Abstract
Extracellular matrix remodelling and accurate spatio-temporal coordination of growth factor expression are two factors that are believed to regulate mitoses and cell migration in developing and regenerating tissues. The present quantitative videomicroscopical study examined the influence of some of the principal components of extracellular matrix and several growth factors that are known to be expressed in dermal wounds on three important facets of human skin cell behaviour in culture. Keratinocytes, melanocytes and dermal fibroblasts (and myofibroblast controls) exhibited varying degrees of substrate adhesion, division and migration depending on the composition of the culture substrate. Substrates that are recognized components of transitional matrices generally accentuated cell adhesion and proliferation, and were motogenic, when compared with serum-treated control surfaces, whereas components of more stable structures such as basement membrane had less influence. Platelet-derived growth factor (PDGF), epidermal growth factor (EGF) and α fibroblastic growth factor (αFGF) all promoted cell proliferation and were chemokinetic to dermal fibroblasts, but not keratinocyte growth factor (KGF) or transforming growth factor β (TGFβ). PDGF, EGF and KGF, but not TGFβ or αFGF, all enhanced proliferation of dermal keratinocytes. The same growth factors, and in addition KGF, all stimulated motility in keratinocytes, but TGFβ and αFGF again had no effect. Developing a better understanding of the interdependency of factors that control crucial cell behaviour may assist those who are interested in the regulation of histogenesis and also inform the development of rational therapeutic strategies for the management of chronic and poorly healed wounds.
Keywords: chemokinesis, extracellular matrix, growth factor, human, skin, wound
Introduction
Development and tissue regeneration during wound healing are underpinned by the innate ability of cells to divide and migrate if given an appropriate stimulus (Martin, 1997; Redd et al. 2004). This impetuous can arise from alteration in the physicochemical composition of the tissue microenviroment (Gailit & Clark, 1996), and another from the effect of cytokines generated by the cells therein (Moulin, 1995). Unravelling the unique and combinatorial effects of the many components of the extracellular matrix and growth factors on mitosis and motility may help to explain why chronic wounds stall during the healing process, and may inform the use of cells in scaffolds for tissue engineering applications.
Cell motility and mitoses in wound healing are initiated and rate-limited, in part, by remodelling of the extracellular matrix (Gailit & Clark, 1996) and alterations in the expression profile for growth factors by constituent and inflammatory cells (Moulin, 1995). There appears to be cross-talk between the two because it has been reported that components of the extracellular matrix may activate cells by signalling through growth factor receptors during wound healing (Tran et al. 2004). Dynamic aspects of cell motility such as orientation and velocity are also substratum-dependent and can be both inhibited and enhanced depending on the composition of the substratum, or provisional matrix (Hynes, 1992). Directional components of cell motility and division are believed to result from chemotaxis (Zigmond, 1973), haptotaxis (Brandley & Schnaar, 1989), contact guidance (Weiss, 1945) and population pressure (Abercrombie & Gitlin, 1965), although there is a paucity of unequivocal evidence that these mechanisms operate in vivo. The principle of interdependency between cell behaviour and connective tissue architecture is, however, something that has been reiterated many times (Stopak & Harris, 1982).
That topical application of growth factors enhances healing of dermal wounds has been claimed (Robson et al. 1992; Greenhalgh & Rieman, 1994; Wu & Mustoe, 1995; Ono et al. 2004a,b) and may, to some extent, be dependent on the known proven chemotaxic and mitogenic effects of fibroblastic growth factor (FGF) (Grant et al. 1992), epidermal growth factor (EGF) (Andresen & Ehlers, 1998; Hudson & Cawley, 1998), hepatocyte growth factor (HGF) (Stoker, 1989; Bevan et al. 2004), Platelet-derived growth factor (PDGF) (Kamiyama et al. 1998) and transforming growth factor (TGF) (Grant et al. 1992) on keratinocytes and fibroblasts (Werner & Grose, 2003). Growth factors and attachment factors can act synergistically in accelerating cell growth (Nickoloff et al. 1988; Kohyama et al. 2002a; Karvinen et al. 2003; Li et al. 2004), although it is recognized that some cytokines present in the wound milieu can also inhibit cell motility (Kohyama et al. 2002b).
Superimposed on the chemotaxic and guidance capabilities of growth factors and substrata is their ability to accelerate cell migration in a non-direction manner, a phenomenon that has been termed chemokinesis (Stoker 1989), but it is important to stress that motility often has other components to it such as persistence and taxis (Anand-Apte & Zetter, 1997). As a ubiquitous event, chemokinesis has been studied in vitro in several cells types (Wilkinson, 1998), but nevertheless descriptions of this behaviour in epithelial cells and fibroblasts are relatively few in number (Uren et al. 1994; Kamiyama et al. 1998) and reports relating specifically to human skin cells are hard to find. The present study has therefore examined the mitogenic and motogenic potential of culture substrates derivatized with different components of the extracellular matrix on human keratinocytes and dermal fibroblasts. In addition, by using time-lapse video microscopy, these same cells were examined after exposure to a selection of relevant growth factors to determine whether they have chemokinetic potential.
It was considered important to include in the experimental design cells that are known to be involved in dermal wound healing, but which have been implicated in abnormal events such as hypertrophic scarring. The myofibroblast is considered to be a differentiated form of fibroblast (Gabbiani et al. 1971), and has been implicated in the pathology of many diseases due to its contractile nature (Gabbiani et al. 1972; Clark, 1993) including hypertrophic scar (Baur et al. 1975), keloid (James et al. 1980), Dupuytren's contracture (Gabbiani & Manjo, 1972) and desmoid tumour (Goellner & Soule, 1980). Normally few in number, the interesting observation that myofibroblasts are again reduced in frequency after wounds heal (Rudolph et al. 1977) suggests that they may be recruited into healing tissues by the same signals that influence the indigenous cells and so may exhibit similar behavioural charateristics in cell culture.
Materials and methods
Patients between the ages of 21 and 87 years and undergoing elective surgery kindly donated tissue samples with informed consent as approved by the Local Ethics Committee. Tissue samples were obtained during auriculoplasty, abdominoplasty and mammoplasty, briefly rinsed in 70% ethanol/30% MilliQ water then washed in three changes of Ca2+/Mg2+-free Hanks balanced salt solution (HBSS, Sigma) containing 100 units mL−1 penicillin and 100 µg mL−1 streptomycin (Sigma). Using sterile forceps, the epidermis was raised and small pieces of tissue trimmed off, leaving behind as much connective tissue as possible. The tissue pieces were then incubated in 0.5 units mg−1 Dispase (Boerhinger Mannheim) overnight at 4 °C.
