Abstract
Cell division serves to distribute chromosomes and organelles into two daughter cells, but the mechanism of rough endoplasmic reticulum (RER) segregation in animal cell mitosis is poorly understood. Here we study the distribution of RER in mitotic HeLa cells and its relation to the cytoskeleton. At metaphase, the RER was located in the cell cortex and was most concentrated in two locations. Close to the plasma membrane the RER was closely associated with cortical actin, and after treatment with Latrunculin A RER elements retracted to the deep cortex and became more tubular. Positioning was therefore dependent on cortical F-actin. Deeper in the cortex cisternae were wrapped tightly around the contours of the spindle body and orientated along microtubules close the spindle poles. Stereology revealed a close correlation between RER volume and cell volume in telophase daughter cells. These results suggest that the RER is positioned at the outer and inner regions of metaphase cortex by association with cytoskeleton. This arrangement combined with a disposition in concentric layers, deep to the plasma membrane, appears to distribute the RER evenly in the cortex and may help to couple quantities of RER and cell constituents.
Keywords: actin, endoplasmic reticulum, mitosis, organelle segregation
Introduction
The main function of cell division is to segregate chromosomes and organelles into the daughter cells. For chromosomes the well-defined strategy involves tethering to microtubules with each chromatid entering one of the daughter cells. For cytoplasmic organelles the mechanisms of segregation are much less clear (Birky, 1983; Warren & Wickner, 1996). They could involve similar tethering mechanisms, anchoring organelles to structures that reliably enter one or other daughter; or they could involve diffusional/stochastic dispersion throughout a cell compartment that itself segregates with a defined probability (Gerace & Burke, 1988; Nigg, 1992; Warren & Wickner, 1996). Such mechanisms may be distinguished by quantitative studies of organelle positioning, tethering and segregation but up to now only a few such studies have been performed (Shima et al. 1997; Nebenfuhr et al. 2000; Bergeland et al. 2001).
The rough endoplasmic reticulum (RER) of interphase cells is a continuous membrane system that synthesizes membrane destined for the Golgi, endolysosomes, secretory vesicles and plasma membrane. RER is distributed as a continuous network composed of tubules and/or cisternae stretching from the nuclear envelope to the cell periphery (Voeltz et al. 2002). At restricted locations, the so-called endoplasmic reticulum exit sites package proteins into COPII-coated vesicles for export into the secretory pathway (Barlowe, 2002) whereas at other locations the RER becomes continuous with the smooth endoplasmic reticulum with more tubular morphology. In mitosis, RER-related biosynthesis is shut down and vesicular export from the exit sites is inhibited allowing some itinerant proteins derived from the early secretory pathway to become trapped in the RER (Zaal et al. 1999; Terasaki, 2000). It has been a matter of debate whether Golgi stack proteins recycle to the RER (Jokitalo et al. 2001) but recent data indicate that a major fraction of Golgi membrane components fragment into numerous vesiculotubular clusters and vesicles (Barr, 2004). Importantly, the fragments of Golgi (Shima et al. 1997; Prescott et al. 2001) remain closely associated with the RER and it is at these locations that the Golgi stacks reassemble during telophase (Lucocq et al. 1989). Taken together the observations indicate that mitotic segregation of the RER determines the fate not only of RER residents, but also of a subset of proteins recycled from the early secretory pathway. As the RER is the primary site of membrane synthesis, the precision of its partitioning during mitosis will determine the synthetic capacity and therefore viability of the early G1 cell.
Important aspects of RER segregation at animal cell mitosis remain to be understood (Barr, 2002; Voeltz et al. 2002). Early work focused on reporting cell types in which the RER appeared to undergo vesiculation, suggesting that uniform dispersion of vesicular RER elements could facilitate equal segregation. More recent studies have suggested that the RER does not consistently disassemble into vesicles in all cell types and remains continuous (Terasaki, 2000; Barr, 2002). Irrespective of RER morphology a key question is whether RER is tethered, bound or restricted to structures or compartments for delivery to daughter cells, or is it distributed uniformly by a diffusion-based mechanism, to be bisected along with the cytoplasm at cytokinesis (Warren & Wickner, 1996)?
In this study we used detailed quantitative measurements of RER distribution in mitotic HeLa cells. We show that the RER of mitotic cells remains cisternal and is limited to a ‘cortical container’ bounded peripherally by the cortical actin and centrally by the mitotic spindle. A peripheral RER cisterna is closely applied to, and depends on, cortical actin for its positioning, while another pool of RER is associated with microtubules at the spindle and its poles. Association with cytoskeletal structures thus appears to play a role in the positioning of RER. We also observe a close coupling of RER volume to daughter cell size, a relationship that matches biosynthetic capacity to growth potential in G1 cells of the next generation.
Materials and methods
Cell culture and drug treatments
HeLa cells were cultured in minimal essential medium (MEM) supplemented with 10% fetal bovine serum, l-glutamine, vitamins, non-essential amino acids and 1% penicillin/streptomycin (Gibco-Brl, Paisley, UK) in a humidified atmosphere of 5% CO2 at 37 °C. To accumulate mitotic cells, cell monolayers were grown either on 5.5-cm-diameter plastic Petri dishes or on 850-cm2 roller bottles to 70% confluency and treated with nocodazole (Sigma Chemical Co., Dorset, UK) at a final concentration of 40 ng mL−1 diluted from 10 mg mL−1 stock in carrier dimethylsulphoxide (DMSO). This concentration does not depolymerize interphase cyoplasmic microtubules but prevents spindle assembly in mitosis. Mitotic cells were isolated from HeLa cell monolayers grown on roller bottles. To release the mitotic cells preferentially the roller bottles were spun at 300 r.p.m. (Zieve et al. 1980). To isolate cells at all mitotic stages (natural mitotics), this shake-off procedure was also performed without prior addition of nocodazole. Latrunculin A (a gift from Kathryn Ayscough, University of Glasgow; Spector et al. 1989) was stored in DMSO carrier at 0.5 mm and added to the culture medium at a final concentration of 1 µm or 5 µm for 2 h.
