Abstract
This study investigates the foot and ankle myology of gibbons and bonobos, and compares it with the human foot. Gibbons and bonobos are both highly arboreal species, yet they have a different locomotor behaviour. Gibbon locomotion is almost exclusively arboreal and is characterized by speed and mobility, whereas bonobo locomotion entails some terrestrial knuckle-walking and both mobility and stability are important. We examine if these differences in locomotion are reflected in their foot myology. Therefore, we have executed detailed dissections of the lower hind limb of two bonobo and three gibbon cadavers. We took several measurements on the isolated muscles (mass, length, physiological cross sectional area, etc.) and calculated the relative muscle masses and belly lengths of the major muscle groups to make interspecific comparisons. An extensive description of all foot and ankle muscles is given and differences between gibbons, bonobos and humans are discussed. No major differences were found between the foot and ankle musculature of both apes; however, marked differences were found between the ape and human foot. The human foot is specialized for solely one type of locomotion, whereas ape feet are extremely adaptable to a wide variety of locomotor modes. Apart from providing interesting anatomical data, this study can also be helpful for the interpretation of fossil (pre)hominids.
Keywords: ankle, bonobo, foot myology, gibbon, locomotion
Introduction
In this study we set out to investigate to what extent the specific locomotor adaptations of apes are reflected in their functional morphology. In primates both hands and feet interact with the environment and are therefore most likely to reflect the locomotor behaviour and habitat of the species (Sigmon & Farslow, 1986). However, we chose to focus on the foot and ankle complex of apes because the hand morphology might show some locomotion-manipulation compromises (Tuttle, 1972) and because we were particularly interested in hind-limb-dominated locomotor modes, such as bipedalism.
The human foot is paradigmatic in reflecting the species’ locomotor adaptations, because of its striking specializations for habitual bipedalism (Morton, 1935). However, the form–function relationship of the foot of non-human primates is undoubtedly as significant in an evolutionary context. Arboreal primates are known to have a flexible foot, with powerful grasping muscles and an opposable hallux (Morton, 1924; Tuttle, 1970, 1972). Terrestrial primates, by contrast, and ultimately humans, possess a more robust and compact foot with lever and shock-absorbing capabilities (Jacob, 2001). Establishing viable form–function relationships in the foot and ankle complex of extant primates is not only crucial for thorough investigation of primate locomotion but can also be helpful in the reconstruction of the locomotor behaviour of extinct hominoids.
Comparisons of the linear proportions of the various foot segments have repeatedly been used to investigate the adaptation of the primate foot (Morton, 1924; Schultz, 1963, 1973; Lessertisseur & Jouffroy, 1973). Clearly, this is of considerable functional relevance, but the importance of the muscles, as actuators of these foot segments, should not be underestimated. In addition, bone is a dynamic structure, sensitive to mechanical loading, and the observed structure might therefore rather be a reflection of activity patterns than of actual adaptations. Gross anatomical features of the musculature, such as the distribution, origin and insertion, and the presence or absence of muscles, are more conservative than bony structure and might thus better reflect the evolutionary pathway and adaptations of the species (Gibbs et al. 2002).
Unfortunately, previous papers investigating the foot and ankle myology of non-human apes are very scarce. Bisschoff (1870) and Kohlbrügge (1890/91) provide a gross anatomical description of the gibbon, and Wilder (1863), Miller (1952), Sokoloff (1972) and Swindler & Wood (1973) give information on the gross anatomical musculature of bonobos and chimpanzees. A more detailed description of the hip and thigh musculature is given by Sigmon (1974, 1975), and Tuttle (1970, 1972) provides a functional analysis of the hand and foot morphology of non-human apes. Other researchers have used EMG to investigate the recruitment of the hind limb muscles during gait (e.g. Tuttle et al. 1978; Stern & Susman, 1981; Shapiro & Jungers, 1988, 1994). More recently, Thorpe et al. (1999) and Payne (2001) have made detailed studies that provide quantitative data on the fore- and hind limb musculature of all ape species. These are all very valuable studies but to date the only information on the foot and ankle myology of primates is provided by Langdon (1990). In this work he combines observations from original dissections (n = 67) and from the literature to investigate the variation in cruropedal musculature throughout different primate taxa (14 families), including the apes. Although this is an extensive and very comprehensible work, a detailed functional description of the hominoid foot and ankle myology is still warranted.
We chose to study gibbons (Hylobates sp.), bonobos (Pan paniscus) and compare them with modern humans because their locomotor anatomy and behaviour are strikingly different. In addition, gibbons, bonobos and humans all belong to the same superfamily Hominoidea (Goodman et al. 1994; Gibbs et al. 2002) and all three species can and do walk bipedally despite their markedly different morphology (Carpenter, 1964; Susman et al. 1980).
Gibbons are lightly built apes, specialized for very fast ricochetal arm-swinging or brachiation (Chang et al. 2000). Beside this, their locomotor repertoire also contains climbing (4–20%), leaping (6–20%) and bipedal walking on large branches (4–11%), all executed at high speeds (Carpenter, 1964; Ellefson, 1967; Tuttle, 1972; Andrew & Groves, 1976; Fleagle, 1976; Gittins, 1983; Sati & Alfred, 2002). They live in the middle to upper levels of the forest canopy and rarely come to the ground (Carpenter, 1964; Tuttle, 1972). Observations of terrestrially walking gibbons are infrequent in the wild and occur predominantly when crossing gaps and roads in fragmented forested regions (Sati & Alfred, 2002; B. Rawson and G. Thampy, personal communication). Thus, the gibbon is characterized by a fast arboreal locomotion. This combination of swift movements and a complex three-dimensional environment requires highly mobile and flexed limbs (Schmitt, 1999). When a gibbon swings at high speed through the forest, it must have the ability to grasp a branch in almost every orientation and it must also be capable of quickly changing direction and speed. Obviously, arm-swinging is a forelimb-dominated locomotion type and the hind limbs are mostly kept flexed at hip and knee (Jungers & Stern, 1976). Nevertheless, mobility of the hind limb and foot are also crucial, as these swinging phases are alternated with short and fast bipedal bouts on large branches, with jumps, and with quadrumanous climbing (Tuttle, 1972). In view of their important prehensile function, flexibility of both hands and feet is essential in gibbon locomotion.
In bonobo locomotion, by contrast, different features can be premised. Although bonobos are larger and heavier than gibbons, they are also gracile and arboreal apes. They most commonly travel using arboreal quadrupedalism, quadrumanous climbing and scrambling (Susman et al. 1985; Doran, 1993), often performed at a slow deliberate pace. Faster locomotion types, such as diving, leaping and arm-swinging, are observed in agitated or fleeing animals but fast ricochetal brachiation as seen in gibbons is absent (Susman et al. 1985; Doran, 1993). In contrast with the fully arboreal gibbons, bonobos regularly come to the ground and travel terrestrially (Susman et al. 1985; Doran, 1993; Doran & Hunt, 1994). They most often do so using quadrupedal knuckle-walking, supporting 40% of their body weight on the knuckles of the forelimbs and 60% on the hind limbs (Reynolds, 1985; Susman et al. 1985; Doran, 1993). Beside this, bipedalism and tripedalism are also occasionally used during terrestrial travel (Susman et al. 1985; Kano, 1992; Doran, 1993). A robust and compact foot is most suitable for terrestrial walking, in order to support high compressive stresses and to generate large propulsive forces (Morton, 1935). However, high foot mobility seems equally important for their arboreal locomotor behaviour. Thus, the bonobo foot combines a prehensile and a propulsive function and its morphology should therefore be a compromise between stability and mobility.
Human locomotion is exclusively terrestrial and, as a consequence, the human foot has lost its prehensile function. However, the generation of propulsion has become extremely important during bipedal locomotion, so stability seems to be the ultimate requisite of the human foot–ankle complex (Morton, 1935).
Based on the above considerations, we hypothesize that gibbons will have relatively slender extrinsic foot muscles, allowing fast contraction and a wide range of motion. In addition, we expect that gibbons will have relatively stronger deep hind flexors and larger intrinsic foot muscles, in view of the important prehensile foot function. Bonobos, by contrast, should have more bulky extrinsic foot muscles, especially the plantar flexors, to generate large propulsive forces but also allowing a wide range of motion. Undeniably, a deeper knowledge of the morphology of the foot and ankle is needed to gain insight into the mechanics of ape and human locomotion. Thus, besides testing the above-mentioned hypothesis, we wish to provide a functional description of the foot and ankle muscles of gibbons and bonobos, useful for further kinesiological and comparative research on primate locomotion.
Materials and methods
Dissection data (Table 1) for the bonobo (Pan paniscus) were obtained from two adult specimens, a male, which is the same individual as in the study of Payne (2001), and a female. The male died of a heart attack, the female from a severe wound at the hand. The female had some arthrosis at her left ankle, but the remaining part of the musculoskeletal system of both cadavers was in good condition. Both bonobos were obtained from the Royal Zoological Society of Antwerp, Belgium. The data for the gibbon were obtained from two Hylobates lar specimens and from one Nomascus leucogenys specimen. The female H. lar (black variant) was put-down because of old age and some severe disorders (distortion of the vertebral column and blindness). The other H. lar (pale brown variant) was a male that died from his injuries after an aggressive attack by its father. Both lar gibbons were put at our disposal by the Royal Zoological Society of Antwerp, Belgium. The white-cheeked gibbon (N. leucogenys) was supplied by the ‘Parc Animalier de Branféré’, Brittany, France, and cause of death was drowning. The presented gibbon data are based on the dissection of the male lar gibbon and the dissections of the other two specimens were used for verification.
Table 1.
Subject data of the dissected specimens
| Species | Specimen | Age | Sex | Body weight (kg) | Foot length (cm) | Origin |
|---|---|---|---|---|---|---|
| Pan paniscus | De | 29 years | M | 60.0 | 25.5 | RZCA, B* |
| Dz | 31 years | F | 36.5 | 25.0 | RZCA, B* | |
| Hylobates lar | ||||||
| (black) | Mo | 25 years | M | 5.6 | 13.3 | RZCA, B |
| (pale brown) | Ya | 6 years | F | 6.3 | 17.3 | RZCA, B |
| Nomascus leucogenys | Br | 1 year 6 months | M | 2.9 | 10.8 | PAB, F |
Wild born specimens; RZCA, B = Royal Zoological Society Antwerp, Belgium; PAB, F = Parc Animalier de Branféré, France.
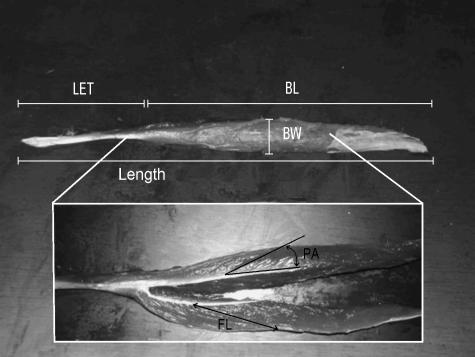
All specimens were eviscerated during post-mortem examination and were stored in freezers until dissection took place. The dissections were performed on fresh, non-fixed cadavers. The gross dissection of the hind limb muscles of the adult bonobo male was executed in cooperation with Dr M. M. Günther from the University of Liverpool. The detailed dissections of the foot–ankle complex of the bonobo and gibbon cadavers were executed by E.E.V. During these dissections the muscles were isolated one by one and their origin and insertion were noted. The action of the muscles was deduced from their sites of attachment, their trajectory and by pulling on them with the foot placed in a neutral position. In addition, several measurements were taken to determine the muscle mechanics of the gibbon and bonobo foot and ankle complex. These measurements are illustrated in Fig. 1, and include the (wet) muscle mass; the muscle length, measured from origin to the insertion of the muscle; the muscle belly length, BL, which is the distance from the origin of the most proximal muscle fibres to the insertion of the most distal muscle fibres; the muscle belly width, BW, i.e. the width of the muscle belly measured perpendicular to the force-generating axis of the muscle; the tendon length, TL, the distance from the most proximal origin of the tendon to the insertion of the tendon on the bone; the length of the external tendon, LET, i.e. the distance from the most distal muscle fibres to the insertion of the tendon on the bone; pennation angle, PA, the average angle of the muscle fibres relative to the force-generating axis; and the fibre length, FL, which is the approximate length of the muscle fibres.
Fig. 1.
Illustration of the different measurements taken on the isolated muscles. Muscle fibre length (FL) and pennation angle (PA) are measured on the longitudinally dissected muscle belly (lower inset). Legend: Length = total muscle–tendon length, BL = muscle belly length, BW = belly width, LET = length of the external tendon, FL = muscle fibre length and PA = pennation angle.