For isolation of epidermal keratinocytes (HK) and melanocytes (HM), the epidermis was digested using 0.25% trypsin-EDTA solution (Sigma) at 37 °C for 10 min then following centrifugation at 500 g for 5 min. Cells were maintained using MCDB 153 medium (Sigma) containing 25 mm HEPES, 10 ng mL−1 EGF (Sigma), 5 µg mL−1 transferrin (Sigma), 5 µg mL−1 insulin (Sigma), 500 ng mL−1 hydrocortisone (Sigma), 2.5 µg mL−1 bovine pituitary extract (Gibco), 100 units mL−1 penicillin and 100 µg mL−1 streptomycin. For human dermal fibroblast (HDF) cell culture, the dermis was washed thoroughly in HBSS, and then macerated using a sterile blade. A minimal volume of serum-containing medium was added to aid collection, and then the macerate was transferred to 75-cm3 tissue culture flasks (Dow Corning). Flasks were tipped to ensure even coverage of the macerate then inverted and 8 mL growth medium added. Flasks were incubated at 37 °C and re-inverted after 48 h. Prior to subculture, dermal explants were maintained in Hams F10 nutrient medium (Sigma) supplemented with 25 mm HEPES, 20% fetal bovine serum (FBS, Sigma), 100 units mL−1 penicillin and 100 µg mL−1 streptomycin. The content of FBS in the media was subsequently reduced to 5%. Human myofibroblasts (HMF) were isolated from Dupuytren's nodules using the method previously described for fibroblast isolation from dermis. As with dermal cultures, following subculture the FBS content was reduced to 5%. Cell suspensions were obtained by detachment of cells by 0.25% trypsin-EDTA solution (Sigma). Trypsinization was stopped by addition of serum-containing medium, the cells counted after centrifugation at 2000 r.p.m. and then re-suspended at the appropriate density.
For analysis of cell-substrate adhesion and cell division culture dishes were derivatized using fibronectin (bovine plasma, Sigma), type 1 collagen (rat tail, prepared in-house), type IV collagen (Sigma), laminin (EHS basement membrane derived, Sigma), vitronectin (human plasma, Sigma) and ECM Gel (Sigma) at 10 µg cm−2 for 2 h at room temperature before rinsing ×3 in sterile RO water. Controls were surfaces onto which cells were plated without prior derivatization. HDF, HK and HMF at passage 1, 2 and 3 were inoculated in triplicate into dishes at 1 × 104 cells cm−2 and, after 6 days had elapsed, the numbers of cells present in six fields of view selected by systematic random sampling were counted. The cell density on commencement of the investigation was taken as 100% and the total number of adherent cells in subsequent analyses taken as multiples of that.
For investigation of growth factor effects, PDGF (10 ng mL−1), acidic fibroblast growth factor (10 ng mL−1), EGF (10 ng mL−1), TGFβ (10 ng mL−1) or keratinocyte growth factor (10 ng mL−1) were included in the growth media. Fibroblasts or keratinocytes were inoculated into dishes at a concentration of 1 × 104 cells cm−2 and analyses carried out as before.
For analysis of cell motility HDF, HK, HMF and HM time-lapse video sequences were made 24–48 h after cells were inoculated into serum-treated dishes at a concentration of 1 × 104 cells cm−2. Cultures were filmed for 24–72 h at a rate of 8 frames h−1 using a CCD camera (Nikon CB-230 H) attached to a phase contrast microscope (Nikon PSM-2120), during which time temperature of the medium was maintained at 37 °C and 100% humidity using an environmental control unit. Time-lapse clips were converted into both movies and still image series and movement of cells was plotted using an image analysis macro developed in-house for use with for Scion Image. Coordinates of individual cell movement were then entered into a motility macro in Microsoft Excel in which various aspects of cell behaviour such as velocity, persistence and total distance travelled were calculated arithmetically.
Consideration of the data obtained from these experiments on tissues derived from a small number of patients suggested that statistical comparison between the subject groups should be done using the Kolmogorov–Smirnov two-sample test.
Results
Substratum composition and level of passage affects the adhesion and proliferation of human fibroblasts, keratinocytes and myofibroblasts
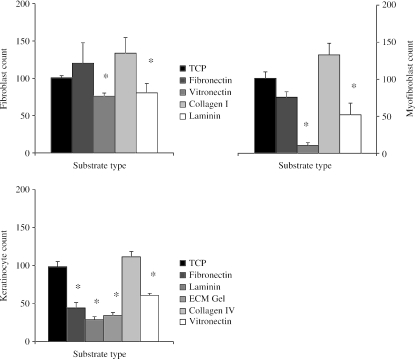
None of the extracellular matrix (ECM)-derivatized substrata increased the adhesion of any of the cells examined over an 8-h period beyond that of tissue culture plastic controls (Fig. 1). There was a tendency for type I collagen substrates to enhance the adhesion of fibroblasts and myofibroblasts but this observation did not prove to be statistically significant. Vitronectin and laminin were significantly less adhesive than control surfaces for both fibroblasts and myofibroblasts (P < 0.05). This was also the case for keratinocytes, but ECM gel and fibronectin were also less adhesive for these cells when compared with tissue culture plastic (TCP) surfaces (P < 0.05).
Fig. 1.
Charts illustrating the adhesion (mean ± SE) of P2 fibroblasts, keratinocytes and myofibroblasts to various ECM molecules. Cells on control TCP are assumed to be 100% adhered; all other ECM components are expressed as a percentage of the control (*P < 0.05 compared with TCP).