Fixation and embedding for EM
Cells grown on dishes were fixed in 0.5% glutaraldehyde in 0.2 m cacodylate/HCl buffer, pH 7.4 (cacodylate buffer), or 0.5% glutaraldehyde in 0.2 m PIPES, pH 7.2, at room temperature for 30 min and scraped off using a rubber policeman before pelleting. Mitotic cells, isolated from roller bottles, were fixed in the same fixative but were pelleted before fixation. All pellets were post-fixed in 1% osmium tetroxide and 1.5% potassium ferrocyanide in cacodylate buffer for 30 min. The cell pellets were then dehydrated and embedded in epoxy resin (Epon 812 equivalent; Agar Scientific Ltd, Stansted, UK). To stain the RER of mitotic cells, cell pellets of natural mitotic cells were treated with osmium tetroxide for up to 2 days at 37 °C before embedding in epoxy resin (see Lucocq & Warren, 1987 for details).
Ultrathin sections were cut using a Reichert Ultracut E Ultramicrotome at a nominal section thickness of 70, 100 or 200 nm. To prepare section stacks, every 15th or 30th section was collected and mounted on pioloform-coated 1-mm copper slot grids to produce approximately ten or five sections per cell. Grids were observed either unstained or stained with uranyl acetate (10 min) followed by lead citrate (5 min). Near-equatorial sections were identified as those that contained the largest cell profile.
Monolayer cultures
Cell monolayers grown to 70% confluency on glass coverslips were incubated with or without nocodazole at 40 ng mL−1 (procedure as before) for 4 h. They were then incubated with or without Latrunculin A (1 or 5 µm) for 2 h in the presence or absence of nocodazole. At the end of 2 h the medium was removed and the cells fixed in 4% or 8% formaldehyde in PBS for 30 min at room temperature. For actin staining the cells were then washed in 1% bovine serum albumin (BSA) in PBS for 30 min and incubated at room temperature in TRITC-labelled phalloidin (gift from Dr Alan Prescott, University of Dundee) for 30 min. Latrunculin A at 5 µm but not at 1 µm induced depolymerization of peripheral actin stained by phalloidin. The cells were washed again in 1% BSA in PBS for 30 min and labelled with Hoechst 33258 to stain DNA before a further wash in 1% BSA in PBS for 30 min and mounting in moviol. For electron microscopy cells were grown on CELLOCATE coverslips (Eppendorf; see Prescott et al. 2001), fixed in 0.5% glutaraldehyde in 0.2 m PIPES for at least 30 min at room temperature and mitotic cells identified using phase contrast microscopy before processing and embedding in Epoxy resin as described above.
Estimates of partitioning fraction
Estimates of partitioning fraction were obtained using an adaptation of the Cavalieri estimator (Gundersen & Jensen, 1987) that used a randomly placed stack of ten systematically spaced sections through whole mitotic cells. The volume of the cell or RER was estimated on selected sections using square lattice grids with point-to-point spacing of 4.98 mm and 1 mm, respectively (micrographs taken at 2000× for telophase cells and 2500× for metaphase cells). The grids were thrown at random onto the cell profile images (magnified 10×) and the number of point hits over the cell or RER was counted and summed for all the sampled sections of the stack. Typically the average number of points counted over a mitotic cell was 185 for the cell and 460 for the RER compartment.
Using the RER as an example, an estimate of the total volume of RER is Σ P · a · t · N where a is the area associated with each point falling on the RER (P), t is the section thickness and N is the number of sections in the stack interval.
So the fraction of RER in each half of a metaphase cell or in each daughter cell at telophase can be obtained as follows:
As the estimates were made on the same stack of sections the t and N terms cancel and the estimate becomes
The fraction of volume in each cell was estimated in the same way as for RER, using point counts over the cytoplasm. A daughter cell at telophase was demarcated by a line bisecting the cleavage furrow. The equator of each metaphase cell was identified by a line bisecting the profiles of the equatorial chromosomes.
To determine the minimum number of sections required to obtain precise estimates of cell or RER partitioning fraction we compared estimates from different section stacks selected from a complete serial section series through the same cell. The first section of each stack was selected using random numbers and stack sections spaced systematically through the whole mitotic cell (as described above) to give either five or ten sections. Remarkably when ten sections were used in each stack, the estimates for RER volume proportion obtained from four such randomly selected section stacks through the same cell were closely clustered together and had a sample standard deviation of just 0.54% (Howard & Reed, 1998). The corresponding value for cytoplasm volume estimated on the same four section stacks had a sample standard deviation of 1.13%. These results indicated that a majority of RER and cell volume estimates that could be obtained from different stacks were within 1–2% of the true value
RER volume and volume density
To estimate the volume density as a function of the distance from the plasma membrane, square lattice grids were cast over cell profiles in equatorial sections from mitotic cells isolated by shake-off (magnification at least 2000×). The shortest distance of each point to the plasma membrane was measured and converted into micrometres. Point hits over the RER and over the cell were grouped according to their distance from the plasma membrane and the local volume density was calculated.
