Abstract
In macaque monkeys, the posterior parietal cortex (PPC) is concerned with the integration of multimodal information for constructing a spatial representation of the external world (in relation to the macaque's body or parts thereof), and planning and executing object-centred movements. The areas within the intraparietal sulcus (IPS), in particular, serve as interfaces between the perceptive and motor systems for controlling arm and eye movements in space. We review here the latest evidence for the existence of the IPS areas AIP (anterior intraparietal area), VIP (ventral intraparietal area), MIP (medial intraparietal area), LIP (lateral intraparietal area) and CIP (caudal intraparietal area) in macaques, and discuss putative human equivalents as assessed with functional magnetic resonance imaging. The data suggest that anterior parts of the IPS comprising areas AIP and VIP are relatively well preserved across species. By contrast, posterior areas such as area LIP and CIP have been found more medially in humans, possibly reflecting differences in the evolution of the dorsal visual stream and the inferior parietal lobule. Despite interspecies differences in the precise functional anatomy of the IPS areas, the functional relevance of this sulcus for visuomotor tasks comprising target selections for arm and eye movements, object manipulation and visuospatial attention is similar in humans and macaques, as is also suggested by studies of neurological deficits (apraxia, neglect, Bálint's syndrome) resulting from lesions to this region.
Keywords: AIP, CIP, cytoarchitecture, equivalent, fMRI, homologue, LIP, macaque, MIP, parietal lobule, VIP
Introduction
The functional organization of the posterior parietal cortex (PPC) has been extensively mapped during the past three decades in both humans and monkeys (Fig. 1). The pioneering electrophysiological experiments by Mountcastle et al. (1975) demonstrated that the PPC contains quite different sets of neurons concerned with specific aspects of goal-directed arm and eye movements, for example reaching, grasping, fixation and rapid eye movements (saccades). Anatomically, the PPC is strategically well situated between the sensorimotor fields around the central sulcus and the visual areas found posteriorly in the occipital cortex (Fig. 2). Numerous neuroimaging studies provide us with information about the role of human PPC in attention processes, planning and execution of movements, and control of rapid eye movements (Culham & Kanwisher, 2001). These data are supported by studies of patients presenting with lesions of the parietal cortex and neuropsychological deficits such as visuospatial neglect, different forms of apraxia and other visuomotor coordination problems (for reviews see, for example, Marshall & Fink, 2001, 2003).
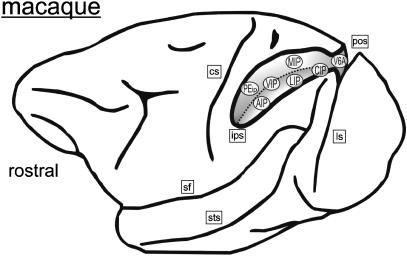
Fig. 1.
Lateral view of a macaque brain with the intraparietal sulcus (IPS) opened up. The arrangement of the IPS areas is schematically depicted as defined in various electrophysiological experiments (see text for references). The bottom of the IPS is indicated by a dashed line. cs, central sulcus; pos, parieto-occipital sulcus; ls, lunate sulcus; sf, Sylvian fissure (lateral sulcus); sts, superior temporal sulcus.
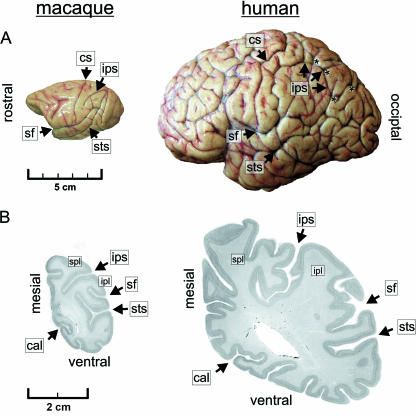
Fig. 2.
Post-mortem anatomy of a macaque and a human brain (specimens taken from the Zilles collection, C. & O. Vogt Institute of Brain Research, Düsseldorf University, Germany). (A) The sulcal anatomy of a macaque brain is much less complicated than the highly gyrified cortex of a human brain. The IPS in humans often has various side branches (indicated here by asterisks in A), and shows a complex folding pattern as demonstrated on the cell-stained coronal sections through posterior portions of the IPS (B). Note the highly gyrified medial aspect of the human IPS (right section) with a large unlabelled intraparietal gyrus. cal, calcarine sulcus; spl; superior parietal lobule; ipl; inferior parietal lobule; other abbreviations as defined in Fig. 1.
In non-human primates such as macaques, anatomical and electrophysiological studies have identified multiple cortical fields in the PPC, each of them being concerned with particular functions for visuomotor processes. Techniques such as single electrode recordings while the animal is performing a specific task (e.g. grasping an object) have helped to delineate functionally different regions based on their neuronal response properties. In particular, the areas around the intraparietal sulcus (IPS), which separates the parietal lobe into a superior (SPL) and an inferior (IPL) part, have been shown to integrate neural signals from different sensory modalities for guiding and controlling action in space (Fig. 1). Anatomically, these areas, which are arranged in a modular fashion, are interconnected with each other, and are thus implemented in a series of neural circuits comprising motor areas in the frontal cortex and visual areas in the occipital cortex (Rizzolatti et al. 1998). Dependent upon the connectivity pattern of a specific IPS area, its respective neurons encode information such as spatial coordinates of objects, the position of body parts in space, eye movement data, or geometrical properties of objects such as shape, size and orientation (see, for example, Duhamel et al. 1992; Colby et al. 1993; Sakata et al. 1995; Rizzolatti et al. 1998)
Although significantly larger and more expanded than in the monkey, the IPS constitutes an anatomically constant sulcus in the human brain (Fig. 2). Owing to its complex folding and branching pattern (Fig. 2A) it is sometimes difficult to localize the main course of the human IPS, especially when the sulcus is split into several anatomical segments (Ono et al. 1990; Grefkes et al. 2001). Microanatomically, e.g. in terms of cyto- or myeloarchitecture, to date little is known about the IPS in the human brain (Zilles & Palomero-Gallagher, 2001; Choi et al. 2002).
The macaque IPS has been used as a model by many neuroscientists for understanding the structure and function of its human equivalent. Furthermore, as functional imaging techniques such as positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) provide limited temporal and spatial information on a neuronal level, the interpretation of neuroimaging experiments frequently draws on the knowledge of the neuronal response properties as established in macaques with invasive electrophysiology. Here we review recent findings with respect to the functional organization of the macaque intraparietal cortex, and present the current evidence for candidate regions that constitute putative functional equivalents in the human brain.
The intraparietal cortex in macaques
As the IPS in macaques is situated between the somatosensory cortex and the visual cortex, anterior parts of the IPS are more concerned with sensorimotor processing whereas posterior parts are more dedicated to visual information processing. Additionally, there is another functional gradient across the IPS: neurons located on the medial bank are more responsive to arm movements, whereas neurons situated in the lateral bank are predominantly involved in eye movements. Importantly, almost each area of the IPS processes information from more than one sensory modality. The systematic testing of IPS neurons for the preferred type of stimulus and behaviour has led to the definition of several distinct cortical areas within the intraparietal cortex named after their topographical position (Fig. 1): the anterior intraparietal area (area AIP), the ventral intraparietal area (area VIP), the medial intraparietal area (area MIP), the lateral intraparietal area (LIP) and the caudal intraparietal area (area CIP).
