Abstract
The structure of the striated urethral sphincter, the so-called rhabdosphincter, remains the subject of controversy. There are two main concepts regarding its structure: either it is a part of the urogenital diaphragm, or it extends from the base of the bladder up to the urogenital diaphragm and is an integral part of the urethra. It is also uncertain whether it possesses a somatic innervation or a mixed innervation (i.e. autonomic and somatic). The purpose of this study was to show the precise location of the nerves running to the urethra, and to try to determine their exact nature. Histology and immunohistochemistry were performed in the external urethral sphincter of ten male fetuses (114–342 mm crown–rump length, or between 14 and 40 weeks of gestation). A three-dimensional (3D) reconstruction of the urethral structure and its innervation was made from serial sections. The 3D reconstruction of the same section levels with different strains allowed us to identify the precise structure of the muscle layers (smooth and striated muscle fibres) and the nature of the nerve elements (myelinated and unmyelinated), their distributions and their relationship to the urethral wall, the prostate and the seminal vesicles. Histological and immunohistochemical 3D reconstruction of the anatomical elements of the urethral sphincter helps us to understand the 3D arrangement of the sphincter muscle layers. It also provides a better understanding of the origin and nature of the nerve elements that play a role in urinary continence.
Keywords: anatomy, innervation, three-dimensional reconstruction, urethral sphincter
Introduction
The innervation of the external sphincter of the male urethra, which is essential for urinary continence, was for many years the subject of numerous conflicting studies. Some authors reported a purely autonomic innervation derived from the inferior hypogastric plexus (Koyanagi, 1980; Delmas et al. 1984), and asserted that there was no connection between any branch of the pudendal nerve and the external sphincter of the urethra (Akita et al. 2003; Gil Vernet, 1964). Most authors, however, have supported the view that the branches of the pudendal nerve innervate the external sphincter of the urethra. Certain branches of this nerve leave the main trunk to follow the course of the inferior hypogastric plexus (Donker et al. 1976; Gosling & Dixon, 1979; Tanagho et al. 1982). A further hypothesis, derived from studies performed on animals, is that the innervation is mixed (i.e. autonomic and somatic) (Elbadawi & Schenk, 1974; Fletcher & Bradley, 1978).
If the nature of the nerves to the external urethral sphincter is controversial; the course and the relationships of these nerves to the pelvic organs and the muscle structure of the perineum are no less debatable. The structure of this sphincter also remains a matter of controversy (Donker et al. 1976; Delmas et al. 1984). It is considered by some to be part of the urogenital diaphragm, situated in a caudal position relative to the prostate (Carrol & Dixon, 1992). Other studies, however, have questioned the existence of the urogenital diaphragm (Oelrich, 1980; Myers et al. 1998; Dorschner et al. 1999); yet others believe that this sphincter extends from the base of the bladder up to the urogenital diaphragm, and that it is an integral part of the urethra. It surrounds the membranous urethra and it is absent on the posterior face of the prostatic urethra (Henle, 1866; Brooks et al. 1998; Burnett & Mostwin, 1998).
The surgical implications, with the possibility that unidentified sphincter nerve fibres may be inadvertently damaged, are major in terms of continence and thus postoperative quality of life after radical pelvic surgery, as well as in terms of technical failure after surgery for incontinence (Akita et al. 2003). The success of the techniques of vesical replacement after cystectomy for bladder cancer, with the preservation of continence, is also based on a better understanding of the anatomy of the sphincter and its innervation. The identification of the nature of these components extending to the urethral sphincter is also essential for the therapeutic approach to incontinence.
We set out to identify the precise location, origin and nature of the nerve fibres extending to the urethral sphincter, and to construct a three-dimensional (3D) representation of the male urethral sphincter and its innervation in the male fetus.
Materials and methods
We studied ten normal male human fetuses (114–342 mm, or between 14 and 40 weeks of gestation) obtained by therapeutic or spontaneous abortions during autopsy. The gestational age of each fetus was determined from the crown–rump length (CRL), as defined by others (Fritsch, 1989; Ludwikowski et al. 2001). We chose ten fetuses, between 14 and 40 weeks of gestation, because at the 15th week of gestation the fetus shows a clear differentiation between striated and smooth muscles, while the fetus at term can be macroscopically dissected and the basic pattern of the sphincter is established at this stage (Oelrich, 1980). All of the specimens contained complete pelvic viscera and surrounding tissues. They were fixed in formalin (formaldehyde 10%) for 24 h, then washed and paraffin-embedded. Tissues were removed and cut into blocks: a block every 4 mm, and serially sectioned at a thickness of 4 µm. These sections were examined under the optical microscope after staining with the following methods.
