Text and Atlas. Philadelphia: J.B. Lippincott Co; 1989. [Google Scholar]
- Ye XD, Laties AM, Stone RA. Peptidergic innervation of the retinal vasculatures and optic nerve head. Invest. Ophthalmol. Vis. Sci. 1990;31:1731–1737. [PubMed] [Google Scholar]
- Yoshida A, Feke GT, Mori F, et al. Reproducibility and clinical application of a newly developed stabilized retinal laser Doppler instrument. Am. J. Ophthalmol. 2003;135:356–361. doi: 10.1016/s0002-9394(02)01949-9. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Murase K, Oku N, et al. Heterogeneity of cerebral blood flow in Alzheimer disease and dementia. Am. J. Neuroradiol. 2003;24:1341–1347. [PMC free article] [PubMed] [Google Scholar]
- Yousen DM, Tasman WS, Grossman RI. Proliferative retinopathy: absence of white matter lesions at MR imaging. Radiology. 1991;179:29–230. doi: 10.1148/radiology.179.1.2006282. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Alder VA, Su EN. Intraretinal oxygen distribution in rats as a function of systemic blood pressure. Am. J. Physiol. 1994;36:H2498–H2507. doi: 10.1152/ajpheart.1994.267.6.H2498. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Ret. Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Yu DY, Su EN, Cringle SJ, Schoch C, Percicot CP, Lambrou GN. Comparison of the vasoactive effects of the docosaoid unoprostone and selected prostanoids on isolated perfused retinal arterioles. Invest. Ophthalmol. Vis. Sci. 2001;42:1499–1504. [PubMed] [Google Scholar]
- Yu DY, Su EN, Cringle SJ, Yu PK. Isolated preparations of ocular vasculature and their applications in ophthalmic research. Prog. Ret. Eye Res. 2003;22:135–169. doi: 10.1016/s1350-9462(02)00044-7. [DOI] [PubMed] [Google Scholar]
- Yu T, Mitchell P, Berry G, Li W, Wang J. Retinopathy in older persons without diabetes and its relationship to hypertension. Arch. Ophthalmol. 1998;116:83–89. doi: 10.1001/archopht.116.1.83. [DOI] [PubMed] [Google Scholar]
- Zamir M. Optimality principles in arterial branching. J. Theor. Biol. 1976;62:227–251. doi: 10.1016/0022-5193(76)90058-8. [DOI] [PubMed] [Google Scholar]
- Zamir M, Medeiros J, Cunningham TK. Arterial bifurcations in the human retina. J. Gen. Physiol. 1979;74:537–548. doi: 10.1085/jgp.74.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir M, Medeiros J. Arterial branching in monkey and man. J. Gen. Physiol. 1982;77:353–360. doi: 10.1085/jgp.79.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xu S, Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience. 1995;69:1195–1204. doi: 10.1016/0306-4522(95)00302-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Stone J. Role of astrocytes in the control of developing retinal vessels. Invest. Ophthalmol. Vis. Sci. 1997;38:1653–1666. [PubMed] [Google Scholar]