Abstract
Functional imaging techniques have allowed researchers to look within the brain, and revealed the cortical representation of pain. Initial experiments, performed in the early 1990s, revolutionized pain research, as they demonstrated that pain was not processed in a single cortical area, but in several distributed brain regions. Over the last decade, the roles of these pain centres have been investigated and a clearer picture has emerged of the medial and lateral pain system. In this brief article, we review the imaging literature to date that has allowed these advances to be made, and examine the new frontiers for pain imaging research: imaging the brainstem and other structures involved in the descending control of pain; functional and anatomical connectivity studies of pain processing brain regions; imaging models of neuropathic pain-like states; and going beyond the brain to image spinal function. The ultimate goal of such research is to take these new techniques into the clinic, to investigate and provide new remedies for chronic pain sufferers.
Keywords: brain, fMRI, functional, noxious, spinal
Introduction
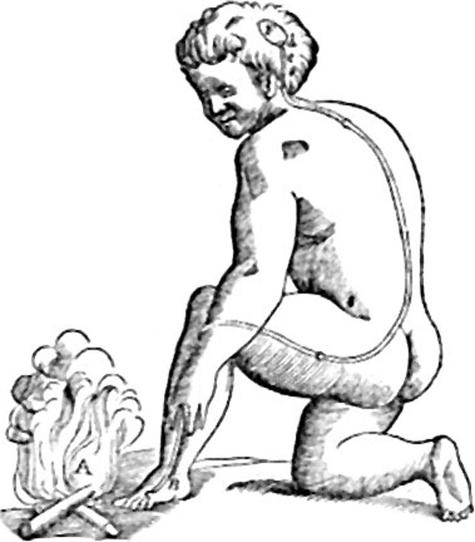
Pain has long been considered to be a submodality of the sense of touch (Kandel et al. 2000); however, advances in immunohistochemistry, histology, genetics and neuroimaging have challenged this widely accepted view. Descartes’ classic picture of pain (Descartes, 1644) provided a framework for the early anatomists (e.g. Bell, 1824) and neurophysiologists (e.g. Muller, 1833; Von Frey, 1896; Sherrington, 1906) to explain how pain was transduced and relayed to the spinal cord and brain (see Fig. 1).
Fig. 1.
Descartes’ view of pain, taken from his treatise De l’homme (Descartes, 1644). With great insight, he wrote: ‘If for example fire comes near the foot, minute particles of this fire, which you know move at great velocity, have the power to set in motion the spot of skin on the foot which they touch, and by this means pulling on the delicate thread which is attached to the spot of the skin, they open up at the same instant the pore against which the delicate thread ends, just as by pulling on one end of a rope one makes to strike at the same instant a bell which hangs at the end.’
Early descriptions of the pain pathways in humans consisted of relatively simple connections from primary nociceptor (the peripheral nerve) to spinal cord to thalamus and finally terminating in cerebral cortex (Willis, 1985). However, even by the early 20th century the view of pain as being transmitted via ‘hard-wired’ circuits was starting to be questioned. Head & Holmes (1911) observed that patients with specific cerebral lesions, particularly those in the parietal lobe disrupting primary somatosensory cortex (S1), were still able to feel pain – an unexpected finding given that the sensory portion of the thalamus was thought to project exclusively to this region. More recently, our view of pain has been dramatically modified from the one-to-one correspondence of nociceptor to specific pain, to a more plastic and integrative model (Melzack & Wall, 1965). However, it is only fairly recently that modern neuroimaging techniques, e.g. positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have allowed us to look inside the brain, and revealed that, as Head and Holmes suspected, pain is so much more than sensation.
In this brief review, we will attempt to describe the recent advances in neuroimaging that have added to our understanding of how pain is perceived and processed by the human brain.
Functional imaging methods: PET and fMRI
There is considerable evidence that local cerebral blood flow (CBF) changes reflect variations in local synaptic activity, as measured using PET (Sokoloff et al. 1977). The dramatic change in blood flow during neuronal activity is utilised by fMRI, in which signal change reflects alteration in local CBF and, more specifically, variations in the ratio of deoxyhaemoglobin to oxyhaemoglobin (Ogawa et al. 1990; Rosen et al. 1998) – this is termed blood oxygenation level dependent (BOLD) contrast. More recently, the neurophysiological basis of the BOLD response has been further investigated and the findings confirm that the BOLD contrast mechanism reflects the input and intracortical processing of a given brain area (Logothetis et al. 2001). A review by Howseman & Bowtell (1999) further describes these techniques, explaining the contrast mechanisms that enable signal detection (see also Jezzard et al. 2002). When comparing the various techniques for recording neuronal activity, PET, fMRI, magnetoencephalography (MEG), electroencephalography (EEG), optical imaging, etc., we find that fMRI has relatively limited temporal resolution (of the order of several 100s of milliseconds), but has relatively high spatial resolution (of the order of millimetres). This combination of adequate temporal and good spatial resolution explains why it has been adopted by numerous research centres as their main tool for neuroimaging research. Furthermore, the non-invasive aspect of fMRI enables longitudinal studies to be performed safely, and patients can be followed and imaged several times during the course of their disease progression or therapeutic intervention.
The broad range of sophisticated cognitive and neurophysiological experiments that have been performed with fMRI has expanded our knowledge of brain function and extended enormously the early PET literature. A review of the results of these experiments, along with the pros and cons of PET vs. fMRI is beyond the scope of this article; however, there are several excellent reviews and books that cover the basic principles, methods and scientific contributions that fMRI and PET have made to neuroscience (Frackowiak et al. 1997; Mazziotta et al. 2000; Mazziotta, 2000; Peyron et al. 2000; Jezzard et al. 2002; Toga & Mazziotta, 2002; Ward & Frackowiak, 2004). For the purposes of this article, we will focus specifically on the application of fMRI to the study of pain-related neuronal activation throughout the entire human central nervous system (CNS).
The biology of pain sensation
The International Association for the Study of Pain (IASP) define pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’, which indicates that, in the conventional sense, pain is caused by noxious stimuli, but may also be experienced in the absence of such stimuli. Here we will discuss the purely physiological aspects of pain perception; the more psychological components (e.g. attention, anxiety, anticipation, empathy to pain) will be discussed later in this article (in relation to other imaging studies).
The concept of a distinct class of peripheral nerve fibres conducting pain signals was first described by Sherrington (1906). Subsequently, several classes of nerve fibre have been described (Raja et al. 1999) that convey information about the type of painful stimulus being experienced. For example, thinly myelinated Aδ fibres respond to changes in temperature and to mechanical stimuli; however, one may further classify Aδ nociceptors to reflect whether they are fast or slowly adapting, and whether they have a high or low threshold for activity (so-called type I or II A-fibre nociceptors). The other major class of nociceptors are C-fibres, or C mechano-heat receptors; these are unmyelinated, and thus are relatively slowly conducting, and convey a sensation of burning. Note that there is large overlap in the types of stimuli that will activate given nociceptors, and hence many are termed polymodal. Following a painful stimulus, if sufficient numbers of a particular type, or types, of nociceptor are activated, an afferent volley will be produced. The afferent volley travels along the peripheral nociceptor and enters the spinal cord via the dorsal horn (Basbaum & Jessell, 2000). Within the dorsal horn, the terminal of the afferent nociceptor synapses with a dorsal horn neuron, and depending on the intensity of stimulation, and hence frequency of firing, this may be sufficient to produce a postsynaptic output. In addition to the significant progress in neurophysiological classification of peripheral nociceptors, there have been considerable advances recently in our understanding of the molecular basis of nociception (Hunt & Mantyh, 2001; Julius & Basbaum, 2001) and thermosensation (Cesare et al. 1999; Peier et al. 2002; Jordt et al. 2003; Patapoutian et al. 2003).