Hematein-eosin-safran (HES)
Every 1 mm was divided into four levels at 250-µm intervals. At the first level a slice 4 µm thick was stained with HES, and was then doubled by adding another slice stained with Luxol Fast Blue (LFB) at 4-µm intervals. At the second level, Protein S100 immunolabelling was used to study a section after neuronal immunomarking, and the thickness was then doubled by adding another slice stained with LFB at 4-µm intervals. At the third level, a section was studied after immunolabelling of the smooth muscle fibres and doubled by two sections of HES and LFB at intervals of 4 µm each. At the fourth level, a section was stained with LFB and joined with another slice stained with HES.
Neuronal immunolabelling
Rabbit Anti-Cow Protein (S100 Code No. Z031: DAKO A/S) is a specific immunomarker (Hollabaugh et al. 1997) for identifying the Schwann's nucleus and cytoplasm in nerve tissues (Ball et al. 1997; Von Heyden et al. 1998). The antibody that we used was obtained after immunization of rabbits, and was diluted at 1 : 200.
Muscle cell immunolabeling (monoclonal mouse anti-human smooth muscle actin: Clone 1 A4, Code No. M 0851)
We used a monoclonal antibody diluted 1 : 150, and intended specifically for the actin of the smooth muscles. For immunohistochemistry, tissues were fixed with 10% neutral buffered formalin; phosphate buffers were used to stabilize the pH between 6.8 and 7.2; and an indirect biotin streptavidin system, for detecting a specific mouse monoclonal or rabbit polyclonal primary antibody, was used (the Ventana Medical System iVIEW™ DAB kit 760–061). This system utilizes universal biotinylated secondary antibody to locate the bound primary antibody followed by the binding of streptavidin–HRP conjugate. The complex is then visualized using hydrogen peroxide and the diaminobenzidine (DAB) chromagen.
Luxol Fast Blue
Solvent blue 38 SIGMA 3382 has been used to identify the myelin (Narayan et al. 1995). This method is based on the use of an alcohol-soluble amino salt of sulphonated copper phthalocyanine as LFB MBS. This dye is characterized by the striking intensity of the blue or bluish-green staining, its specificity for myelin, and its remarkable fastness to light, heat acids and alkalies. It has the main advantage of being applicable to formalin-fixed tissues without the usual mordants required for the traditional myelin methods. Our interest in comparing the slice stained by HES with that stained by LFB, and thus which the Protein S100 studied after a neuronal immunolabelling, was to distinguish the nervous myelinated fibres from the unmyelinated fibres. After studying the capacity and the qualities of the software for 3D reconstruction, we chose to create sections at four levels within a thickness of 1 mm. With this software we can study and compare a precise element on four levels before and after the section that carries this element.
Three-dimensional image reconstruction
Preliminary microscopic examination of all the slides using optical microscopy allowed us to identify the various anatomical structures to be reconstructed, and to distinguish between the somatic nerve fibres of the autonomic nerve fibres and the smooth muscular fibres of the striated muscular fibres. The computer software used was as follows: digitalization system: Minolta Dimâge Scan Dual II; numeration software: Minolta DS Dual2 Easy Scan Utility; image treatment: Adobe Photoshop; image reconstruction: Surf Driver 3.5.3 Macintosh version 3D image reconstruction software (D. Moody and S. Lozanoff of the University of Hawaii, and the University of Alberta, respectively).
Results
The muscular structure of the male urethra
At the level of the bladder neck, smooth muscle fibres are of oblique and longitudinal orientation, and longitudinal fibres run parallel to the longitudinal smooth fibres of the prostatic urethra (Fig. 1B,I). At this level we identified some striated muscle fibres, which rise obliquely up to the detrusor (M. detrusor vesicae).