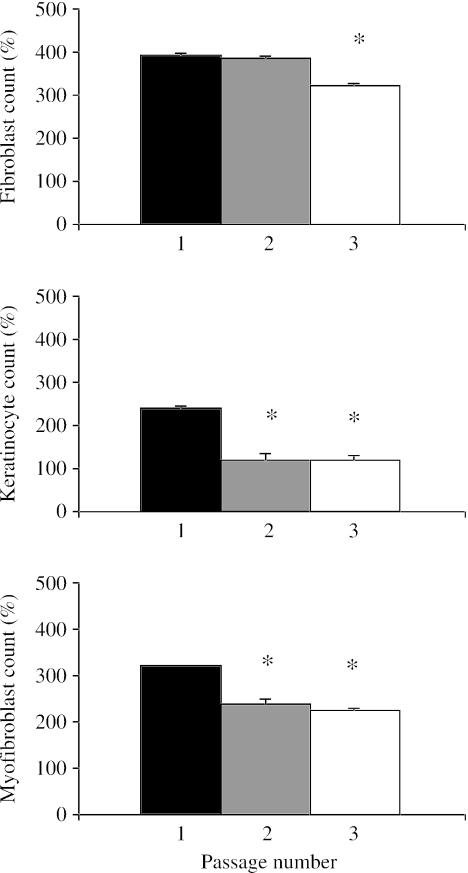
Early serial passage did not appear to affect the ability of fibroblasts to replicate (Fig. 2), but by the third passage (P3) the rate had dropped significantly (P < 0.05). Keratinocytes lost their proliferative potential following the first passage (P < 0.05). This was coincident with a change in cell morphology where after each passage the cells became larger and more spread. Early passaging of myofibroblasts also resulted in a decrease in cell proliferation after P2, although this was not as pronounced as for keratinocytes.
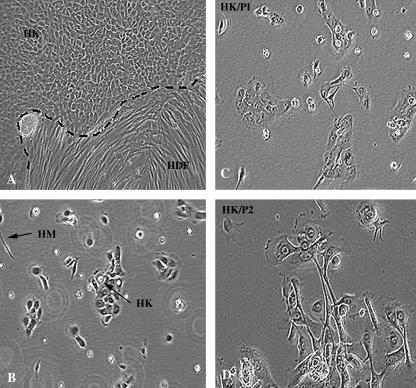
Fig. 2.
Phase contrast micrographs illustrating (A and B) the morphology of primary human epidermal keratinocytes (HK) and fibroblasts (HDF) grown from explants of whole skin and dissociated from epidermis (B). Panels C and D demonstrate an alteration in the morphology of keratinocytes between passage 1 and 2. Original magnification ×100 for all panels (HK, human keratinocytes; HDF, human dermal fibroblasts; HM, human melanocytes).
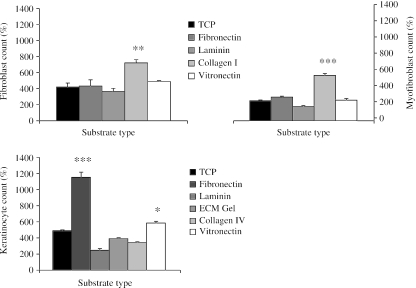
Proliferation of fibroblasts was enhanced only on type I collagen (P < 0.05) as compared with control substrates (Fig. 3). Proliferation of keratinocytes was accelerated on fibronectin, which induced a ten-fold increase in cell numbers by day 6 (P < 0.01), and on vitronectin (P < 0.05), but the remaining substrates had no siginificant effect. Type I collagen was the substrate having the greatest effect on myofibroblast proliferation (P < 0.01) with the remaining substrates having no significant growth-enhancing effect.
Fig. 3.
Charts illustrating the effects of passage on proliferation of dermal fibroblasts, keratinocytes and myofibroblasts over a 6-day period. Expressed as a percentage increase of the initial cell count (mean ± SE) that was taken to be 100%. *P < 0.05 by day 6 compared with P1.
Proliferation of human fibroblasts and keratinocytes is modulated by growth factors
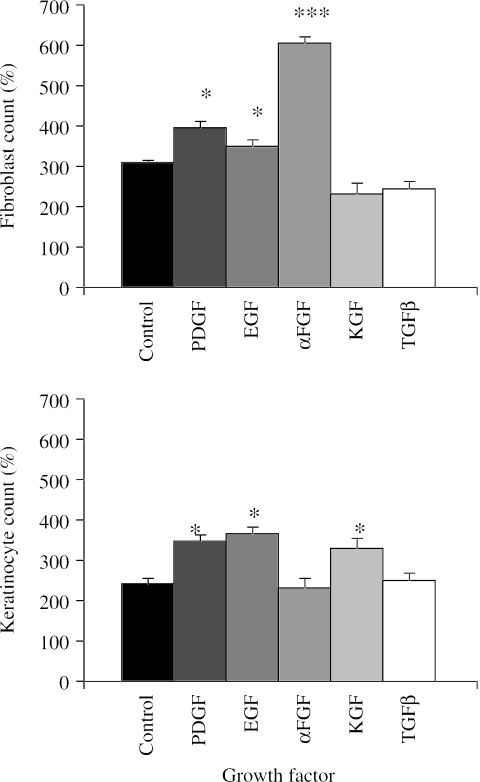
Fibroblast proliferation was enhanced by αFGF (P < 0.01), EGF (P < 0.05) and PDGF (P < 0.05) (Fig. 4). This effect was most marked with αFGF, which induced more than a six-fold increase in cell numbers by day 6 compared with only a three-fold increase in controls. Keratinocyte growth factor (KGF) and TGFβ did not influence proliferation of fibroblasts. Keratinocyte proliferation was accelerated by PDGF, EGF and KGF (P < 0.05). TGFβ and αFGF did not enhance keratinocyte proliferation as compared with control cultures.
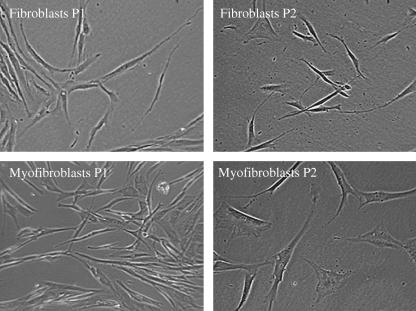
Fig. 4.
Phase contrast micrographs illustrating the alteration in the morphology of primary human dermal fibroblasts and myofibroblasts between P1 and 2 in primary dissociated culture. Original magnification ×100 for all panels.