To estimate total volume of RER we used the rotator (Jensen & Gundersen, 1993). In epoxy resin sections of mitotic cells isolated by shake-off, we identified cells that had been cut through the centrosomes at the spindle poles (micrographs taken at 2000× or more and scanned at 2000 d.p.i.). As these sections were from randomly sectioned pellets of spherical cells, we were able to estimate the volume of RER using the locally applied and discretized version of the rotator (Mironov & Mironov, 1998). In these cells a central axis line bisecting the cell profile and running through the spindle poles was drawn in Adobe Photoshop. A square lattice grid (spacing 0.5 µm) was randomly drawn over the profiles and each point hit over cell or RER was recorded and the distance measured to (i) the plasma membrane and (ii) the central axis in a direction orthogonal to it. Point hits were grouped into shells according to their distance from the plasma membrane in 0.2- or 0.4-µm intervals. From the Pappus theorem the product of the distance travelled by the centre of gravity of a figure and its area can be used to estimate its total volume in rotation (Jensen & Gundersen, 1993). Volumes were estimated using the point hits over all RER in each shell, the distance of each point hit from the axis and the area associated with one point (Mironov & Mironov, 1998). To verify the estimates were valid, volumes of the mitotic cell cortical cytoplasm were also estimated and corresponded closely to values obtained using the Cavalieri method (Lucocq et al. 1989).
Orientation of the RER
Central (equatorial) sections through metaphase and telophase cells were identified as the largest cell profiles obtained from section stacks already prepared for Cavalieri estimates. Point locations on the RER were then identified by line intersects obtained using a systematic square lattice grid thrown onto the cell profiles (grid spacing was 2 cm, which at a magnification of 2500× represents 8 µm) and approximately 10–20 intercepts were identified per mitotic cell profile. A line was then drawn along the shortest distance from the RER intersection to the plasma membrane to identify a plasma membrane intercept. Finally, the orientation of the tangents at the RER cisterna intercept and the plasma membrane intercept were compared using an orientation rose with a spacing of 10°. The orientation parallel to the plasma membrane at the plasma membrane intercept was set at 0° and results were plotted as a frequency distribution in intervals of 10° (90° being vertical to the plasma membrane). It should be emphasized that in each case these orientations were measured on plane sections that were partially orientated along cells that showed central chromosomes (metaphase) or cleavage furrow/elongated nucleus (telophase/G1).
Results
Interconnected RER cisternae with non-uniform distribution in mitotic cell cortex
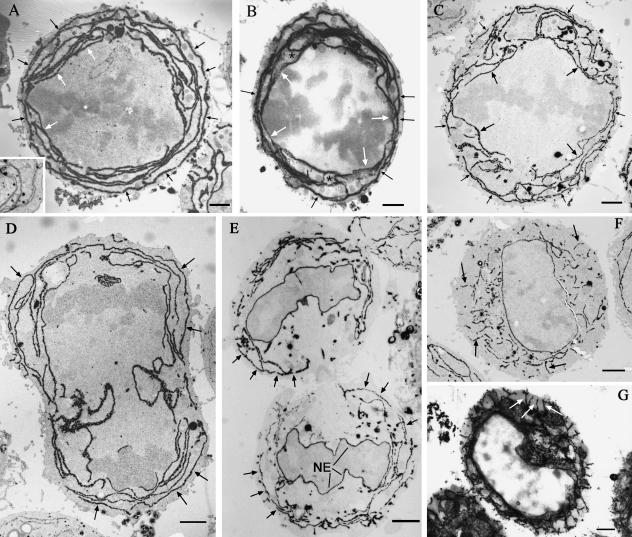
The RER was identified by its coating of ribosomes, positive immunogold staining for protein disulphide isomerase (cryosection data, not shown), or cytochemical staining with prolonged osmium treatment and remained cisternal throughout mitosis (Fig. 1). As detected by these methods the RER of metaphase cells isolated by shake-off (Fig. 1A–C) was exclusively cisternal and limited to the cell cortex (some cells contained smooth membranes clustered inside the spindle near the spindle poles but these were not coated with ribosomes). In cells with large amounts of RER the cisternae were disposed in successive shells roughly concentric to the plasma membrane. These RER cisternae often encircled the cell completely and were more continuous than observed in interphase cells (compare Fig. 1A and 1F). In cells that contained least RER (Fig. 1C), the outermost and innermost cisternae followed the contours of the plasma membrane and spindle closely. Between these cisternae the RER was less ordered with mixed radial and annular orientations. Semithin, 0.5-µm sections observed at 120 kV revealed that cortical RER cisternae were extensively interconnected (Fig. 1B) and continuity could be traced from outermost to innermost cisternae; this is in agreement with previously published photobleaching experiments showing the RER of mitotic cells to be a continuous reticulum (Ellenberg et al. 1997; Terasaki, 2000).
Fig. 1.
Distribution of RER in mitotic HeLa cells. Mitotic cells isolated by shake-off were exposed to prolonged osmium tetroxide treatment to highlight the mitotic RER cytochemically (black reaction product). In thin sections of metaphase cells (A and C) an RER cisterna was consistently observed following closely the plasma membrane at a depth of about 150–200 nm (small arrows). Conversely, a single cisterna was found to follow the contour of the spindle body (larger arrows). When the quantity of RER in the cortex was low, the peripheral and spindle cisternae were better seen (C) and the interconnecting cisternae were more randomly orientated. In 0.5-µm semithin sections (B) the peripheral cisternae (small arrows) and spindle-related cisternae (large arrows) could be seen connected via a network of radial and concentric cisternae (asterisks). The peripheral cisterna was observed in early telophase (arrows in D) and later telophase (arrows in E) but was absent in late telophase/early G1 (F, thin section and G, semithin 0.5-µm section). At this stage the disposition of RER had changed from mainly concentric and peripheral in mitotic cells to radial and central. The inset to A is from conventionally processed epoxy-embedded material and shows the extended, non-fragmented, nature of RER cisternae in a mitotic HeLa cell.