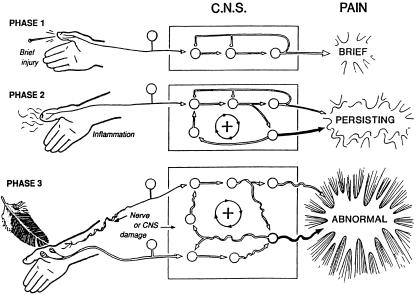
Dorsal horn pain processing is an enormous field of research (Doubell et al. 1999; Woolf & Salter, 2000), a full discussion of which is well beyond the scope of the current article; however, one aspect of dorsal horn dynamics deserves consideration. In Fig. 2, the processing of acute and prolonged painful stimulation is depicted, and provides the physiological basis for two major characteristics of clinical pain: hyperalgesia and allodynia. An acute stimulus will trigger a series of events leading to excitatory pain signals reaching the brain via the spinal cord; as the stimulus is short lived, so is the neuronal response. However, given a longer, more chronic stimulus, sensitization may occur at either the peripheral and/or the central level. Localized inflammation in the tissues leads to hyperexcitability of peripheral nociceptors, and may cause exaggerated responses to normally painful mechanical or thermal stimuli – this is termed primary hyperalgesia. Alternatively, sensitization may occur at the level of the dorsal horn neuron following, for example, a burn or cut injury (so-called central sensitisation). Amplification mechanisms, which are still not fully understood, then enable peripheral neurons not normally associated with pain to evoke painful sensations. Such centrally mediated sensitization is thought to explain the phenomenon of secondary hyperalgesia, whereby mechanical stimulation around the initial injury site (i.e. in normal skin) produces pain. Another related symptom of peripheral nerve injury is depicted in phase 3 of Fig. 2. Similar to secondary hyperalgesia, damage to the peripheral nerve induces plastic changes in the CNS (i.e. central sensitization), which are maintained by continuing discharge from the damaged afferent, and enables recruitment of low-threshold mechanoreceptors (e.g. Aβ fibres), which, when brushed, evoke pain. Here, because pain is produced following a normally non-painful stimulus (e.g. light brush), the pain evoked is referred to as allodynia.
Fig. 2.
Schematic representation of the three phases of pain, proposed by Cervero & Laird (1991). Phase 1: an acute phase, with equally short-lived response in the central nervous system (CNS). Phase 2: prolonged noxious stimulation leading to an inflammatory response, and continued discharge of peripheral nociceptors, which in turn lead to changes in excitability of dorsal horn neurons. Phase 3: peripheral nerve damage may lead to spontaneous discharge, which modifies the behaviour of dorsal horn neurons, and allows non-nociceptive peripheral nerves (e.g. brush-sensitive Aβ fibres) access to the ascending pain system. Reproduced with permission from Cervero & Laird (1991).
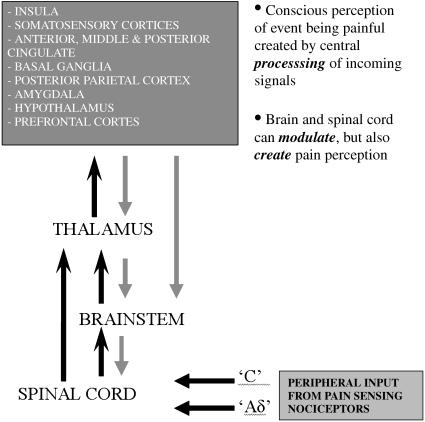
Beyond the peripheral nociceptor and dorsal horn, depending on the type of nociceptor activated, pain-related information ascends in the contralateral spinothalamic tract (STT), but there are also direct connections to the medulla and brain stem via the spinoreticular (SRT) and spinomesencephalic (SMT) tracts and to the hypothalamus via the spinohypothalamic tract (SHT). Numerous animal studies have been performed using anatomical tracers, and indicate that functionally differentiated nociceptors form synaptic connections within eight distinct laminae of the dorsal horn. Generally, cells within these laminae send their ascending axonal projections across the dorsal or ventral commissure of the spinal cord and form white matter bundles or funiculae, which connect to the brainstem and thalamus. By recording from electrodes placed within the brainstem or thalamus, it is possible to measure the response characteristics of these projections site and determine from which lamina they receive projections. Using this knowledge, it is possible to define functional and anatomical divisions of the thalamus, which, through their connections to specific laminae, may be ascribed certain roles in pain sensation (Craig & Dostrovsky, 1999). For instance, the ventral posterior nucleus (VP) of the thalamus primarily receives input from laminae IV–V (the target for nociceptors of low threshold and wide dynamic range), and in turn projects to primary somatosensory cortex (S1). Outside of the thalamus, there are spinal projections to the ventrolateral medulla, parabrachial nucleus, periaqueductal grey (PAG) and brainstem reticular formation. Of particular interest is the role of these structures in both inhibiting and facilitating nociceptive transmission and subsequent pain perception (Mayer & Price, 1976; Heinricher et al. 1989; Tracey & Dunckley, 2004). A brief summary of the projections involved from the periphery to the CNS is shown in Fig. 3.
Fig. 3.
Simple schematic of nociceptive pathways from the periphery to supraspinal regions. Black arrows represent transmission of pain signals supraspinally, which is integrated at several levels along the neuroaxis, and at almost every level influenced by descending fibres (grey arrows).
The pain matrix
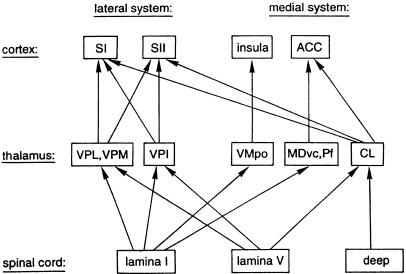
With the arrival of neuroimaging methods, the picture of how pain is represented in the cortex was further refined. The early studies were performed using PET, and immediately led to a revolution in our understanding of the involvement of the cortex in pain sensation. For instance, rather than pain primarily being represented in S1, large distributed brain networks were found to be active during painful stimulation (Talbot et al. 1991; Jones et al. 1992; Casey et al. 1994; Davis et al. 1995; Porro et al. 1998; Craig et al. 2000; Price, 2000; Tracey et al. 2000). The cortical and subcortical brain regions found to be commonly activated from these early studies by nociceptive stimulation included: anterior cingulate cortex, insula, frontal cortices, S1, second somatosensory cortex (S2) and amygdala (Peyron et al. 2000) – and are often referred to as the ‘pain matrix’ (Ingvar, 1999). A summary of the pain matrix is given in Fig. 4.