Cellular components of human skin are differentially motile in primary culture
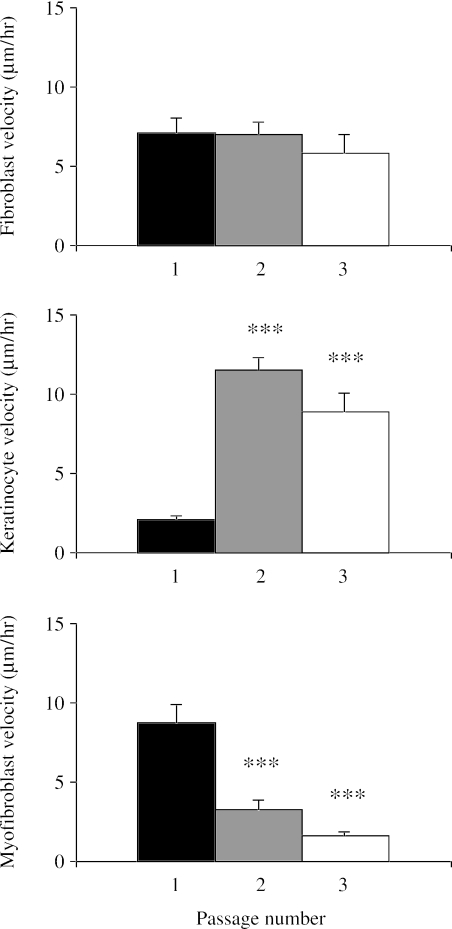
Fibroblasts, myofibroblasts and keratinocytes were all motile in cell culture to the extent that some cells were able to move large distances if unimpeded (Figs 5 and 6). Keratinocytes were initially slower than fibroblasts or myofibroblasts, but with passage became the most motile of the skin cells with a peak mean velocity of around 12 µm h−1. Qualitative observations suggested that motile behaviour of keratinocytes differed depending on whether the cells were isolated or clustered, and whether the cells had a spread or rounded morphology. Fibroblasts and myofibroblasts did not display as great a variation in morphology, being uniformly stellate although different in size, and so had less variation in velocity, 3.55 ± 0.46 and 3.67 ± 1.02 µm h−1, respectively, and both were significantly slower then keratinocytes (P < 0.05). Melanocytes, by contrast, were virtually stationary, with a mean velocity of only 0.47 ± 0.04 µm h−1 (see supplementary Video 7). This time-lapse video of human dermal melanocytes grown in low-density culture illustrates that the melanocytes are neuron-like with slender projections from the cell body and move very little compared with the flattened keratinocytes. However in high-density culture the melanocytes move far more vigorously (Video 8), but the process seems to involve pulling and pushing past their nearest neighbour, rather than being a substratum-dependent event. Video 9 shows human dermal melanocytes growing inside a living skin equivalent. The melanocytes probe their surroundings in a manner that is very similar to their behaviour in high-density dissociated culture and become increasingly pigmented with time. The pattern of movement is characteristic of exploratory activity rather than obvious translocation. There was a slight but not significant drop in fibroblast motility with passage. By contrast, keratinocytes showed increased motility with passage, with mean velocity increasing several fold between P1 and P2 (P < 0.01) and P1 and P3 (P < 0.01). Velocity of myofibroblasts decreased dramatically with passage; between P1 and P3 these cells had much reduced motility (P < 0.01). Videos 1–4 illustrate the differences in motility in fibroblasts and keratinocytes grown on control surfaces (Videos 1 and 2) and surfaces derivatized with collagen (Video 3) and fibronectin (Video 4). In the case of keratinocytes, the measured increase in motility is reflected by the alteration in morphology of the cells, with the smallest and most rounded cells appearing the most active.
Fig. 5.
Charts illustrating the influence of several extracellular matrix molecules on the proliferation of fibroblasts, keratinocytes and myofibroblasts in culture. Expressed as a percentage increase of the initial cell count (mean ± SE), which was taken to be 100% (*P < 0.05, **P < 0.025 by day 6 compared with TCP).
Fig. 6.
Charts illustrating the effects of PDGF, EGF, αFGF, KGF and TGFβ on proliferation of primary dermal fibroblasts, keratinocytes and myofibroblasts over a 6-day period. Expressed as a percentage increase of the initial cell count (mean ± SE) that was taken to be 100% (*P < 0.05, ***P < 0.01 compared with control cultures).
Substratum composition can be motogenic and growth factors chemokinetic for human fibroblasts, keratinocytes and myofibroblasts
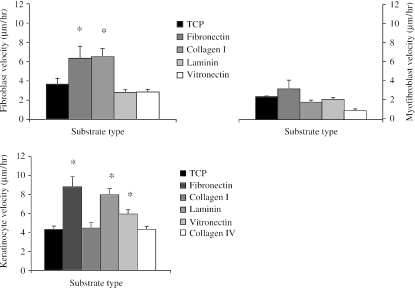
The velocity of fibroblasts was accelerated on fibronectin and type I collagen substrates (P < 0.05) but on laminin and vitronectin motility was no different from controls (Fig. 7). Keratinocyte migration was accelerated on fibronectin (P < 0.01), laminin (P < 0.02) and vitronectin (P < 0.05), but was similar to controls on collagen types I and IV. Myofibroblast velocity did not appear to be affected by substrate type. None of the substrates investigated seemed to have any unusual or adverse effects on the morphology of any of the cell types.
Fig. 7.
Charts illustrating the effects of passage on the motility (mean velocity ± SE) of dermal fibroblasts, keratinocytes and myofibroblasts (*P < 0.05, **P < 0.025, ***P < 0.01 compared with P1 cultures).
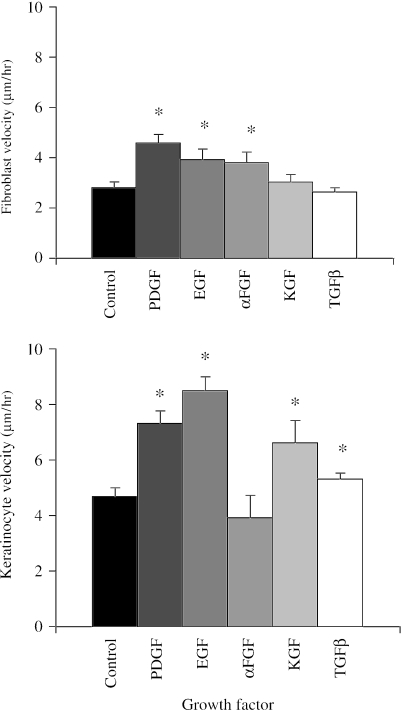
Fibroblast chemokinesis was induced by αFGF, EGF and PDGF (P < 0.05), but not by KGF or TGFβ when compared with control cultures (Figs 8 and 9). Chemokinesis of keratinocytes was induced by PDGF (P < 0.02), EGF (P < 0.01), KGF (P < 0.02) and TGFβ (P < 0.05), but not by αFGF. Of these, EGF was most effective, with cells having a mean velocity almost twice that of controls. Videos 5 and 6 demonstrate the motogenic effect of PDGF on fibroblasts (Video 5) and EGF on keratinocytes (Video 6). Both cell types appear to move more vigorously under the influence of PDGF, especially the isolated and rounded keratinocytes which are far more motile than any other cell type, or any of the spread keratinocytes.