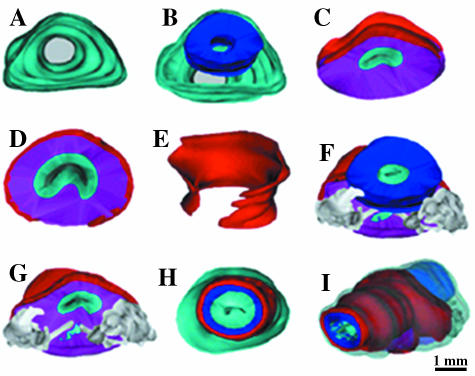
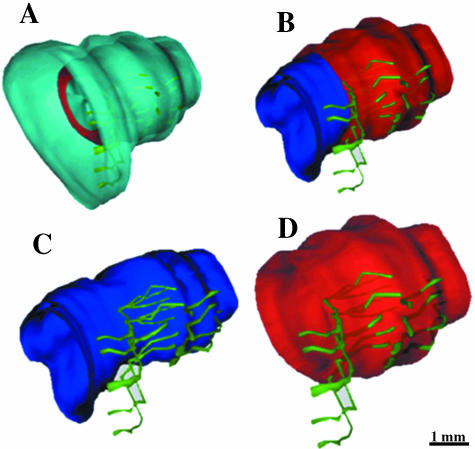
Fig. 1.
Three-dimensional reconstruction from transverse sections of a 30-week-old fetus (282 mm CRL). (A–E) The connective tissue surrounding all the reconstructed structures (in cyan), the bladder neck (in blue), the prostate (in magenta) and striated muscles (in red). At the level of the seminal colliculus (veru-montanum, colliculus seminalis): striated fibres (in red) surround, almost completely, the prostatic tissue. (G,F) Seminal vesicles and ejaculatory ducts (in grey). The prostatic utricle (in cyan). (H) The striated muscles form complete circles at the level of the membranous urethra. (I) A global view, of all the reconstructed elements, shows (by transparency) the continuance between the prostatic urethral smooth muscles and the membranous urethral smooth muscles (in blue).
At the level of the prostatic and membranous urethra, the prostatic capsule constitutes a peripheral condensation of the non-glandular part of the prostate. It surrounds the prostate and is inseparable from the fibro-muscular stoma of the prostate. It contains smooth and striated muscular fibres, vascular elements and nerve fibres. It does not appear to be bounded by either the parenchyma or the prostatic fascia. The striated muscle fibres are present in the anterior and the lateral faces of the prostatic capsule (Fig. 1C,D). The majority of these fibres are concentrated at the level of the apex of the prostate, and expand laterally in the ischio-rectal fossa.
At the level of the external urethral sphincter, the anterior wall of the striated urethral sphincter appears to be twice the length of the posterior wall and it invests the whole apex of the prostate. The striated urethral sphincter was inseparable from the internal muscle layer of the prostatic and membranous urethra (Fig. 2A,B). Three-dimensional reconstructed images showed that the external urethral sphincter continued from the prostatic base to the membranous urethra in the form of a crescent shape above the seminal colliculus and a horseshoe shape below the seminal colliculus (Fig. 1E,H), and covering the membranous urethra evenly at all sides (Fig. 1I). Transverse sections also revealed the relationship of the external sphincter muscles encircling the seminal colliculus. Beneath the striated muscles of the external sphincter, smooth muscles in a circular formation are evident (Fig. 2C). The striated muscle fibres of the external sphincter were arranged in a circular pattern in the membranous urethra (Fig. 1H,I), and were intermingled with the smooth muscle fibres in the posterior and lateral part of the sphincter (Fig. 2B–D). The relationship between the prostate, sphincteric complex and urethra did not change as a function of gestational age in the specimens studied.
Fig. 2.
Immunohistochemical images of smooth muscular alpha-actin; transverse sections of a 24-week-old male fetus (220 CRL mm). (A,B) Striated muscles in the prostatic capsule (anterior and lateral faces), surrounding the smooth muscles at the level of seminal colliculus (C). (C,D) They are interspersed with the smooth muscles in the posterior and lateral faces of the sphincter.
The innervation of the male urethra
At the level of the bladder neck, the nerve fibres run under the pelvic fascia on either side of the recto-vesical pouch, lateral and cranial to the rectum and the seminal vesicles, and penetrate into the bladder neck at 5 o'clock and at 7 o'clock positions (Figs 3A and 4A,B). The autonomic nerves, originating from the inferior hypogastric plexus, run beneath the fascia of the levator ani muscle (Fritsch et al. 2004) along the lateral surface of the rectum (Fig. 3A,D), around the anterolateral aspects of the seminal vesicles and over the inferolateral aspect of the prostate (Fig. 3A,B). At this level there are myelinated and unmyelinated nerve fibres on the posterior face of the bladder neck (Fig. 3C). A section of the unmyelinated fibres follow the ejaculatory ducts of the cranial prostate to reach the prostate and the prostatic urethra (Fig. 3C). Myelinated nerves follow the same course as the autonomic nerves and end in striated muscle fibres of the prostatic capsule (Figs 4C,D and 8C).