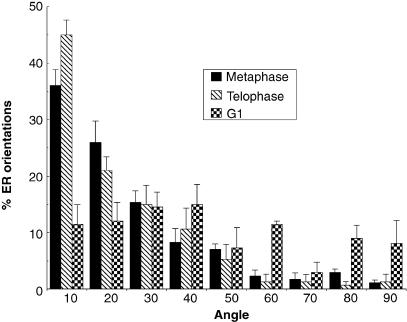
The largely annular arrangement of cortical RER was maintained throughout most of telophase (Fig. 1D,E). After cytokinesis the RER profiles became shorter, and the orientation became much more radial (Fig. 1F, G). A quantitative analysis of orientation (Fig. 2) confirmed the cisternae generally followed the orientation of the plasma membrane in metaphase and telophase and this changed to a more radial arrangement after cytokinesis was complete and nuclear decondensation had started.
Fig. 2.
Orientation of RER in mitotic and interphase cells. The RER in equatorial EM sections was sampled and the orientation of the RER compared with that of local tangents at the plasma membrane using an orientation rose (see Materials and methods). The angle of the RER relative to the plasma membrane is expressed here as a frequency distribution. The orientation of RER in metaphase and telophase is similar to the plasma membrane but it becomes more radially orientated in G1. Between 40 and 50 intercepts were analysed for each condition over a minimum of three cells (bars represent standard error of the mean).
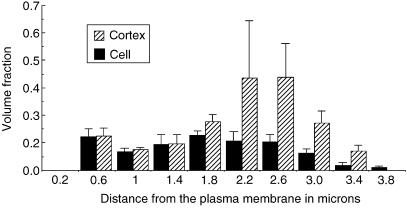
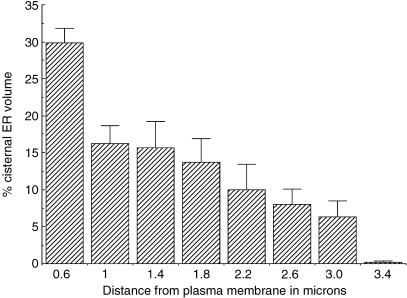
We next tested whether the distribution of RER in the cortex was uniform, as might be consistent with a diffusional/stochastic model for partitioning. To do this we estimated the fraction of cytoplasmic volume occupied by RER (volume fraction) as a function of its distance from the plasma membrane (Fig. 3). This was performed in equatorial sections of cells isolated by shake-off from roller bottles. The results showed that the distribution was clearly inhomogeneous with two peaks of density, one in the periphery close to the plasma membrane and one deep in the cortex. Estimates of the contribution to the total volume of RER in different regions of metaphase cells are presented in Fig. 4 (see Materials and methods). This shows the most peripheral RER elements contribute a major proportion of the total (approximately 30%). This large contribution to the total RER is probably due to the fact that the peripheral RER forms a sphere with the largest possible radius.
Fig. 3.
The fraction of cytoplasm volume occupied by RER cisternae as a function of distance from the plasma membrane in metaphase cells. Volume fraction of RER in the cortex (excluding the spindle region) or the cell as a whole (including the spindle region) was estimated using point counting and the location of each sampling point was recorded with respect to the nearest plasma membrane segment (values on the x-axis show the innermost border of each distance class with respect to the plasma membrane). Data are from equatorial sections of four cell profiles. Error bars are standard error of the mean.
Fig. 4.
Proportion of RER volume as a function of distance from the plasma membrane in metaphase HeLa cells. Volume of RER in shells of cytoplasm inside the plasma membrane was estimated using the rotator method (see Materials and methods) and expressed as a percentage of total cellular RER. The lower border of the first category is 0.2 µm. Error bars are standard error of means from four metaphase cells.
Peripheral RER associated with, and dependent on, cortical F-actin
Further quantitative and qualitative analysis showed that the concentration of RER in the periphery was due to a peripheral cisterna that did not generally approach the plasma membrane closer than approximately 150–200 nm, thereby delineating a zone of exclusion of constant thickness (Fig. 1A–C). This relationship was even more marked in monolayer cells examined in situ by conventional electron microscopy (Fig. 5A). TRITC-phalloidin staining and electron microscopy identified the zone of exclusion as containing cortical F-actin (Fig. 5A, inset). The close spatial relationship between cortical actin and the peripheral cisterna was observed as early as prophase and was maintained through prometaphase, metaphase and anaphase and telophase up until completion of cytokinesis (Fig. 1E, F). Following cytokinesis, during chromosome decondensation and radial reorientation of RER the peripheral RER cisterna was no longer observed (Fig. 1F, G).
Fig. 5.