Fig. 8.
Charts illustrating the effect of various extracellular matrix molecules on motility (mean velocity ± SE) of P2 human dermal fibroblasts, keratinocytes and myofibroblasts (*P < 0.05, **P < 0.025 compared with velocity on TCP).
Fig. 9.
Charts illustrating the effects of PDGF, EGF, αFGF, KGF and TGFβ on the motility (mean velocity ± SE) of dermal fibroblasts and keratinocytes (*P < 0.05, **P < 0.025, ***P < 0.01 compared with control cultures).
Discussion
The present study has confirmed that cell adhesion, proliferation and motility in dissociated cultures of human skin can be accentuated or diminished depending on substratum composition and the type and concentration of growth factors. The magnitude of the variation of the cells’ responses to substratum-derived or tropic stimulus was vastly greater than any apparent differences between cell populations obtained from different patients or from different parts of the body, despite the fact that donors covered a wide range of age and phenotype. That does not preclude the possibility that aspects of cell behaviour might correlate with donor age. However, corroborating this theory would have required far larger numbers of biopsies and with present-day circumstances was therefore largely impractical.
The mitogenic effects of growth factors are well known, for example enhancement of fibroblast proliferation by FGF and PDGF (Shipley et al. 1989), but reports on the chemokinetic effects of growth factors on primary cells of human origin are rare. Seppa et al. (1982) demonstrated that growth factors induce chemotaxis at mitogenic concentrations, but growth factors do not obey classical dose–response properties even with regard to cell proliferation (Cordeiro et al. 2000). The selection of growth factor concentration here was informed by observation of mitogenic effects. It is possible that chemokinetic growth factors could further accelerate motility, or even retard cells, if applied at other concentrations. Barrandon & Green (1987) reported a correlation between cell migration and cell proliferation in colonies of epidermal keratinocytes treated with EGF and TGFα and suggested that the two processes were interdependent. Zicha et al. (1999) elaborated on this by reporting that TGFβ-dependent increase in motility was associated with alteration in the relative duration of the phases of the cell cycle, the crucial factor being the duration of G2 (growth phase 2).
The motogenic potential of ECM molecules has often featured in theories regarding the mechanism of accelerated wound healing. By way of example, Donaldson & Mahan (1983) reported that epidermal cell migration from a wound edge in adult newt skin occurred far more readily across glass slides derivatized with fibronectin than untreated surfaces or surfaces coated with allogeneic serum or bovine serum albumin. This is not surprising because re-epithelialization in cutaneous wounds is known to take place over a provisional matrix containing fibronectin, vitronectin and fibrin (Redd et al. 2004). In addition to confirming that culture substrata consisting of fibronectin, vitronectin and laminin all accelerate keratinocyte motility, the present study showed that motility remained unchanged on type IV collagen, a component of stable epithelial basement membrane, suggesting that cell responsiveness is conditioned, at least in part, by the level of differentiation. Given that the laminin used here was tumour-derived, and previous studies have shown that the normal basement membrane component laminin-5 slows keratinocyte migration (O'Toole et al. 1997), it seems that substratum composition is pivotal to the control of cell behaviour. So is the timing of expression, as Zhang & Kramer (1996) have reported that laminin-5 is the first ECM component expressed by pro-migratory keratinocytes and which actually promotes early migration of keratinocytes in cell culture. Taken together, these two reports suggest that latent migratory potential of cells is held in check by balancing the timing and level of expression of matrix components. It has been reported that speed of migration in keratinocytes is correlated with morphology and that this in turn is influenced by substratum composition (Sutherland et al. 2000).
Greiling & Clark (1997) developed a wound-healing model to examine the mechanism of fibroblast migration from connective tissue towards and into the fibrin clot. Fibronectin was found to be critical to transmigration of fibroblasts by providing a conduit from a collagen matrix into a provisional fibrin matrix. Removal of fibronectin, or blocking binding using arg-gly-asp amino acid sequence (RGD) peptide or monoclonal antibodies against the subunits of the α5β1 and α5β3 integrin receptor, prevented cell migration. The results of this study suggest that fibroblast migration in that model would be accelerated by fibronectin as a provisional matrix, but is also subject to synergistic effects of growth factors.
Previous reports have concluded that fibronectin and vitronectin also accelerate keratinocyte motility (Kim et al. 1992) through a mechanism that is transduced through the α5β1 integrin for fibronectin and via the α5β5 for vitronectin (Kim et al. 1994). There is evidence that keratinocyte chemokinesis by growth factors, as reported here for PDGF, EGF, KGF and TGFβ, may operate by up-regulating integrins for motogenic substrata. Chen et al. (1993) have reported that receptor EGF and TGFα promote human keratinocyte locomotion on collagen and fibronectin, coincident with increased expression of the α2-integrin subunit, concluding that cell growth-independent stimulation of keratinocyte locomotion via regulation of integrin expression might underpin accelerated re-epithelialization during wound healing. The present study not only reinforces this but also suggests that interaction between growth factors and motogenic substrata may be wide-ranging, although we acknowledge that statistical analysis of interactions between motogenic substrata and chemokines was not attempted. Examples of this include reports describing time- and concentration-dependent KGF stimulation of keratinocyte migration on fibronectin and collagen types I and IV, but not laminin, vitronectin or tensacin (Putnins et al. 1999), and human platelet-derived growth factor-BB (PDGF-BB) promoting dermal fibroblast motility on type I collagen (Li et al. 2004).