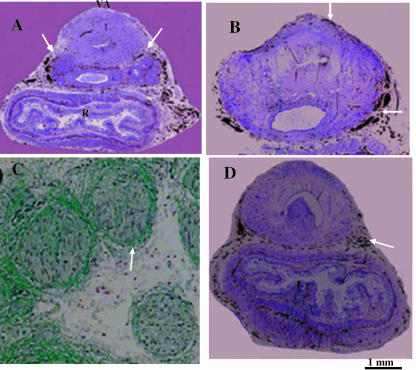
Fig. 3.
Immunohistochemical images of nerve fibres; transverse sections of 25-week-old male fetus (241 mm CRL). (A) The unmyelinated nerves are situated on the lateral and anterior faces of seminal vesicles and penetrate into the bladder neck at 5 o'clock and at 7 o'clock (arrows). (B,D) They are situated outside and behind the prostatic capsule and run behind the cavernous nerves of the penis (arrows). (C) Luxol Fast Blue identification of myelinated nerves on the posterior face of the bladder neck.
Fig. 4.
Three-dimensional reconstruction of nerve fibres from transverse sections of a 36-week-old male fetus (330 mm CRL). (A,B) The unmyelinated nerve fibres (in yellow) are situated in anterolateral position with respect to seminal vesicles and they penetrate into the bladder neck mainly by its posterolateral faces but also by the anterolateral faces. (C,D) The myelinated nerve fibres (in green) follow the course of the unmyelinated fibres, and end in striated muscles of the prostatic capsule.
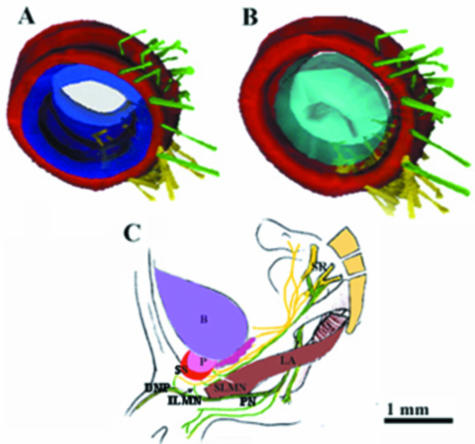
Fig. 8.
(A,B) Three-dimensional reconstruction from transverse sections of a 36-week old male fetus (CRL 330 mm). The unmyelinated nerve fibres reached the smooth muscles via the zone between the striated and smooth muscles. (C) A schematic representation of the supralevator myelinated nerves (SLMN) and infralevator myelinated nerves (ILMN); SR, sacral roots; PN, pudendal nerve; DNP, dorsal nerve of the penis; LA, levator ani muscle; B, bladder; P, prostate; SS, striated sphincter.
At the level of the prostate and the prostatic apex, unmyelinated nerved fibres are situated outside and behind the prostatic capsule. Numerous branches follow the ejaculatory ducts through the prostate gland until they reach the urethra at the level of the smooth muscle fibres of the infra-montanal prostatic urethra and the submucosa (Fig. 5B,D). The cavernous nerves of the penis (Fig. 5A) are situated dorsolaterally between the rectum and prostate (Fig. 6A–C, arrows). Lateral to the membranous urethra, these nerve fibres pass the urogenital diaphragm near the muscular wall of the urethra (Fig. 6D, arrow), finally reaching the corpora cavernosa (Fritsch, 1989).
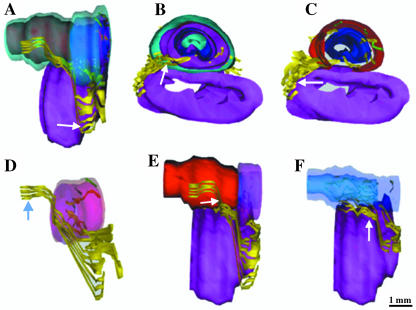
Fig. 5.
