Abstract
We investigated the angiogenic response induced by acellular femoral matrices implanted in vivo on to the chick embryo chorioallantoic membrane (CAM), a useful model for such investigation. The results showed that acellular matrices were able to induce a strong angiogenic response, comparable with that of fibroblast growth factor-2 (FGF-2), a well-known angiogenic cytokine. The angiogenic response was further increased when exogenous FGF-2 or transforming growth factor beta-1 (TGF-β1) was added to the matrices and inhibited by the addition of anti-FGF-2 or anti-TGF-β1 antibodies. The response may be considered to be dependent on a direct angiogenic effect exerted by the matrices, and also in part by the presence of FGF-2 and TGF-β1 in the acellular matrices.
Keywords: acellular matrices, angiogenesis, chorioallantoic membrane, femur, fibroblast growth factor-2, transforming growth factor-beta
Introduction
Natural collagenous extracellular scaffolds and chemically or physically decellularized matrices have been used in many studies of tissue reconstitution for vessels, nerves, the intestine and the urinary tract, and some studies have demonstrated that cells infiltrating into the matrix regenerated a novel functional tissue therein. Endogenous growth factors such as fibroblast growth factor-2 (FGF-2) and transforming growth factor beta (TGF-β) remained in naturally derived scaffolds (Voytik-Harbin et al. 1997; Koizumi et al. 2000). Acellular matrices are the non-cellular part of a tissue and consist of proteins such as collagen and carbohydrate structures secreted by the resident cells. They can be transplanted without rejection and provide an environment conducive to normal cellular growth, differentiation and angiogenesis, and also provide a framework for tissue regeneration, because they are completely replaced by the host tissue (Hodde, 2002). The integration of these matrices into the host tissue may benefit from a microvascular network capable of anastomosing with the host microvasculature, thereby ensuring an adequate supply of blood and nutrients to the implants. The decellularization procedure of the aorta resulted in a complete loss of all cellular structures with minimal damage to the extracellular matrix. Several authors have developed decellularization techniques followed by in vitro reseeding with autologous cells (Steinhoff et al. 2000; Cebotari et al. 2002) or allowing ingrowth of host cells into the graft after transplantation (Wilson et al. 1995; Goldstein et al. 2000; Steinhoff et al. 2000; Dohmen et al. 2002).
There is a close link between bone formation and angiogenesis, specifically in vascular regression occurring during mesenchymal cell condensation and chondrogenesis and in angiogenesis occurring during the subsequent transition from hypertrophic cartilage to bone. FGF-2 and TGF-β are among the angiogenic cytokines involved in bone formation. Injection of FGF-2 into intact bone stimulates bone formation (Nakamura e al. 1995, 1998). The exact mechanism by which FGF-2 stimulates bone repair remains uncertain, but FGF-2 induces angiogenesis and stimulates mitogenesis of mesenchymal cells and osteoblasts (Globus et al. 1998), which might be mediated and modulated by TFG-β (Nakamura et al. 1995). Otherwise, TGF-β stimulates bone formation when injected into rodent bones (Joyce et al. 1990); it could stimulate recruitment and proliferation of mesenchymal cells (stem cells, chondroblasts and osteoprogenitors) and may affect inflammation and angiogenesis.
In this study we investigated the angiogenic response induced by acellular matrices obtained from rat femoral diaphysis implanted in vivo on to the chick embryo chorioallantoic membrane (CAM), a useful model for studying angiogenesis and anti-angiogenesis (Ribatti et al. 2001) and the effects of other acellular scaffolds, such as those obtained from the brain and aorta (Ribatti et al. 2003; Conconi et al. 2004).
Materials and methods
Preparation of the acellular matrix
Rat femurs were rinsed four times for 15 min in phosphate-buffered saline (PBS) containing 1% antibiotic and antimycotic solution (Sigma Chemical Co., St Louis, MO, USA), and treated according to the methods of Meezan et al. (1975) to obtain an acellular matrix. Briefly, samples were frozen and thawed four times and were then processed three times as follows: distilled water for 72 h at 4 °C, 4% sodium deoxycholate (Sigma Chemical Co.) for 4 h, and 2000 kU Dnase I (Sigma Chemical Co.) in 1 m NaCl for 2 h. The absence of cellular elements was confirmed histologically (haematoxylin–eosin staining) (Merck, Darmstadt, Germany), and acellular matrices were stored in PBS at 4 °C until use.