All linear measurements were taken with a digital calliper (Mitutuyo) and pennation angles were measured on digital images in CorelDraw! 9 (see also Ledoux et al. 2001). The data provided for fibre length and pennation angle are average values of at least three independent measurements taken on different places on the longitudinally dissected muscle belly. Pennation angle and LET values are lacking for the bonobo, because these were not taken during dissection and recovery of the data from the preserved muscles was impossible. Additionally, we have also calculated the physiological cross-sectional area (PCSA), using the formula provided by Mendez & Keys (1960): PCSA = muscle mass * cos (PA)/1060 kg m−3 * fibre length. However, because the largest pennation angle (PA) was 30°, the cosine of which is 0.87, we omitted the PA-factor in our calculations of the PCSA (see also Payne, 2001). To allow comparison between gibbons, bonobos and humans, the PCSA data were scaled to body mass to the two-thirds. The PCSA was not calculated for the smallest muscles, because accurate fibre lengths were not available for these muscles. The abbreviations of the foot and ankle muscles are given in Table 2 and the raw muscle data of the gibbon (Table A1) and bonobo (Table A2) dissections are given in the Appendix. The anatomical data of the female lar gibbon are not included in these tables because we are not confident about the accuracy of these data. Apart from severe distortions of the spine we also observed marked modifications in the appendicular skeleton of the cadaver and it is thus not unlikely that the soft tissue characteristics (muscles masses, PCSA, etc.) of this specimen are also affected. Therefore, the dissection of this specimen was only used to check the attachment sites and the presence or absence of the lower leg muscles, and muscle dimension data were omitted.
Table 2.
Abbreviations used for muscles
| Muscle | Code |
|---|---|
| m. gastrocnemius lateralis | Galat |
| m. gastrocnemius medialis | Gamed |
| m. soleus | Soleus |
| m. extensor hallucis longus | EHL |
| m. extensor digitorum longus II–III | EDL I |
| m. extensor digitorum longus IV–V | EDL II |
| m. tibialis anterior | TA |
| m. flexor fibularis | FF |
| m. flexor tibialis | FT |
| mm. lumbricales | lumbr |
| m. peroneus longus | Plong |
| m. peroneus brevis | Pbrev |
| m. tibialis posterior | TP |
| m. abductor hallucis, pars I | AbdH I |
| m. abductor hallucis, pars II | AbdH II |
| m. adductor hallucis c. transversum | AddHt |
| m. adductor hallucis c. obliquum | AddHo |
| m. extensor hallucis brevis | EHB |
| m. extensor digitorum brevis | EDB |
| m. flexor hallucis brevis | FHB |
| m. flexor digitorum brevis, pars I | FDB I |
| m. flexor digitorum brevis, pars II | FDB II |
| m. abductor digiti minimi | AbdV |
| m. flexor digiti minimi | FlexV |
| m. opponens digiti minimi | ODM |
| m. interossei plantares | iPlant |
| m. interossei dorsales | iDors |
We have calculated the relative masses and belly lengths of the major muscle groups of the gibbon and bonobo specimens. The relative muscle belly length is determined as the proportion of the muscle belly length to total muscle tendon length. The relative muscle masses are calculated as percentages of the total extrinsic or total intrinsic foot muscle mass to allow comparison between the different sized species. We have also included human data for the extrinsic foot muscle masses, which are provided by Wickiewicz et al. (1983).
Results and discussion
Gross anatomical description of the foot and ankle complex of gibbons and bonobos
This article focuses on the musculature of the foot and ankle complex of gibbons and bonobos but we consider that it is appropriate first to summarize the main skeletal features of gibbons and bonobos that were investigated previously by Schultz (1963, 1973) and Tuttle (1970, 1972). We also provide an illustration of the major anatomical landmarks of the lower hind limb and foot skeleton of both apes to clarify the attachment sites of the muscles that are described in the following paragraphs (Fig. 2).
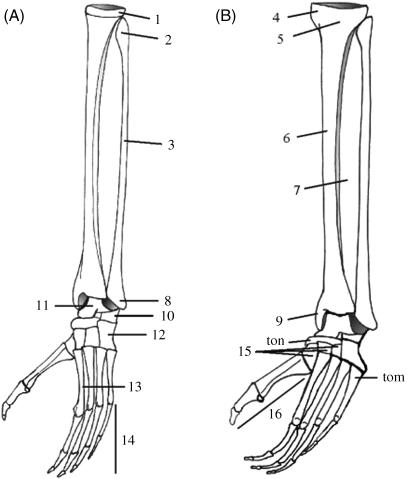
Fig. 2.
Illustration of the skeleton of the lower hind limb and foot of a gibbon (A) and a bonobo (B) with indication of the major anatomical landmarks. Legend: (1) lateral condyle; (2) fibula head; (3) fibula shaft; (4) medial condyle; (5) tibial head; (6) tibial shaft; (7) membrana interossea; (8) lateral malleolus; (9) medial malleolus; (10) calcaneus; (11) talus; (12) cuboid; (13) the metatarsus, consisting of five metatarsal bones (I–V); (14) the digits, each consisting of three phalanges; (15) the cuneiform bones (laterale, intermedium and mediale); and (16) the hallux. Note also the presence of sesamoid bones on the hallux and a prominent tuberosity on the naviculare (ton) and on the fifth metatarsal (tom).
The gibbon is a small ape with long arms, relatively long hind limbs and very slender feet. Owing to a great elongation of the limb bones, without a corresponding increase in thickness, these bones have become extremely gracile (Schultz, 1973). The foot skeleton is narrow and has a short heel, indicating a lessened leverage of the calf muscles (the relative length of the heel is the functional power arm of the foot; Morton, 1924; Schultz, 1963). The phalanges of the toes are curved and strikingly elongated, accounting for over 40% of the total foot length, and the hallux, i.e. the first metatarsal and digit, is long and is not enclosed in the foot sole (Tuttle, 1972; Schultz, 1973). The bonobo skeleton is clearly more robust and the foot has a relatively large heel or power arm (cf. common chimpanzee: Tuttle, 1970). The phalanges are relatively shorter than in gibbons and the tarsal region is relatively more elongated although the mid-tarsal bones (i.e. the navicular, the cuboid and the cuneiforms) are compressed in an antero-posterior direction (Morton, 1924). Both apes lack a longitudinal foot arch and have a rather robust fibula (Tuttle, 1970).
A gross anatomical description of the extrinsic and intrinsic foot muscles of gibbons and bonobos is provided in Tables 3a and 4a. Details on the morphological appearance of the different muscles are provided in the next paragraph.
Table 3a.
Origin, insertion and function of the extrinsic foot muscles of Pan paniscus
| Muscles | Origin | Insertion | Form | Function |
|---|---|---|---|---|
| (a) Triceps surae | ||||
| Gamed | short tendon at posterior side of the medial femoral condyle | common short Achilles tendon onto the posterior side of the tuber calcanei | large, unipennate with extensive tendon sheet at origin and insertion | foot plantar flexion |
| Galat | short tendon at posterior side of the lateral femoral condyle | |||
| Soleus | broad tendon at posterior side of the fibular head and the membrana interossea | large, unipennate with extensive tendon sheet at origin and insertion | ||
| Plant | short tendon at posterior side of the lateral femoral condyle | small, pennate; proximally fused with Galat | ||
| (b) Dorsiflexors | ||||
| EDL | proximal 1/3 of the antero-medial side of the fibular shaft and the membrana interossea | 4 tendons to the dorsal aponeuroses of digits II, III, IV and V | long, slender, unipennate | dorsiflexion of ankle and toes; foot eversion |
| EHL | middle 1/3 of the antero-medial side of the fibular shaft and the membrana interossea | tendon to dorso-medial side of the distal phalanx of digit I | thin, unipennate; tendon through lig. naviculare–metatarse I | extension and abduction of hallux; foot inverson |
| TA | antero-lateral side of tibial head and proximal 1/2 of the tibial shaft and the membrana interossea | 2 separate tendons to medio-plantar side of the medial cuneiform and first metatarsal | bipennate; proximally divided in large and small muscle head | foot dorsiflexion and inversion |
| (c) Deep hind flexors | ||||
| FT | proximal 1/3 of the posterior side of the tibial shaft | 2 tendons to the plantar side of the distal phalanges of digits II and V | superficial, pennate head; tendons fused with FF tendons, m. lumbricale II, and FDB | digital flexion; foot plantar flexion |
| FF | proximal 1/2 of the posterior side of the fibular head and shaft and the membrana interossea | 3 tendons to the plantar side of the distal phalanges of digits I, III and IV | deep, unipennate head; fused with FT tendons and mm. Lumbricales III, IV and V | hallucal and digital flexion; foot plantar flexion and inversion |
| (d) Invertors and evertors | ||||
| TP | proximal 1/2 of the postero-lateral side of the tibia and the postero-medial of the fibula and the membrana interossea | strong tendon to plantar side of the medial cuneiform and naviculare | large, pennate | foot plantar flexion and inversion |
| Plong | antero-lateral side of the fibular head and prox. 1/2 of the fibular shaft | tendon to the plantar base of first metatarsal | bipennate; proximally fused with Pbrev | foot eversion and plantar flexion; hallucal flexion and adduction |
| Pbrev | distal 1/2 of the anterior side of the fibular shaft | tendon to the lateral tuberosity of metatarsal V | unipennate; proximally fused with Plong | foot eversion and plantar flexion |
Table 4a.
Origin, insertion and function of the extrinsic foot muscles of Hylobates lar
| Muscles | Origin | Insertion | Form | Function |
|---|---|---|---|---|
| (a) Triceps surae | ||||
| Gamed | short tendon at posterior side of the medial femoral condyle | common Achilles tendon onto the posterior side of the tuber calcanei + sesamoid bone | unipennate; (sesamoid bone at origin variable) | foot plantar flexion; stabilization of the ankle joint |
| Galat | short tendon at posterior side of the lateral femoral condyle | fusiform; two-headed or fused with Plant; sesamoid bone at origin | ||
| Soleus | short tendon at postero-lateral side of the fibular head and lateral side of the knee joint | unipennate; strongly fused with Achilles tendon | ||
| Plant | short tendon at posterior side of the lateral femoral condyle | fusiform; fused with Galat; infrequent | ||
| (b) Dorsiflexors | ||||
| EDL I | fibular head and antero-lateral side of tibial head; proximal 3/4 of the antero-medial fibular shaft | tendons to the dorsal aponeuroses of digits II and III | fusiform; fused with EDL II | dorsiflexion of ankle and toes, foot eversion |
| EDL II | tendons the dorsal aponeuroses of digits IV and V | long, unipennate; fused with EDL I and EHL | ||
| EHL | middle 1/3 of the membrana interossea and the antero-medial side of the fibular shaft | tendon to dorso-medial side of the distal phalanx of digit I | long, thin, unipennate; fused with EDL; tendon through lig. naviculare-metatarsus I | extension and abduction of hallux; foot inversion |
| TA | antero-lateral side of the tibial head and proximal 1/2 of anterior tibial shaft | tendon(s) to medial foot border (metatarsal I base, naviculare or medial cuneiform) | thick, bipennate; strong, broad tendon at insertion | foot dorsiflexion and inversion |
| (c) Deep hind flexors | ||||
| FT | proximal 1/2 or medial 1/3 of the postero-lateral side of the tibial shaft | 2 long, flat tendons to the plantar side of the distal phalanges of digits I and V | long, unipennate; tendons fused with FF tendons, lumbricale V and FDB II muscle; variable organization | hallucal and digital flexion; foot plantar flexion |
| FF | proximal 2/3 of the posterio-medial fibular shaft | 4 long, flat tendons to the plantar side of the distal phalanges of digits I, II, III and IV | thick, unipennate; tendons fused with FT tendons and lumbricales II, III, IV; variable organization | hallucal and digital flexion; foot plantar flexion |
| (d) Invertors and evertors | ||||
| TP | proximal 1/2 of the posterior side of the membrana interossea, the lateral border of the tibial shaft and the medial border of the fibular shaft | tendon(s) to the plantar side of the cuneiforme intermedium (and laterale and naviculare) | short, unipennate; long tendon with sesamoid bone at insertion | foot plantar flexion and inversion |
| Plong | from the fibular head to the proximal 1/2 of the antero-lateral side of the fibular shaft | long tendon to the medio-plantar base of the first metatarsal | bipennate; fused with Pbrev; sesamoid bone in tendon, near cuboid | foot eversion; hallucal flexion and adduction |
| Pbrev | distal 1/2 of the lateral side of the fibular shaft (to malleolus lateralis) | tendon to the lateral tuberosity of metatarsal V | unipennate; proximally fused with Plong | foot eversion and plantar flexion |
Functional morphological comparison of the foot and ankle muscles of gibbons, bonobos and humans
The extrinsic foot muscles
The triceps surae
The triceps surae, or calf muscles, consist of the gastrocnemius, plantaris and soleus muscle and their main action is plantar flexion of the ankle joint. In all three species the m. gastrocnemius and m. soleus are very large as they are important power generators during locomotion (see also Morton, 1924). The plantaris muscle, however, is small and is frequently absent in bonobos (32–48%; Loth, 1913; Langdon, 1990) and humans (7–10%; Loth, 1913; Langdon, 1990) and is rare in gibbons (Kohlbrügge, 1890/91; Loth, 1913; Sigmon & Farslow, 1986; Langdon, 1990). The gastrocnemius, plantaris and soleus muscle are fused distally into the Achilles tendon, which shows a different development in the three species. Bonobos have a short Achilles tendon, which accounts for only up to 10% of the total muscle length (Table 5). In gibbons, the tendon is remarkably long compared with the other apes and accounts for 45% of the muscle length, although variation within the Hylobatidae is high (Bisschof, 1870; Kohlbrügge, 1890/91; Table 5). In humans, the strong, well-developed Achilles tendon accounts for up to 65% of the muscle length (Prejzner-Morawska & Urbanowicz, 1981) and it functions as an energy-saving mechanism, acting like a spring during running (Alexander, 1992; Hof et al. 2002).