Area AIP in macaques
Area AIP is located on the lateral bank of the anterior IPS (Fig. 1). Electrophysiological recordings within this area have shown that AIP neurons are active during fixation and manipulation of objects (Sakata et al. 1995). These neurons are highly responsive to size, shape and orientation of objects, sometimes even highly selective in their response to the presentation of a specific object geometry, e.g. selective responses for plates but not for cylinders (Murata et al. 2000).
AIP neurons can be subdivided into three groups according to their visuomotor discharge properties (Sakata et al. 1995). ‘Motor dominant neurons’ fire to fairly similar degrees during object manipulation in the light and in the dark. ‘Visual dominant neurons’ discharge during the manipulation of objects in the light but not in the dark. The ‘visual-and-motor neurons’ show an intermediate behaviour with less activation during object manipulation in the dark than in the light. Some of the latter two types of neurons also fire during object observation when no object manipulation is preformed (‘object type neurons’, Murata et al. 1996). Furthermore, some neurons show sustained activity after brief presentation of an object, which has been interpreted as a short-term memory function for three-dimensional (3D) objects (Murata et al. 1996).
Anatomically, area AIP is connected to the ventral premotor cortex (Matelli et al. 1986), especially to motor area F5, the neurons of which also discharge during specific object-related hand movements and even only on presentation of a 3D object without subsequent manipulation (Murata et al. 1997). It seems that area AIP in combination with area F5 transforms 3D properties of an object into appropriate finger formations and hand orientation for visually guided grasping movements (Rizzolatti et al. 1998; Murata et al. 2000).
Putative human AIP equivalent
The relevance of anterior IPS in object manipulation and grasping movements has been demonstrated in patients with lesions affecting anterior intraparietal cortex (Binkofski et al. 1998). Likewise, PET studies with healthy persons showed that an area in anterior IPS is involved in a tactile shape-processing network when subjects discriminate different objects according to changes in their 3D structure (Bodegard et al. 2001). Jäncke et al. (2001) also found activations in anterior (and posterior) IPS when subjects constructed or recognized 3D shapes with their hands. The same region has also been implicated in the discrimination of the orientation of visual stimuli (Shikata et al. 2001).
Therefore, it seems that human anterior intraparietal cortex is concerned with tactile and visual object processing just as macaque area AIP. Furthermore, that area was shown to play a crucial role for the cross-modal transfer of object information between the visual and sensorimotor systems (Grefkes et al. 2002; Fig. 3). When subjects encoded and subsequently recognized abstract 3D objects by visual inspection or tactile exploration (rendering a delayed matching-to-sample task), the lateral aspect of anterior IPS was specifically active when 3D object information had to be transferred between the visual and the sensorimotor systems for successful object recognition (Fig. 3B). It is important to note that unimodal (visual, tactile) object recognition also increased neural activity in this area (compared with the low-level baseline) albeit to a lesser degree than cross-modal object information processing. Because macaque area AIP contains neurons that discharge during object manipulation (‘motor dominant neurons’) or visual inspection (‘visual dominant neurons’) and that have short-term memory functions for 3D shapes (Sakata et al. 1995; Murata et al. 1996), the putative human AIP candidate (hAIP) in lateral IPS exhibits similar functional response properties in an equivalent position (Grefkes et al. 2002). The cross-modal enhancement of activity in hAIP may facilitate the interplay of visual object data and finger movements for object grasping and exploration.
Fig. 3.
Putative human AIP equivalent as assessed with fMRI (Grefkes et al. 2002). (A) Subjects had to encode and subsequently to recognize the shape of 3D objects either by visual inspection (as presented on a video screen) or by tactile manipulation (using their right hand).(B) When 3D information had to be transferred between the visual and the sensorimotor systems for successful object recognition (cross-modal transfer), increased neural activity was measured in the lateral anterior aspect of left intraparietal sulcus (P < 0.05, small volume corrected for multiple comparisons, n = 12). That region was significantly less active when objects were encoded and recognized within the same modality (visual, tactile). L, left; R, right; A, anterior; P, posterior; pocs, postcentral sulcus; other abbreviations as in Fig. 1. (Adapted from Grefkes et al. 2002, with permission.)
Area VIP in macaques
Macaque VIP is located in the fundus of the IPS (Maunsell & Van Essen, 1983), and constitutes a polymodal association zone responding to visual, tactile, vestibular and auditory stimuli (Colby et al. 1993; Bremmer et al. 1997; Duhamel et al. 1998; Klam & Graf, 2003). Neurons in VIP receive projections from several visual areas (especially from the middle temporal area (MT) and the middle superior temporal area (MST) complex), from motor, somatosensory, auditory, vestibular areas and from other polysensory cortices (Maunsell & Van Essen, 1983; Lewis & Van Essen, 2000). In particular, stimuli conveying motion information have been demonstrated to activate VIP neurons (Colby et al. 1993; Bremmer et al. 1997). The locations of these stimuli are frequently represented in head-centred coordinates, which means that when the position of the eyes is changed, the receptive fields of VIP neurons maintain the representation of a certain spatial location (Colby et al. 1993). Interestingly, most receptive fields for somatosensory stimuli are restricted to the head and the face, and match those for visual information in location, size and stimulus direction (Duhamel et al. 1998). For example, a typical VIP neuron may discharge when a moving visual stimulus is detected in the upper left visual quadrant, but also when the left eyebrow of the animal is touched. Likewise, the preferred direction of VIP neurons for visual and vestibular stimulation (horizontal rotation of the animal on a turntable) is congruent, i.e. the neurons are preferentially responsive to visual motion and head rotation in the same direction (Bremmer et al. 2002). Furthermore, a vast majority of VIP neurons simultaneously code for head velocity, acceleration and position (Klam & Graf, 2003).
Therefore, the functions of VIP may encompass the perception of self movements and object movements in near extrapersonal space (Bremmer et al. 2002). For example, when an animal is heading for fruits, visual (approaching branches of trees), tactile (touching leaves) and vestibular information (perception of head movement) may guide the monkey to move through the forest. Interestingly, electrical stimulation of VIP neurons evokes avoidance behaviour such as eye closure, contraction of facial muscles, shoulder shrugs and arm movements, which do not occur when the cortex surrounding VIP is stimulated (Cooke et al. 2003). Thus, VIP may also contribute to the control of defensive movements triggered by stimuli on or near the head (Cooke et al. 2003).