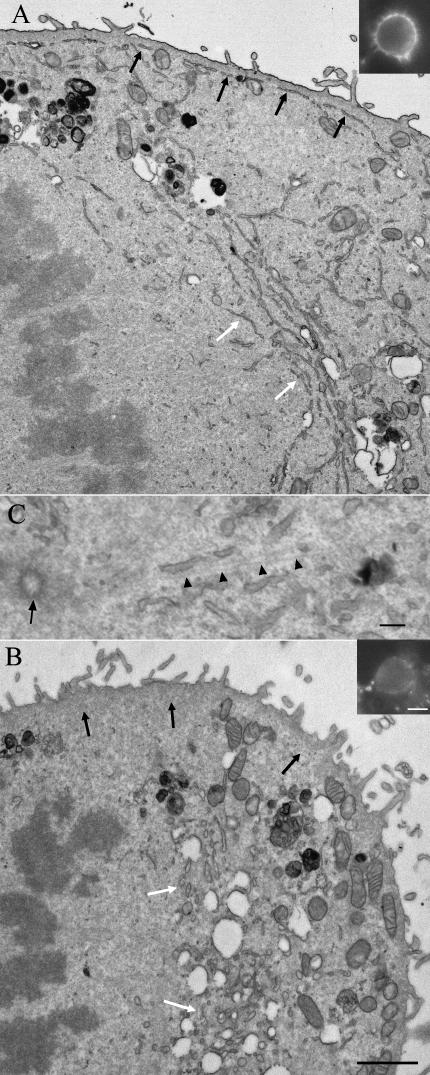
Endoplasmic reticulum in thin sections of epoxy-embedded metaphase monolayer HeLa cells. The peripheral RER in untreated cells (A) was found at a constant distance from the plasma membrane and closely applied to the cortex (black arrows), which was shown to contain actin using fluorescently labelled phalloidin (inset). Deeper in the cell and close to the spindle there is a concentration of parallel RER cisternae (white arrows). In cells treated for 2 h with 5 µm Latrunculin A (B) peripheral RER is no longer present (region of the black arrows) but the RER is visible in the juxta-spindle region and is now present as shorter, more rounded section profiles (region of the white arrows). The peripheral actin staining is now very weak (inset to B). Mitochondria (labelled M in A and B) become concentrated in the periphery after Latrunculin A treatment. (C) Shows close association of ER cisternae with microtubules (arrow heads) radiating from the centriole (arrow). Scale bars: A and B, 2 µm; insets, 10 µm; C, 150 nm.
To test whether the location of the peripheral cisterna was dependent on F-actin, monolayer cells were pretreated for 2 h with Latrunculin A and fixed and mitotic cells analysed by electron microscopy in situ. Mitotic cells lacked the peripheral RER cisterna (compare Fig. 5A and 5B) and RER elements were now centrally located deep in the cortex (see below) and were more tubular in form. In interphase cells the RER remained cisternal during this treatment (data not shown). Cortical actin revealed using TRITC-phalloidin was now greatly decreased in both interphase (data not shown) and mitotic cells (Fig. 5B, inset). In some metaphase cells mitochondria that appeared to be distributed throughout the cortex in untreated cells were now clustered close to the plasma membrane (Fig. 5A,B). The relocation of RER on Latrunculin A treatment appeared not to be related simply to a shape of the mitotic cells because the mitotic cells retained their near spherical shape during treatment with the drug. These results indicate an actin-dependent localization of RER cisternae in the periphery of mitotic cells.
RER associated with, and dependent on, the mitotic spindle
Quantitative and qualitative results showed clear evidence for concentration of the RER deep in the cortex close to the spindle (Figs 1, 3 and 5A). Here there were two principal populations of RER cisternae: a single RER cisterna that, as determined by serial section analysis, followed the contours of the spindle (Fig. 1C); and aggregates of parallel RER cisternae profiles that appeared to emanate from the spindle pole region (white arrows in Fig. 5A, C) – close examination of sections through the centrosomal region showed the aggregates to be orientated along microtubules that radiated from the centrosomal region (Fig. 5C). RER deep in the cortex was therefore related to microtubule-rich structures. In three dimensions, these deep RER cisternae formed conical sheaths extending around the apex of each spindle cone (Fig. 1) and were connected to the peripheral cisterna via RER elements with more radial orientations (Fig. 1B). In cells treated with Latrunculin A in which the peripheral cisterna was absent, the tubular RER remained concentrated in this juxta-spindle location (Fig. 5A). The spindle RER and deep aggregates were observed from anaphase up until the nuclear envelope started to reassemble and cytokinesis had commenced (data not shown). Thereafter, possible remnants of these cisternae were found in the region of the interzonal fibres as anaphase cell elongation progressed (Fig. 1D).
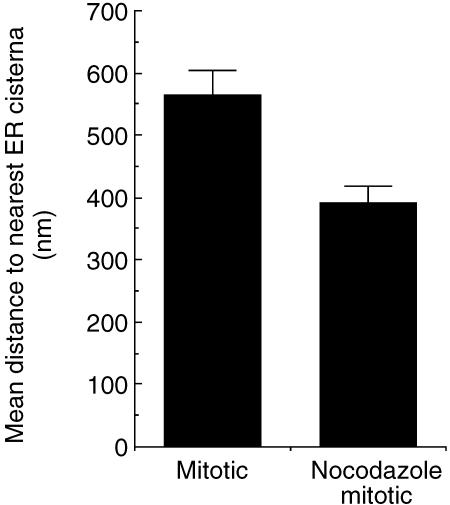
We next tested whether the distribution of spindle RER was related to the presence of the spindle by analysing mitotic cells accumulated in pseudoprometaphase using low-dose nocodazole (40 ng mL−1). In these cells the peripheral RER remained closely associated with the actin cortex. Other cortical RER cisternae remained orientated annular with the plasma membrane but were now more crowded (Fig. 6), with the deepest RER cisternae positioned relatively closer to the plasma membrane than in the metaphase cells of natural mitotics. Now the deepest cisterna often followed the contours of the disordered pseudoprometaphase chromosomes. Sheets of RER could be observed passing deep into the cell between the chromosomes, suggesting that it was free to move there (results not shown). We attempted to use green fluorescent protein/confocal live cell imaging to follow movements of RER in metaphase or nocodazole-arrested cells but resolution of individual cisternae was not possible. Overall these results indicate that in metaphase cells the distribution of RER deep in the cortex is closely associated with, and determined by, the spindle. However, in nocodazole-arrested cells the distribution of RER is now determined by the positioning of chromosomes. Thus the annular and cisternal disposition of RER is not dependent on the spindle but its distribution is defined by central cellular structures. A summary of the RER distribution in mitotic HeLa cells is shown in Fig. 7.