Fig. 4.
Cortical areas that receive information from the spinothalamic tract. Main spinothalamic and thalamocortical projections were summarized and simplified from several reports on the central nociceptive pathways in the monkey (see Treede et al. 1999, for references: Vogt et al. 1979; Willis, 1985; Apkarian & Shi, 1994; Craig, 1996). Cortico-cortical connections are not shown. ACC, anterior cingulate cortex; CL, centrolateral nucleus; MDvc, ventrocaudal part of medial dorsal nucleus; Pf, parafascicular nucleus; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; VMpo, posterior part of ventromedial nucleus; VPI, ventral posterior inferior nucleus; VPL, ventral posterior lateral nucleus; VPM, ventral posterior medial nucleus. (Note that the insula is now considered to lie between the lateral and medial systems, since it has both a sensory-discriminative and a cognitive-evaluative role in pain sensation). Reproduced with permission from Treede et al. (1999).
In Fig. 4, the pain matrix is subdivided into a medial and a lateral pain system; this distinction, which is based on the projection sites from medial or lateral thalamic structures to the cortex, is probably an oversimplification of the networks involved, but is a useful means for grouping brain regions that appear to have similar roles in pain perception. For instance, the lateral pain system (S1, S2) is primarily thought to have a role in discriminating the location and intensity of painful stimuli (Bushnell et al. 1999; Kanda et al. 2000), whereas the ACC (Rainville et al. 1997; Vogt et al. 2003) is involved in the affective (cognitive–evaluative) component of pain. The insula, however, encodes both the intensity (Coghill et al. 1999; Craig et al. 2000) and the laterality (Brooks et al. 2002; Bingel et al. 2003) of painful and non-painful thermal stimuli, but may also have a role in affective pain processing (Craig, 2003b; Critchley et al. 2004; Seymour et al. 2004; Singer et al. 2004). Thus, the insula probably occupies a space between the medial and lateral systems, facilitating integration of information from both (Peyron et al. 2002).
As imaging and analysis techniques have improved (Jezzard et al. 2002), and more evidence from neurophysiological methods such as EEG (Garcia-Larrea et al. 2003) and MEG (Maihofner et al. 2002) has been acquired, the cortical networks comprising the pain matrix have been further resolved and many different brain regions found to be active during pain processing. Of particular interest are activations in and around the frontal operculum, which includes S2 and insula. These brain regions, which are some of the most robustly activated in studies of pain (Peyron et al. 2000; Treede et al. 2000), are strongly implicated in pain sensation and comprise the only cortical areas in which direct electrical stimulation produces a perception of pain (Ostrowsky et al. 2002; Frot & Mauguiere, 2003).
Disruption of the cortical and subcortical brain regions that form the pain matrix, and the pathways between them, can have serious implications for an individual's well-being, but may also improve our understanding of the networks involved. In a study of patients with an ischaemic injury (i.e. stroke) affecting the operculo-insula region, Greenspan et al. (1998) observed that lesions affecting both the posterior insula and S2 tended to lead to thermesthesia and loss of pain sensation. However, similar lesions may also cause pain asymbolia (Berthier et al. 1988), whereby patients perceive painful stimulation on the body surface, but fail to react appropriately (e.g. lack of withdrawl, or absence of an emotional response). Paradoxically, these lesions may also cause pain (Bowsher et al. 2004), as is observed in the phenomenon of central post-stroke pain (CPSP; Boivie et al. 1989; Bainton et al. 1992). In a recent study of CPSP following from an ischaemic brainstem injury (Willoch et al. 2004), patients were found to experience pain in the body side contralateral to their lesion. Furthermore, by studying the patients using PET and a radiolabelled opioid receptor agonist, it was possible to demonstrate dramatic reductions in opioid receptor binding in both the posterior thalamus and the posterior insula (which was incorrectly identified as S2) ipsilateral to the lesion (see Fig. 5). One possible interpretation of these findings is that the lesion disrupts the normal pain processing pathway, from brainstem to posterior thalamus to posterior insula – thus leading to pain in these patients (Craig, 2003a).
Fig. 5.
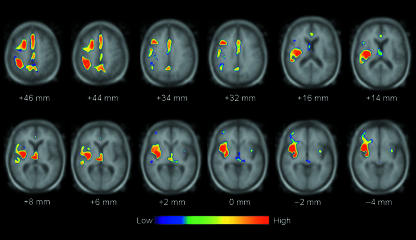
The clusters of reduced [11C]diprenorphine binding in the patient group in comparison with controls are superimposed on to a magnetic resonance image arrayed in a standard stereotactic space (see Willoch et al. 2004 for reference: Talairach & Tournoux, 1988). Only differences passing the significance threshold of P < 0.006 are displayed. The distance note beneath each slice is distance in relation to the bicommissural line (AC–PC line). Left side of image is contralateral to the painful hemibody side. (Note that the main reductions in opioid receptor binding in the patient group are contralateral to the lesion, and are located in the contralateral posterior thalamus and posterior insula: x = 38, y = −16, z = 14.) Reproduced with permission from Willoch et al. (2004).
Descending control of pain
Just as pain signals are important for survival, it is as important to regulate pain signalling in the nervous system. Head and Holmes postulated very early the existence of a descending pain modulatory system (Head & Holmes, 1911). Later this postulation was empirically confirmed (Hagbarth & Kerr, 1954) and provided a theoretical framework with the gate-control theory of Melzack & Wall (1965). Wall (1967) also demonstrated a tonic regulatory influence from the brainstem on the spinal cord dorsal root level. The concept of the descending analgesic system was further developed when Mayer & Price (1976) demonstrated that stimulation in the PAG produced analgesia without any concurrent effects on alertness or motor performance, so called stimulus-produced analgesia. In the PAG, ascending pain stimuli are integrated with descending influences from the diencephalon and the limbic forebrain. Important regions are the hypothalamus, amygdala, rostral components of the anterior cingulate cortex, insula and orbitofrontal cortex. PAG also receives influence from nearby nuclei of the catecholaminergic tone setting systems. Of interest is that micro-injections of opioids into the amygdala produce analgesia, and analgesia that can be blocked by interference locally in the PAG (Helmstetter et al. 1998). The PAG has strong bidirectional connections to the rostral medulla and this could be viewed as part of the pain modulation process given the role of the medulla in autonomic control. There are also strong suggestions that the analgesic system is heavily related to the endogenous opioid systems (Yaksh, 1999). Indeed, this is the commonest explanation for pain relief produced via acupuncture, which is believed to recruit descending pain control systems (Liu et al. 2004). By studying how the brainstem integrates information from autonomic, homeostatic, affective and limbic brain centres, we are now beginning to acquire a better understanding of the descending control of pain (Suzuki et al. 2004). Imaging studies of the brainstem structures involved in descending control of pain are just beginning (Tracey et al. 2002; Tracey & Iannetti, 2005a), but also of their role in ‘clinical’ pain states (Zambreanu et al. 2005) and the role of these structures in pain facilitation or pronociception rather than pain inhibition or antinociception (Tracey & Dunckley, 2004).