The precise effect of growth factors on cell behaviour may depend on the particular isoform that is used. For example, it has been reported that only TGFβ3, but not TGFβ1 and 2, can restore depressed motility in fibroblasts cultured from skin (Qui et al. 2004) but that all three TGFβ isoforms have similar mitogenic effects on fibroblasts. Fergusson & O’Kane (2004) have suggested that growth factors may have some promiscuous, or blanket, actions and some isoform-specific effects such as control of motility. This has important functional significance given that stimulated fibroblast migration into a healing wound results in better restitution of dermal architecture and reduction in scarring (Fergusson & O’Kane, 2004). Exogenous administration of TGFβ3 culminating in levels similar to that found in scar-free embryonic wounds has been shown to improve or even remove scarring during adult wound healing in rats (Shah et al. 1995). In addition to the TGF superfamily, several other growth factors are known to have a beneficial influence on cell behaviour in skin wounds, including scatter-factor (Bevan et al. 2004), FGF (Ono et al. 2004a), PDGF (Li et al. 2004), KGF (Karvinen et al. 2003) and EGF (Shirakata et al. 2003).
The various factors affecting keratinocyte proliferation and motility may operate partly by up-regulating production of molecular components of the ECM. Recent observations of cross-talk between receptors and signal transduction pathways for ECM molecule binding domains and growth factors support this theory (Howe et al. 1997). The ECM has domains that interact with and activate receptors with intrinsic tyrosine kinase activity and recognized as strong mediators of cell proliferation, migration, differentiation and dedifferentiation. Unlike traditional growth factor effects, these domains within tenascin-C, laminin, collagen and decorin possess relatively low binding affinity and are often presented in multiple valencies. It has been suggested that these ‘matrikine’ ligands may be critical for wound healing, as the majority of known ECM components possessing matrikines play a strong role, or are presented uniquely, during skin repair (Tran et al. 2004). It is important to reiterate the observation that certain growth factors could accelerate motility in primary human skin cells, properly defined as chemokinesis and not chemotaxis as no directional preference was evident. No attempt was made to determine the transduction events involved in this effect, but ‘matrikine’ mechanisms may be important.
Data interpretation from cell culture model systems and extrapolation of findings to inform the mechanisms underpinning tissue regeneration in vivo should be attempted with caution. An example of this is the report by Brown et al. (1991) that vitronectin inhibits collagen-induced human keratinocyte motility. This apparent effect of vitronectin (also called serum-spreading factor, epibolin and S protein) could indeed have been a bone fide inhibitory mechanism affecting the expression and/or distribution of intergrins but equally it could also have been indicative of inadvertent alteration in substratum chemical composition that is known to occur in some circumstances (Kasemo & Gold, 1999). If extracellular components can indeed modulate cell responses to particular substrata this is highly significant because cells themselves are producers of matrix molecules (O’Keefe et al. 1984) and matrix enzymes such as collagenase (Scharffetter et al. 1991) and metalloproteinase (Ghahary et al. 2001).
In considering the results of the present study, an issue worthy of consideration is the manner in which ECM components adsorb to the culture surface and subsequently present binding sites to cell-surface receptors. Gaudet et al. (2003) examined three variables associated with cell-surface interaction (projected area, migration speed, traction force) at various type I collagen surface densities in a population of fibroblasts. Cell area increased with ligand density up to a transition level, at which point further increases in collagen cause the cell area to decline. The threshold was approximately 160 molecules µm−2, equal to the cell surface density of integrin molecules. At low density, the availability of collagen binding sites was limited and the cells became flattened. Because the size and morphology of cells is likely to influence migration and proliferation, the biomolecular composition of substrata either in vitro or in vivo is therefore likely to be an important determinant of cell behaviour.
Given that the subjects of this study were primary cells of human origin, it is noteworthy that certain aspects of their behaviour varied with the level of passage. Although this may be indicative of normal progressive differentiation in keratinocytes affecting their behaviour, the possibility that the cells were in fact dedifferentiating cannot be excluded. If the state of differentiation did influence growth factor-dependent aspects of cell behaviour in culture this would in any case have been superimposed on their intentional, or programmed, response. This observation is consistent with the finding reported by Albini et al. (1988) that reduction in the proliferative capacity of fibroblasts is associated with reduced chemotaxis. This only became apparent after P25 in embryo-derived tissue whereas it occurred after P15 in cells from 70- to 90-year-old donors. The present study found changes in cell motility much earlier, after P1 in all the cell types studied. A more precise interpretation of the proliferative and motile behaviour of keratinocytes in the context of differentiation could be achieved by monitoring the expression of cytokeratins and markers such as filaggrin, involucrin, keratin 2e and transglutaminase (Eichner et al. 1984;Stark et al. 1999). By way of example, Nickoloff et al. (1988) reported that human keratinocytes maintained in an undifferentiated state are more motile than cells differentiated using calcium supplementation. Nickoloff et al. (1988) also reported that TGFβ, KGF and fibronectin all stimulate motility in keratinocytes emerging from agarose gels or migrating in Boyden's chambers but is indicative of a chemotaxic component to the increase in motility.
The observed enhancement of cell motility here was appropriately described as chemokinesis but it must be pointed out that contact inhibition and cell proliferation must also have been involved as collisions between cells were unavoidable. Contact inhibition has long been recognized as affecting any interpretation of cell motility (Abercrombie, 1967), suggesting that any comparison between the present results with more traditional in vitro investigations of cell motility using scratched monolayer wound models, for example, (Albrecht-Buehler, 1977) may not be straightforward. In that model cells migrating way from the edges of a wounded monolayer have a strong directional component to motility that originates from population pressure. This suggests that the motogenic and chemokinetic effects of substrata and growth factors here may further accentuate cell motility if superimposed on to other directional and stimulatory effects.
Acknowledgments
This study was part-funded by the Wellcome Trust. Thanks to Professor Sharp, Bradford Royal Infirmary, for skin biopsies and Professor Tony Thody for collaboration leading to the videomicrospical images of the living skin equivalent.
Supplementary material
Supplementary material is available in the full text version of this article online at http://www.blackwell-synergy.com.