Fig. 6.
Increased density of RER cisternae in the periphery of nocodazole-arrested mitotics. The spacing between RER cisternae was measured in equatorial sections and plotted as mean values. Data are from four metaphase cells with a total of 139 and 142 measurements in natural and nocodazole mitotics, respectively (error bars indicate standard error of the mean).
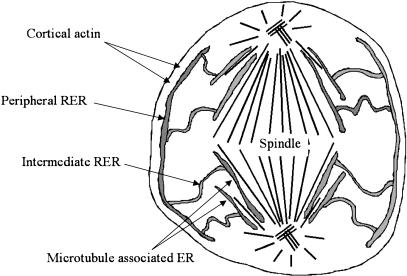
Fig. 7.
Model of RER distribution in metaphase HeLa cells. Peripheral RER is anchored at the actin-rich cortex and this stretches and extends the intermediate cisternae from the spindle associated RER. The spindle cisterna wraps itself tightly around the spindle body and other RER cisternae appear localized along microtubules emanating from the spindle poles. When RER is abundant the intermediate RER forms concentric shells of cisternae spread throughout the cortex (not shown). When RER is less abundant it tends to stretch more radially across the cortex from the peripheral to spindle RER (as illustrated here). Association of RER with symmetrical structures such as plasma membrane and spindle can provide balanced partitioning while stretching RER between these sites may facilitate uniform distribution of the remainder of the RER in the cortex.
Segregation of RER matched to daughter cell volume
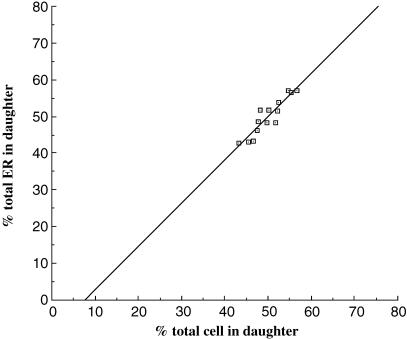
We have found no previous quantitative studies on the precision of RER segregation in the literature. We took advantage of the highly efficient Cavalieri method (as described in Materials and methods; Gundersen & Jensen, 1987) to estimate the proportion of RER entering each daughter. In this method, volume is estimated on stacks of sections and we first assessed the optimum number of sections to obtain precise estimates; a ten-section stack gave results that were clustered within a range of 1–2% (see Materials and methods). When data from telophase daughters were analysed in this way we found considerable variation of the daughter cell volumes from equality with some cells showing a deviation of up to 7%. Remarkably, however, the proportion of RER entering each daughter was closely correlated to the proportions of cytoplasm (Fig. 8). Although telophase cells are bisected by the cleavage furrow, RER segregation could be attained as early as metaphase when the chromosomes line up on the metaphase plate and effectively close off two portions of cytoplasm. In metaphase cells estimates of RER and cytoplasm volumes on each side of the metaphase plate were similar. Thus for the smaller future daughter cell the mean (± SD) proportion of cytoplasm volume was 47.7 ± 3.8% (n = 3 cells), and the mean proportion of RER volume was 47.3 ± 1.99% (n = 3 cells). As in telophase, there was, however, a significant departure from equal partitioning and in one case only 43.3% of RER and 45% of cytoplasm were found on one side of the metaphase plate. The mean difference between RER and cytoplasm proportions in metaphase cells was relatively small (1.46 ± 1.1%, n = 3 cells), again indicating a correlation between cell and RER volume. We conclude that there is close matching of RER and cytoplasm in telophase and possibly metaphase.
Fig. 8.
Close correlation between RER and cell volume partitioning. The fraction of total cell or RER volume in telophase daughter cells was estimated using the Cavalieri volume estimator as described under Materials and methods.
Discussion
Our results show the RER of mitotic HeLa cells remains cisternal and limited to the cell cortex where it encircles much of the cell. The distribution across the cortex is markedly non-uniform. In the periphery a single RER cisterna is closely associated with cortical actin and is dependent on it to maintain its position. Deeper in the cell the deepest RER cisterna wraps itself closely around the whole spindle or chromosomes. The rest of the RER in the cortex (which we term intermediate RER) is interconnected with the peripheral and spindle RER cisternae and is variably orientated depending on its abundance. Finally, we find that the amount of segregation RER that enters a daughter is very closely linked to the amount of cytoplasm in that daughter.