Modulation of pain
The relationship between reported pain intensity and the peripheral stimulus that evokes it depends on many factors such as the level of arousal, anxiety, depression, attention and expectation or anticipation. These factors are in the process of being characterized on the physiological and pharmacological levels by means of functional imaging. These ‘psychological’ factors are in turn regulated by overt and covert information as well as more general contextual cues that establish the significance of the stimulus and help determine an appropriate response to it. Simple manipulations with attention alter the subjective pain experience as well as the corresponding pattern of activation during painful stimulation. The main effect of distracting subjects during pain appears to be increased activity within the medial pain system, e.g. orbitofrontal, dorso and medial prefrontal cortex and rostral cingulate cortices, and a corresponding reduction in activation in the lateral pain system, i.e. thalamus and insula (Petrovic et al. 2000; Longe et al. 2001; Bantick et al. 2002; Brooks et al. 2002; see Fig. 6). Recent work using functional and connectivity analyses suggest that the increased activity within prefrontal and cingulate cortices during distraction decreases pain perception via the descending pain modulatory system, presumably via antinociceptive pathways (Valet et al. 2004). How these ‘medial’ brain regions are affected by distraction or hypervigilance to a nociceptive input, and subsequently connect to and influence descending inhibitory or facilitatory circuits, is only recently being appreciated, but clearly provide an additional route by which pain perception can be modulated. A more complete understanding of these mechanisms is potentially important for interpreting cognitive behavioural therapies.
Fig. 6.
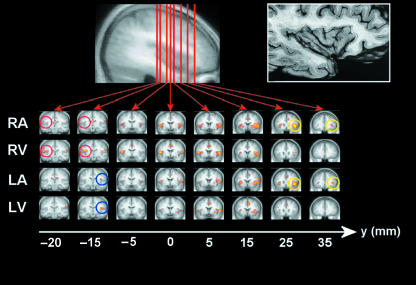
The effect of stimulus lateralization and attention on statistical maps for each experimental condition. RA = attend to pain on right hand, RV = attend to visual stimulus during pain to right hand. L replaces R for experiments with pain delivered to the left hand. The distribution of activation sites is shown on coronal sections taken through the insula and Talairach y-coordinates shown below each image [activated voxels are significant at P (corrected) < 0.05]. Images are displayed using neurological convention, i.e. right is right, left is left. Attended painful stimulation activated more rostral regions of the anterior insula than distracted stimulation (see yellow circles on anterior coronal sections). Also demonstrated is the effect of stimulus laterality on activation of posterior insula cortex (foci of activity are highlighted with coloured circles: right-hand stimulation, red; left-hand stimulation, blue). Posterior insula activity switched sides when the stimulus was transferred from hand to hand and did not depend on the attentional context during stimulation. Reproduced with permission (Brooks et al. 2002).
Other experimenters (e.g. Ploghaus et al. 1999; Porro et al. 2002) have investigated the effect of anticipation of an impending painful stimulus on regional brain activity. Ploghaus and colleagues performed such a study by using a novel conditioning protocol in 12 healthy volunteers, who underwent fMRI while being presented with a pseudo-random sequence of two intensities of thermal stimulation (painful hot or non-painful warm). Coloured lights signalled in advance the two kinds of thermal stimulation and subjects learned during the imaging session which colour signalled pain and which signalled warmth. The high temporal resolution of fMRI was ideally suited to this protocol, and was exploited to identify brain regions involved in the experience of pain itself by comparing brain activation during pain with activation during warm stimulation. In addition, however, brain regions involved in the anticipation of pain were identified by comparing brain activation during the coloured light preceding pain with activation during the coloured light preceding warm stimulation. The main effects of anticipation were found to be activation of rostral anterior insula and medial prefrontal cortices during the anticipation of pain, whilst during pain itself insula activity was more caudal, and the prefrontal focus was replaced by activity within the anterior cingulate cortex (see Fig. 7).
Fig. 7.
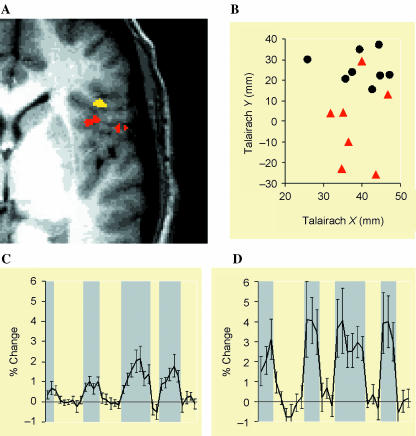
Insular cortex. (A) Group-combined activation map showing volumes selectively activated during pain (red) and anticipation of pain (yellow). (B) Individual subject's activation centres during pain (red triangles) and anticipation of pain (black circles). Centres associated with the anticipation of pain (black circles; mean Talairach coordinates x = 40 mm, y = 26 mm, z = 10 mm) were significantly more anterior than those associated with pain (red triangles; mean Talairach coordinates x = 38 mm, y = −1 mm, z = 11 mm) (P < 0.05). (C) Time course of fMRI signal intensity change over the period of the scan averaged across subjects. Epochs related to anticipation of pain are shaded in grey (mean ± SEM). (D) Time course of fMRI signal intensity change over the period of the scan averaged across subjects (mean ± SEM). Epochs of pain are shaded in grey. Reproduced with permission from Ploghaus et al. (1999).
Ploghaus and colleagues took this work further, to determine how increases in anxiety produced an increased pain perception and whether this effect was the same as ‘turning the heat up’. We know from recent studies that a large nociceptive drive, or indeed pain perception to the same nociceptive drive, reliably produces increased brain activation across most of the pain matrix as measured by PET or fMRI, respectively (Coghill et al. 1999, 2003). The study investigated whether anxiety-induced increased pain perception produced a generalized increase in brain activation, similar to that produced by increased nociceptive input. Generally this was found not to be the case, except for the hippocampal formation (entorhinal complex), which was responsible for producing anxiety-induced increased pain perception by Ploghaus and colleagues (Ploghaus et al. 2001) that was different to the increased pain produced by an increased nociceptive drive. This supports data from earlier studies (Prado & Roberts, 1985; Gray & McNaughton, 2000) and provides novel anatomical targets for subsequent therapies aimed at alleviating pain that is largely anxiety invoked.
Studies of attention and anticipation have demonstrated one common finding: when subjects actively attend to their pain or anticipate an upcoming painful stimulus, activity within the anterior insula (AI) appears to be more rostral than during pain itself. Therefore, the AI appears to provide a neurological substrate for monitoring the state of the body during pain, or possibly for preparing oneself in advance. The rostral AI has recently been proposed as an interoceptive brain centre, i.e. a region that constantly monitors the state of the body for changes in temperature, pain or other homeostatic function (Craig, 2002). In line with this hypothesis, Critchley et al. (2004) recently demonstrated that subjects who were better able to perceive changes in their own heart rate were likely to have both more strongly correlated activation in right AI in response to an interoceptive task (heart rate monitoring), and also to have increased grey matter density within this region. These findings suggest new avenues for future research, and highlight the importance of recognizing that structures such as the insula have important functional subdivisions yet to be fully characterized.