Immunohistochemistry
Immunohistochemical analysis was carried out on femoral acellular matrices, formalin-fixed and embedded in paraffin according to standard procedures. Five-micrometre-thick sections were deparaffinized and treated with methanol : H2O2 for 20 min to inhibit endogenous peroxidase. They were then washed in PBS. Non-specific sites were saturated with normal goat serum for 20 min at room temperature. Sections were incubated at 37 °C with primary rabbit polyclonal antibodies anti-TGFβ1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-FGF-2 (Chemicon International, Temecula, CA, USA) diluted 1 : 200 in 1% bovine serum albumin (BSA) for 60 min. After washing in PBS the sections were exposed to biotinylated goat anti-rabbit Ig (Dako, Glostrup, Denmark), diluted 1 : 300 in PBS supplemented with 10% heat-inactivated fetal calf serum (FCS) for 15 min at room temperature, and streptavidin-peroxidase conjugate (Vector, Burlingame, CA, USA) diluted 1 : 250 in PBS for 15 min at room temperature. The developing reaction was performed with 0.05 m acetate buffer, pH 5.1, 0.02% 3-amino-9-ethylcarbazole grade 2 (Sigma) and 0.05% H2O2.
Finally, sections were counterstained with Harris’ haematoxylin, mounted in buffered glycerin (Glycergel, Dako), and photographed using a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany). A pre-immune rabbit serum (Dako) replacing the primary antibody served as negative control.
CAM assay
Fertilized White Leghorn chicken eggs (ten for each experimental group) were incubated at 37 °C at constant humidity. On day 3 of incubation a square window was opened in the eggshell after removal of 2–3 mL of albumen in order to detach the developing CAM from the shell. The window was sealed with glass and the eggs were returned to the incubator. On day 8, a 1-mm-thick cross-section cut with scissors of an acellular femoral matrix was implanted on top of the growing CAMs under sterile conditions. In some experiments, matrices were mixed before implantation with: (1) 500 ng per embryo of recombinant FGF-2 (R & D Systems, Abingdon, UK); (2) 500 ng per embryo of anti-FGF-2 antibody (Santa Cruz Biotechnology); (3) 500 ng per embryo of recombinant TGF-β1 (Santa Cruz Biotechnology); (4) 500 ng per embryo of anti-TGF-β1 antibody (Santa Cruz Biotechnology); (5) a mixture of the two antibodies at the same concentrations. Moreover, 1-mm3 sterilized gelatin sponges (Gelfoam, Upjohn, Kalamazoo, MI, USA) adsorbed with 500 ng per embryo of FGF-2 dissolved in 1 µL PBS or with PBS alone, used as positive and negative controls, respectively, were implanted on day 8 on top of some CAMs, as previously reported (Ribatti et al. 1997).
CAMs were examined daily until day 12 and photographed in ovo with a stereo microscope equipped with a Camera System MC 63 (Zeiss, Oberkochen, Germany). Blood vessels entering the implants or the sponges within the focal plane of the CAM were counted by two observers in a double-blind fashion at 50× magnification.
Macroscopic digital images were captured using a stereo microscope connected to an image analyser system (Olympus Italia, Italy). The largest blood vessels in the CAM (primary vessels) have branches called secondary vessels. The number of branches from two secondary vessels was measured for each of the CAMs in the experimental and control groups. The number of branches per millimetre was calculated for each vessel and used to determine the mean number of branches per millimetre for each group.
Statistical analysis
Means ± SD were determined for all variables. The statistical significance of the differences between mean values was determined by the Student's t-test for unpaired data.
Results
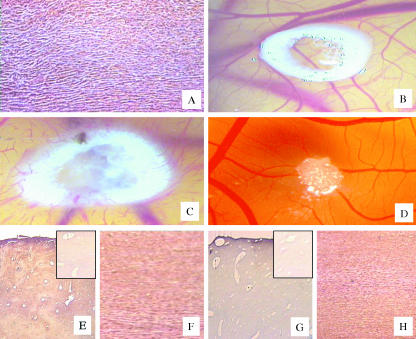
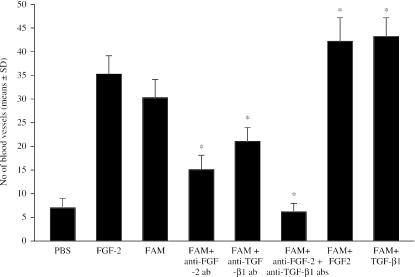
A section of the femoral acellular matrix obtained following the detergent-enzymatic treatment is shown in Fig. 1(A); histological examination revealed an absence of cells. Macroscopic observation of CAMs treated with femoral acellular matrices showed that the implants on day 12 of incubation were surrounded by allantoic vessels (mean number of vessels around the implants = 30 ± 4; P < 0.001 vs. the vehicle alone) that developed radially towards the implant in a ‘spoked-wheel’ pattern, as compared with the same implants on day 8 of incubation (mean number of vessels around the implants = 8 ± 2) (Fig. 1B,C). The angiogenic response was similar to that exerted by a well-known angiogenic cytokine, FGF-2 (mean number of vessels around the implants = 35 ± 4; P < 0.001 vs. the vehicle alone), whereas on day 12 of incubation no vascular reaction was detectable around the sponge treated with the vehicle alone (mean number of vessels around the implants = 7 ± 2) (Fig. 1D). Moreover, femoral acellular matrices increase the number of branches coming off the secondary vessels, as compared with the CAMs treated with the vehicle alone (Fig. 2).