Putative human VIP equivalent
Many studies have aimed at demonstrating that cortical regions are concerned with motion processing in different sensory modalities. Visual motion detection (Corbetta et al. 1991; Zeki et al. 1991), perception of auditory movements (Griffiths et al. 1994, 2000), vestibular processing (Dieterich & Brandt, 2000; Fasold et al. 2002; Fink et al. 2003) and processing of tactile movements (Bodegard et al. 2000; Downar et al. 2000; Kitada et al. 2003) have all been shown to recruit both modality-specific and supramodal areas. Many of these studies report the involvement of PPC, implicating that this region is part of a network concerned with polymodal information processing (Macaluso et al. 2003). For example, the neural networks for visual and auditory motion processing encompass distinct but partially overlapping activations in or near the IPS (Lewis et al. 2000). Likewise, vestibular stimulation increases neural activity in intraparietal cortex (Suzuki et al. 2001). The polymodal nature of the PPC is further demonstrated by patient studies showing that lesions to the PPC may cause disorders such as neglect or extinction affecting more than one modality. Interestingly, these deficits are often organized in a head-centred frame of reference (Vallar et al. 1999, 2003). Therefore damage to a putative human VIP equivalent (hVIP) may be responsible for some of the symptomatology observed in visuospatial neglect.
As reported above, the main features of macaque area VIP encompass polymodal motion processing in predominantly head-centred coordinates. Accordingly, in order to identify a putative human equivalent, Bremmer et al. (2001) conducted an fMRI study using a polymodal motion protocol (Fig. 4). In that study, healthy subjects experienced either a visual (moving starfield pattern), tactile (air flow across forehead) or auditory (binaural beats) motion stimulus, or stationary controls (Bremmer et al. 2001; Fig. 4A). Testing for common activations in all three tasks conveying motion information (relative to the perception of a stationary modality-matched stimulus) revealed a bilateral activation in either intraparietal cortex (Fig. 4B). The single subject data confirmed that the region activated lay indeed in the fundus of IPS. As the only area in macaque IPS responsive to visual, auditory and tactile motion stimulation is area VIP (Bremmer et al. 2000), it can be concluded that the human VIP equivalent is found at a similar topographical position as the macaque area VIP (Bremmer et al. 2001).
Fig. 4.
Putative human VIP equivalent as assessed with fMRI (Bremmer et al. 2001). (A) Subjects experienced either a visual (large random dot pattern), a tactile (air flow across forehead) or an auditory (binaural beats) motion stimulus, or a stationary modality-specific control. (B) A conjunction analysis (P < 0.05, corrected for multiple comparisons, n = 8) testing for increased neural activity common to all three conditions conveying motion information revealed significant activations in three circumscribed regions, one of which was located in parietal cortex (labelled ‘1’). The superimposition of the functional images with the mean anatomical volume showed that the area activated lay in the depth of either intraparietal sulcus (IPS). (Adapted from Bremmer et al. 2001, with kind permission.)
Area MIP in macaques
Neurons in the macaque MIP are implemented in neural circuits mediating planning, execution and monitoring of reaching movements. This area has been much less studied than area AIP or other IPS areas, and thus only little information about its specific function for reaching movements is available. Area MIP is part of the parietal reaching region (PRR; Cohen & Andersen, 2002), which includes also other areas such as area V6A and the intraparietal parts of area PE (area PEip) (Fig. 1).
The anterior parts of MIP are differentially concerned with somatosensory information compared with more posterior and ventral portions of medial IPS, which are preferentially responsive to visual information processing (Colby & Duhamel, 1991). Neurons in MIP specifically discharge dependent on the direction of hand movements towards a visual target. For example, increased neuronal activity was recorded in MIP when monkeys used a joystick to guide a spot to a visual target presented on a computer monitor (Eskandar & Assad, 1999; Fig. 5A). The activity was much less when monkeys just watched a play-back version of a trial without moving the joystick (Eskandar & Assad, 2002), which indicates that MIP neurons are involved in the coordination of hand movements and visual targets. It has been claimed, accordingly, that MIP neurons transform the spatial coordinates of the target to be reached (e.g. an object) into a representation that can be used by the motor system for computing the respective movement vector (Cohen & Andersen, 2002). These coordinate transformation processes may take place even before an arm movement is initiated (Johnson et al. 1996). Such cross-modal representations of the spatial relations between a motor effector (e.g. the hand) and a nearby visual stimulus (e.g. an object) are also likely to contribute to monitoring a limb position during an actual reaching movement (Eskandar & Assad, 2002). MIP neurons are also considered to be part of a neural network that detects movement errors or position changes of a target so that fast movement corrections can occur (Desmurget & Grafton, 2000; Kalaska et al. 2003). These automatic and unconscious correction mechanisms do not necessarily require a visual feedback of the moving hand, which implies that the correction mechanisms may also rely on efference copy information, proprioceptive feedback and on an internal representation of the target location (Kalaska et al. 2003).
Fig. 5.
Putative human MIP equivalent as assessed with fMRI (Grefkes et al. 2004). (A) Electrophysiological MIP experiments in the macaque brain (Eskandar & Assad, 2002). MIP neurons were recorded while the monkey performed a visuomotor joystick task. MIP neurons responded irrespective of whether a visual movement feedback was present on the video screen but much less when joystick movements were absent (playback trials). (From Eskandar & Assad, 2002, with permission.) (B) Adopted protocol used for the fMRI study with human subjects (Grefkes et al. 2004). Fixating volunteers were asked to guide the black square from the white to the black circle using an MRI-compatible joystick. (C) Visually directed joystick movements (with or without movement feedback) elicited significantly higher activity in medial IPS than the control conditions consisting of stereotype-intransitive joystick movements. (Adapted from Grefkes et al. 2004, with permission.)
Putative human MIP equivalent
Although only a few imaging studies have investigated the cortical regions involved in reaching and visuomotor transformation in the human brain, it has been demonstrated that human medial IPS also plays a crucial role in tasks requiring a visuomotor coordination of hand movements and targets (Chaminade & Decety, 2002; Simon et al. 2002). The same region was shown to be involved in the spatial updating of pointing targets (Medendorp et al. 2003). Likewise, transcranial magnetic stimulation (TMS) over left posterior IPS may interfere with the adjustment of reaching movements to a dynamic environment (della Maggiore et al. 2004), and may also disturb sudden switches between different grip apertures for different target sizes (Glover et al. 2005), indicating that this region is relevant for online-control and correction of movement errors. Indeed, some patients suffering from reaching movement disorders such as optic ataxia present with lesions located within or near medial intraparietal cortex (Pisella et al. 2000; Roy et al. 2004). However, other studies testing for areas involved in pointing movements found increased neural activity outside the IPS, for example around the mesial parietal cortex near the precuneus or the posterior cingulate cortex (Astafiev et al. 2003; Connolly et al. 2003). Because electrophysiological experiments in macaques do not usually employ pointing tasks for studying area MIP, it is difficult to determine whether these discrepant findings are due to differences in interspecies anatomy or rather result from differences in the behavioural protocols used (Astafiev et al. 2003).