Table 5.
Relative muscle belly lengths for the extrinsic foot muscles of the gibbon and bonobo
| Gibbon | Bonobo | |||
|---|---|---|---|---|
| Muscle | adult | juvenile | male | female |
| Triceps surae | ||||
| Galat | 0.72 | 0.55 | 0.92 | – |
| Gamed | 0.62 | 0.50 | 0.92 | – |
| Soleus | 0.67 | 0.84 | 0.96 | – |
| Deep hind flexors | ||||
| FT | 0.64 | 0.69 | 0.49 | 0.65 |
| FF | 0.52 | 0.52 | 0.56 | – |
| Dorsiflexors | ||||
| TA | 0.86 | 0.77 | 0.70 | 0.74 |
| EDL | 0.45* | 0.61 | 0.61 | 0.72 |
| EHL | 0.58 | 0.59 | 0.66 | 0.70 |
| Evertors | ||||
| Plong | 0.62 | 0.65 | 0.65 | 0.72 |
| Pbrev | 0.69 | 0.67 | 0.83 | 0.87 |
| Invertors | ||||
| TP | 0.57 | 0.49 | 0.77 | 0.74 |
Mean of both EDL heads
The attachment sites of the gastrocnemius muscle are similar in gibbons, bonobos and humans. In gibbons, we observed sesamoid bones at the posterior side of the lateral femoral condyle, in the tendon of the lateral head (i.e. the lateral fabella) and near the calcaneus in the Achilles tendon. Some authors have also described the presence of a sesamoid bone in the medial head of the m. gastrocnemius (i.e. the medial fabella; Sigmon & Farslow, 1986; Lewis, 1989; Payne, 2001), although Kohlbrügge (1890/91) found none. Sesamoid bones in the medial and lateral gastrocnemius head are present in common chimpanzees (Sigmon & Farslow, 1986; Lewis, 1989) but were not seen in our bonobo specimens. In humans, a lateral fabella is infrequent (13–21%; Lewis, 1989) and a medial one is very rare (Sigmon & Farslow, 1986; Lewis, 1989; Sarin et al. 1999).
The soleus muscle is slender in gibbons and is closely associated with the m. gastrocnemius. In bonobos, the m. soleus is very large and has a broad attachment site onto the fibular head. In humans, there is an extra attachment of the m. soleus onto the tibia, which is sometimes also present in Pan (i.e. the popliteal line; Sigmon & Farslow, 1986; Lewis, 1989; Gibbs et al. 2002).
Although a plantaris muscle is frequently absent in common chimpanzees (Wilder, 1863; Loth, 1913; Sigmon & Farslow, 1986; Langdon, 1990; Deloison, 1993; Thorpe et al. 1999; Gibbs et al. 2002), we did find a plantaris muscle in both bonobo specimens (also described by Miller, 1952). It originates together with the lateral head of the m. gastrocnemius but it is clearly distinct distally and has a long, thin tendon that merges distally into the Achilles tendon. According to several researchers (Kohlbrügge, 1890/91; Bisschoff, 1870; Sigmon & Farslow, 1986; Langdon, 1990) a plantaris muscle is absent in gibbons. Groves (1972), however, noticed the absence of a plantaris muscle in Hylobates syndactylus and H. lar but he did find an m. plantaris in H. hoolock. We found a small plantaris muscle in our adult lar specimen, which was fused with the large lateral head of the m. gastrocnemius, but in the juvenile and adult male gibbon a distinct plantaris muscle was absent. However, in the latter specimens the lateral head of the m. gastrocnemius could be divided into two parts, possibly including a firmly fused plantaris muscle. In Homo, a plantaris muscle is present but is reduced compared with the plantaris of the non-hominoid primates (Sigmon & Farslow, 1986).
The dorsiflexors
The extensor digitorum longus, the extensor hallucis longus and the tibialis anterior muscle are grouped into the dorsiflexors, pointing to their main function. They are located in the anterior compartment of the lower leg and all have very long tendons. The three muscles have a similar distribution and function in gibbons, bonobos and humans and there is little variation in the organization of the long extensors (see also Langdon, 1990). The tibialis anterior muscle, however, shows some muscular variation and is much larger than the long extensors.
In gibbons and bonobos, the m. extensor digitorum longus (EDL) can be split up to a varying degree. In the male lar gibbon, the EDL muscle was divided in two small muscle heads, a short head with two long external tendons inserting onto digits II and III, and a long head with two tendons inserting onto digits IV and V. In the other gibbon specimen no such separation was found and Kohlbrügge (1890/91) did not refer to a two-headed EDL muscle in his cadavers. Sometimes a tendon to the fifth digit may be lacking (Payne, 2001). In bonobos, the four tendons of the EDL are sometimes proximally grouped in two larger tendons (Miller, 1952) but in our specimen we found four separate tendons originating from one muscle head, as observed in humans.
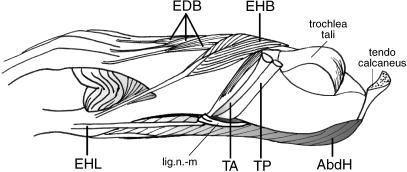
In gibbons and bonobos, the tendon of the m. extensor hallucis longus (EHL) passes through the short naviculo-metatarsal ligament at the medial side of the foot, together with the TA and TP tendons (Fig. 3). This ligament keeps the tendon in position during abduction of the hallux. In gibbons, the EHL muscle is slightly fused with the extensor digitorum longus muscle at the medial fibular shaft.
Fig. 3.
Medial view of a bonobo foot. Lig. n-m = naviculo-metatarsal ligament.
The tibialis anterior (TA) muscle runs obliquely over the anterior side of the tibia and passes through the transverse crural ligament and in both apes also through the naviculo-metatarsal ligament, before inserting at the medial side of the foot. Because of this medial insertion, the tibialis anterior muscle acts also as an invertor. There is some variation in attachment sites and structure of the muscle between gibbons, bonobos and humans. In gibbons, the muscle inserts with one or two strands onto the navicular bone, the base of the first metatarsal and/or the medial cuneiform bone. The tendon contains a sesamoid bone near insertion, the so-called ‘prehallux’, but we did not observe a divided muscle belly as has been described by Lewis (1989). In bonobos (and in common chimpanzees; Wilder, 1863), the tibialis anterior muscle is divided into a large and a small muscle belly, sometimes referred to as the m. abductor hallucis longus (Deloison, 1993). Both heads are slightly fused at their origin but have a separate tendon inserting onto the medial sesamoid bone of the first metatarsal and onto the medial cuneiform bone. The presence of a two-headed TA muscle in Pan, and in other non-human primates, points to a powerful and prehensile hallux (Deloison, 1993). In humans, the tibialis anterior muscle is usually one-headed but it inserts also onto the first metatarsal and the medial cuneiform bone.
The deep hind flexors and the mm. lumbricales
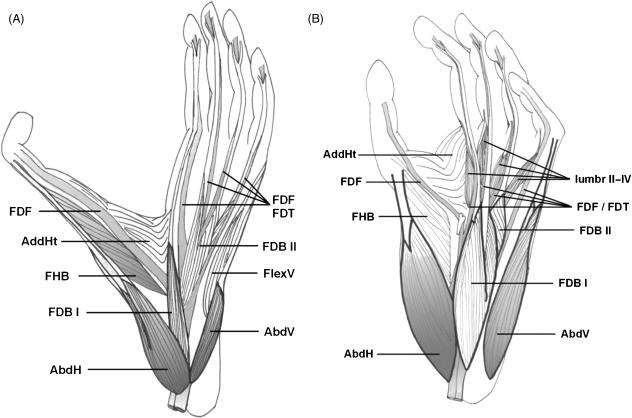
The deep hind flexors, which include the m. flexor fibularis (FF) and the m. flexor tibialis (FT), are both strong digital flexors and plantar flexors of the foot. In the ancestral mammalian condition the tendons of the two muscles were fused at the sole of the foot before dividing in separate strands for insertion onto each of the digits (Lewis, 1964, 1989). In the extant apes, however, both muscles have lost some tendons and there is considerable variation in the specific distribution of the tendons towards the digits (Langdon, 1990). In gibbons and bonobos, the tendons of deep hind flexors are arranged in a superficial (FT) and a deep (FF) plantar layer, which are slightly interconnected and which might allow independent flexion of the toes. Below, we describe the most common organization observed in both apes (Fig. 4; see also Sokoloff, 1972; Lewis, 1989; Langdon, 1990; Deloison, 1993). In humans these muscles have undergone a functional division in a hallucal (FF) and digital (FT) flexor and are therefore called m. flexor hallucis longus and m. flexor digitorum longus in human anatomy (Lewis, 1989).
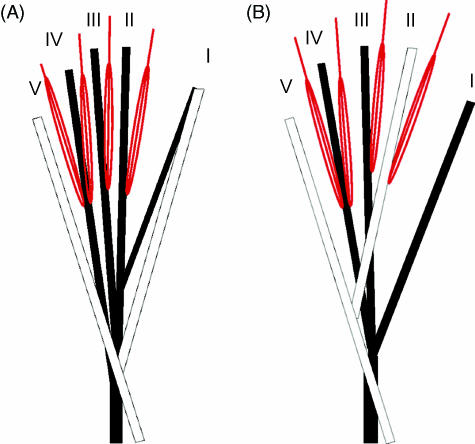
Fig. 4.
Schematic distribution of the m. flexor fibularis (FF, in black) and m. flexor tibialis (FT, in white) tendons and the mm. lumbricales (red) in the gibbon (A) and bonobo (B) foot.
In gibbons (Fig. 4A), the FF muscle has lost its contribution to the tendon of digit V and splits into four tendons at the plantar side of the foot, inserting onto the phalanges of digits I, II, III and IV. The mm. lumbricales II, III and IV originate from these tendons. In our specimens, the FT muscle had retained two tendons inserting onto the plantar side of the phalanges of digits I and V but other patterns have been described as well (see Langdon, 1990). The lumbricale V muscle originates from the FT tendon towards digit V. The two muscle bellies of the deep layer of the FDB originate also from the FT tendon. The FT tendon towards digit I is fused with the FF tendon and the long tendon inserting onto the fifth digit sends some fibres to the tendons of digits II and IV of the FF muscle. In bonobos (Fig. 4B), the FF muscle has lost its contribution to the tendons of digits II and V and retains the tendons inserting onto digits I, III and IV. These FF tendons are fused with the tendons of the FT, which insert onto digit II and V and which are also fused with the FDB muscle. A similar tendon distribution has been observed in common chimpanzees (Langdon, 1990). The mm. lumbricales are closely associated with both long flexors. In humans, the homologues of FT and FF, the m. flexor digitorum longus and the m. hallucis longus, act as separate flexors of the lateral toes and the hallux.