Of course, we are not limited to psychological intervention when trying to modulate pain, and several studies have investigated whether it is possible to monitor changes in brain activation following administering analgesic drugs. In a study performed in our laboratory, the action of a fast-acting µ-opioid, remifentanil, was investigated using conventional psychophysics and fMRI. When given at a steady-state effecter site concentration at three different doses, remifentanil produced an increasing behavioural report of pain relief, both on the sensory and on the affective axes of pain measurement. This is no surprise as the drug is a very good analgesic compound. The increasing drug–dose regime was performed simultaneously with fMRI data collection during noxious heat stimulation to the dorsum of the left hand, so that the modulation of brain regions by remifentanil, as the dose increases, could be quantified and compared with the subjective verbal report. Not only did this identify which pain processing brain regions are affected by the drug, but also that the patterns of change – the ‘fMRI dose–response’ curves – are different across these brain regions (see Fig. 8). In fact, the dose–response curves obtained using fMRI appear to show greater sensitivity than the conventional ‘subjective dose–response’ curves, potentially showing which brain regions are more involved with modulating the intensity or affective dimensions of the pain experience. In essence, in this remifentanil experiment the fMRI signal highlighted drug efficacy and modulation before the subject consciously perceived pain relief. Further work in our laboratory has extended these observations to determine which key brain regions are involved in generating pharmacologically induced analgesia (Wise et al. 2002, 2004; Rogers et al. 2004). These pharmacological fMRI (or phfMRI) studies are providing novel tools for the drug discovery process so that potential efficacy of a new compound can be determined early in phase II studies (Tracey et al. 2005b). Most pain medications used for the treatment of chronic pain (e.g. anti-depressants or anti-epileptics) were not necessarily discovered using biochemical and neurophysiological knowledge of pain mechanisms, but rather via trial and error and good clinical observation. In this respect, the conventional route of targeting specific mechanisms and use of animal pain models to determine efficacy has proved spectacularly unsuccessful. Combining our knowledge of the neural correlates of pain processing using tools that simultaneously provide sensitive readouts of drug efficacy gives us the opportunity to target specific components of the pain experience (i.e. the affective component) pharmacologically.
Fig. 8.
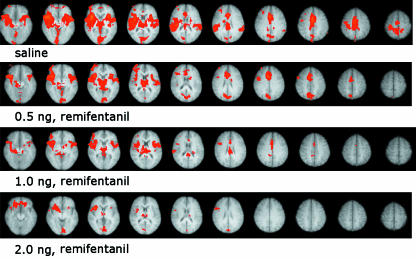
Pharmacological fMRI. In healthy subjects, the response to painful stimulation during three different infusion rates of the µ-opioid agonist remifentanil was compared with that under saline infusion. Results represent a mixed-effects group analysis (Z > 2.0, cluster corrected P < 0.05), and demonstrate broad activation across the pain matrix during saline infusion (bilateral insula, thalamus, ACC and contralateral S1). With increasing dose of remifentanil we see a progressive reduction in activity throughout the pain matrix. Images are displayed using radiological convention, i.e. left side of images is right side of brain and vice versa.
It is well known with both practising medicine and the pharmaceutical industry that the placebo effect is both real and in some instances dominant. In an excellent study, Petrovic et al. (2002) investigated whether the placebo and opioid analgesia share a neuronal network. Placebo analgesia is thought to involve both higher order cognitive networks and endogenous opioid systems. It is known that the rostral anterior cingulate cortex (rACC) and the brainstem are implicated in opioid analgesia, and therefore it makes some sense that both these structures play a similar role in placebo analgesia. Using PET, Petrovic and colleagues confirmed that both opioid and placebo analgesia are associated with increased activity in the rACC. They also observed a covariation between the activity in the rACC and the brainstem during both opioid and placebo analgesia, but not during the pain-only condition. More recently, Wager et al. (2004) extended these observations and examined placebo-induced changes in fMRI in the anticipation and experience of pain.
Beyond simple psychological and drug modulation of the brain's response to pain, we are beginning to see new studies investigating the subjective and empathetic components of the pain experience. Coghill et al. (2003) have recently addressed the issue that some individuals claim to be ‘sensitive’ to pain, whereas others claim they tolerate pain well. As it is difficult to determine whether these subjective reports reflect true interindividual differences in the experience, Coghill and colleagues combined psychophysical ratings to define pain report with fMRI to assess brain activity in 17 normal, healthy subjects. They found that highly ‘sensitive’ individuals exhibited more frequent and more robust pain-induced activation of the primary somatosensory cortex, anterior cingulate cortex and prefrontal cortex than did less ‘sensitive’ individuals. At least in normal healthy controls, this study validates the subjective report as a reliable indicator of what is going on within the brain.
Furthermore, Singer et al. (2004) found that empathy for pain involves the affective but not sensory components of the pain matrix. It is known that our ability to experience another's pain is a characteristic of empathy. Singer and colleagues used fMRI to assess brain activity while volunteers experienced a painful stimulus and compared it with that elicited when they observed a signal indicating that their loved one, present in the same room and whose hand was observable to the subject being imaged, was receiving a similar pain stimulus. Bilateral AI, rACC, brainstem and cerebellum were activated when subjects received pain, but also by the signal indicating that the loved one had just experienced pain. AI and ACC activation correlated with individual empathy scores, whilst activity in the posterior insular/secondary somatosensory cortex, the sensorimotor cortex (S1/M1) and the caudal ACC was specific to receiving pain. The authors conclude that a neural response in AI and rACC, activated in common for ‘self’ and ‘other’ conditions, suggests that the neural substrate for empathic experience does not involve the entire ‘pain matrix’ but that only part of the pain network associated mainly with its affective qualities, but not its sensory qualities, mediates empathy (Singer et al. 2004). This study and others highlight what we have suspected for years, and is encapsulated in the IASP definition of pain: ‘An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’– that the subjective experience of pain or a feeling of unpleasantness can arise without any peripheral nociceptive input, and may in fact be generated centrally. An imaging experiment by Eisenberger et al. (2003) examined the neural correlates of social exclusion to test the hypothesis that the brain bases of social pain are similar to those of physical pain. By scanning subjects as they played a virtual ball-tossing game in which they were ultimately excluded, they found that similar to results from physical pain studies, the ACC was more active during exclusion than during inclusion and correlated positively with self-reported distress. Right ventral prefrontal cortex (RVPFC) was active during exclusion and correlated negatively with self-reported distress. ACC changes mediated the RVPFC–distress correlation, suggesting that RVPFC regulates the distress of social exclusion by disrupting ACC activity (Eisenberger et al. 2003).