As reviewed above, not only reaching movements but also visuomotor coordination tasks using a joystick may activate MIP neurons in macaques. For example, neurons in MIP show increased activity when fixating monkeys use a joystick to move a cursor to a target, even when the cursor is transiently hidden during the joystick movements (Fig. 5A; Eskandar & Assad, 1999, 2002). Neural activity in these neurons is reduced, however, when the animal sees a visual play-back of a trial and no joystick movements related to the moving stimuli are performed (Eskandar & Assad, 2002). Accordingly, an fMRI study employing a similar joystick task with human subjects may identify the neural correlates for similar processes in the human brain, therefore possibly activating the human MIP equivalent (hMIP; Grefkes et al. 2004). Indeed, when subjects performed object-guided precision movements using an MRI-compatible joystick (Fig. 5B), neural activity in medial intraparietal cortex (Fig. 5C) was significantly higher when subjects were required to compute accurate movement vectors (and corrections thereof) to reach the target object than in the control conditions. These control conditions consisted of stereotype intransitive joystick movements (i.e. pushing the joystick in the direction indicated by an arrow) not requiring that movements closed in precisely on a target. As observed for macaque area MIP (Fig. 5A), there was no statistical difference in IPS activity whether or not subjects experienced a direct visual feedback of their joystick movements on the video screen. Rather, modality-specific areas were additionally recruited, i.e. differential activation of visual area V5/MT was observed when a direct visual movement feedback was present, and differential activation of sensorimotor areas was demonstrated when subjects had to rely predominantly on proprioception and efference copy information.
The data suggest that human medial intraparietal cortex is crucial for transforming visual coordinates into motor programs, and for the online control of goal-directed precision movements. Because such processes are computed in the PRR and area MIP of macaque monkeys, the functions of human medial intraparietal cortex seem to resemble closely those observed for monkey area MIP (Eskandar & Assad, 2002; Grefkes et al. 2004).
Area LIP in macaques
Area LIP is found in the lateral wall of monkey IPS, and is part of a network of areas mediating saccades. LIP receives input from several visual areas, and is interconnected with the frontal eye field (FEF) and the superior colliculus (Lynch et al. 1985; Blatt et al. 1990).
Neurons in area LIP fire during the detection of a salient visual stimulus such as a flashing spot in the visual periphery, but also in the delay period when the location of a stimulus has to be memorized, and finally when the animal is actually performing the saccade to that location (Gnadt & Andersen, 1988). In the double step saccade task (Hallett & Lightstone, 1976), the monkeys have to perform two successive saccades to previously encoded targets. It has been shown that LIP neurons encode the correct movement vector for the second saccade even before the first saccade is executed (Duhamel et al. 1992). Such predictive response characteristics of LIP neurons have been interpreted to represent fast remapping mechanisms in order to correct for the anticipated displacement of the eyes in relation to a visual target (Duhamel et al. 1992; Heide et al. 2001; Bisley & Goldberg, 2003a).
Other studies have indicated that LIP neurons fire more in saccade trials than in reach trials, supporting the view that area LIP is specifically involved in the representations of motor plans for saccades (Mazzoni et al. 1996; Snyder et al. 1997). Likewise, auditory cues may modulate activity in LIP (Stricanne et al. 1996) albeit to a lesser degree than visual cues (Cohen et al. 2004). Other studies, however, suggest that LIP is not directly concerned with generating saccades as the activity of LIP neurons does not predict where, when or if at all a saccade will occur (Goldberg et al. 2002). Rather, the neural activity in LIP seems to describe accurately the locus of attention, hence representing a ‘salience map’ (Bisley & Goldberg, 2003b). For example, the spike activity of an LIP neuron significantly correlates with the level of attention at a specific location, but not with whether a saccade is actually attended or performed to that location (Bisley & Goldberg, 2003b). However, more recent studies (Janssen & Shadlen, 2005) suggest that LIP activity does predict saccadic reaction times, albeit not as well as FEF or superior colliculus activity (Everling & Munoz, 2000). Whether LIP neurons directly influence reaction time or simply mirror with great fidelity the activity of the other oculomotor areas (i.e. FEF and superior colliculus) remains to be elucidated (Janssen & Shadlen, 2005). Nonetheless, neural activity of LIP neurons is significantly modulated by the anticipation of a saccade to a potential target, and this anticipatory modulation is strongest in LIP neurons that represent the locus of the intended eye movement (Janssen & Shadlen, 2005). The inactivation of LIP by injecting the GABAA agonist muscimol produces deficits in saccadic target selection whereas saccade programming and execution remain unaffected (Wardak et al. 2002).
It has also been suggested that LIP uses the saccade signal (driven by the FEF or other prefrontal areas) to create a representation of a saccadic end point in its salience map (Bisley & Goldberg, 2003a). Additionally, the predictive visual response behaviour of LIP neurons for anticipated saccades may help to maintain a spatially accurate representation of the visual field despite a moving eye and a resulting change in the eye-centred frame of reference (Duhamel et al. 1992; Bisley & Goldberg, 2003a). It has also been suggested that LIP encodes the elapsed time between two events (e.g. between the target onset and the ‘go’ signal) affecting the meaningfulness of visual objects that are potential gaze targets (Janssen & Shadlen, 2005).
In summary, the exact functions of LIP are still a matter of debate. Furthermore, complex cognitive functions such as decision processes, reward expectancy and perception of time have also been related to activity of LIP neurons (Platt & Glimcher, 1999; Roitman & Shadlen, 2002; Dickinson et al. 2003; Leon & Shadlen, 2003; Cohen et al. 2004).
Putative human LIP equivalent
There are numerous functional imaging studies that have shown that human IPS is involved in attention and control of eye movements (e.g. Fink et al. 1997; Corbetta et al. 1998; Corbetta & Shulman, 2002; Pierrot-Deseilligny et al. 2004; Thiel et al. 2004). For example, shifts in visuospatial attention are associated with activation in IPS (Corbetta et al. 1995; Connolly et al. 2000). Searching for a visual target while fixating activates both intraparietal cortex and FEF whereas the detection of that target increases neural activity only in IPS but not in FEF (Shulman et al. 2003). The higher sensitivity to sensory salience in human IPS parallels the situation found for the neurons in macaque area LIP, as reviewed above (Bisley & Goldberg, 2003a). However, many of the activations observed in human IPS in these tasks were located in the medial wall of IPS (Shulman et al. 2003). Likewise, saccade protocols using variations of the double step saccade task – similar to that used to study the predictive visual responses in LIP neurons – showed activations predominantly in superior parietal cortex and medial portions of IPS, albeit also extending into the lateral wall and into the adjacent inferior parietal cortex (Heide et al. 2001). Another study using several delayed saccade protocols in a retinotopic mapping design identified a putative LIP equivalent in superior parietal cortex just beyond the medial end of posterior IPS (Sereno et al. 2001). The same region was involved in remapping of the contralateral space when fixating subjects had to perform two subsequent saccades to remembered target locations in another variation of the double step saccade task (Medendorp et al. 2003).