The m. quadratus plantae (or m. flexor accessorius) was only found in one foot of the adult male bonobo and was absent in all gibbon specimens. In the bonobo, the muscle was weakly developed and one-headed. It originated from the latero-plantar side of the calcaneus and was distally fused with the FT tendon towards digit V. The muscle is also often reduced or absent in other higher primates (Sokoloff, 1972; Lewis, 1989). In humans, however, it is a strong, double-headed muscle originating from both sides of the calcaneus and it provides a firm base for the m. flexor digitorum longus when contracted. This allows simultaneous contraction of the long and short digital flexors during toe-off and it also assists in foot eversion, which is important in terrestrial (bipedal) walking (Sigmon & Farslow, 1986).
The invertors and evertors of the foot
The m. tibialis posterior (TP) is the main invertor of the foot in both apes and in humans. In apes, it is important for inversion during arboreal locomotion and grasping. In humans, the muscle has a broad insertion and is particularly well developed because it has an important role in supporting the medial longitudinal foot arch (Langdon, 1990).
A sesamoid bone is sometimes present near the insertion of the TP tendon of gibbons (our personal observation; Kohlbrügge, 1890/91). In bonobos (and in common chimpanzees; Deloison, 1993), a sesamoid bone is absent but there is a strong tendon with a broad attachment site, which is related to the presence of a prominent tuberosity of the navicular bone in bonobos (Fig. 2). The m. tibialis posterior of humans has sometimes a sesamoid bone in its tendon, near the talus or near the navicular bone (Gray, 1918). In humans, it is a strong muscle with two or three strands inserting onto the navicular bone and onto the three cuneiform bones. Other attachments, onto the cuboid, the metatarsal bases and onto the tendon sheet of the m. peroneus longus, can occur and fusion with the m. flexor hallucis brevis is variable (Otis & Gage, 2001). These multiple insertions are bipedal specializations, which provide powerful action of the m. tibialis posterior and stabilize the longitudinal arch with help from the m. flexor hallucis brevis (Lewis, 1964).
The peronei are powerful foot evertors in apes and humans. The m. peroneus longus acts also as a hallucal flexor and adductor in apes. Organization of the peronei is similar in gibbons, bonobos and humans.
The m. peroneus longus (Plong) has a long tendon that runs downward along the lateral side of the fibula and lies above the m. peroneus brevis tendon at the ankle joint. It runs behind the lateral malleolus and crosses the plantar side of the foot through a canal (i.e. the sulcus tendinis m. peronei longi). At the entrance of the canal, near the cuboid bone, the tendon often contains a sesamoid bone. Such sesamoid bone was lacking in our bonobo specimens but was observed by Miller (1952). In gibbons and bonobos, the peroneus longus muscle is fused with the muscle belly of m. peroneus brevis at its origin and the tendon inserts onto the first metatarsal. In humans, there is also an insertion onto the medial cuneiform and a sesamoid bone is rare (Macalister, 1875).
Both in apes and in humans, the m. peroneus brevis (Pbrev) is much smaller than the m. peroneus longus and its attachment onto the fibula extends to the malleolus lateralis. At this point the external tendon emerges and inserts laterally onto the tuberosity of metatarsal V.
The m. peroneus tertius (or m. fibularis tertius; Eliot & Jungers, 2000) is usually present in Homo (95%) but variable in Hylobates (30–50%) and rare in Pan (0–5%; Miller, 1952; Deloison, 1993; Jungers et al. 1993; Thorpe et al. 1999; Gibbs et al. 2000). However, in our dissections we found an m. peroneus tertius in the adult female bonobo and none in the gibbon specimens. The muscle arises from the lower third of the anterior surface of the fibula and from the lower part of the interosseous membrane. The tendon, after passing under the transverse and cruciate crural ligaments, inserts into the dorsal surface of the base of the metatarsal bone of the little toe (Gray, 1918). The m. peroneus tertius functions as an evertor and dorsiflexor of the foot during the swing phase. The muscle works in concert with the m. tibialis anterior and the EDL muscle to level the foot and to cause toe clearance during bipedal walking (Jungers et al. 1993). The function of the m. peroneus tertius in apes is, however, questionable, considering the highly variable occurrence of the muscle.
The intrinsic foot muscles
The hallucal muscles
The muscles that move the hallux are closely associated and well-developed in non-human apes. But the hallucal muscles are also relatively large in humans, despite the adducted position of the hallux in the human foot. This might be related to the important propulsive function of the hallux during bipedal walking.
In gibbons, the m. abductor hallucis (AbdH) consists of two muscle bellies that are fused proximally and insert separately onto the hallux (Fig. 5A). Although a two-headed m. abductor hallucis has also been described for common chimpanzees (Sokoloff, 1972), we did not observe such an organization in the bonobo foot. In bonobos and humans, the m. abductor hallucis is a thick, one-headed muscle, with a broad insertion onto the medial sesamoid bone and hallux (Fig. 5B).
Fig. 5.
Muscles in the upper plantar layer of a gibbon (A) and bonobo (B) foot.
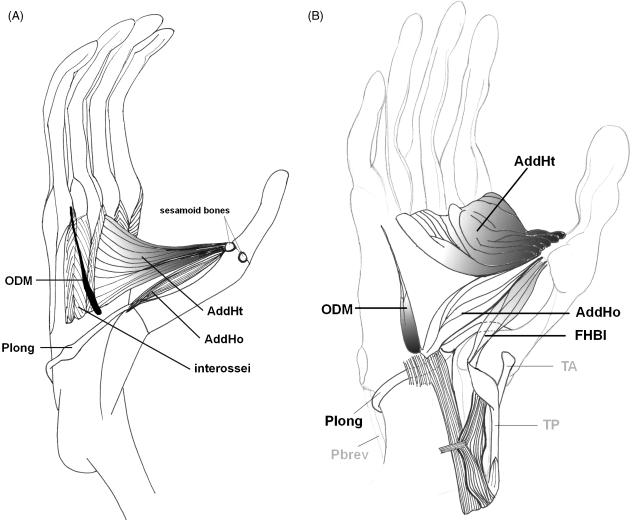
In non-human apes, the m. adductor hallucis is a large two-headed muscle, consisting of a small ‘oblique head’ (AddHo) and a massive ‘transverse head’ (AddHt), which are closely associated (Fig. 6). Insertion of both heads is similar in gibbons, bonobos and humans but the place of origin is different. In humans, the two heads of the m. hallucal adductor are not fused and the transverse head is weakly developed, reflecting the absence of an opposable hallux.
Fig. 6.
Hallucal muscles in the upper plantar layer of a gibbon (A) and bonobo (B) foot.
In gibbons, the m. flexor hallucis brevis (FHB) is a rather broad and flat muscle with a complex organization. The muscle belly is proximally fused with the AddHo muscle and distally with the AbdH I tendon. It has a sesamoid bone near the site of origin (in the annular ligament) and at insertion. In bonobos, the m. flexor hallucis brevis is a two-headed muscle, lying just beneath the AbdH. There is also a lig. annulare near its base, through which the tendon of the FF runs, but a sesamoid bone is absent (Figs 4B and 5B). The FHB has a similar organization in humans and bonobos; the medial head is fused with the AbdH and the lateral head is fused with the AddH at insertion (Fig. 5B). This muscle is larger in humans than in apes and reflects the importance of hallucal flexion during bipedal locomotion (Aiello & Dean, 1990).
In gibbons, the muscle belly of m. extensor hallucis brevis (EHB) has parallel orientated fibres and is not fused with the muscle bellies of the m. extensor digitorum brevis. In bonobos, the muscle belly of m. extensor hallucis brevis is bipennate and is slightly fused at is base with the muscle bellies of the m. extensor digitorum brevis. A similar organization is found in humans.
The short digital extensors
These are small muscles with thin tendons that work in concert with the EDL to extend the digits. However, contraction of the short digital extensors permits extension of the toes independently of ankle dorsiflexion (Langdon, 1990). In gibbons, bonobos and humans there is a clear division between the hallucal extensor and the digital extensors (inserting onto digits II–IV) but the amount of association between both intrinsic extensors differs. The distribution and function of the short extensors is similar in gibbons, bonobos and humans but the fibre architecture of the muscle bellies is variable. A tendon to the fifth toe is usually lacking.
The m. extensor digitorum brevis (EDB) has three thin muscle bellies lying on the dorsum pedis, each of which sends a small tendon to digits II, III and IV. In gibbons, they are unipennate muscles, which are slightly interconnected but the belly to the fourth digit was separate in one specimen. A tendon to the fifth toe was reported for H. syndactylus and H. hoolock (Groves, 1972; see also Lewis, 1989) but was absent in all our specimens (and in the specimens reported by Langdon, 1990). In bonobos, they are bipennate muscles and in the left foot of the adult male a tendon towards the third digit was lacking. In humans, the EDH and EDB are proximally fused, as in bonobos, and occasionally one or more tendons are lacking.
The short digital flexors and associated muscles
The m. flexor digitorum brevis (FDB) is a small muscle lying in the upper plantar muscle layer. The FDB tendons are perforated by the tendons of the deep hind flexors before insertion onto the distal phalanges (Fig. 5). The muscle has a different organization in apes and humans and there is a high intraspecific, and even intra-individual, variation in both apes in the distribution of the tendons towards the digits (see also Wilder, 1863; Kohlbrügge, 1890/91; Sokoloff, 1972; Langdon, 1990). Even in humans some variation in tendon distribution is present (Macalister, 1875).
In both apes, the muscle is arranged into a deep and superficial head. The superficial head of the FDB has a strong origin onto the medial calcaneal process and can contract separately from the other flexors. Thus, phalangeal flexion of the middle toes (II and III) is independent from the position of the foot, plantar flexion in particular, which strengthens the grasping capability of the ape foot. The deep layer, however, is fused with the FT tendon and flexion of the fourth (and fifth) toe will be accompanied by plantar flexion of the foot due to the simultaneous contraction of FT and FDB II.
In gibbons, the superficial layer has one muscle belly and the deep layer has two or three smaller muscle bellies (Fig 5A). The tendons of the deep layer insert onto digits III, IV and V but the fifth tendon is not perforated and is frequently absent (Kohlbrügge, 1890/91; Langdon, 1990). In one specimen the tendon to the fourth digit was not perforated either. The superficial head has a long tendon inserting onto the second digit. In bonobos, the arrangement of the tendons is variable, even between the left and right foot of the same specimen (see also Wilder, 1863 and Sokoloff, 1972 on P. troglodytes). The tendon of the superficial layer runs towards digit II and the tendon of the deep layer runs towards digit IV (Fig. 5B). Insertion onto digit III can be either by a tendon of the superficial layer or by a tendon of the deep layer. A tendon towards the fifth digit is absent in bonobos and is also frequently lacking in common chimpanzees (Sokoloff, 1972; Langdon, 1990; Deloison, 1993) and humans (in 23% of the cases, Gray, 1918). The FDB muscle of humans has 3–4 muscle bellies but is arranged in one (superficial) layer, which is closely connected with the plantar aponeurosis (Sigmon & Farslow, 1986; Deloison, 1993). However, a deep head is found in some human populations, e.g. the South African Bushmen, and is associated with a weakly developed superficial head and a small medial process of the calcaneal tuberosity or ‘heel process’ (Sarmiento, 1983; Lewis, 1989).
The mm. lumbricales are very small muscles located in the middle plantar layer of the foot and are closely associated with the deep hind flexors (see above; Figs 4 and 5). They assist in metatarso-phalangeal flexion.
The muscles of the fifth toe
There are several separate muscles that assist in both flexion and abduction of the fifth digit. These are mostly tiny muscles that are closely associated with each other and hence difficult to identify separately.
In gibbons, the m. abductor digiti minimi (AbdV) is thin and its entire tendon is fused with the FlexV muscle (Fig. 5A). In bonobos, the m. abductor digiti minimi is distally fused with the FlexV muscle, and has two long, separate tendons at insertion. The same organization has been described for common chimpanzees (Wilder, 1863). It is a very thick muscle, which forms the lateral part of the sole of the foot (Fig. 5B). In humans, the muscle is even more prominent, and originates from both calcaneal processes. It stabilizes the human foot during bipedal walking (Mann & Inman, 1964).
In gibbons, the m. flexor digiti minimi brevis (FlexV) is a small muscle located at the metatarsal V shaft that is fused at its whole length with the AbdV tendon (Fig. 5A). In bonobos, the FlexV muscle consists of two parallel muscle bellies, running over the whole length of the fifth metatarsal, and both bellies are fused at origin and insertion with the AbdV tendon. The FlexV muscle is one-headed in humans but is more prominent than in the apes and is only slightly fused with the AbdV tendon at insertion.