References
- Albini A, Pontz B, Pulz M, Allavena G, Mensing H, Muller PK. Decline of fibroblast chemotaxis with age of donor and cell passage number. Coll Relat Res. 1988;8:23–37. doi: 10.1016/s0174-173x(88)80033-5. [DOI] [PubMed] [Google Scholar]
- Abercrombie M, Gitlin G. The locomotory behaviour of small groups of fibroblasts. Proc Roy Soc. 1965;162:289–302. [Google Scholar]
- Abercrombie M. Contact inhibition: the phenomenon and its biological implications. Nat Cancer Inst Monogr. 1967;26:249–277. [PubMed] [Google Scholar]
- Albrecht-Buehler G. The phagocytic tracks of 3T3 cells. Cell. 1977;11:395–404. doi: 10.1016/0092-8674(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Anand-Apte B, Zetter B. Signaling mechanisms in growth factor-stimulated cell motility. Stem Cells. 1997;15:259–267. doi: 10.1002/stem.150259. [DOI] [PubMed] [Google Scholar]
- Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res. 1998;17:79–87. doi: 10.1076/ceyr.17.1.79.5261. [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987;25:1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Baur PS, Larson DL, Stacey TR. The observation of myofibroblasts in hypertrophic scars. Surg Gynecol Obstet. 1975;141:22–26. [PubMed] [Google Scholar]
- Bevan D, Gherardi E, Fan TP, Edwards D, Warn R. Diverse and potent activities of HGF/SF in skin wound repair. J Pathol. 2004;203:831–838. doi: 10.1002/path.1578. [DOI] [PubMed] [Google Scholar]
- Brandley BK, Schnaar RL. Tumor cell haptotaxis on covalently immobilized linear and exponential gradients of a cell adhesion peptide. Dev Biol. 1989;135:74–86. doi: 10.1016/0012-1606(89)90159-0. [DOI] [PubMed] [Google Scholar]
- Brown C, Stenn KS, Falk RJ, Woodley DT, O'Keefe EJ. Vitronectin: effects on keratinocyte motility and inhibition of collagen-induced motility. J Invest Dermatol. 1991;96:724–728. doi: 10.1111/1523-1747.ep12470960. [DOI] [PubMed] [Google Scholar]
- Chen JD, Kim JP, Zhang K, et al. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res. 1993;209:216–223. doi: 10.1006/excr.1993.1304. [DOI] [PubMed] [Google Scholar]
- Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993;306:42–48. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT. TGF-beta1, -beta2, and -beta3 in vitro: biphasic effects on Tenon's fibroblast contraction, proliferation, and migration. Invest Ophthalmol Vis Sci. 2000;41:756–763. [PubMed] [Google Scholar]
- Donaldson DJ, Mahan JT. Fibrinogen and fibronectin as substrates for epidermal cell migration during wound closure. J Cell Sci. 1983;62:117–127. doi: 10.1242/jcs.62.1.117. [DOI] [PubMed] [Google Scholar]
- Eichner R, Bonitz P, Sun TT. Classification of epidermal keratins according to their immunoreactivity, isoelectric point, and mode of expression. J Cell Biol. 1984;98:1388–1396. doi: 10.1083/jcb.98.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson MWJ, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Phil Trans R Soc Lond. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wopund contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Hirschel BJ, Ryan GB, Statov PR, Manjo G. Granulations tissue as a contractile organ: a study of structure and function. J Exp Med. 1972;135:719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Manjo G. Dupytren's contracture; fribroblast contraction? An ultrastructural study. Am J Pathol. 1972;66:131–146. [PMC free article] [PubMed] [Google Scholar]
- Gailit J, Clark RA. Studies in vitro on the role of alpha v and beta 1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin and fibrinogen. J Invest Dermatol. 1996;106:102–108. doi: 10.1111/1523-1747.ep12328177. [DOI] [PubMed] [Google Scholar]
- Gaudet C, Marganski WA, Kim S, et al. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys J. 2003;85:3329–3335. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahary A, Marcoux Y, Karimi-Busheri F, Tredget EE. Keratinocyte differentiation inversely regulates the expression of involucrin and transforming growth factor beta1. J Cell Biochem. 2001;83:239–248. doi: 10.1002/jcb.1223. [DOI] [PubMed] [Google Scholar]
- Goellner JR, Soule EH. Desmoid tumours, an ultrastructural study of eight cases. Human Pathol. 1980;11:43–50. doi: 10.1016/s0046-8177(80)80104-3. [DOI] [PubMed] [Google Scholar]
- Grant MB, Khaw PT, Schultz GS, Adams JL, Shimizu RW. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest. Ophthalmol Vis Sci. 1992;33:3292–3301. [PubMed] [Google Scholar]
- Greenhalgh DG, Rieman M. Effects of basic fibroblastic growth factor in the healing of patial thickness donor sites: a prospective, randomised, double-blind trial. Wound Repair Regen. 1994;2:113–121. doi: 10.1046/j.1524-475X.1994.20205.x. [DOI] [PubMed] [Google Scholar]
- Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997;110:861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1997;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Hudson LG, McCawley LJ. Contributions of the epidermal growth factor receptor to keratinocyte motility. Microsc Res Techn. 1998;43:444–455. doi: 10.1002/(SICI)1097-0029(19981201)43:5<444::AID-JEMT10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- James WD, Besanceney CD, Odum RB. The ulstrastructure of a keloid. J Am Acad Dermatol. 1980;3:50–57. doi: 10.1016/s0190-9622(80)80224-6. [DOI] [PubMed] [Google Scholar]
- Kamiyama K, Iguchi I, Wang X, Imanishi J. Effects of PDGF on the migration of rabbit corneal fibroblasts and epithelial cells. Cornea. 1998;17:315–325. [PubMed] [Google Scholar]
- Karvinen S, Pasonen-Seppanen S, Hyttinen JM, et al. Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J Biol Chem. 2003;278:49495–49504. doi: 10.1074/jbc.M310445200. [DOI] [PubMed] [Google Scholar]
- Kasemo B, Gold J. Implant surfaces and interface processes. Adv Dental Res. 1999;13:8–20. doi: 10.1177/08959374990130011901. [DOI] [PubMed] [Google Scholar]
- Kim JP, Zhang K, Chen JD, Wynn KC, Kramer RH, Woodley DT. Mechanism of human keratinocyte migration on fibronectin: unique roles of RGD site and integrins. J Cell Physiol. 1992;151:443–450. doi: 10.1002/jcp.1041510303. [DOI] [PubMed] [Google Scholar]
- Kim JP, Zhang K, Chen JD, Kramer RH, Woodley DT. Vitronectin-driven human keratinocyte locomotion is mediated by the alpha v beta 5 integrin receptor. J Biol Chem. 1994;269:26926–26932. [PubMed] [Google Scholar]