Taken together, most of the studies cited above suggest that the human equivalent for monkey area LIP (hLIP) is situated in medial IPS rather than lateral IPS. Compelling evidence for that conclusion comes from a recent fMRI study in which the same saccade task was performed in both humans and monkeys (Koyama et al. 2004; Fig. 6). In that study, BOLD (blood oxygenation level dependent) signal changes were compared in response to visually guided leftward and rightward saccades (Fig. 6A). In monkeys, as in humans, a functionally equivalent region could be identified within IPS that was highly selective to the direction of saccades (Fig. 6B). However, whereas the monkeys most significantly activated the lateral intraparietal cortex (dorsal portion of area LIP, LIPd), the corresponding activations in human IPS were found in the posterior part of medial intraparietal cortex (Fig. 6BC). As that study used identical tasks and identical methods, it must be concluded that the functional organization of the IPS in terms of area LIP is different between monkeys and humans with hLIP found on the medial wall of IPS.
Fig. 6.
Putative human LIP equivalent as assessed with fMRI (Koyama et al. 2004). (A) The oculomotor task consisted of horizontal saccades in response to the shift of the fixation target. Upper row: horizontal target displacement. Lower rows: eye movement recordings in horizontal and vertical direction. (B) BOLD signal associated with saccades in monkeys. Increased neural activity is found in the lateral wall of IPS (left image: coronal section; right image: horizontal section). (C) BOLD signal associated with saccades in humans. Increased neural activity is found in the medial wall of IPS (horizontal section). (D) Saccade-related activity superimposed on 3D renderings of a macaque brain (left) and a human brain (right). Monkey area LIP is situated in lateral IPS, the putative human LIP equivalent is located in medial aspects of posterior IPS. (Adapted from Koyama et al. 2004, with kind permission.)
Area CIP in macaques
The macaque area CIP is situated in the lateral bank of the caudal IPS posterior to area LIP, and receives fibre projections from visual areas V3, V3A and probably V4 (Cavada & Goldman-Rakic, 1989; Tsutsui et al. 2003). Neurons in area CIP are involved in the analysis of 3D object features, and are especially responsive to axis and surface orientations of objects in space (Sakata, 2003; Tsutsui et al. 2003). Axis orientation-selective neurons preferentially discharge upon the presentation of elongated stimuli and show tuning to the orientation of the longitudinal axes of objects in 3D space (Sakata et al. 1998). Other neurons within area CIP are selective for the 3D surface orientation of a flat surface defined by binocular disparity (Shikata et al. 1996; Sakata et al. 1998; Taira et al. 2000). However, not only binocular but also monocular depth cues are processed in macaque area CIP. For example, the specific 3D orientation of texture gradients consisting of dots or lines with perspective cues (e.g. texture spacing, size and shape cues) constitutes an effective stimulus for activating CIP neurons (Tsutsui et al. 2002; Fig. 7A). Importantly, texture gradient-sensitive neurons are not responsive to the local features of the dot patterns, but rather respond to the gradient signals extracted from the texture patterns (Tsutsui et al. 2002). The majority of surface-orientation-selective neurons are sensitive to both disparity and perspective cues, and neural responses are the strongest when both cues are present (summation effect) (Tsutsui et al. 2001). Because the preference of CIP neurons for texture and disparity gradients is significantly correlated in terms of surface orientation selectivity, area CIP may integrate these different (binocular, monocular) spatial depth cues to compute a 3D surface representation of the perceived object (Taira et al. 2000; Tsutsui et al. 2002). Additionally, it has recently been demonstrated that a high proportion of CIP neurons show delay activity and match enhancement after presentation of a specific surface orientation, possibly reflecting neural substrates for short-term memory of 3D surface orientations (Tsutsui et al. 2003). Inactivation of CIP neurons through intracortical injections of the GABAA agonist muscimol leads to a significant impairment of 3D surface orientation discrimination without affecting the ability to discriminate 2D shapes (Tsutsui et al. 2001).
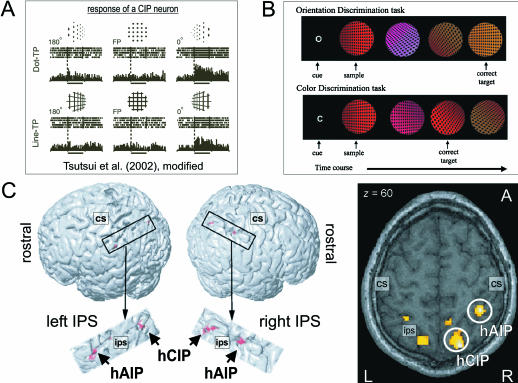
Fig. 7.
Putative human CIP equivalent as assessed with fMRI (Shikata et al. 2001). (A) Electrophysiological recordings from a texture gradient-sensitive neuron in macaque area CIP (adapted from Tsutsui et al. 2002, with permission). Responses to three orientations [180° tilt, frontoparallel (FP), 0° tilt) are shown for dot texture patterns (dot-TP) and line texture patterns (line-TP) aligned at the onset of sample stimulus presentation. Insets above rasters indicate stimuli presented. The neuronal responses are selective to texture gradients of patterns defining 0° tilt (or right-side-nearer orientation). (B) Protocol of the fMRI study with human subjects (Shikata et al. 2001). After a cue stimulus (‘O’ for orientation discrimination, ‘C’ for colour discrimination), sample and test stimuli composed of dot-TPs and different colours were sequentially presented. Subjects were asked to press a button as soon as the matching stimulus appeared. (C) Surface orientation discrimination vs. colour discrimination significantly activated two regions in human IPS bilaterally (single subject brain, P < 0.05, corrected; detailed images thresholded at P < 0.001, uncorrected). The posterior activation (putative human CIP, hCIP) is stronger positively modulated by the orientation discrimination performance of the subjects than the anterior region (putative human AIP, hAIP). (Adapted from Shikata et al. 2001, with kind permission.)
Conceptually, area CIP is probably part of a neural circuit for visually guided grasping movements (Sakata, 2003). It has been proposed that area CIP analyses the 3D shape and 3D orientation of objects by integrating binocular and monocular depth cues and information from the dorsal and ventral visual streams (Sakata, 2003; Tsutsui et al. 2003). Object information is then fed forward to visual-dominant and visual-and-motor neurons in area AIP (see above), which further projects to motor-related neurons in area F5 in the ventral premotor cortex coding for finger shaping and grasping movements (Rizzolatti et al. 1998; Sakata, 2003).
Putative human CIP equivalent
PET and fMRI studies have demonstrated that also in humans, as in macaques, posterior portions of the IPS are activated during the analysis of surface and pattern orientation (Taira et al. 1998; Faillenot et al. 1999, 2001). In an fMRI experiment, neural activity was increased in caudal parts of right IPS when subjects had to discriminate whether the central part of a surface was protruded or recessed based on shading cues (Taira et al. 2001).