The m. opponens digiti minimi (ODM) and the m. contrahens V are very small muscles, located in the deep plantar layer of the foot. The ODM and contrahens V muscles have been described for most non-human primates but observations in hominoids are infrequent (Kohlbrügge, 1890/91; Miller, 1952; Sokoloff, 1972; Sigmon & Farslow, 1986; Lewis, 1989; Deloison, 1993). These small muscles are often fused (Jouffroy, 1962; Grand, 1967; Lewis, 1989). In the male lar gibbon we have identified a very small muscle, originating from the lateral cuneiform bone and inserting onto the plantar side of the fifth metatarso-phalangeal joint, which is probably the ODM muscle (Fig. 6A). We have also found a presumed ODM muscle in the deep plantar layer of one bonobo foot, running obliquely from the cuboid-metatarsal IV joint to the metatarso-phalangeal V joint (Fig. 6B). The muscle was lying on top of the mm. plantar interossei and had a small tendon at its insertion. The ODM, which is sometimes described as a deep part of the FlexV muscle, is often present in the human foot and is a minor flexor of the fifth metatarsal (Gray, 1918). An m. contrahens V is rarely seen in modern humans.
The mm. interossei
These are small, bipennate muscles that are located between the metatarsal bones and run from the bases of the metatarsal bones to the bases of the first phalanges of the same toe. They are divided into the dorsal and ventral mm. interossei in humans but this distinction is less clear in gibbons and bonobos (and P. troglodytes; Sokoloff, 1972). The mm. interossei are very small and the dorsal and ventral group are located very close to each other. Thus, it is practically difficult to study the exact origin and insertion of these groups (see also Wilder, 1863; Grand, 1967; Sigmon & Farslow, 1986). There are four dorsal mm. interossei that are arranged around the functional axis of the foot. In higher non-human primates the axis is the third digit (mesaxonic pattern); in humans it is the second digit (entaxonic pattern; Sigmon & Farslow, 1986; Lewis, 1989). The dorsal mm. interossei abduct digits II and IV from the third digit and also cause metatarso-phalangeal flexion of digits II, III and IV. There are three plantar mm. interossei, at the lateral side of metatarsal II and the medial sides of metatarsals IV and V. They adduct digits II, IV and V towards the third ray and also cause metatarso-phalangeal flexion. In the juvenile gibbon, we found two additional mm. interossei, one at the medial side of the second digit, inserting distally onto the proximal phalanx I, and one at the latero-plantar side of the third metatarsal.
The organization of the mm. interossei in apes appears to be different from the typical human pattern and therefore we suggest that another nomenclature should be used for the description of the interosseus muscles of non-human primates. It might be beneficial to abandon the prevailing distinction into a plantar and dorsal interosseus muscle group and instead adopt a nomenclature in which the mm. interossei are grouped per digital unit (digits II–V; see also Sokoloff, 1972).
The planta pedis
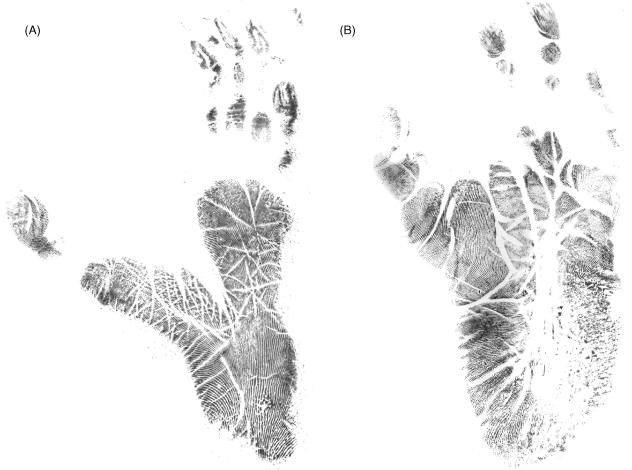
The bonobo foot has a broad heel region compared with the slender gibbon foot, as can clearly be seen in Fig. 7. On these footprints you can also observe the deep cleft between the first and second toe in the gibbon foot and the apparent flatness of both ape feet, which is due to the absence of a longitudinal arch. Between the plantar epidermis and the plantar aponeurosis there is a layer of fat tissue. In the human foot this is a thick layer, which is particularly dense in the heel region, i.e. the so-called ‘heel pad’. Both apes lack such a well-developed plantar fat layer but in gibbons regions of accumulated adipose tissue are observed at the heel, at the lateral foot border, at the base of the hallux and at the metatarsal heads. In bonobos, fat tissue was only found in the heel region and lateral foot border. This is an interesting difference, because the distribution of fat tissue on the foot sole gives information on the position of the foot during locomotion. Bonobos strike the ground with the heel and lateral midfoot (Vereecke et al. 2003), whereas gibbons do not heel-strike but exert high impact forces at the middlemost metatarsal heads (Schmitt & Larson, 1995; Vereecke et al. in press). In line with the marked heel-strike in humans, there is a particularly thick heel pad in the human foot.
Fig. 7.
Footprint of a gibbon (A) and bonobo (B), scaled to the same length.
The human plantar aponeurosis is a tight network of collagen fibres, reaching from the calcaneus to the base of the phalanges of the five digits, which helps to maintain the longitudinal foot arch. It functions as a shock absorber (Jacob, 2001) and as an elastic recoil mechanism that saves up to 17% of energy during human bipedalism (Alexander, 1992). This plantar aponeurosis is also present in gibbons and bonobos but is not as extensive and strong as in humans and a longitudinal foot arch is lacking. In gibbons and bonobos, the plantar aponeurosis originates from the calcaneal tuberosity and from the intermuscular septum between the hallucal and digital flexors. It runs over the foot sole towards the metatarso-phalangeal joints of digits II–IV, towards the navicular bone, to the lateral side of the first metatarsal head, and to the lateral tuberosity of metatarsal V. It consists of strong and parallel orientated fibres that are closely associated with the foot sole and that are connected with the fascia of the superficial plantar foot muscles. The plantar aponeurosis of both apes might assist in digital flexion (Sokoloff, 1972) but an energy-saving function is presumably absent, due to the lack of a longitudinal foot arch.
The relative importance of the foot and ankle muscles in gibbons, bonobos and humans
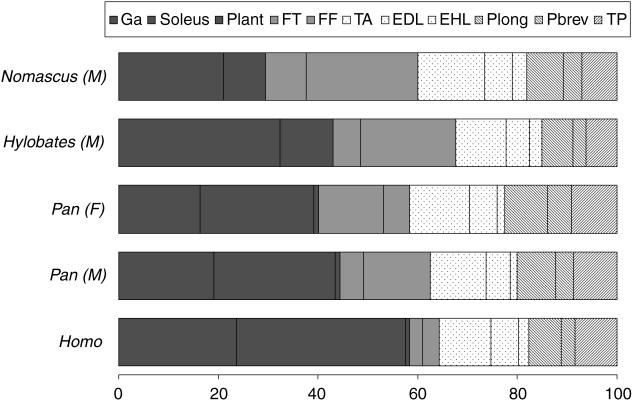
We compared the relative amount of extrinsic and intrinsic foot muscle mass in gibbons and bonobos and for both apes we have found that they account for, respectively, 3.0% and 0.6% of the total body mass. Thus, gibbons do not have relatively larger intrinsic foot muscles and bonobos do not have relatively heavier extrinsic foot muscles.
The relative mass distribution of the extrinsic foot muscles is shown in Fig. 8 and the triceps are clearly the largest muscle group in humans, accounting for up to 60% of the extrinsic foot muscles. This is not too surprising given that plantar flexion is very important during bipedal walking. Large propulsive forces have to be generated prior to toe-off, which explains the need for large plantar flexors (Hof et al. 2002). But, the triceps are also the largest muscle group in both apes, accounting for more than 40% of the extrinsic muscle mass (Fig. 8). Although these are not yet comparable with the huge human triceps, it appears that plantar flexion is also important in gibbon and bonobo locomotion. Looking in more detail at the relative mass distribution and the scaled PCSA of the different triceps muscles we do find some differences between the two apes. The m. soleus is the largest and strongest plantar flexor in bonobos, whereas the m. gastrocnemius is the largest and most powerful plantar flexor in gibbons and an m. plantaris is frequently absent (Fig. 8; Tables A1 and A2).
Fig. 8.
Relative distribution of the extrinsic muscles in the gibbon (Nomascus and Hylobates sp.), bonobo (Pan paniscus) and human (Homo sapiens) foot. M: male and F: female specimen.
Other differences are found in the relative amount of invertor/evertors and deep hind flexors. We have observed that bonobos have a relatively larger and stronger m. tibialis posterior, acting as a powerful invertor of the foot (see the scaled PCSAs of Tables A1 and A2). By contrast, gibbons have a relatively stronger m. peroneus brevis, which is an important foot evertor (see the scaled PCSAs of Tables A1 and A2). Gibbons also have slightly heavier and stronger deep hind flexors than bonobos (see the scaled PCSAs of Tables A1 and A2), pointing to more powerful digital flexion in the gibbon foot.
A last difference is found in the relative strength of the dorsiflexors. The m. tibialis anterior and the EDL muscle of bonobos have a relatively larger PCSA than observed in the adult gibbon, although no difference was found in the relative mass of these dorsiflexors (Fig. 8; Tables A1 and A2). Apparently, these muscles are more elongated, and hence less forceful, in gibbons. There is, however, considerable individual variation in the PCSA of the extrinsic muscles, especially between the adult and juvenile gibbon, so we should be cautious when looking at these data.
If we compare the extrinsic foot muscles of humans with those of both apes, we find that the sizes of the human dorsiflexor and invertor/evertor muscle group are similar to these of both apes (Fig. 8). However, the human deep hind flexors are very small, which can be related to the absence of a prehensile function in the human foot.
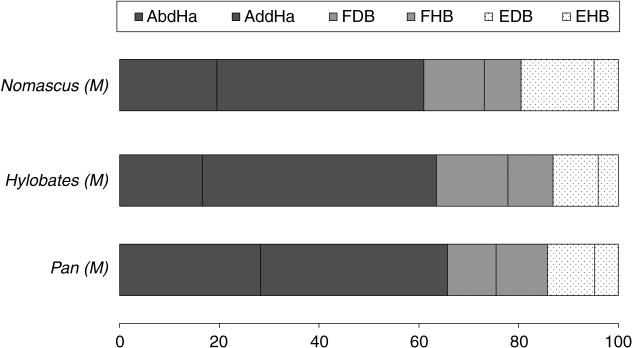
The mass distribution of the intrinsic foot muscles of gibbons and bonobos is depicted in Fig. 9 and is very similar in both apes. The hallucal abductors and adductors are clearly the largest intrinsic foot muscle group in gibbons and bonobos, accounting for more than 60% of the intrinsic foot muscles. This points to a powerful hallux and is related to the prehensile ape foot. Gibbons have a relatively larger m. hallucal adductor and a smaller abductor than bonobos but apart from this no significant size differences are observed. In both apes, the short flexors are somewhat larger than the short extensors, which is in accordance with the importance of digital flexion during arboreal locomotion. Unfortunately, comparison with the human foot was not possible because we could not obtain data from the intrinsic foot muscles of humans. When we compare the scaled PCSA of the intrinsic foot muscles of both apes we find that gibbons have somewhat stronger hallucal extensors and flexors than bonobos. This might point to a stronger hallucal grasp in gibbons compared with bonobos. However, we have to be cautious when interpreting these, and the other, muscle mass data, as they only come from two gibbon specimens and one bonobo specimen.
Fig. 9.
Relative distribution of the intrinsic muscles in the gibbon (Nomascus and Hylobates sp.) and bonobo (Pan paniscus) foot. M: male and F: female specimen.
The importance of tendon in the locomotion of non-human apes can be estimated by calculating the ratio of muscle belly length to total muscle–tendon unit length (see Table 5). We found some differences between the relative muscle belly lengths of gibbons and bonobos, but again, we have to be aware that there might be some intraspecific variation as well. The most apparent difference is found in the relative length of the Achilles tendon. The Achilles tendon comprised a greater proportion of the muscle–tendon unit of the triceps in gibbons (28–38%) than in bonobos (4–8%) and other non-human apes (Payne, 2001). As a consequence the m. gastrocnemius and m. soleus have shorter muscle bellies and the mass of the triceps is more proximally distributed in gibbons than in bonobos and other great apes. The importance of the Achilles tendon as an energy-saving mechanism during high-speed locomotion has been well documented in human and non-human animals (Alexander & Vernon, 1975; Alexander et al. 1982; Ker et al. 1988; Alexander, 1991; Biewener, 1998; Payne, 2001; Hof et al. 2002). As gibbons have a well-developed Achilles tendon and their bipedal locomotion is often very fast and bouncing (our personal observations; Tuttle, 1972), it is very likely that a similar energy-saving mechanism is also active during high-speed locomotion of gibbons. As a consequence, gibbon locomotion might be more (energetically) efficient than the locomotion of other non-human apes. However, this still needs to be confirmed by a detailed analysis of the energetic costs of hylobatid locomotion.