Our results confirm the conclusions of a number of studies that show elements of ER in animal cells remain cisternal in mitosis (reviewed in Barr, 2002; Voeltz et al. 2002) but this is the first description of actin-based positioning in mitotic mammalian cells. In interphase mammalian cells RER positioning and extension is known to be dependent on microtubules. However, in plants, actin is important for formation and maintenance of RER (Terasaki, 1990; Voeltz et al. 2002) and in eggs/oocytes a number of reports have emphasized accumulation of RER in the actin-rich regions of oocytes (Houliston & Elinson, 1991; Mehlmann et al. 1995). Budding yeast also has two RER pools: central nuclear related RER and peripheral RER associated with cortical actin. In this case peripheral RER is dependent on actin for motility but not for its positioning (Prinz et al. 2000). A recent study has provided strong evidence that the RER membranes can move along F-actin and fuse during mitosis, and that the increase in motility was driven by myosin V (Woellert et al. 2002). It is conceivable that myosin V could mediate the spreading of the RER and fusion over the inner aspect of the cortical actin shell. We found that when F-actin was depolymerized the RER relocated to the spindle, suggesting that the RER cisternae may be under some form of tension. The RER could be stretched from the spindle region to the actin cortex and when the peripheral tether is broken the RER recoils. Furthermore, the observation that the peripheral cisterna was largely absent from early G1 cells and interphase cells in general suggests the binding to the actin cortex is mitosis specific. It will therefore be important to investigate the cell cycle regulation of actin-associated RER binding in vitro. The relocation of mitochondria from the cortex to the cell periphery on actin depolymerization is of interest. A close association between RER and mitochondria has been described before in mammalian cells (Voeltz et al. 2002) and so actin could be important for maintaining a mitochondrial reticulum/assemblage in the cortex of mitotic cells as well. The movement of mitochondria to the periphery could therefore simply be a consequence of exclusion by the accumulated RER close to the spindle. Another possibility is that the independent repositioning of these organelles could reflect distinct segregation mechanisms.
We observed RER intimately associated with the mitotic spindle surface and polar microtubules close to the centrosome and this has been observed during early sea-urchin development (Terasaki, 2000). Possible links between RER and the spindle/microtubules might be microtubule binding proteins, which have been described in RER, and these include the integral membrane protein p63 (Klopfenstein et al. 1998, 2001). Other links might include microtubule-based motors (Allan, 1996) or even RER-associated organelles, which themselves can associate with microtubules, such as Golgi (Shima et al. 1997; Prescott et al. 2001) or RER export sites that have been found clustered deep in the cell in the spindle region (Prescott et al. 2001). Recent evidence has documented a mechanism for nuclear envelope disassembly in which cytoplasmic dynein anchored on the outside of the nucleus generates tension, tearing the nuclear envelope (Beaudouin et al. 2002; Gonczy, 2002; Salina et al. 2002). Dynein is a motor that is directed toward the minus-end of microtubules and it is possible that the RER we observed close to the spindle poles is positioned there by a negative-end directed motor. Indeed, this pool of RER might be a direct descendant of the nuclear envelope.
So what are the possible functions for positioning the RER close to the plasma membrane and spindle elements (see Fig. 7)? Tethering RER to the outer and inner walls of the cortical cytoplasmic compartment could force extension of RER throughout the cortex, producing a uniform distribution and the close match between segregation of cell cytoplasm and RER we observed. The plasma membrane and spindle are near symmetric structures and association with these structures could ensure a major portion of RER is divided equally on each side of the chromosome mass prior to cell division. Our own unpublished data suggest that the amount of segregating plasma membrane is (as we have found for the RER) also closely correlated with cell volume. The plasma membrane is an important ‘end product’ of RER synthesis and matching RER to amounts of endomembrane and plasma membrane entering each daughter could be a way of maximizing the efficiency of plasma membrane and organelle growth/function during the ensuing G1. Conversely, the greater the discrepancy between RER and plasma membrane, the greater the defect in membrane synthesis in one of the daughters would be. Alternatively, the precise RER positioning could play a role in organelle reassembly. The spindle-related RER is found close to the separating chromosomes during anaphase and is found connected to the reassembling nuclear envelope soon after. If, as has been suggested, the nuclear envelope reassembles by adsorbing RER cisternae (Ellenberg et al. 1997; Barr, 2002), the centrally positioned RER could provide a pool of appropriate membranes for this process. The RER at this location might even be specifically tethered at the spindle region by nuclear envelope components, although this seems unlikely in view of the widespread distribution of these components in mitotic cells (Mattaj, 2004).
Partitioning accuracy has been measured for other membranous organelles in a limited number of fluorescence studies. In the case of endosomes, the partitioning fraction departed strongly from equality (Bergeland et al. 2001) in accordance with a more random mechanism. In the case of a Golgi protein, partitioning was more even, suggesting a tethering mechanism (Shima et al. 1997). The deviation of Golgi partitioning in that study was similar to the values we have reported here for RER, although a possible correlation with cell mass was not investigated. Given the precision of the present technique it will be possible to extend these analyses to estimate the segregation fraction of a wide range of organelles, perhaps by combining Cavalieri estimation with sophisticated volume rendering light microscopy techniques to improve the overall efficiency. The principle of this method was generated centuries ago by Bonaventura Cavalieri and re-introduced by Gundersen & Jensen (1987). We have shown here that only ten sections will provide accurate quantitative information on segregation in other organelles. Our results illustrate the power of these methods to generate precise and unbiased data from slice information.
In summary, we have shown that the RER of mitotic HeLa cells maintains its cisternal nature and is restricted to the cell cortex in mitotic HeLa cells. The RER is non-uniformly distributed as indicated by (1) a peripheral RER cisterna adherent to, and dependent on, cortical actin, (2) central cisterna that associate with the spindle and (3) intermediate cisternae that stretch between the two. During mitosis there appears to be a switch from microtubule-based organization to an actin- and microtubule-based organization in mitosis of animal cells. Tethering of RER to the walls of the mitotic cell cortex could play a role in matching synthetic potential of daughter cells to the size of their organelles.
Acknowledgments
J.M.L. was supported by a Research Leave Fellowship from the Wellcome Trust (059767/Z/99/Z) and by Tenovus Scotland. Technical help was provided by John James of the Centre for High Resolution Imaging and Processing (CHIPS) at Dundee University of Dundee. We thank Lijun Wang for critical reading of the manuscript.