Three-dimensional reconstruction of nerve fibres from transverse sections of a 36-week old male fetus (CRL 330 mm). (A,B,D) The unmyelinated nerves give off branches, which penetrate into the prostate and emerge from the seminal colliculus to innervate the prostate, the smooth muscules and the submucosa. These nerves receive behind the cavernous nerves (A, arrows), which stay in the posterolateral position. The striated muscular fibres, situated at the anterolateral faces of the prostate, are innervated by myelinated nerve fibres (C,D, arrows).
Fig. 6.
Three-dimensional reconstruction of nerve fibres from transverse sections of a 34-week old male fetus (CRL 325 mm). The cavernous nerves run along the anterior face of the rectum and the posterior face around the sphincter (E,F, arrows) to become anterior and to cross the perineal floor to join the corpus cavernosum penis (D, arrow).
The membranous urethra is the thickest segment of the urethra. It is a muscular organ that contains smooth muscle fibres. At this level, the majority of the autonomic nerve fibres penetrate the urethral sphincter muscle from the posterolateral surface (Fig. 6E,F), and most of the myelinated nerves enter the urethral sphincter from the anterolateral surface (Fig. 7A,B). The myelinated nerve fibres penetrate the striated muscular layer, and some of these fibres reach the smooth muscle layer and the submucosa; are these the sensory nerve fibres? (Figs 7C,D and 8B). The unmyelinated nerve fibres, crossing the zone of intricacy between the striated and smooth muscle fibres, end in the smooth muscle layer and the submucosa (Fig. 8A,B). We noticed a certain spatial arrangement between the striated and the smooth muscles of the sphincteric complex, which implies that there is one specific location of the nerves destined for these muscles. The unmyelinated nerve fibres reached smooth muscle fibres via the posterior and lateral surfaces, where they crossed the space created by the dissociation of the striated muscle fibres, which formed incomplete circles on the posterior surface of the urethra, at the level of the apex of the prostate and the proximal portion of the membranous urethra (Fig. 2C,D).
Fig. 7.
Three-dimensional reconstruction of nerve fibres from transverse sections of a 40-week old male fetus (CRL 342 mm). The majority of myelinated nerve fibres penetrate into the sphincter of its anterolateral faces (B,D). These fibres penetrate into the striated muscular layer and reach the smooth muscular layer and the submucousa (C, where the striated muscles were made completely transparent, and D by transparency).
Discussion
The external urethral sphincter is an integral part of the male urethra, and is situated at the level of the membranous urethra (Gosling & Dixon, 1979; Delmas et al. 1984). It represents the thickest muscular structure of the male urethra. It possesses one smooth muscle component and one striated muscle component, their respective muscle fibres intermingling on the lateral face of the urethra (Koyanagi, 1980). The striated muscles are shaped like incomplete circles on the anterior and lateral faces, and they run parallel to the striated musculature of the prostatic capsule (Elbadawi et al. 1997).
Our morphological investigation of the urethral musculature revealed a striated muscle structure going from the bladder neck to the proximal bulbar urethra and closely associated with the smooth muscle structure. The dissociation of the striated muscle fibres, which form incomplete rings on the lateral and posterior faces of the urethra, represents a space through which the unmyelinated nerve fibres can pass on their way to the smooth muscle layer and submucosa. This phenomenon explains, in our opinion, the presence of unmyelinated nerve fibres in the deep part of the striated muscles (Elbadawi & Schenk, 1974; Fletcher & Bradley, 1978; Kumagai et al. 1987). They are heading towards the smooth muscular fibres, but they can be found on 2D images as they pass.
The striated part of the sphincter is the only mechanism of continence after prostectomy where the smooth muscles will be divided and muscular vesico-prostatico-urethral continuance will be interrupted. Conservation of the sphincter during vesico-prostatic and pelvic surgery is a basic element in avoiding postoperative incontinence (Steiner, 2000). It means that we must minimize trauma during dissection of the prostate, in particular its apex; great attention should be given to the posterior continuation of the striated sphincter and its attachments with the pelvic fascia, and also to the striated muscles on the anterolateral face of the sphincter during the ligature of the dorsal venous complex and during the vesico-urethral anastomosis (Walsh, 1998).