Future directions
From the initial thoughts of Descartes, to the identification of nociceptors (Sherrington, 1906), their connections with the spinal cord (Doubell et al. 1999) and finally to the neuromatrix (Albe-Fessard et al. 1985; Melzack, 1990), we are now arriving at a point in time when we are able to perform non-invasive imaging studies of the majority of the pain pathway. To this end, we are exploring the central nervous system from the level of the spine (Brooks et al. 2004), through the brainstem (Tracey et al. 2002; Zambreanu et al. 2005) and thalamus to the cerebral cortex (Brooks et al. 2005).
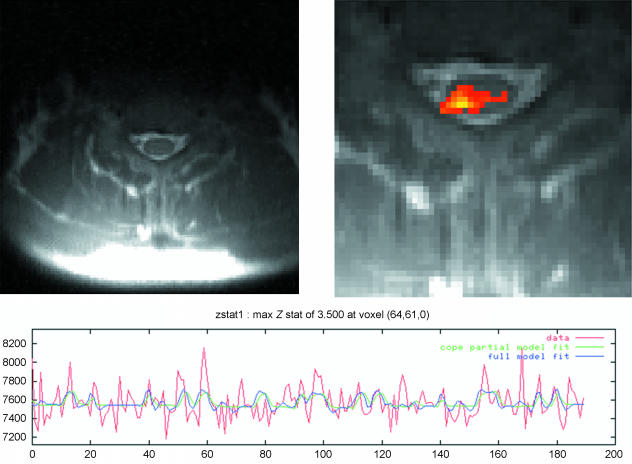
We have been building on the earlier work of Stroman and colleagues (Kornelsen & Stroman, 2004; Lawrence et al. 2004; Stroman et al. 2004), to develop techniques for acquiring functional images of spinal activity. Crucially, the analysis technique used depends on correction for pulsation of cerebrospinal fluid in the subarachnoid space, and for respiratory motion associated with movement of the chest wall (Glover et al. 2000; Friese et al. 2004), and whilst technically challenging, we are able to present data acquired using a painful thermal stimulus applied to the C7 dermatome of the hand (see Fig. 9). Significantly, these statistical maps are corrected for multiple comparisons and cluster corrected – that is, they are plausible given the inherent variability (i.e. noise) in the images. The activation site observed in this and other subjects appears in the dorsal portion of the spinal cord ipsilateral to the stimulus site, potentially reflecting dorsal horn processing of afferent pain signals.
Fig. 9.
Painful thermal stimulation of the C7 dermatome (hand) gives rise to activation in the ipsilateral dorsal portion of the spinal cord. Thermal stimulation was delivered using a block design (30 s off, 30 s on). Images were acquired using a single-shot fast spin-echo pulse sequence, which is shown at the top left. Physiological monitoring of electrocardiogram and respiratory motion allows retrospective correction for movement due to pulsation of CSF in the subarachnoid space (SAS) and for movement of the chest wall. Incorporating this information in the statistical model allows detection of activations unrelated to physiological noise. The statistical map (Z > 1.8, cluster corrected P < 0.05), top right, was obtained by masking the spine and SAS in the image, and has an associated time course shown below.
Although functional imaging of peripheral nerves may be feasible (Bozza et al. 2004) it may not be necessary to help advance the field of pain research. For instance, development of new experimental models allow access to investigating key symptoms of chronic pain states in healthy controls, such as hyperalgesia (e.g. Valeriani et al. 2003), and the development of modern laser stimulators has allowed selective stimulation of distinct nociceptor classes (e.g. Iannetti et al. 2004).
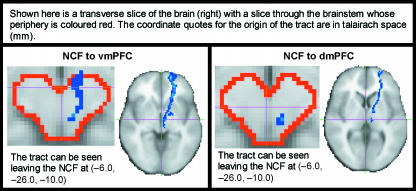
From an anatomical perspective, we are currently acquiring high-resolution diffusion tensor imaging (DTI) data, which are capable of non-invasively demonstrating anatomical connections between brain centres (Basser et al. 1994; Behrens et al. 2003). Although we are able to demonstrate plausible anatomical connections between brain regions that process pain (see Fig. 10), new analytical methods (functional and effective connectivity analyses) are helping to close the loop, by demonstrating that these regions do appear to ‘communicate’ with one another to mediate specific effects on pain perception (Lorenz et al. 2003; Valet et al. 2004).
Fig. 10.
Diffusion tensor imaging (DTI) reveals connections between brainstem structures and prefrontal cortex, both of which are known to be involved in descending control of pain. In particular, these data show putative connections between the nucleus cuneiformis (NCF) to ventromedial (vm) and dorsomedial (dm) prefrontal cortex (PFC). Diffusion-weighted EPI images were acquired with 60 diffusion gradient directions on a 1.5-T Siemens Sonata scanner, and are the average of three acquisitions. The processed images are in standard space and were constructed by binarizing the tracts for each subject and adding them together across the group (i.e. to create a frequency map). Voxel values represent the total number of subjects for which tracts pass through that given voxel. Data were processed with FMRIB's diffusion tools (http://www.fmrib.ox.ac.uk/fsl; see also Behrens et al. 2003).
Although these techniques are providing insight into the connectivity of different brain regions, they do so on a time scale far removed from activity of the local neuron. To help bridge the spatial and temporal gap, we are beginning to utilize simultaneous EEG and fMRI to help provide a solution to the temporal/spatial limitations of these two techniques, and address fundamental issues regarding neuronal–haemodynamic coupling (Iannetti et al. 2005). The final goal is to apply these developments and knowledge towards obtaining a better understanding of pain processing in chronic pain patients, who often have a constellation of physiological changes leading to plasticity or central sensitization, and associated psychological changes. Early studies have confirmed results from control studies but have yet to inform us of the ‘anatomical signature’ of chronic pain (Hsieh et al. 1995; Peyron et al. 1998, 2004; Petrovic et al. 1999; Garcia-Larrea et al. 2002; Valeriani et al. 2003; Zambreanu et al. 2005). However, the pain imaging community is slowly turning its attention to clinical pain and advances in our knowledge are expected within the next few years.
Conclusions
The advent of modern neuroimaging and electrophysiological techniques has enabled researchers to examine non-invasively the pain processing network. In particular, studies using PET and fMRI have helped resolve the major components of the pain matrix. However, the use of pain matrix as a construct has its disadvantages. For example, the insula, which is a fundamental component of this network, has too often been considered as a single functional unit. Only now are studies revealing the separate functional subunits of the insula and their individual roles, and will allow a greater understanding of pain processing at multiple levels. The combination of new techniques to demonstrate anatomical and functional connectivity will be of great help in this task. Although the majority of functional imaging studies have focused on processing at the supraspinal level, a new horizon is emerging which ultimately may prove more important in the study of clinical pain. The first level of pain processing, and the site of significant reorganization/sensitization in clinical pain, is in the dorsal horn. The ability non-invasively to image and track changes longitudinally via spinal fMRI is an exciting prospect for pain research.
Acknowledgments
J.B. kindly acknowledges the financial support of the Dr Hadwen Trust for Humane Research. I.T. wishes to acknowledge the support of the Higher Education Funding Council of England, and the Medical Research Council, which provides financial support for the FMRIB Centre.