References
- Allan V. Role of motor proteins in organizing the endoplasmic reticulum and Golgi apparatus. Sem. Cell Dev. Biol. 1996;7:335–342. [Google Scholar]
- Barlowe C. COPII-dependent transport from the endoplasmic reticulum. Curr. Opin. Cell Biol. 2002;14:417–422. doi: 10.1016/s0955-0674(02)00348-4. [DOI] [PubMed] [Google Scholar]
- Barr FA. Inheritance of the endoplasmic reticulum and Golgi apparatus. Curr. Opin. Cell Biol. 2002;14:496–499. doi: 10.1016/s0955-0674(02)00345-9. [DOI] [PubMed] [Google Scholar]
- Barr FA. Golgi inheritance: shaken but not stirred. J. Cell Biol. 2004;164:955–958. doi: 10.1083/jcb.200402011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- Bergeland T, Widerberg J, Bakke O, Nordeng TW. Mitotic partitioning of endosomes and lysosomes. Curr. Biol. 2001;11:644–651. doi: 10.1016/s0960-9822(01)00177-4. [DOI] [PubMed] [Google Scholar]
- Birky CW. The partitioning of the cytoplasmic organelles at cell division. Int. Rev. Cyt. Suppl. 1983;15:45–89. doi: 10.1016/b978-0-12-364376-6.50009-0. [DOI] [PubMed] [Google Scholar]
- Ellenberg J, Siggian ED, Moreira JE, et al. Nuclear membrane dynamics and reassembly in living cells. targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L, Burke B. Functional organization of the nuclear envelope. Ann. Rev. Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Gonczy P. Nuclear envelope: torn apart at mitosis. Curr. Biol. 2002;12:R242–R244. doi: 10.1016/s0960-9822(02)00781-9. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Houliston E, Elinson RP. Evidence for the involvement of microtubules, ER, and kinesin in the cortical rotation of fertilized frog eggs. J. Cell Biol. 1991;114:1017–1028. doi: 10.1083/jcb.114.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology – Three Dimensional Measurement in Microscopy. Oxford: Bios Scientific Publishers; 1998. [Google Scholar]
- Jensen EB, Gundersen HJG. The rotator. J. Microsc. 1993;170:35–44. [Google Scholar]
- Jokitalo E, Cabrera-Poch N, Warren G, Shima DT. Golgi clusters and vesicles mediate mitotic inheritance independently of the endoplasmic reticulum. J. Cell Biol. 2001;154:317–330. doi: 10.1083/jcb.200104073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Kappeler F, Hauri HP. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Klumperman J, Lustig A, Kammerer RA, Oorschot V, Hauri HP. Subdomain-specific localization of CLIMP-63 (p63) in the endoplasmic reticulum is mediated by its luminal alpha-helical segment. J. Cell Biol. 2001;153:1287–1300. doi: 10.1083/jcb.153.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq JM, Warren G. Fragmentation and partitioning of the Golgi apparatus in HeLa cells. EMBO J. 1987;6:3239–3246. doi: 10.1002/j.1460-2075.1987.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq JM, Berger EG, Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J. Cell Biol. 1989;109:463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. Soring out the nuclear envelop from the endoplasmic reticulum. Nature Rev. Mol. Cell Biol. 2004;5:1–5. doi: 10.1038/nrm1263. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev. Biol. 1995;170:607–615. doi: 10.1006/dbio.1995.1240. [DOI] [PubMed] [Google Scholar]
- Mironov AA, Jr, Mironov AA. Estimation of subcellular organelle volume from ultrathin sections through centrioles with a discretized version of the vertical rotator. J. Microsc. 1998;192:29–36. doi: 10.1046/j.1365-2818.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- Nebenfuhr A, Frohlick JA, Staehelin LA. Redistribution of Golgi stacks and other organelles during mitosis and cytokinesis in plant cells. Plant Physiol. 2000;124:135–151. doi: 10.1104/pp.124.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA. Assembly–disassembly of the nuclear lamina. Curr. Opin. Cell Biol. 1992;4:105–109. doi: 10.1016/0955-0674(92)90066-l. [DOI] [PubMed] [Google Scholar]
- Prescott AR, Farmaki T, Thomson C, et al. Evidence for prebudding arrest of ER export in animal cell mitosis and its role in generating Golgi partitioning intermediates. Traffic. 2001;2:321–335. doi: 10.1034/j.1600-0854.2001.002005321.x. [DOI] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J. Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins-novel marine macrolides that disrupt microfilament organization and affect cell growth. I. Comparison with cytochalasin. D. Cell Motil. Cytoskel. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- Terasaki M. Recent progress on structural interactions of the endoplasmic reticulum. Cell Motil. Cytoskel. 1990;15:71–75. doi: 10.1002/cm.970150203. [DOI] [PubMed] [Google Scholar]
- Terasaki M. Dynamics of the endoplasmic reticulum and Golgi apparatus during early sea urchin development. Mol Biol. Cell. 2000;11:897–914. doi: 10.1091/mbc.11.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Report. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Woellert T, Weiss DG, Gerdes HH, Kuznetsov SA. Activation of myosin V-based motility and F-actin-dependent network formation of endoplasmic reticulum during mitosis. J. Cell Biol. 2002;159:571–577. doi: 10.1083/jcb.200204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal KJ, Smith CL, Polishchuk RS, et al. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell. 1999;99:589–601. doi: 10.1016/s0092-8674(00)81548-2. [DOI] [PubMed] [Google Scholar]
- Zieve GW, Turnbull D, Mullins JM, McIntosh JR. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Exp. Cell Res. 1980;126:397–405. doi: 10.1016/0014-4827(80)90279-7. [DOI] [PubMed] [Google Scholar]