The origin, the course and nature of the nerve contingents destined for the external sphincter of the male urethra remain the subject of controversy (Zvara et al. 1994; Hollabaugh et al. 1997; Akita et al. 2003). Some authors have described three types of branches derived from the nerve pudendal destined for the external urethral sphincter: branches arising from the pudendal nerve before Alcock's canal and following the fibres of the inferior hypogastric plexus, the branches originating from the pudendal canal and the branches derived from the dorsal nerve of the penis (Narayan et al. 1995). Others have reported that this sphincter may receive contingents above and below the levator ani muscles (Juenemann et al. 1987; Hollabaugh et al. 1997; Brooks et al. 1998; Colombel et al. 1999). Strasser et al. (1996) did not include evidence of such branches. However, the nature of these fibres remains to be determined (Shafik & Doss, 1999).
In this study we identified, at the level of the bladder neck and the proximal part of the male urethra, unmylinated nerve fibres destined for the smooth muscular fibres, which run alongside the mylinated nerve fibres. They possess very narrow reports with the lateral and anterior faces of the seminal vesicles and the lateral and posterior faces of the prostate. The majority of the unmylinated nerve fibres approach the smooth muscular layers at angles of 5 o'clock and 7 o'clock. Concerning the myelinated nerve fibres, our results confirm the data of Fritsch (1989) showing that the majority of the myelinated nerve fibres penetrate the sphincter at angles of 9 o'clock and 3 o'clock. The identification of myelinated nerve fibres following the course of the unmyelinated nerve fibres, from the bladder neck to the striated muscular complex (prostatic and membranous), suggests that some intrapelvic (supralevator) courses of the somatic nerve fibres could be present. In the present study, it was difficult to confirm that the myelinated nerve fibres of the striated sphincter are derived from the pudendal nerve. However, the anterolateral orientation of these fibres and their identification after the level of the apex of the prostate indicates that they do not have the same origin as the intrapelvic (supralevator) somatic nerve fibres (Fig. 8C), and they probably originated from the perineal nerve or the dorsal nerve of the penis.
We performed this study in the fetus where the size of the pelvis does not complicate attempts at 3D reconstruction (Kokoua et al. 1993; Ludwikowski et al. 2001). The 3D reconstruction, based on a histological and immunohistochemical approach, is a novel method but one which has been used with success in previous studies (Benoît et al. 1994; Colleselli et al. 1995; Myers et al. 1998). The relationship between the pelvic viscera, prostate, sphincteric complex and urethra does not show a radical change as a function of age, and in post-fetal stages the general topography of the inferior hypogastric plexus does not undergo fundamental changes as a result of growth (Fritsch, 1989). The use of the fetus as a model for representing the morphological aspects of the musculature and innervation of the male urethra provides a unique opportunity for understanding the pelvic nerve-sparing techniques. In the fetus, relatively thick nerves are surrounded by a small amount of connective tissue, and these nerves are much easier to identify than those of adult specimens. In fact, pelvic nerve-sparing techniques applied in adults are based largely on microscopic investigations of fetuses (Colleselli et al. 1995; Walsh et al. 1998) and knowledge of the topographical anatomy of autonomic and somatic nerves of the sphincter help the surgeon to visualize and dissect them in detail at the macroscopical level (Schlegel & Walsh, 1987; Myers 1991).
This method allowed us to clarify the course, location and distribution of the nerve fibres destined for smooth and striated muscles of the urethral sphincter. Further histofluorescent staining, histochemical analysis and electron microscopic studies are needed to determine the detailed nature of the sphincteric nerve fibres and also to identify the neurotransmitters implied in the neurological control of the external urethral sphincter. Knowledge of a mixed innervation controlling the continence and having narrow anatomical reports with the lateral faces of the prostate is of major importance in the prevention of incontinence following surgical and cœlioscopic operations of the prostate and bladder (Lepor et al. 1985; Walsh et al. 1998). The surgical implications of the unidentified sphincteric nerve fibres are considerable in term of continence, as well as in terms of technical failure after surgery for incontinence (Benoît et al. 1994; Ball et al. 1997).