Shikata et al. (2001) used event-related fMRI to identify the neural correlates for surface orientation discrimination with monocular depth cues such as texture gradients, similar to those employed in macaques for studying area CIP. In that experiment, subjects had to encode and subsequently to recognize the orientation of a sample stimulus consisting of black dots assembled into a perspective array (rendering a slanted plane in virtual 3D space) in a delayed matching-to-sample (DMS) task (Fig. 7B, upper row). The control conditions consisted of colour discrimination between the sample and the test stimuli (Fig. 7B, lower row). Comparing surface orientation discrimination vs. colour discrimination showed increased neural activity in two distinctive IPS regions, bilaterally, comprising anterior and posterior intraparietal cortex, respectively (Fig. 7C). In particular, the posterior activation was strongly modulated by the discrimination performance of the subjects, which implies that the putative human CIP equivalent (hCIP) is primarily related to the surface orientation discrimination task per se whereas the anterior activation (putative hAIP) possibly reflects a more general visual representation of 3D object features that can be used for grasping movements (see above). This hypothesis is supported by a recent fMRI experiment by Shikata et al. (2003) showing that the activity in hAIP is highly modulated during the surface orientation discrimination task when subjects were asked to imagine or pantomime grasping movements related to the perceived 3D surface with their right index finger and thumb. By contrast, the activity in hCIP during surface orientation discrimination is not influenced by imagined or executed spatial finger adjustments, indicating that hCIP is likely to be concerned with coding 3D features of objects whereas hAIP is more involved in visually guided hand movements, as has been observed in macaques (Shikata et al. 2003).
Interestingly, in both experiments (Shikata et al. 2001, 2003) the anatomical position of the putative human CIP equivalent was found on the medial bank of IPS, and not laterally, as described for macaque area CIP, resembling the situation reported for the human equivalent of macaque area LIP (Koyama et al. 2004).
Conclusions
The studies reviewed here show that the human IPS is involved in visuomotor and attentional processes similar to those observed in macaques. Likewise, human IPS comprises various functionally different highly specialized areas that are likely to reflect the human equivalents for macaque areas AIP, VIP, MIP, LIP and CIP (Bremmer et al. 2001; Shikata et al. 2001; Grefkes et al. 2002, 2004; Koyama et al. 2004). However, the anatomical arrangement of these functionally defined areas is not entirely equivalent, especially for the lateral wall of IPS comprising area hLIP and hCIP in macaques, which appear to have ‘crossed’ the bottom of the sulcus to the medial bank in humans.
That a functional area may be encountered at different topographical positions in humans and monkeys is a well-known phenomenon. For example, the motion-sensitive area MT/V5 is found in the superior temporal sulcus in the macaque and in the inferior temporal sulcus in humans (Zeki et al. 1991; Watson et al. 1993; Wunderlich et al. 2002). Such differences in functional topography may result from disproportionate expansion of cortical regions in humans (Orban et al. 2004). Warping techniques that register the macaque cortex to the human brain using anatomical and functional landmarks suggest that in humans the parietal visual cortex underwent greater expansion than the ventral visual cortex (Denys et al. 2004; Orban et al. 2004). Therefore, the evolution of the dorsal visual stream representing areas concerned with action in space (Ungerleider & Mishkin, 1982) and adjacent cortical regions comprising language areas (Ungerleider et al. 1998) might have led to a ‘medialization’ of the human LIP and CIP equivalents. The functional centre of gravity of hLIP is found posterior to hMIP (Grefkes et al. 2004; Koyama et al. 2004). In addition, the putative human equivalents for area CIP and the V6 complex within the parieto-occipital sulcus (Fig. 1) are found more medially in humans than in macaques Shikata et al. 2001, 2003; Dechent & Frahm, 2003). By contrast, the location of anterior regions of human IPS comprising areas hAIP and hVIP seems to be relatively well preserved across the species (Bremmer et al. 2001; Grefkes et al. 2002).
These interspecies differences in the functional organization of the IPS are also paralleled by structural differences in the anatomy of the intraparietal region. For example, the IPS in humans – especially in its medial and posterior aspects – is often markedly gyrified, sometimes containing various subsulci or even a large intraparietal gyrus (as depicted in Fig. 2B, left to the ‘ips’ arrowhead), which is not the case in the macaque brain. The degree to which this apparent anatomical enlargement reflects interspecies differences in the organization of the IPS areas is unknown. Although various functional imaging studies have consistently demonstrated that human areas MIP and LIP are both located medially to the main (i.e. deepest) branch of the IPS (Sereno et al. 2001; Medendorp et al. 2003; Grefkes et al. 2004; Koyama et al. 2004), there is still some evidence for a relative dorso-medial and ventro-lateral separation between the functional zones associated with visual attentional and motor intentional processes (Rushworth et al. 2001), indicating that both areas (hLIP and hMIP) may also be anatomically separated in the human brain.
There remains a great number of questions that need to be addressed in future experiments. For example, the precise topography of human areas hAIP, hVIP, hMIP, hLIP and hCIP within the intraparietal cortex is not yet clear. In particular, the anatomical relationship between hLIP and hMIP both closely situated on the medial wall has to be examined further. A promising approach to that question may be found in monkey fMRI as this method fills the missing link between neurophysiology in macaques and fMRI in humans, and may therefore provide direct comparisons of fMRI-based functional anatomy between humans and monkeys (Koyama et al. 2004; Orban et al. 2004). Such studies have already suggested that human IPS contains probably more visuospatial processing areas than macaque IPS (Vanduffel et al. 2002). In addition, preliminary data from structural analyses indicate that that there might be more areas in human IPS than in the monkey (Zilles & Palomero-Gallagher, 2001; Choi et al. 2002). Because little is known in terms of the microstructural anatomy of the human intraparietal cortex, further studies using cyto-, myelo- or receptorarchitectonical approaches may provide useful information to establish equivalencies in the organization of the IPS in humans and macaques. And finally, human IPS and its surrounding cortical areas are strongly implicated in many higher cognitive functions, e.g. spatial orientating and re-orientating (Corbetta et al. 1998; Thiel et al. 2004) or local/global processing (Fink et al. 1996, 1999). Some of these cognitive processes have been investigated in the macaque (Horel, 1994; Robinson et al. 1995; Tanaka et al. 2001), but more studies comparing well-established protocols in humans and macaque are needed. Only by such a multifaceted approach we will obtain a clearer picture with regard to interspecies differences in the functional and structural organization of the parietal cortex.