- Kohyama T, Liu X, Wen FQ, et al. Nerve growth factor stimulates fibronectin-induced fibroblast migration. J Lab Clin Med. 2002a;140:329–335. doi: 10.1067/mlc.2002.128347. [DOI] [PubMed] [Google Scholar]
- Kohyama T, Liu XD, Wen FQ, Kim HJ, Takizawa H, Rennard SI. Prostaglandin D2 inhibits fibroblast migration. Eur Respir J. 2002b;19:684–689. doi: 10.1183/09031936.02.01272001. [DOI] [PubMed] [Google Scholar]
- Li W, Fan J, Chen M, et al. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell. 2004;15:294–309. doi: 10.1091/mbc.E03-05-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Moulin V. Growth factors in skin wound healing. Eur J Cell Biol. 1995;68:1–7. [PubMed] [Google Scholar]
- Nickoloff BJ, Mitra RS, Riser BL, Dixit VM, Varani J. Modulation of keratinocyte motility. Correlation with production of extracellular matrix molecules in response to growth promoting and antiproliferative factors. Am J Pathol. 1988;132:543–551. [PMC free article] [PubMed] [Google Scholar]
- O’Keefe EJ, Woodley DT, Castilo G, Russell N, Payne RE. Production of soluable and cel-associated fibronectin by cultured keratinocytes. J Invest Dermatol. 1984;82:150–155. doi: 10.1111/1523-1747.ep12259708. [DOI] [PubMed] [Google Scholar]
- O'Toole EA, Marinkovich MP, Hoeffler WK, Furthmayr H, Woodley DT. Laminin-5 inhibits human keratinocyte migration. Exp Cell Res. 1997;233:330–339. doi: 10.1006/excr.1997.3586. [DOI] [PubMed] [Google Scholar]
- Ono I, Yamashita T, Hida T, et al. Local administration of hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. J Surg Res. 2004a;20:47–55. doi: 10.1016/j.jss.2003.08.242. [DOI] [PubMed] [Google Scholar]
- Ono I, Yamashita T, Hida T, et al. Combined administration of basic fibroblast growth factor protein and the hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. Wound Repair Regen. 2004b;12:67–79. doi: 10.1111/j.1067-1927.2004.012113.x. [DOI] [PubMed] [Google Scholar]
- Putnins EE, Firth JD, Lohachitranont A, Uitto VJ, Larjava H. Keratinocyte growth factor (KGF) promotes keratinocyte cell attachment and migration on collagen and fibronectin. Cell Adhes Commun. 1999;7:211–221. doi: 10.3109/15419069909010803. [DOI] [PubMed] [Google Scholar]
- Qui CX, Brunner G, Fergusson MGW. Abnormal wound healing and scarring in the TGFβ3 null mouse embryo. Development. 2004 [Google Scholar]
- Redd MJ, Cooper L, Wood W, Strammer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Phil Trans R Soc Lond. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MC, Phillips LG, Lawrence WT, et al. The safety and effect of topically-applied recombinant basic fibroblastic growth factor on the healing of chronic pressure sores. Ann Surg. 1992;216:401–408. doi: 10.1097/00000658-199210000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph R, Guber S, Suzuki M, Woodward M. The life cycle of the myofibroblast. Surg Gynecol Obstet. 1977;145:389–394. [PubMed] [Google Scholar]
- Scharffetter K, Wlaschek M, Hogg A, et al. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch Dermatol Res. 1991;283:506–511. doi: 10.1007/BF00371923. [DOI] [PubMed] [Google Scholar]
- Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982;92:584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Fergusson MWJ. Neutralisation of TGFβ1 and TGFβ2 or exogenous addition of TGFβ3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Shipley GD, Keeble WW, Hendrickson JE, Coffey RJ, Jr, Pittelkow MR. Growth of normal human keratinocytes and fibroblasts in serum-free medium is stimulated by acidic and basic fibroblast growth factor. J Cell Physiol. 1989;138:511–518. doi: 10.1002/jcp.1041380310. [DOI] [PubMed] [Google Scholar]
- Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hashimoto K. So-called biological dressing effects of cultured epidermal sheets are mediated by the production of EGF family, TGF-beta and VEGF. J Dermatol Sci. 2003;32:209–215. doi: 10.1016/s0923-1811(03)00103-8. [DOI] [PubMed] [Google Scholar]
- Stark HJ, Baur M, Breitkreutz D, Mirancea N, Fusenig NE. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J Invest Dermatol. 1999;112:681–691. doi: 10.1046/j.1523-1747.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- Stoker M. Effect of scatter factor on motility of epithelial cells and fibroblasts. J Cell Physiol. 1989;139:565–569. doi: 10.1002/jcp.1041390316. [DOI] [PubMed] [Google Scholar]
- Stopak D, Harris AK. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982;90:383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- Sutherland J, Robertson M, Monaghan W, Riehle M, Britland ST. Human keratinocytes in primary culture display three distinct phenotypes with differential motility. J Anat. 2000;198:A66.. [Google Scholar]
- Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- Weiss P. Experiments on cell and axon orientation in vitro: the role of colloidal exudates in tissue organisation. J Exp Zool. 1945;100:253–386. doi: 10.1002/jez.1401000305. [DOI] [PubMed] [Google Scholar]
- Uren A, Yu JC, Gholami NS, Pierce JH, Heidaran MA. The alpha PDGFR tyrosine kinase mediates locomotion of two different cell types through chemotaxis and chemokinesis. Biochem Biophys Res Commun. 1994;204:628–634. doi: 10.1006/bbrc.1994.2505. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wilkinson PC. Assays of leukocyte locomotion and chemotaxis. J Immunol Meth. 1998;216:139–153. doi: 10.1016/s0022-1759(98)00075-1. [DOI] [PubMed] [Google Scholar]
- Wu L, Mustoe TA. Effect of ischaemia upon growth factor enhancement of incisional wound healing. Surgery. 1995;117:570–576. doi: 10.1016/s0039-6060(05)80257-0. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kramer RH. Laminin 5 deposition promotes keratinocyte motility. Exp Cell Res. 1996;227:309–322. doi: 10.1006/excr.1996.0280. [DOI] [PubMed] [Google Scholar]
- Zicha D, Genot E, Dunn GA, Kramer IM. TGFbeta1 induces a cell-cycle dependant increase in motility of epithelial cells. J Cell Sci. 1999;112:447–454. doi: 10.1242/jcs.112.4.447. [DOI] [PubMed] [Google Scholar]
- Zigmund SH. Cell locomotion and chemotaxis. Curr Opin Cell Biol. 1973;1:800–886. doi: 10.1016/s0955-0674(89)80041-9. [DOI] [PubMed] [Google Scholar]