The results for the other extrinsic foot muscles are less definite. Some muscles are more tendinous in gibbons, such as the EHL, the Pbrev and the TP muscle, whereas other muscles are more tendinous in bonobos, such as the TA muscle (Table 5). Previously obtained data on the hind limb muscles of hominoids (Thorpe et al. 1999; Payne, 2001) have emphasized the remarkable slender and tendinous thigh muscles of gibbons, but apparently this is less pronounced in the more distal muscle groups.
The sesamoid bones
We have observed a markedly higher occurrence of sesamoid bones in the gibbon foot compared with the bonobo and human foot. Most sesamoid bones are embedded in the tendons, near the attachment site, but some are found proximally, e.g. in the gastrocnemius muscle. The two sesamoid bones of the hallux are present in gibbons, bonobos and humans but gibbons (and common chimpanzees; Deloison, 1993) also have sesamoid bones at the other metatarso-phalangeal joints. Nearly all sesamoid bones that we have observed in gibbons have also been described for humans, but most of them are very uncommon (Pfitzner, 1896; Gray, 1918). Probably, a similar number of sesamoid bones are present as cartilaginous nodules in ape and human fetuses but different physical demands may determine which sesamoid bones persist in the adult (Gray, 1918; Sarin et al. 1999). Apparently, gibbon locomotion selects for the retention of many sesamoid bones, which offers several benefits to the musculoskeletal system:
(1) First, they can improve the joint mechanics, by increasing the lever arm of the muscle and, hence, increasing the flexion torque. They can also change the direction of pull and can diminish friction, which also enhances the joint mechanics. (2) Secondly, the reduction of friction also enhances tendon sliding, which prevents wear and tear in tendon. Thus, sesamoid bones can also provide mechanical protection to the tendon. (3) Finally, they can disperse forces and modify pressure, by acting as a shock absorber and in transferring loads from the substrate to the bones (David et al. 1989; Perlman, 1994).
But why have most sesamoid bones not been retained in bonobos and humans, if they are indeed so advantageous? This might be related to the more tendinous muscles of gibbons compared with other apes and humans (Payne, 2001), as it is probably more crucial for longer tendons to reduce friction and to obtain mechanical protection. This might also be the reason why horses, which have extremely long tendons, have numerous sesamoid bones (Nickel et al. 1986).
Conclusion
The foot and ankle musculature follows the same general ‘bauplan’ in gibbons, bonobos and humans, which is not so surprising in view of their close phylogenetic relationship. The human foot is most deviant, owing to its bipedal specializations, but the foot–ankle complex of gibbons and bonobos is remarkably similar. Both apes have strong plantar flexors and large hallucal muscles, which are related to a propulsive and prehensile foot function. Thus, although gibbons and bonobos have a clearly different ecological niche and locomotor behaviour, the myology of their foot–ankle complex is largely similar. Both apes have a very adaptable foot–ankle complex with a generalized structure, which enables them to use a wide variety of locomotor modes and substrates. Whether the similarities in the foot myology of gibbons and bonobos are homoplasies or synapomorphies remains unresolved but we hope that additional research on primate foot myology might help to clarify this question in the (near) future.
This study gives a clear and detailed description of the functional morphology of the foot–ankle complex of two extant ape species and provides viable form–function relationships. This can be used in studies on primate locomotion but might also be helpful for the reconstruction of the locomotor behaviour of (pre)hominid fossils.
Table 3b.
Origin, insertion and function of the intrinsic foot muscles of Pan paniscus
| Muscles | Origin | Insertion | Form | Function |
|---|---|---|---|---|
| (a) Hallucal muscles | ||||
| AbdH | medial side of the tuber calcanei, tuberosity of the naviculare, and plantar aponeurosis | strong, broad tendon to medial sesamoid bone and base of proximal phalanx of the hallux | thick, pennate; fused with FHBm | hallucal abduction and flexion |
| AddHo | plantar side of the metatarsal III base | lateral sesamoid bone of the first metatarsal | pennate; short tendon at insertion | hallucal adduction and flexion |
| AddHt | plantar side of the metatarsal II, III and IV heads | massive, parallel head | ||
| (b) Short flexors | ||||
| FHBm | plantar side of the medial cuneiform bone | tendon to the medial sesamoid bone of the first metatarsal | thick, pennate, superficial head; fused with AbdH tendon | slight flexion of the first metatarsal |
| FHBl | lateral sesamoid bone of the first metatarsal | elongated, pennate, deep head; tendon at origin | ||
| FDB I | medio-plantar side of the tuber calcanei | perforated tendons to the plantar side of the middle phalanges of digits II (and III) | superficial head; unipennate (proximally fused bellies); variable distribution | flexion of digit(s) II (and III) |
| FDB II | from the latero-plantar side of the FT tendon | perforated tendons to the plantar side of the middle phalanges of digits (III and) IV | deep head; slender, unipennate; variable distribution | flexion of digit(s) (III and) IV; foot plantar flexion |
| (c) Short extensors | ||||
| EHB | dorso-lateral side calcaneus; trochlea peronealis, near the sinus tarsi | tendon into the dorsal aponeuroses of digit I | bipennate; proximally fused with EDB | hallucal extension and foot supination |
| EDB | 2–3 tendons into the dorsal aponeuroses of digits II (III) and IV | 2–3 bipennate muscle bellies; proximally fused with FDB | digital extension (digits II–IV) | |
| (d) Other | ||||
| Lumbr II | medial side of the FT tendon to digit II | tendons to the dorsal side of the proximal phalanges of digit II, III, IV and V | small, unipennate | assists in flexion of digital (proximal) phalanges |
| Lumbr III and IV | medial side of the FF tendons to digit III and IV | |||
| Lumbr V | lateral side of the FF tendon to digit IV | |||
| AbdV | plantar side of the tuber calcanei; ligament between the tuberosity of metatarsal V and the lateral malleolus | 2 tendons to the plantar base of the proximal phalanx of digit V | thick, bipennate; distally fused with FlexV | flexion and abduction of digit V |
| FlexV | along metatarsal V and from the AbdV belly | separate insertion onto the AbdV tendon and to the metatarso-phalangeal joint | pennate; 2 separate muscle bellies; fused with AbdV | slight flexion of digit V |
Table 4b.
Origin, insertion and function of the intrinsic foot muscles of Hylobates lar
| Muscles | Origin | Insertion | Form | Function |
|---|---|---|---|---|
| (a) Hallucal muscles | ||||
| AbdH I | medial side of the tuber calcanei (and lig. calcaneonaviculare) | long tendon to the medial sesamoid bone of the first metatarsal | fusiform; proximally fused with AbdH II; distally fused with FHB | hallucal abduction and flexion |
| AbdH II | short tendon to the proximo-medial side of the first metatarsal | pennate; proximally fused with AbdH I | ||
| AddHo | tendon at plantar side of the naviculare–cuneiform I joint; sesamoid bone (in lig. annulare) | lateral sesamoid bone of the first metatarsal | small, pennate; proximally fused with FHB; internal tendons | hallucal adduction and flexion |
| AddHt | plantar side of the metatarsal III shaft (and metatarsal II base) | parallel; no tendons | ||
| (b) Short flexors | ||||
| FHB | (tendon) at plantar side of the metatarso-cuneiform (or naviculare-cuneiform) joint | broad tendon to the medial sesamoid bone of the first metatarsal | flat (multi)pennate; distally fused with AbdH I tendon | hallucal flexion |
| FDB I | medial side of the tuber calcanei | long, perforated tendon to the plantar side of the middle phalanx of digit II | superficial, unipennate head; proximally fused with AbdV; variable | flexion of digit II |
| FDB II | originating from the FT tendons at the plantar midfoot | perforated tendons to the plantar sides of the middle phalanges of digits III, IV and V | 2–3 deep heads; proximally fused with each other and with the FDT tendons; variable | flexion of the digits III, IV and V; foot plantar flexion |
| (c) Short extensors | ||||
| EHB | dorso-lateral side of the neck of the calcaneum and the calcaneo-cuboid joint, near the sinus tarsi | tendon to the proximal phalanx of digit I | fusiform | hallucal extension |
| EDB | 3 tendons into the dorsal aponeuroses of digits II, III and IV | 3 thin, unipennate, slightly fused muscle bellies | digital extension (digits II–IV) | |
| (d) Other | ||||
| lumbr II– | medial side of the FT tendons to digits II, III and IV | tendons into the dorsal aponeuroses of digits II, III and IV | thin, elongate, fusiform; tiny tendons at insertion | assist in flexion of the digital proximal phalanges |
| III–IV | ||||
| lumbr V | lateral side of the FT tendon to digit IV | tendons into the dorsal aponeurosis of digit V | ||
| AbdV | medio-plantar side of the tuber calcanei and at the latero-plantar side of the tuberosity of metatarsal V (and cuboid) | tendon to the plantar base of the proximal phalanx of digit V; fused with FlexV | long, thin, unipennate; proximally fused with FlexV and FDB I | |
| FlexV | tendon fused with AbdV tendon at metatarsal V shaft | tendon at lateral plantar side of the metatarsal V head; fused with AbdV tendon | very small, thin, unipennate | flexion of digit V |
| ODM | lateral cuneiform bone | short tendon to the plantar side of metatarsal V and to proximal phalanx of digit V | very small, pennate; infrequent | flexion of digit V |
Acknowledgments
This study was supported by a research assistant grant to E.E.V. and a research project to P.A. (G.0209.99) from the Fund for Scientific Research, Flanders (Belgium). We thank the Flemish Government for structural support to the Centre for Research and Conservation (CRC), and the Royal Zoological Society of Antwerp and the Parc Animalier de Branféré for placing the gibbon and bonobo cadavers at our disposal. We also want to thank Michael M. Günther, University of Liverpool, for introducing us to primate anatomy, and acknowledge Gayatri Thampy, Vincent Nijman, Susan Cheyne, Ben Rawson and Fitriah Usman for their personal communications on the locomotor behaviour of wild gibbons. Finally, we are also grateful to Prof. Dr Bernard Wood for his helpful comments on the original manuscript.
Appendix
Table A1.