References
- Akita K, Sakamato H, Sato T. Origins and courses of the nervous branches to male urethral sphincter. Surg. Radiol. Anat. 2003;25:387–392. doi: 10.1007/s00276-003-0151-9. [DOI] [PubMed] [Google Scholar]
- Ball TP, Teichman J, Jr, Sharkey FE, Rogenes VJ, Adrian EK. Terminal nerve distribution to the urethra and bladder neck: considerations in the management of stress urinary incontinence. J. Urol. 1997;158:827, 829. doi: 10.1097/00005392-199709000-00037. [DOI] [PubMed] [Google Scholar]
- Benoît G, Merlaud L, Medori G. Anatomy of the prostatic nerves. Surg. Radiol. Anat. 1994;16:23–29. doi: 10.1007/BF01627917. [DOI] [PubMed] [Google Scholar]
- Brooks JD, Chao WM, Kerr J. Male pelvic anatomy reconstructed from the Visible Human data set. J. Urol. 1998;159:868. [PubMed] [Google Scholar]
- Burnett AL, Mostwin JL. In situ anatomical study of the male urethral sphincteric complex: relevance to continence preservation following major pelvic surgery. J. Urol. 1998;160:1301. [PubMed] [Google Scholar]
- Carrol PR, Dixon CM. Surgical anatomy of the male and female urethra. Urol. Clin. North Am. 1992;19:339–346. [PubMed] [Google Scholar]
- Colleselli K, Strasser H, Poisel S, Stenzl A, Bartsch G. Continence after anterior exenteration and radical prostatectomy: anatomical approach. J. Urol. 1995;153:305A. [Google Scholar]
- Colombel M, Droupy S, Paradis V, Lassau JP, Benoit G. Caverno-pudendal nervous communicating branches in the penile hilum. Surg. Radiol. Anat. 1999;21:273–276. doi: 10.1007/BF01631399. [DOI] [PubMed] [Google Scholar]
- Delmas V, Benoit G, Gillot C, Hureau J. Anatomical basis of the surgical approach to the membranous urethra. Anat. Clin. 1984;6:69–78. doi: 10.1007/BF01773158. [DOI] [PubMed] [Google Scholar]
- Donker PJ, Droes JTh, van Ulden BM. Anatomy of the musculature and innervation of the bladder and the urethra. In: Williams DI, Chilshon GD, editors. Scientific Foundations of Urology. Vol. 2. London: Heinemann Medical Books Ltd; 1976. pp. 32–39. [Google Scholar]
- Dorschner W, Biesold M, Schmidt F, Stolrenburg JU. The dispute about the external sphincter and the urogenital diaphragm. J. Urol. 1999;162:1942–1945. doi: 10.1016/S0022-5347(05)68074-3. [DOI] [PubMed] [Google Scholar]
- Elbadawi A, Schenk EA. A new theory of the innervation of the bladder musculature. Part 4. Innervation of the vesico-urethral junction and external urethral sphincter. J. Urol. 1974;111:613. doi: 10.1016/s0022-5347(17)60028-4. [DOI] [PubMed] [Google Scholar]
- Elbadawi A, Mathews R, Light JK, Wheeler TM. Immunohistochemical and ultrastructural study of rhabdo-sphincter component of the prostate capsula. J. Urol. 1997;158:1819. doi: 10.1016/s0022-5347(01)64138-7. [DOI] [PubMed] [Google Scholar]
- Fletcher TF, Bradley WE. Neuroanatomy of the bladder-urethra. J. Urol. 1978;119:153. doi: 10.1016/s0022-5347(17)57419-4. [DOI] [PubMed] [Google Scholar]
- Fritsch H. Topography of the pelvic autonomic nerves in human fetuses between 21 and 29 weeks of gestation. Anat. Embryol. 1989;180:57–64. doi: 10.1007/BF00321900. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Lienemann A, Brenner E, Ludwikowski B. Clinical anatomy of the pelvic floor. Adv. Anat. Embryol. Cell Biol. 2004;175:1–64. doi: 10.1007/978-3-642-18548-9. [DOI] [PubMed] [Google Scholar]
- Gil Vernet S. Innervation de la vessie et de l'urètre postérieur. Arch. Anat. Pathol. 1964;12:A119–A125. [PubMed] [Google Scholar]
- Gosling JA, Dixon JS. Light and electron microscopic observations on the human external urethral sphincter. J. Anat. 1979;129:216. [Google Scholar]
- Henle J. Handbuch der Systematischen Anatomie des Menschen. Braunschweig: Vieweg; 1866. [Google Scholar]