Fig. 1.
(A) Absence of cells in a section of the acellular femoral matrix obtained following the detergent enzymatic treatment. (B,C) Macroscopic pictures of an implant of an acellular matrix obtained from a femoral diaphysis on to the chick embryo CAM on day 8 of incubation in B and after 4 days in C. Note in C numerous allantoic vessels developing radially toward the implant. (D) CAM on day 12 of incubation, incubated for 4 days with a sponge adsorbed with PBS: no vascular response is detectable around the sponge. (E–H) Sections of femoral diaphysis (E,G) and of corresponding acellular matrices (F,H) immunoreactive to FGF-2 (E,F) and to TGF-β (G,H). (E, G, inset) Two negative controls in which pre-immune rabbit serum replacing the primary antibody has been used. Original magnifications: A, ×250; B,D, ×30; C, ×50; E,G, ×250; F,H, ×400.
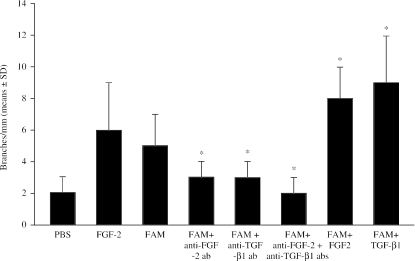
Fig. 2.
Macroscopic evaluation of the angiogenic activity of femoral acellular matrices (FAM) in the chick embryo CAM, evaluated as the number of branches coming off secondary vessels. *P < 0.001 vs. FAM.
Histological examination of the acellular matrices revealed an absence of cells and a less compact structure (Fig. 1F,H) compared with untreated tissues (Fig. 1E,G). The detergent–enzymatic treatment preserved the presence of FGF-2 and TGF-β1 from tissues, as demonstrated by a diffuse strong immunoreactivity in both whole (Fig. 1E,G) and acellular matrices (Fig. 1F,H).
To assess whether the angiogenic response was due to an increased mobilization of endogenous FGF-2 or TGF-β, matrices were added to the CAM in the presence of anti-FGF-2 or anti-TGF-β antibodies. The results shown in Fig. 3 demonstrated that anti-FGF-2 antibodies reduced the angiogenic response by 50%, whereas anti-TGF-β antibodies reduced it by 30%. When the two antibodies were added together, they acted in a synergistic way, reducing the angiogenic response by 80%. Otherwise, when the acellular matrices were added to the CAM in the presence of FGF-2 or TGF-β, the angiogenic response increased by 40%. Anti-FGF-2, anti-TGF-β and a mixture of two antibodies significantly decreased the number of branches coming off secondary vessels, whereas FGF-2 and TGF-β significantly increase their number, as compared with acellular matrix alone (P < 0.001) (Fig. 2).
Fig. 3.
Macroscopic evaluation of the angiogenic activity of femoral acellular matrices (FAM) in the chick embryo CAM, evaluated as the number of blood vessels around the implants. *P < 0.001 vs. FAM.
Discussion
In this study we demonstrated for the first time that acellular femoral matrices are able to induce a strong angiogenic response, comparable with that of FGF-2, when implanted on to the chick CAM. The newly formed blood vessels grow radially around the matrices, invading them at some points, and the angiogenic response may be considered to be dependent on direct angiogenic activity exerted by the matrices.
The advantage of using acellular matrices, rather than exogenously added growth factors such as FGF-2, may be attributable to direct angiogenic activity exerted by the matrices. It is conceivable that extraction techniques may not remove all the factors that impact on angiogenesis. In fact, immunohistochemical staining showed that the angiogenic activity may also be dependent on the presence of FGF-2 and TGF-β, as demonstrated in other experimental conditions (Taipale et al. 1996; Ribatti et al. 2003; Conconi et al. 2004). Moreover, acellular matrices may induce the release of endogenous angiogenic factors such as FGF-2 (Ribatti et al. 1995) and TGF-β (Yang & Moses, 1990) stored in the extracellular matrix of the developing CAM. This finding is confirmed by the fact that application of anti-FGF-2 antibody reduced the angiogenic response by 50%, whereas anti-TGF-β antibody reduced it by 30%. When the two antibodies were added together, they acted in a synergistic way, reducing the angiogenic response by 80%. Overall, these data indicate that the femoral acellular matrices induce an angiogenic response in vivo, which may be considered to be a consequence of direct angiogenic activity exerted by the growth factors retained in the matrix through processing, and indirect induction of the angiogenic cytokines released from the CAM extracellular matrix as a result of the matrix being in contact with it.