References
- Albe-Fessard D, Berkley KJ, Kruger L, Ralston HJ, 3rd, Willis WD., Jr Diencephalic mechanisms of pain sensation. Brain Res. 1985;356:217–296. doi: 10.1016/0165-0173(85)90013-x. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci. 1994;14:6779–6795. doi: 10.1523/JNEUROSCI.14-11-06779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton T, Fox M, Bowsher D, Wells C. A double-blind trial of naloxone in central post-stroke pain. Pain. 1992;48:159–162. doi: 10.1016/0304-3959(92)90052-D. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Basbaum A, Jessell T. The perception of pain. In: Kandel E, Schwartz J, Jessell T, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. pp. 472–491. [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bell C. An Exposition of the Natural System of the Nerves of the Human Body. London: Spottiswoode.; 1824. [Google Scholar]
- Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory–limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage. 2003;18:740–748. doi: 10.1016/s1053-8119(02)00033-2. [DOI] [PubMed] [Google Scholar]
- Boivie J, Leijon G, Johansson I. Central post-stroke pain – a study of the mechanisms through analyses of the sensory abnormalities. Pain. 1989;37:173–185. doi: 10.1016/0304-3959(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Bowsher D, Brooks J, Enevoldson P. Central representation of somatic sensations in the parietal operculum (SII) and insula. Eur Neurol. 2004;52:211–225. doi: 10.1159/000082038. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Brooks J, Nurmikko T, Bimson W, Singh K, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Brooks JCW, Robson M, Schweinhardt P, Wise R, Tracey I. Functional magnetic resonance imaging (fMRI) of the spinal cord: a methodological study. Ann Meeting Am Pain Soc. Vancouver, Canada. 2004:Abstract #667. [Google Scholar]
- Brooks JCW, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation human insula painful heat studied with high resolution func imaging. NeuroImage. 2005 doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Bushnell M, Duncan G, Hofbauer R, Ha B, Chen J, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JMA. One pain or many pains? News Physiol Sci. 1991;6:268–273. [Google Scholar]
- Cesare P, Moriondo A, Vellani V, McNaughton PA. Ion channels gated by heat. Proc Natl Acad Sci USA. 1999;96:7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill R, Sang C, Maisog J, Iadorola M. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci USA. 2003;100:8538–8542. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Dostrovsky JO. Medulla to thalamus. In: Wall P, Melzack R, editors. Textbook of Pain. London: Harcourt Publishers Ltd; 1999. pp. 183–214. [Google Scholar]
- Craig A, Chen K, Bandy D, Reiman E. Thermosensory activation of insular cortex. Nature Neurosci. 2000;3:184–189. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Craig AD. An ascending general homeostatic afferent pathway originating in lamina l. Prog Brain Res. 1996;107:225–242. doi: 10.1016/s0079-6123(08)61867-1. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003a;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003b;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davis KD, Wood ML, Crawley AP, Mikulis DJ. fMRI of human somatosensory and cingulate cortex during painful electrical nerve stimulation. Neuroreport. 1995;7:321–325. [PubMed] [Google Scholar]
- Descartes R. Lectures on the History of Physiology During the 16th, 17th and 18th Centuries. Cambridge: Cambridge University Press; 1644. L’Homme. [Google Scholar]
- Doubell TP, Mannion RJ, Woolf CJ. The dorsal horn: state-dependent sensory processing, plasticity and generation of pain. In: Wall P, Melzack R, editors. Textbook of Pain. London: Harcourt Publishers Ltd; 1999. pp. 165–182. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC. Human Brain Function. California: Academic Press; 1997. [Google Scholar]
- von Frey M. Abhandlungen der mathematischphysischen Klasse der Königichen Sächsischen Gesellschaft der Wissenschaften. Untersuchung über die Sinnesfunctionen der menschlichen Haut. 1896 [Google Scholar]
- Friese S, Hamhaber U, Erb M, Kueker W, Klose U. The influence of pulse and respiration on spinal cerebrospinal fluid pulsation. Invest Radiol. 2004;39:120–130. doi: 10.1097/01.rli.0000112089.66448.bd. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguiere F. Dual representation of pain in the operculo-insular cortex in humans. Brain. 2003;126:438–450. doi: 10.1093/brain/awg032. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Convers P, Magnin M, et al. Laser-evoked potential abnormalities in central pain patients: the influence of spontaneous and provoked pain. Brain. 2002;125:2766–2781. doi: 10.1093/brain/awf275. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiol Clin. 2003;33:279–292. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety. Oxford: Oxford University Press; 2000. [Google Scholar]
- Greenspan JD, Lee RR, Lenz FA. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain. 1999;81:273–282. doi: 10.1016/S0304-3959(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Hagbarth K, Kerr D. Central influences on spinal afferent conduction. J Neurophysiol. 1954;17:295–307. doi: 10.1152/jn.1954.17.3.295. [DOI] [PubMed] [Google Scholar]
- Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain Res. 1911;34:102–254. [Google Scholar]
- Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- Helmstetter F, Tershner S, Poore L, Bellgowan P. Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaquaductal gray and rostral ventromedial medulla. Brain Res. 1998;119:104–118. doi: 10.1016/s0006-8993(97)01104-9. [DOI] [PubMed] [Google Scholar]
- Howseman AM, Bowtell RW. Functional magnetic resonance imaging: imaging techniques and contrast mechanisms. Philos Trans R Soc Lond B Biol Sci. 1999;354:1179–1194. doi: 10.1098/rstb.1999.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Leandri M, Truini A, Zambreanu L, Cruccu G, Tracey I. Adelta nociceptor response to laser stimuli: selective effect of stimulus duration on skin temperature, brain potentials and pain perception. Clin Neurophysiol. 2004;115:2629–2637. doi: 10.1016/j.clinph.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Niazy RK, Wise RG, et al. Recording of laser-evoked brain potentials during continuous, high-field functional magnetic resonance imaging in humans. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Ingvar M. Pain and functional imaging. Phil Trans R Soc Lond B. 1999;354:1347–1358. doi: 10.1098/rstb.1999.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard P, Matthews PM, Smith SM. Functional MRI: an Introduction to the Methods. Oxford: Oxford University Press; 2002. [Google Scholar]
- Jones AK, Friston K, Frackowiak RS. Localization of responses to pain in human cerebral cortex. Science. 1992;255:215–216. doi: 10.1126/science.1553549. [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum A. Molecular mechanisms of nociception. Nature. 2001;413:20–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kanda M, Nagamine T, Ikeda A, et al. and others Primary somatosensory cortex is actively involved in pain processing in human. Brain Res. 2000;853:282–289. doi: 10.1016/s0006-8993(99)02274-x. [DOI] [PubMed] [Google Scholar]
- Kandel E, Schwartz JH, Jessell TM. Principles of Neural Science (International Edition) New York: McGraw-Hill; 2000. [Google Scholar]