- Hollabaugh RS, Jr, Dmochowski RR, Steiner MS. Neuroanatomy of the male rhabdosphincter. Urology. 1997;49:426–434. doi: 10.1016/S0090-4295(96)00497-9. [DOI] [PubMed] [Google Scholar]
- Juenemann KP, Schmidt RA, Melchior H, Tanagho EA. Neuroanatomy and clinical significance of the external urethral sphincter. Urol. Int. 1987;42:132. doi: 10.1159/000281871. [DOI] [PubMed] [Google Scholar]
- Kokoua A, Homsy Y, Lavigne JF, et al. Maturation of the external urinary sphincter: a comparative histotopographic study in humans. J. Urol. 1993;150:617–622. doi: 10.1016/s0022-5347(17)35563-5. [DOI] [PubMed] [Google Scholar]
- Koyanagi T. Studies on the sphincteric system located distally in the urethra: the external urethal sphincter revisited. J. Urol. 1980;124:400. doi: 10.1016/s0022-5347(17)55468-3. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Koyanagi T, Takahashi Y. The innervation of the external urethral sphincter; an ultrastructural study in male human subjects. Urol. Res. 1987;15:39–43. doi: 10.1007/BF00256334. [DOI] [PubMed] [Google Scholar]
- Lepor H, Gregermann M, Crosby R, Mostofi FK, Walsh PC. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J. Urol. 1985;133:207. doi: 10.1016/s0022-5347(17)48885-9. [DOI] [PubMed] [Google Scholar]
- Ludwikowski B, Oesch Hayward I, Brenner E, Fritsch H. The development of the external urethral sphincter in humans. BJU Int. 2001;87:565–568. doi: 10.1046/j.1464-410x.2001.00086.x. [DOI] [PubMed] [Google Scholar]
- Myers RP. Male urethral sphincteric anatomy and radical prostatectomy. Urol. Clin. N. Am. 1991;18:211–227. [PubMed] [Google Scholar]
- Myers RP, Cahill DR, Devine RM, King BF. Anatomy of radical prostatectomy as defined by magnetic resonance imaging. J. Urol. 1998;159:2148–2158. doi: 10.1016/S0022-5347(01)63297-X. [DOI] [PubMed] [Google Scholar]
- Narayan P, Konety B, Aslam K, Aboseif S, Blumenfeld W, Tanagho E. Neuroanatomy of the external urethral sphincter: Implication for urinary continence preservation during radical prostate surgery. J. Urol. 1995;153:337. doi: 10.1097/00005392-199502000-00012. [DOI] [PubMed] [Google Scholar]
- Oelrich TM. The urethral sphincter muscle in the male. Am. J. Anat. 1980;158:229–246. doi: 10.1002/aja.1001580211. [DOI] [PubMed] [Google Scholar]
- Schlegel PN, Walsh PC. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J. Urol. 1987;138:1402. doi: 10.1016/s0022-5347(17)43655-x. [DOI] [PubMed] [Google Scholar]
- Shafik A, Doss S. Surgical anatomy of the somatic terminal innervation the anal and urethral sphincters: role in anal and urethral surgery. J. Urol. 1999;161:85–89. [PubMed] [Google Scholar]
- Steiner MS. Continence-preserving anatomic radical retropubic prostatectomy: the ‘No-Touch’ technique. Curr. Urol. Report. 2000;1:20–27. doi: 10.1007/s11934-000-0031-3. [DOI] [PubMed] [Google Scholar]
- Strasser H, Klima G, Poisel S, Horninger W, Bartsch G. Anatomy and innervation of the rabdomyosphincter of the male urethra. The Prostate. 1996;28:24–31. doi: 10.1002/(SICI)1097-0045(199601)28:1<24::AID-PROS4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tanagho EA, Schmidt RA, De Araujo CG. Urinary striated sphincter: What is its nerve supply? Urology. 1982;20:415. doi: 10.1016/0090-4295(82)90468-x. [DOI] [PubMed] [Google Scholar]
- Von Heyden B, Jordan U, Hertle L. Neurotransmitters in the human urethral sphincter in the absence of voiding dysfunction. Urol. Res. 1998;26:299–310. doi: 10.1007/s002400050061. [DOI] [PubMed] [Google Scholar]
- Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J. Urol. 1998;160:2418–2424. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- Zvara P, Carrier S, Kour NW. The detailed neuroanatomy of the human striated urethral sphincter. Br. J. Urol. 1994;74:182–187. doi: 10.1111/j.1464-410x.1994.tb16583.x. [DOI] [PubMed] [Google Scholar]