Acknowledgments
This work was supported in part by a grant from Ministero dell’Istruzione, dell’Università e della Ricerca (FIRB), Rome, Italy, to D.R. and G.G.N.
References
- Cebotari S, Mertsching H, Kallenbach K, et al. Construction of autologous human heart valves based on an acellular allograft matrix. Circulation. 2002;106:163–168. [PubMed] [Google Scholar]
- Conconi MT, Nico B, Mangieri D, et al. Angiogenic response induced by acellular aortic matrix in vivo. Anat Rec. 2004;281A:1303–1307. doi: 10.1002/ar.a.20137. [DOI] [PubMed] [Google Scholar]
- Dohmen PM, Lembcke A, Hotz H, Kivelitz D, Konertz WF. Ross operation with a tissue-engineered heart valve. Ann Thorac Surg. 2002;74:1438–1442. doi: 10.1016/s0003-4975(02)03881-x. [DOI] [PubMed] [Google Scholar]
- Globus RK, Patterson-Buckendahl P, Gospodarowicz D. Regulation of bovine cell proliferation by fibrobast growth factor and transforming growth factor beta. Endocrinology. 1998;123:98–105. doi: 10.1210/endo-123-1-98. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Clarke DR, Walsh SP, Black KS, O'Brien MF. Transpecies heart valve transplant: advanced studies of a bioengineered xeno-autograft. Ann Thorac Surg. 2000;70:1962–1969. doi: 10.1016/s0003-4975(00)01812-9. [DOI] [PubMed] [Google Scholar]
- Hodde J. Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Engineering. 2002;8:295–308. doi: 10.1089/107632702753725058. [DOI] [PubMed] [Google Scholar]
- Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- Meezan E, Hjelle JT, Brendel K. A simple, versatile, non disruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975;17:1721–1732. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hanada K, Tamura M, et al. Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor. Endocrinology. 1995;136:1276–1284. doi: 10.1210/endo.136.3.7867582. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hara Y, Tagawa M, et al. Recombinant human basic fibroblast growth factor accelerates fracture healing by enhancing callus remodeling in experimental dog tibial fracture. J Bone Miner Res. 1998;13:942–949. doi: 10.1359/jbmr.1998.13.6.942. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Urbinati C, Nico B, Rusnati M, Roncali L, Presta M. Endogenous basic fibroblast growth factor is implicated in the vascularization of the chick embryo chorioallantoic membrane. Dev Biol. 1995;170:39–49. doi: 10.1006/dbio.1995.1193. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Gualandris A, Bastaki M, et al. New model for the study of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane: the gelatin sponge/chorioallantoic membrane assay. J Vasc Res. 1997;34:455–463. doi: 10.1159/000159256. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A, Roncali L, Burri PH, Djonov V. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat Rec. 2001;264:317–324. doi: 10.1002/ar.10021. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Conconi MT, Nico B, et al. Angiogenic response induced by acellular brain scaffolds grafted onto the chick embryo chorioallantoic membrane. Brain Res. 2003;989:9–15. doi: 10.1016/s0006-8993(03)03225-6. [DOI] [PubMed] [Google Scholar]
- Steinhoff G, Stock U, Karim N, et al. Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: in vivo restoration of valve tissue. Circulation. 2000;102(Suppl. 3):III50–55. doi: 10.1161/01.cir.102.suppl_3.iii-50. [DOI] [PubMed] [Google Scholar]
- Taipale J, Saharinen J, Hedman K, Keski-Oja JJ. Latent transforming growth factor-beta 1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem. 1996;44:875–879. doi: 10.1177/44.8.8756760. [DOI] [PubMed] [Google Scholar]
- Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478–491. [PubMed] [Google Scholar]
- Wilson GJ, Courtman DW, Klement P, Lee JM, Yeger H. Acellular matrix: a biomaterials approach for coronary artery bypass and heart valve replacement. Ann Thorac Surg. 1995;60:S353–S358. doi: 10.1016/0003-4975(95)98967-y. [DOI] [PubMed] [Google Scholar]
- Yang EY, Moses HL. Transforming growth factor beta-1 induces changes in cell migration, proliferation and angiogenesis in chicken chorioallantoic membrane. J Cell Biol. 1990;2:731–741. doi: 10.1083/jcb.111.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]