- Kornelsen J, Stroman PW. fMRI of the lumbar spinal cord during a lower limb motor task. Magn Reson Med. 2004;52:411–414. doi: 10.1002/mrm.20157. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Stroman PW, Bascaramurty S, Jordan LM, Malisza KL. Correlation of functional activation in the rat spinal cord with neuronal activation detected by immunohistochemistry. Neuroimage. 2004;22:1802–1807. doi: 10.1016/j.neuroimage.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Liu WC, Feldman SC, Cook DB, et al. fMRI study of acupuncture-induced periaqueductal gray activity in humans. Neuroreport. 2004;15:1937–1940. doi: 10.1097/00001756-200408260-00021. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Longe SE, Wise R, Bantick S, et al. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport. 2001;12:2021–2025. doi: 10.1097/00001756-200107030-00047. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey K. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Kaltenhauser M, Neundorfer B, Lang E. Temporo-spatial analysis of cortical activation by phasic innocuous and noxious cold stimuli – a magnetoencephalographic study. Pain. 2002;100:281–290. doi: 10.1016/S0304-3959(02)00276-2. [DOI] [PubMed] [Google Scholar]
- Mayer D, Price D. Central nervous system mechanisms of analgesia. Pain. 1976;2:379–404. doi: 10.1016/0304-3959(76)90080-4. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC. Imaging: window on the brain. Arch Neurol. 2000;57:1413–1421. doi: 10.1001/archneur.57.10.1413. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Frackowiak RSJ. Brain MappingThe Disorders. California: Academic Press; 2000. [Google Scholar]
- Melzack R, Wall P. Pain mechanisms: a new theory. Science. 1965;150:971–999. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Melzack R. Phantom limbs and the concept of a neuromatrix. Trends Neurosci. 1990;13:88–92. doi: 10.1016/0166-2236(90)90179-e. [DOI] [PubMed] [Google Scholar]
- Müller J. Handbuch der Physiologie Des Menschen für Vorlesungen. Koblenz: Hölscher; 1833–1840. [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M, Stone-Elander S, Petersson K, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85:19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia – imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire M, et al. Allodynia afer lateral-medullary (Wallenberg) infact. A PET study. Brain. 1998;121:345–356. doi: 10.1093/brain/121.2.345. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Peyron R, Frot M, Schneider F, et al. Role of operculoinsular cortices in human pain processing: converging evidence from PET, fMRI, dipole modeling, and intracerebral recordings of evoked potentials. Neuroimage. 2002;17:1336–1346. doi: 10.1006/nimg.2002.1315. [DOI] [PubMed] [Google Scholar]
- Peyron R, Schneider F, Faillenot I, et al. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati J, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284 doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro C, Cettolo V, Francescato M, Baraldi P. Temporal and intensity coding of pain in human cortex. J Neurophysiol. 1998;80:3312–3320. doi: 10.1152/jn.1998.80.6.3312. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, et al. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22:3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado WA, Roberts MH. An assessment of the antinociceptive and aversive effects of stimulating identified sites in the rat brain. Brain Res. 1985;340:219–228. doi: 10.1016/0006-8993(85)90917-5. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan G, Price D, Carrier B, Bushnell M. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, Ringkamp M, Campbell JN. Peripheral neural mechanisms of nociception. In: Wall P, Melzack R, editors. Textbook of Pain. London: Harcourt Publishers Ltd; 1999. pp. 11–58. [Google Scholar]
- Rogers R, Wise RG, Painter DJ, Longe SE, Tracey I. An investigation to dissociate the analgesic and anesthetic properties of ketamine using functional magnetic resonance imaging. Anesthesiology. 2004;100:292–301. doi: 10.1097/00000542-200402000-00018. [DOI] [PubMed] [Google Scholar]
- Rosen BR, Buckner RL, Dale AM. Event related functional MRI: past, present and future. Proc Natl Acad Sci USA. 1998;95:773–780. doi: 10.1073/pnas.95.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Sherrington C. The Integrative Action of the Nervous System. Oxford: Oxford University Press; 1906. [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, et al. The [14C] deoxyglucose method for the measurement of local cerebral glucose utilisation: theory, procedure and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Kornelsen J, Bergman A, et al. Noninvasive assessment of the injured human spinal cord by means of functional magnetic resonance imaging. Spinal Cord. 2004;42:59–66. doi: 10.1038/sj.sc.3101559. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. New York: Thieme Medical Publishers; 1988. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers. [Google Scholar]
- Talbot J, Marrett S, Evans A, Meyer E, Bushnell M, Duncan G. Multiple representations of pain in human cerebral cortex. Science. 1991;15:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Toga AW, Mazziotta JC. Brain Mapping. The Methods. 2nd. California: Elsevier Science, Academic Press; 2002. [Google Scholar]
- Tracey I, Becerra L, Chang I, et al. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2000;288:159–162. doi: 10.1016/s0304-3940(00)01224-6. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Dunckley P. Importance of anti- and pro-nociceptive mechanisms in human disease. Gut. 2004;53:1553–1555. doi: 10.1136/gut.2004.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Iannetti G. Functional imaging of the human brainstem. Clin Neurophysiol. 2005a doi: 10.1016/s1567-424x(09)70059-5. [DOI] [PubMed] [Google Scholar]
- Tracey I, Schweinhardt P, Bountra C. Brain FMRI in clinical pharmacological studies. In: Beckmann N, Rudin M, editors. In Vivo MR Techniques in Drug Discovery. Boca Raton: CRC Press; 2005b. [Google Scholar]
- Treede R-D, Kenshalo D, Gracely R, Jones A. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Treede R-D, Apkarian A, Bromm B, Greenspan J, Lenz F. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain. 2000;87:113–119. doi: 10.1016/S0304-3959(00)00350-X. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Arendt-Nielsen L, Le Pera D, et al. Short-term plastic changes of the human nociceptive system following acute pain induced by capsaicin. Clin Neurophysiol. 2003;114:1879–1890. doi: 10.1016/s1388-2457(03)00180-9. [DOI] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain – an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wall P. The laminar organisation of dorsal horn and effects of descending impulses. J Physiol. 1967;188:403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Towards a new mapping of brain cortex function. Cerebrovasc Dis. 2004;17:35–38. doi: 10.1159/000075303. [DOI] [PubMed] [Google Scholar]
- Willis WD. The Pain System: the Neural Basis of Nociceptive Transmission in the Mammalian Nervous System. Basel: Karger; 1985. [PubMed] [Google Scholar]
- Willoch F, Schindler F, Wester HJ, et al. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 2004;108:213–220. doi: 10.1016/j.pain.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Wise RG, Rogers R, Painter D, et al. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage. 2002;16:999–1014. doi: 10.1006/nimg.2002.1146. [DOI] [PubMed] [Google Scholar]
- Wise RG, Williams P, Tracey I. Using fMRI to quantify the time dependence of remifentanil analgesia in the human brain. Neuropsychopharmacology. 2004;29:626–635. doi: 10.1038/sj.npp.1300364. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Yaksh T. Central pharmacology of nociceptive transmission. In: Wall P, Melzack R, editors. Textbook of Pain. London: Harcourt Publishers Ltd; 1999. pp. 253–308. [Google Scholar]
- Zambreanu L, Wise R, Brooks J, Iannetti G, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]