References
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. The role of the parietal cortex in the neural processing of saccadic eye movements. Adv Neurol. 2003a;93:141–157. [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003b;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Bodegard A, Geyer S, Naito E, Zilles K, Roland PE. Somatosensory areas in man activated by moving stimuli: cytoarchitectonic mapping and PET. Neuroreport. 2000;11:187–191. doi: 10.1097/00001756-200001170-00037. [DOI] [PubMed] [Google Scholar]
- Bodegard A, Geyer S, Grefkes C, Zilles K, Roland PE. Hierarchical processing of tactile shape in the human brain. Neuron. 2001;31:317–328. doi: 10.1016/s0896-6273(01)00362-2. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Duhamel JR, Ben Hamed S, Graf W. The representation of movement in near extra-personal space in the macaque ventral intraparietal area (VIP) In: Thier P, Karnath HO, editors. Parietal Lobe Contributions to Orientation in 3D Space. Heidelberg: Springer Verlag; 1997. pp. 619–630. [Google Scholar]
- Bremmer F, Duhamel JR, Ben Hamed S, Graf W. Stages of self-motion processing in primate posterior parietal cortex. Int Rev Neurobiol. 2000;44:173–198. doi: 10.1016/s0074-7742(08)60742-4. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, et al. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Klam F, Duhamel JR, Ben Hamed S, Graf W. Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP) Eur J Neurosci. 2002;16:1569–1586. doi: 10.1046/j.1460-9568.2002.02206.x. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey. I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Decety J. Leader or follower? Involvement of the inferior parietal lobule in agency. Neuroreport. 2002;13:1975–1978. doi: 10.1097/00001756-200210280-00029. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Amunts K, Mohlberg H, Fink GR, Schleicher A, Zilles K. Cytoarchitectonic mapping of the anterior ventral bank of the intraparietal sulcus in humans. Neuroimage. 2002;E.40401.01 doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Cohen IS, Gifford GW., III Modulation of LIP activity by predictive auditory and visual cues. Cereb Cortex. 2004;14:1287–1301. doi: 10.1093/cercor/bhh090. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia. 1991;29:517–537. doi: 10.1016/0028-3932(91)90008-v. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol. 2000;84:1645–1655. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a ‘parietal reach region’ in the human brain. Exp Brain Res. 2003;153:140–145. doi: 10.1007/s00221-003-1587-1. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Taylor CS, Moore T, Graziano MS. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci USA. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Dechent P, Frahm J. Characterization of the human visual V6 complex by functional magnetic resonance imaging. Eur J Neurosci. 2003;17:2201–2211. doi: 10.1046/j.1460-9568.2003.02645.x. [DOI] [PubMed] [Google Scholar]
- Denys K, Vanduffel W, Fize D, et al. The processing of visual shape in the cerebral cortex of human and nonhuman primates: a functional magnetic resonance imaging study. J Neurosci. 2004;24:2551–2565. doi: 10.1523/JNEUROSCI.3569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Dickinson AR, Calton JL, Snyder LH. Nonspatial saccade-specific activation in area LIP of monkey parietal cortex. J Neurophysiol. 2003;90:2460–2464. doi: 10.1152/jn.00788.2002. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Brain activation studies on visual-vestibular and ocular motor interaction. Curr Opin Neurol. 2000;13:13–18. doi: 10.1097/00019052-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Eskandar EN, Assad JA. Dissociation of visual, motor and predictive signals in parietal cortex during visual guidance. Nat Neurosci. 1999;2:88–93. doi: 10.1038/4594. [DOI] [PubMed] [Google Scholar]
- Eskandar EN, Assad JA. Distinct nature of directional signals among parietal cortical areas during visual guidance. J Neurophysiol. 2002;88:1777–1790. doi: 10.1152/jn.2002.88.4.1777. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faillenot I, Decety J, Jeannerod M. Human brain activity related to the perception of spatial features of objects. Neuroimage. 1999;10:114–124. doi: 10.1006/nimg.1999.0449. [DOI] [PubMed] [Google Scholar]
- Faillenot I, Sunaert S, Van Hecke P, Orban GA. Orientation discrimination of objects and gratings compared: an fMRI study. Eur J Neurosci. 2001;13:585–596. doi: 10.1046/j.1460-9568.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, et al. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17:1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;15:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Fink GR, Dolan RJ, Halligan PW, Marshall JC, Frith CD. Space-based and object-based visual attention: shared and specific neural domains. Brain. 1997;120:2013–2028. doi: 10.1093/brain/120.11.2013. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Dolan RJ. Hemispheric asymmetries in global/local processing are modulated by perceptual salience. Neuropsychologia. 1999;37:31–40. doi: 10.1016/s0028-3932(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, et al. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: an fMRI study with clinical implications. Neuroimage. 2003;20:1505–1517. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Glover S, Miall RC, Rushworth MF. Parietal rTMS disrupts the initiation but not the execution of online adjustments to a perturbation of object size. J Cogn Neurosci. 2005;17:124–136. doi: 10.1162/0898929052880066. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bisley J, Powell KD, Gottlieb J, Kusunoki M. The role of the lateral intraparietal area of the monkey in the generation of saccades and visuospatial attention. Ann NY Acad Sci. 2002;956:205–215. doi: 10.1111/j.1749-6632.2002.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland PE, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Weiss PH, Zilles K, Fink GR. Crossmodal processing of object features in human anterior intraparietal cortex: an fMRI study implies equivalencies between humans and monkeys. Neuron. 2002;35:173–184. doi: 10.1016/s0896-6273(02)00741-9. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Ritzl A, Zilles K, Fink GR. Human medial intraparietal cortex subserves visuomotor coordinate transformation. Neuroimage. 2004;23:1494–1506. doi: 10.1016/j.neuroimage.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Bench CJ, Frackowiak RS. Human cortical areas selectively activated by apparent sound movement. Curr Biol. 1994;4:892–895. doi: 10.1016/s0960-9822(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Green GG, Rees A, Rees G. Human brain areas involved in the analysis of auditory movement. Hum Brain Mapp. 2000;9:72–80. doi: 10.1002/(SICI)1097-0193(200002)9:2<72::AID-HBM2>3.0.CO;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE, Lightstone AD. Saccadic eye movements towards stimuli triggered by prior saccades. Vision Res. 1976;16:99–106. doi: 10.1016/0042-6989(76)90083-3. [DOI] [PubMed] [Google Scholar]
- Heide W, Binkofski F, Seitz RJ, et al. Activation of frontoparietal cortices during memorized triple-step sequences of saccadic eye movements: an fMRI study. Eur J Neurosci. 2001;13:1177–1189. doi: 10.1046/j.0953-816x.2001.01472.x. [DOI] [PubMed] [Google Scholar]
- Horel JA. Local and global perception examined by reversible suppression of temporal cortex with cold. Behav Brain Res. 1994;65:157–164. doi: 10.1016/0166-4328(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Kleinschmidt A, Mirzazade S, Shah NJ, Freund HJ. The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb Cortex. 2001;11:114–121. doi: 10.1093/cercor/11.2.114. [DOI] [PubMed] [Google Scholar]
- Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci. 2005;8:234–241. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cisek P, Gosselin-Kessiby N. Mechanisms of selection and guidance of reaching movements in the parietal lobe. Adv Neurol. 2003;93:97–119. [PubMed] [Google Scholar]