Muscle data of the adult and the juvenile gibbon (juvenile data in parentheses).
| Muscle | Mass (g) | Length (cm) | BL (cm) | BW (cm) | FL (cm) | PA (°) | PCSA (cm2) | Scaled PCSA | LT (cm) | LET (cm) | Oss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Galat | 17.50 (3.49) | 18.10 (14.08) | 13.00 (7.72) | 2.70 (1.40) | 3.30 (3.60) | 18 (–) | 5.00 (0.91) | 1.47 (0.45) | 14.00 (10.50) | 6.50 (6.00) | v |
| Gamed | 14.50 (3.66) | 20.20 (14.80) | 12.50 (7.40) | 2.10 (1.37) | 2.70 (2.40) | 28 (–) | 5.07 (1.44) | 1.49 (0.71) | 13.80 (11.80) | 7.50 (7.00) | v |
| Soleus | 10.40 (2.87) | 17.40 (11.17) | 11.70 (9.33) | 1.80 (1.00) | 2.50 (2.15) | 23 (–) | 3.92 (1.26) | 1.15 (0.62) | 13.70 (8.67) | 5.20 (1.67) | v |
| EHL | 2.40 (0.95) | 23.10 (13.84) | 13.43 (8.15) | 1.30 (0.40) | 5.20 (3.60) | 0.44 (0.25) | 0.13 (0.12) | 14.40 (7.50) | 8.80 (4.20) | – | |
| EDL I | 3.30 (1.91) | 24.60 (20.50) | 15.30 (12.43) | 0.90 (0.70) | 5.60 (1.73) | 0.56 (1.04) | 0.16 (0.51) | 17.40 (12.00) | –(4.50) | – | |
| EDL II | 1.40 (–) | 28.40 (–) | 8.10 (–) | 0.50 (–) | 6.35 (–) | 18 (–) | 0.21 (–) | 0.06 (–) | 25.30 (–) | – | |
| TA | 10.00 (4.55) | 18.80 (13.50) | 16.20 (10.40) | 1.50 (1.40) | 5.80 (2.50) | 20 (–) | 1.63 (1.72) | 0.48 (0.84) | 4.90 (4.85) | –(2.60) | – |
| FF | 18.80 (7.74) | 31.60 (29.90) | 16.50 (11.50) | 1.60 (1.30) | 3.80 (3.10) | 27 (–) | 4.67 (2.36) | 1.37 (1.16) | 25.20 (15.40) | –(15.40) | – |
| FT | 5.50 (2.77) | 28.30 (24.55) | 18.00 (10.15) | 0.90 (1.00) | 2.70 (2.30) | 32 (–) | 1.92 (1.14) | 0.57 (0.56) | 21.80 (14.40) | –(14.40) | – |
| Plong | 6.20 (2.49) | 21.50 (16.00) | 13.40 (10.40) | 1.40 (1.00) | 2.00 (2.10) | 20 (–) | 2.92 (1.12) | 0.86 (0.55) | 18.20 (12.70) | 10.20 (5.40) | v |
| Pbrev | 2.50 (1.33) | 13.10 (9.90) | 9.00 (6.60) | 1.20 (0.80) | 1.30 (1.83) | 26 (–) | 1.81 (0.69) | 0.53 (0.34) | 9.30 (7.70) | 3.70 (3.20) | – |
| TP | 6.20 (2.40) | 22.00 (14.50) | 12.60 (7.10) | 1.30 (1.25) | 2.10 (1.40) | 2.79 (1.62) | 0.82 (0.80) | 15.20 (12.15) | 6.90 (7.10) | v | |
| AbdH I | 0.97 (0.56) | 8.10 (6.25) | 4.00 (3.17) | 0.80 (0.77) | 3.35 (1.70) | 0.27 (0.31) | 0.08 (0.15) | 3.90 (3.90) | 3.90 (3.30) | v | |
| AbdH II | 1.20 (0.20) | 4.70 (3.70) | 4.70 (2.00) | 0.80 (0.65) | 2.15 (–) | 0.53 (–) | 0.16 (–) | 2.90 (2.15) | –(1.60) | – | |
| AddHt | 5.70 (1.49) | 6.10 (4.20) | 6.10 (4.20) | 4.10 (1.90) | 4.40 (3.00) | 1.22 (0.47) | 0.36 (0.23) | – | |||
| AddHo | 0.54 (0.15) | 3.20 (2.55) | 3.20 (2.10) | 1.20 (1.50) | –(0.40) | –(0.35) | –(0.17) | –(1.60) | –(0.40) | v | |
| EHB | 0.54 (0.24) | 8.80 (6.00) | 3.20 (3.25) | 0.60 (0.50) | 0.30 (–) | 1.70 (–) | 0.50 (–) | 6.20 (3.69) | –(3.19) | – | |
| EDB | 1.20 (0.58) | 9.60 (8.35) | 4.10 (3.73) | 5.70 (0.35) | 1.80 (1.50) | 0.63 (0.36) | 0.19 (0.18) | 7.60 (5.70) | –(4.20) | – | |
| FHB | 1.20 (0.32) | 4.20 (3.25) | 4.20 (3.25) | 0.90 (0.90) | 1.00 (–) | 1.13 (–) | 0.33 (–) | v | |||
| FDB I | 0.54 (0.15) | 11.90 (6.85) | 6.70 (3.00) | 0.55 (0.30) | 2.00 (–) | 0.25 (–) | 0.08 (–) | 8.10 (4.65) | 7.80 (–) | – | |
| FDB II | 0.60 (0.31) | 7.00 (5.53) | – | ||||||||
| AbdV | 0.40 (0.24) | 7.90 (6.80) | 4.10 (4.35) | 0.40 (0.45) | –(0.81) | –(0.28) | –(0.14) | 7.90 (5.80) | –(2.80) | – | |
| FlexV | 0.20 (0.07) | 4.80 (2.70) | 2.90 (1.70) | 0.30 (0.30) | –(1.00) | –(0.07) | –(0.03) | 3.60 (1.00) | –(0.50) | – | |
| ODM | 0.06 (–) | 2.60 (–) | 1.80 (–) | 1.50 (–) | – |
Abbreviations: BL = muscle belly length; BW = muscle belly width; FL = muscle fibre length; PA = pennation angle; PCSA = physiological cross-sectional area; LT = tendon length; LET = length of the external tendon; Oss = ossification (v = presence and – = absence of a sesamoid bone).
Table A2.
Muscle data of the male and female bonobo (female data in parentheses). Abbreviations as in Table A1.
| Muscle | Mass (g) | Length (cm) | BL (cm) | BW (cm) | FL (cm) | PA (°) | PCSA (cm2) | Scaled PCSA | LT (cm) | LET (cm) | Oss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Galat | 105.17 (36.00) | 31.20 (33.00) | 28.60 (–) | –(3.50) | 9.50 (7.00) | 10.44 (4.85) | 0.68 (0.44) | – | |||
| Gamed | 141.45 (64.00) | 32.50 (31.00) | 29.80 (–) | –(3.00) | 9.67 (7.50) | 13.80 (8.05) | 0.90 (0.73) | – | |||
| Soleus | 220.21 (140.00) | 29.30 (28.00) | 28.00 (–) | –(5.00) | 6.20 (7.10) | 33.51 (18.60) | 2.19 (1.69) | – | |||
| Plant | 8.66 (6.00) | 32.80 (32.00) | 14.50 (12.00) | –(1.60) | 5.40 (8.50) | 1.51 (0.67) | 0.10 (0.06) | –(20.00) | – | ||
| EHL | 12.05 (10.00) | 32.00 (25.00) | 21.00 (17.50) | –(1.50) | 8.30 (8.00) | 1.37 (1.18) | 0.09 (0.11) | –(7.50) | – | ||
| EDL | 43.23 (34.00) | 46.00 (37.00) | 28.00 (26.50) | –(2.00) | 9.00 (10.50) | 4.53 (3.05) | 0.30 (0.28) | –(10.50) | – | ||
| TA | 101.20 (74.00) | 34.00 (29.00) | 23.80 (25.00) | –(2.00) | 9.50 (19.00) | 10.05 (4.99) | 0.66 (0.45) | –(4.00) | – | ||
| FF | 121.34 (32.00) | 45.30 (41.00) | 25.30 (–) | 8.00 (–) | 14.31 (–) | 0.94 (–) | – | ||||
| FT | 41.52 (80.00) | 41.90 (38.50) | 20.40 (25.00) | 6.30 (–) | 6.22 (–) | 0.41 (–) | –(13.50) | – | |||
| lumbr | –(7.70) | 13.80 (–) | 7.00 (–) | 0.65 (–) | 7.80 (–) | 6.47 (–) | – | ||||
| Plong | 70.58 (52.00) | 33.00 (32.50) | 21.50 (23.50) | 5.40 (6.00) | 12.33 (8.18) | 0.81 (0.74) | –(9.50) | – | |||
| Pbrev | 31.44 (30.00) | 23.00 (31.00) | 19.00 (27.00) | –(2.50) | 5.30 (6.50) | 5.60 (4.35) | 0.37 (0.40) | –(4.00) | – | ||
| TP | 79.25 (56.00) | 27.20 (31.00) | 21.00 (23.00) | –(2.50) | 4.40 (4.00) | 16.99 (13.21) | 1.11 (1.20) | – | |||
| AbdH | 21.80 | 12.61 | 9.72 | 2.41 | 4.80 | 4.29 | 0.28 | 2.94 | v | ||
| AddHt | 24.38 | 5.76 | 5.76 | 4.64 | 4.50 | 5.34 | 0.35 | – | v | ||
| AddHo | 5.53 | 5.53 | 2.78 | 3.10 | 5.02 | 0.33 | – | v | |||
| EHB | 16.30 | 7.49 | 1.77 | 4.20 | 3.05 | 0.20 | 12.79 | – | |||
| EDB | 13.60 | 17.80 | 8.20 | 0.55 | 5.10 | 2.51 | 0.16 | 9.77 | – | ||
| FHBm | 4.71 | 5.52 | 5.52 | 2.02 | 1.80 | 2.47 | 0.16 | – | v | ||
| FHBl | 1.12 | 4.86 | 3.87 | 1.70 | 1.80 | 0.59 | 0.04 | 1.76 | v | ||
| FDB I | 7.17 | 19.08 | 8.20 | 1.62 | 5.30 | 1.28 | 0.08 | 14.38 | – | ||
| FDB II | 1.69 | 13.59 | 5.20 | 1.02 | 2.00 | 0.80 | 0.05 | 9.59 | – | ||
| AbdV | 7.79 | 12.66 | 7.55 | 1.85 | 4.30 | 1.71 | 0.11 | 7.56 | – | ||
| FlexV | 5.03 | 5.00 | – | ||||||||
| iPlant | 17.56 | 5.34 | 5.34 | 1.51 | – | – | |||||
| CH | 0.17 | 5.09 | 5.09 | – | – |
References
- Aiello L, Dean C. An Introduction to Human Evolutionary Anatomy. London: Academic Press; 1990. [Google Scholar]
- Alexander RMcN, Vernon A. The dimensions of the knee and ankle muscles and the forces they exert. J. Hum. Mov. Stud. 1975;1:115–123. [Google Scholar]
- Alexander RMcN, Maloiy GMO, Ker RF, Jayes AS, Warui CN. The role of tendon elasticity in the locomotion of the camel (Camelus dromedarius) J. Zool. 1982;198:293–313. [Google Scholar]
- Alexander RMcN. Elastic mechanisms in primate locomotion. Z. Morph. Anthropol. 1991;78:315–320. [PubMed] [Google Scholar]
- Alexander RMcN. Exploring Biomechanics: Animals in Motion. New York: Scientific American Library; 1992. [Google Scholar]
- Andrew P, Groves CP. Gibbons and brachiation. In: Rumbaugh DM, editor. Gibbon and Siamang: a Series of Volumes on the Lesser Apes Vol. 4: Suspensory Behavior, Locomotion, and Other Behaviors of Captive Gibbons: Cognition. Basel: Karger; 1976. pp. 167–218. [Google Scholar]
- Biewener AA. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;120:73–87. doi: 10.1016/s0305-0491(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Bisschoff ThLW. Beiträge zur Anatomie des Hylobates leuciscus und zu einer vergleichende Anatomie der Muskeln der Affen und des Menschen. Abd. D. Math–Phys. KlDKönigl. Bayer. Akad. D. Wiss. 1870;10:199–297. [Google Scholar]
- Carpenter CR. A field study in Siam of the behavior and social relations of the gibbon (Hylobates lar) In: Carpenter CR, editor. Naturalistic Behavior of Nonhuman Primates. University Park: The Pennsylvania State University Press; 1964. pp. 145–271. [Google Scholar]
- Chang Y-H, Bertram JEA, Lee DV. External forces and torques generated by the brachiating white-handed gibbon (Hylobates lar) Am. J. Phys. Anthropol. 2000;113:201–216. doi: 10.1002/1096-8644(200010)113:2<201::AID-AJPA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- David RD, Delagoutte JP, Renard MM. Anatomical study of the sesamoid bones of the first metatarsal. J. Am. Podiat. Med. Assn. 1989;79:536–544. doi: 10.7547/87507315-79-11-536. [DOI] [PubMed] [Google Scholar]
- Deloison Y. Etude des Restes Fossils des Pieds des Premiers Hominids: Australopithecus et Homo Habilis. Essai d’interpretation de Leur Mode de Locomotion. Paris: PhD thesis, Université Réné Descartes; 1993. [Google Scholar]
- Doran DM. Comparative locomotor behavior of chimpanzees and bonobos: the influence of morphology on locomotion. Am. J. Phys. Anthropol. 1993;91:83–98. doi: 10.1002/ajpa.1330910106. [DOI] [PubMed] [Google Scholar]
- Doran DM, Hunt KD. Comparative locomotor behavior of chimpanzees and bonobos: species and habitat differences. In: Wrangham RW, McGrew WC, de Waal FBM, et al., editors. Chimpanzee Cultures. Cambridge: Harvard University Press; 1994. pp. 93–108. [Google Scholar]
- Eliot DJ, Jungers WL. Fifth metatarsal morphology does not predict presence or absence of fibularis tertius muscle in hominids. J. Hum. Evol. 2000;38:333–342. doi: 10.1006/jhev.1999.0337. [DOI] [PubMed] [Google Scholar]
- Ellefson JO. A natural history of gibbons in the Malay Peninsula. Berkeley: University of California; 1967. PhD thesis. [Google Scholar]
- Fleagle JG. Locomotion and posture of the Malayan siamang and implications for hominid evolution. Folia Primatol. 1976;26:245–269. doi: 10.1159/000155756. [DOI] [PubMed] [Google Scholar]
- Gibbs S, Collard M, Wood B. Soft-tissue characters in higher primate phylogenetics. Proc. Natl. Acad. Sci. USA. 2000;97:11130–11132. doi: 10.1073/pnas.190252697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs S, Collard M, Wood B. Soft-tissue anatomy of the extant hominoids: a review and phylogenetic analysis. J. Anat. 2002;200:3–49. doi: 10.1046/j.0021-8782.2001.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittins PS. Use of forest canopy by the agile gibbon. Folia Primatol. 1983;40:134–144. doi: 10.1159/000156095. [DOI] [PubMed] [Google Scholar]