- Kitada R, Kochiyama T, Hashimoto T, Naito E, Matsumura M. Moving tactile stimuli of fingers are integrated in the intraparietal and inferior parietal cortices. Neuroreport. 2003;14:719–724. doi: 10.1097/00001756-200304150-00012. [DOI] [PubMed] [Google Scholar]
- Klam F, Graf W. Vestibular signals of posterior parietal cortex neurons during active and passive head movements in macaque monkeys. Ann NY Acad Sci. 2003;1004:271–282. doi: 10.1196/annals.1303.024. [DOI] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Beauchamp MS, DeYoe EA. A comparison of visual and auditory motion processing in human cerebral cortex. Cereb Cortex. 2000;10:873–888. doi: 10.1093/cercor/10.9.873. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Graybiel AM, Lobeck LJ. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol. 1985;235:241–254. doi: 10.1002/cne.902350207. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Driver J, Frith CD. Multimodal spatial representations engaged in human parietal cortex during both saccadic and manual spatial orienting. Curr Biol. 2003;13:990–999. doi: 10.1016/s0960-9822(03)00377-4. [DOI] [PubMed] [Google Scholar]
- della Maggiore V, Malfait N, Ostry DJ, Paus T. Stimulation of the posterior parietal cortex interferes with arm trajectory adjustements during the learning of new dynamics. J Neurosci. 2004;3:9971–9976. doi: 10.1523/JNEUROSCI.2833-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JC, Fink GR. Spatial cognition: where we were and where we are. Neuroimage. 2001;14:S2–S7. doi: 10.1006/nimg.2001.0834. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Fink GR. Cerebral localization, then and now. Neuroimage. 2003;20:S2–S7. doi: 10.1016/j.neuroimage.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Bracewell RM, Barash S, Andersen RA. Motor intention activity in the macaque's lateral intraparietal area. I. Dissociation of motor plan from sensory memory. J Neurophysiol. 1996;76:1439–1456. doi: 10.1152/jn.1996.76.3.1439. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T, Crawford JD. Gaze-centered updating of visual space in human parietal cortex. J Neurosci. 2003;23:6209–6214. doi: 10.1523/JNEUROSCI.23-15-06209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Kaseda M, Sakata H. Parietal neurons related to memory-guided hand manipulation. J Neurophysiol. 1996;75:2180–2186. doi: 10.1152/jn.1996.75.5.2180. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol. 1997;78:2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the Cerebral Sulci. Stuttgart: Thieme Verlag; 1990. [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17:17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Pisella L, Grea H, Tilikete C, et al. An ‘automatic pilot’ for the hand in human posterior parietal cortex, toward reinterpreting optic ataxia. Nat Neurosci. 2000;3:729–736. doi: 10.1038/76694. [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Bowman EM, Kertzman C. Covert orienting of attention in macaques. II. Contributions of parietal cortex. J Neurophysiol. 1995;74:698–712. doi: 10.1152/jn.1995.74.2.698. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AC, Stefanini S, Pavesi G, Gentilucci M. Early movement impairments in a patient recovering from optic ataxia. Neuropsychologia. 2004;42:847–854. doi: 10.1016/j.neuropsychologia.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Krams M, Passingham R. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y, Tsutsui K. Neural coding of 3D features of objects for hand action in the parietal cortex of the monkey. Phil Trans Roy Soc Lond B. 1998;353:1363–1373. doi: 10.1098/rstb.1998.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H. The role of the parietal cortex in grasping. Adv Neurol. 2003;93:121–139. [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Shikata E, Tanaka Y, Nakamura H, Taira M, Sakata H. Selectivity of the parietal visual neurones in 3D orientation of surface of stereoscopic stimuli. Neuroreport. 1996;7:2389–2394. doi: 10.1097/00001756-199610020-00022. [DOI] [PubMed] [Google Scholar]
- Shikata E, Hamzei F, Glauche V, et al. Surface orientation discrimination activates caudal and anterior intraparietal sulcus in humans: an event-related fMRI study. J Neurophysiol. 2001;85:1309–1314. doi: 10.1152/jn.2001.85.3.1309. [DOI] [PubMed] [Google Scholar]
- Shikata E, Hamzei F, Glauche V, et al. Functional properties and interaction of the anterior and posterior intraparietal areas in humans. Eur J Neurosci. 2003;17:1105–1110. doi: 10.1046/j.1460-9568.2003.02540.x. [DOI] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, et al. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the parietal lobe. Neuron. 2002;33:475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Stricanne B, Andersen RA, Mazzoni P. Eye-centered, head-centered, and intermediate coding of remembered sound locations in area LIP. J Neurophysiol. 1996;76:2071–2076. doi: 10.1152/jn.1996.76.3.2071. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitano H, Ito R, et al. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2001;12:441–449. doi: 10.1016/s0926-6410(01)00080-5. [DOI] [PubMed] [Google Scholar]
- Taira M, Kawashima R, Inoue K, Fukuda H. A PET study of axis orientation discrimination. Neuroreport. 1998;9:283–288. doi: 10.1097/00001756-199801260-00020. [DOI] [PubMed] [Google Scholar]
- Taira M, Tsutsui KI, Jiang M, Yara K, Sakata H. Parietal neurons represent surface orientation from the gradient of binocular disparity. J Neurophysiol. 2000;83:3140–3146. doi: 10.1152/jn.2000.83.5.3140. [DOI] [PubMed] [Google Scholar]
- Taira M, Nose I, Inoue K, Tsutsui K. Cortical areas related to attention to 3D surface structures based on shading: an fMRI study. Neuroimage. 2001;14:959–966. doi: 10.1006/nimg.2001.0895. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Onoe H, Tsukada H, Fujita I. Attentional modulation of neural activity in the macaque inferior temporal cortex during global and local processing. Neurosci Res. 2001;39:469–472. doi: 10.1016/s0168-0102(01)00202-4. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. Neuroimage. 2004;21:318–328. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Jiang M, Yara K, Sakata H, Taira M. Integration of perspective and disparity cues in surface-orientation-selective neurons of area CIP. J Neurophysiol. 2001;86:2856–2867. doi: 10.1152/jn.2001.86.6.2856. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Sakata H, Naganuma T, Taira M. Neural correlates for perception of 3D surface orientation from texture gradient. Science. 2002;298:409–412. doi: 10.1126/science.1074128. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Jiang M, Sakata H, Taira M. Short-term memory and perceptual decision for three-dimensional visual features in the caudal intraparietal sulcus (Area CIP) J Neurosci. 2003;23:5486–5495. doi: 10.1523/JNEUROSCI.23-13-05486.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual system. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci USA. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Lobel E, Galati G, Berthoz A, Pizzamiglio L, Le Bihan D. A fronto-parietal system for computing the egocentric spatial frame of reference in humans. Exp Brain Res. 1999;124:281–286. doi: 10.1007/s002210050624. [DOI] [PubMed] [Google Scholar]
- Vallar G, Bottini G, Paulesu E. Neglect syndromes: the role of the parietal cortex. Adv Neurol. 2003;93:293–319. [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Peuskens H, et al. Extracting 3D from motion: differences in human and monkey intraparietal cortex. Science. 2002;298:413–415. doi: 10.1126/science.1073574. [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002;22:9877–9884. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JDG, Myers R, Frackowiak RSJ, et al. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wunderlich G, Marshall JC, Amunts K, et al. The importance of seeing it coming: a functional magnetic resonance imaging study of motion-in-depth towards the human observer. Neuroscience. 2002;112:535–540. doi: 10.1016/s0306-4522(02)00110-0. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N. Cyto-, myelo-, and receptor architectonics of the human parietal cortex. Neuroimage. 2001;14:S8–S20. doi: 10.1006/nimg.2001.0823. [DOI] [PubMed] [Google Scholar]