- Goodman M, Bailey WJ, Hayasaka K, Stanhope MJ, Slighton J, Czelusniak J. Molecular evidence on primate phylogeny from DNA sequences. Am. J. Phys. Anthropol. 1994;94:3–24. doi: 10.1002/ajpa.1330940103. [DOI] [PubMed] [Google Scholar]
- Grand TI. The functional anatomy of the ankle and foot of the slow loris (Nycticebus coucang) Am. J. Phys. Anthropol. 1967;26:207–218. [Google Scholar]
- Gray H. In: Anatomy of the Human Body. Lewis WH, editor. Philadelphia: Lea & Febiger; 1918. [Google Scholar]
- Groves CP. Systematics and phylogeny of gibbons. In: Rumbaugh DM, editor. Gibbon and Siamang: a Series of Volumes on the Lesser Apes. Vol. 1. Basel: Karger; 1972. pp. 1–89. [Google Scholar]
- Hof AL, Van Zandwijk JP, Bobbert MF. Mechanics of human triceps surae muscle in walking, running and jumping. Acta Physiol. Scand. 2002;174:17–30. doi: 10.1046/j.1365-201x.2002.00917.x. [DOI] [PubMed] [Google Scholar]
- Jacob HAC. Forces acting in the forefoot during normal gait – an estimate. Clin. Biomech. 2001;16:783–792. doi: 10.1016/s0268-0033(01)00070-5. [DOI] [PubMed] [Google Scholar]
- Jouffroy FK. La musculature des membres chez les lemuriens de Madagascar, étude descriptive et comparative. Mammalia. 1962;26:1–386. [Google Scholar]
- Jungers WL, Stern JT. Kinesiological aspects of brachiation in lar gibbons. In: Rumbaugh DM, editor. Gibbon and Siamang: a Series of Volumes on the Lesser Apes Vol. 4: Suspensory Behavior, Locomotion, and Other Behaviors of Captive Gibbons; Cognition. Basel: Karger; 1976. pp. 119–135. [Google Scholar]
- Jungers WL, Meldrum DJ, Stern JT. The functional and evolutionary significance of the human peroneus tertius muscle. J. Hum. Evol. 1993;25:377–386. [Google Scholar]
- Kano T. The Last ApePygmy Chimpanzee Behavior and Ecology. Stanford, CA: Stanford University Press; 1992. [Google Scholar]
- Ker RF, Alexander RMcN, Bennett MB. Why are mammalian tendons so thick? J. Zool., Lond. 1988;216:309–324. [Google Scholar]
- Kohlbrügge JHF. Versuch einer Anatomie des Genus Hylobates. In: Weber M, editor. Zoologische Ergebnisse Einer Reise in Niederländisch Ost-Indien. Leiden: Verlag von EJ Brill; 18901891. pp. 211–354. [Google Scholar]
- Langdon JH. Variations in cruropedal musculature. Int. J. Primatol. 1990;11:575–606. [Google Scholar]
- Ledoux WR, Hirsch BE, Church T, Caunin M. Pennation angles of the intrinsic muscles of the foot. J. Biomech. 2001;34:339–403. doi: 10.1016/s0021-9290(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Lessertisseur J, Jouffroy FK. Tendances locomotrices des primates traduites par les proportions du pied. Folia Primatol. 1973;20:125–160. doi: 10.1159/000155573. [DOI] [PubMed] [Google Scholar]
- Lewis OJ. The tibialis posterior tendon in the primate foot. J. Anat. 1964;98:209–218. [PMC free article] [PubMed] [Google Scholar]
- Lewis OJ. Functional Morphology of the Evolving Hand and Foot. Oxford: Clarendon Press; 1989. [Google Scholar]
- Loth E. Etude anthropologique sur l'aponeurose plantaire. Bull. Mem. Soc. Anthropol. Paris. 1913;4:601–609. [Google Scholar]
- Macalister A. Observations on muscular anomalies in the human anatomy. Third series with a catalogue of the principal muscular variations hitherto published. Trans. Roy. Irish Acad. Sci. 1875;25:1–130. [Google Scholar]
- Mann RA, Inman VT. Phasic activity of intrinsic muscles of the foot. J. Bone Joint Surg. 1964;46A:469–481. [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Miller RA. The musculature of Pan paniscus. Am. J. Anat. 1952;91:183–232. doi: 10.1002/aja.1000910202. [DOI] [PubMed] [Google Scholar]
- Morton DJ. Evolution of the human foot II. Am. J. Phys. Anthropol. 1924;VII:1–52. [Google Scholar]
- Morton DJ. The Human Foot, its Evolution, Physiology and Functional Disorders. New York: Columbia University Press; 1935. [Google Scholar]
- Nickel R, Schummer A, Seiferle E. The Anatomy of the Domestic Animals Vol. 1: the Locomotor System of the Domestic Mammals. Berlin: Parey; 1986. [Google Scholar]
- Otis JC, Gage T. Function of the posterior tibial tendon muscle. Foot Ankle Clin. 2001;6:1–14. doi: 10.1016/s1083-7515(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Payne RC. PhD theis, University of Liverpool; 2001. Musculoskeletal adaptations for climbing in hominoids and their role as exaptations for the acquisition of bipedalism. [Google Scholar]
- Perlman P. First metatarsal sesamoid pain. Australian Podiatrist. 1994;March:18–23. [Google Scholar]
- Pfitzner W. Beiträge zur Kentnntniss des menschlichen Extremitätenskelets. VII. Die Variationen im Aufbau des Fussskelets. Morph. Arb. 1896;6:245–527. [Google Scholar]
- Prejzner-Morawska P, Urbanowicz M. Morphology of some of the lower limb muscles in primates. In: Chlarelli AB, Corruccini RS, editors. Primate Evolutionary Biology. Berlin: Springer Verlag; 1981. pp. 60–67. [Google Scholar]
- Reynolds TR. Stresses on the limbs of quadrupedal primates. Am. J. Phys. Anthropol. 1985;67:351–362. doi: 10.1002/ajpa.1330670407. [DOI] [PubMed] [Google Scholar]
- Sarin VK, Erickson GM, Giori NJ, Bergman AG, Carter DR. Coincident development of sesamoid bones and clues to their evolution. Anat. Rec. (New Anat.) 1999;257:174–180. doi: 10.1002/(SICI)1097-0185(19991015)257:5<174::AID-AR6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sarmiento EE. The significance of the heel process in anthropoids. Int. J. Primatol. 1983;4:127–138. [Google Scholar]
- Satie JP, Alfred JRB. Locomotion and posture in Hoolock gibbon. Ann. For. 2002;10:298–306. [Google Scholar]
- Schmitt D, Larson SG. Heel contact as a function of substrate type and speed in primates. Am. J. Phys. Anthropol. 1995;96:39–50. doi: 10.1002/ajpa.1330960105. [DOI] [PubMed] [Google Scholar]
- Schmitt D. Compliant walking in primates. J. Zool. 1999;247:149–160. [Google Scholar]
- Schultz AH. Relations between the lengths of the main parts of the foot skeleton in primates. Folia Primatol. 1963;1:150–171. [Google Scholar]
- Schultz AH. The skeleton of the Hylobatidae and other observations on their morphology. In: Rumbaugh DM, editor. Gibbon and Siamang: a Series of Volumes on the Lesser Apes Vol. 2: Anatomy, Dentition, Taxonomy, Molecular Evolution, and Behavior. Basel: Karger; 1973. pp. 1–54. [Google Scholar]
- Shapiro LJ, Jungers WL. Back muscle function during bipedal walking in chimpanzee and gibbon: Implications for the evolution of human locomotion. Am. J. Phys. Anthropol. 1988;77:201–212. doi: 10.1002/ajpa.1330770208. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Jungers WL. Electromyography of back muscles during quadrupedal and bipedal walking in primates. Am. J. Phys. Anthropol. 1994;93:491–504. doi: 10.1002/ajpa.1330930408. [DOI] [PubMed] [Google Scholar]
- Sigmon BA. A functional analysis of pongid hip and thigh musculature. J. Hum. Evol. 1974;3:164–185. [Google Scholar]
- Sigmon BA. Functions and evolution of hominid hip and thigh musculature. In: Tuttle RH, editor. Primate Functional Morphology and Evolution. The Hague: Mouton; 1975. pp. 235–252. [Google Scholar]
- Sigmon BA, Farslow DL. The primate hindlimb. In: Swindler DR, Erwin J, editors. Comparative Primate Biology Vol. 1: Systematics, Evolution, and Anatomy. New York: Alan R. Liss, Inc; 1986. pp. 671–718. [Google Scholar]
- Sokoloff S. The muscular anatomy of the chimpanzee foot. Gegenbaurs Morph. Jb. 1972;1:86–125. [PubMed] [Google Scholar]
- Stern JT, Susman RL. Electromyography of the gluteal muscles in Hylobates, Pongo, and Pan: implications for the evolution of hominid bipedality. Am. J. Phys. Anthropol. 1981;55:153–166. [Google Scholar]
- Susman RL, Badrian NL, Badrian AJ. Locomotor behavior of Pan paniscus in Zaire. Am. J. Phys. Anthropol. 1980;53:69–80. [Google Scholar]
- Susman RL, Badrian NL, Thompson-Handler L. Positional behavior and feeding ecology of the pygmy chimpanzee (Pan paniscus): first year results of the Lomako Forest Pygmy Chimpanzee Project. Natl Geographic Res. 1985;20:725–739. [Google Scholar]
- Swindler DR, Wood CD. An Atlas of Primate Gross Anatomy, Baboon, Chimpanzee, and Man. Seattle: University of Washington Press; 1973. [Google Scholar]
- Thorpe SKS, Crompton RH, Günther MM, Ker RF, Alexander RMcN. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (Pan troglodytes) Am. J. Phys. Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Tuttle RH. Postural, propulsive, and prehensile capabilities in the cheiridia of chimpanzees and other great apes. Chimpanzee. 1970;2:167–253. [Google Scholar]
- Tuttle RH. Functional and evolutionary biology of hylobatid hands and feet. In: Rumbaugh DM, editor. Gibbon and Siamang: a Series of Volumes on the Lesser Apes. Vol. 1. Basel: Karger; 1972. pp. 136–206. [Google Scholar]
- Tuttle RH, Basmajian JV, Ishida H. Electromyography of pongid gluteal muscles and hominid evolution. In: Chivers DJ, Joysey KA, editors. Recent Advances in Primatology: Evolution. Vol. 3. London: Academic Press; 1978. pp. 463–468. [Google Scholar]
- Vereecke E, D’Août K, De Clercq D, Van Elsacker L, Aerts P. Dynamic plantar pressure distribution during terrestrial walking of bonobos (Pan paniscus) Am. J. Phys. Anthropol. 2003;120:373–383. doi: 10.1002/ajpa.10163. [DOI] [PubMed] [Google Scholar]
- Vereecke E, D’Août K, De Clercq D, Van Elsacker L, Aerts P. Functional analysis of the gibbon foot during terrestrial bipedal walking: plantar pressure distributions and 3D ground reaction forces. Am. J. Phys. Anthropol. 2005 doi: 10.1002/ajpa.20158. in press. [DOI] [PubMed] [Google Scholar]
- Wickiewicz TL, Roy RR, Powel PL, Edgerton VR. Muscle architecture of the human lower limb. Clin. Orthop. Relat. Res. 1983;179:275–283. [PubMed] [Google Scholar]
- Wilder BG. Contributions to the comparative myology of the chimpanzee. Journal of the British Society for Natural History. 1863:352–384. [Google Scholar]