Abstract
This study shows that segmental expression of alkaline phosphatase (ALP) activity by the notochord of the Atlantic salmon (Salmo salar L.) coincides with the initial mineralization of the vertebral body (chordacentrum), and precedes ALP expression by presumed somite-derived cells external to the notochordal sheath. The early expression of ALP indicates that the notochord plays an instructive role in the segmental patterning of the vertebral column. The chordacentra form segmentally as mineralized rings within the notochordal sheath, and ALP activity spreads within the chordoblast layer from ventral to dorsal, displaying the same progression and spatial distribution as the mineralization process. No ALP activity was observed in sclerotomal mesenchyme surrounding the notochordal sheath during initial formation of the chordacentra. Our results support previous findings indicating that the chordoblasts initiate a segmental differentiation of the notochordal sheath into chordacentra and intervertebral regions.
Keywords: ALP, Atlantic salmon, chordacentrum, notochord, segmentation, vertebral body
Introduction
The body axis of vertebrates is laid down during gastrulation and results in the formation of the notochord, flanked by two rows of somites. Each somite gives rise to specific cell populations that are precursors of dermis/muscle (dermatomyotome) and skeleton (sclerotome). The differentiation of the sclerotomes is a multistage process, initiated by Sonic hedgehog signalling from the notochord (Fan & Tessier-Lavigne, 1994; Fan et al. 1995), which induces expression of transcription factors from the Pax and Sox families (Balling et al. 1996; Akiyama et al. 2002; Smits & Lefebvre, 2003) and Bapx (Lettice et al. 1999; Tribioli & Lufkin, 1999). Although the nature of the molecular programs controlling the morphogenesis of the different structures of the vertebral column is being unravelled (for a review see Christ et al. 2000), a solid understanding of the relationship between the segmental pattern of the somite rows and that of the vertebral column has yet to be established.
The segmental pattern of the vertebral column has a shift in register vis-à-vis the muscle segments, being displaced by half a segment (Remak, 1855; Verbout, 1976) so that each muscle segment spans two adjacent vertebrae. Numerous studies on amniotes such as chick and mouse indicate that the metamerism within the vertebral column is derived from the somite rows. The proposed mechanism for the frame-shift is that each sclerotome is divided into cranial and caudal cell-lineage-restricted compartments. The vertebral segments arise through a strict resegmentation process whereby the caudal compartment of one sclerotome fuses with the cranial compartment of the adjacent (caudal) sclerotome (for a review see Brand-Saberi & Christ, 2000). This implies that each sclerotome-half may only contribute to one vertebral segment (Bagnall et al. 1988; Goldstain & Kalcheim, 1992; Huang et al. 1996, 2000; Aoyama & Asamoto, 2000). Although substantial evidence supports resegmentation, especially in avian species, notochord excision in chick embryos and in amphibian larvae results in the formation of an unsegmented gutter-shaped rod of vertebral cartilage, indicating that the notochord plays a role in segmental patterning (Kitchin, 1949; Holtzer, 1952; Holtzer & Detwiler, 1953; Strudel, 1955; Hall, 1977; Keynes & Stern, 1988).
In teleosts, such as the Atlantic salmon, the first mineralized element of the vertebral body (chordacentrum) forms as an integrated notochordal structure, in which both notochordal cells and the sheath seem to be involved. Prior to the formation of the vertebral column, the notochord of the Atlantic salmon larvae develops into a prominent and specialized axial fibrewound hydraulic skeleton (Fig. 1). The ring-shaped chordacentra form through mineralization of preformed matrix within the outer half of the notochordal sheath (Grotmol et al. 2003) (Fig. 2A,B). The mineralization process in each chordacentrum starts along the ventral midline of the notochordal sheath and proceeds towards the dorsal side of the notochord in a bilateral, symmetrical manner, finally forming a complete mineralized ring (Fig. 2A). The mineralization process is immediately preceded by the formation of segmental cellular patterns within the chordoblast layer (Grotmol et al. 2003). In zebrafish, ablation of notochordal cells has shown that the formation and metameric pattern of the chordacentra is dependent on notochord integrity, and furthermore that the notochord may be a source of bone matrix (Fleming et al. 2004). In addition, a cell clonal study on zebrafish has shown that single sclerotome halves contribute to more than one vertebra (leaky resegmentation) (Morin-Kensicki et al. 2002), which is incompatible with the theory of resegmentation. In fused somites mutants of zebrafish, somite boundaries are malformed and irregular (van Eeden et al. 1996). Here the vertebral segments develop normally, while the neural and haemal arches are ectopically positioned. One interpretation of these observations is that the metamerism of the vertebral bodies is generated by a different source than that of arches (Fleming et al. 2001, 2004; Grotmol et al. 2003). To sum up, these studies suggest that vertebral segmentation, at least in teleosts, is orchestrated by the notochord.
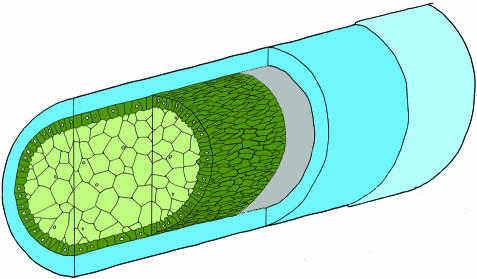
Fig. 1.
Schematic view of the main structural components of the notochord in the Atlantic salmon. The notochord comprises a core of epitheloid cells that is enclosed by an acellular fibrous sheath, which consists of a thin external elastic membrane (light blue), covering a thicker collagenous layer (blue). The cellular core is composed of an inner tube of chordocytes (light green), each with a large, fluid-filled vacuole. The chordocytes are surrounded by a monolayer of chordoblasts (green). The chordoblasts rest on a basal lamina (grey), are germinal and produce the notochordal sheath. For comparison, the notochord of the zebrafish has a similar general structure, except for the chordoblast layer, which consists of thin squamous cells.
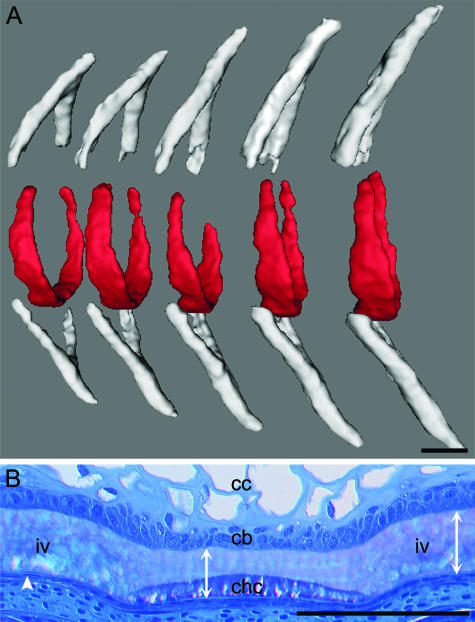
Fig. 2.
The chordacentra develop as mineralized rings within the notochord in the period from approximately 630 to 700 day°. Cranial to the left and dorsal side up. (A) X-ray microcomputed tomographic image of the chordacentra (red) and the neural and haemal arches (grey). Chordacentrum mineralization is initiated at the ventral midline of the notochordal sheath, and they pass through a ‘horseshoe’ shape finally to form complete rings. (B) A sagittal section of the ventral region of the notochord stained with toluidine blue that shows a chordacentrum (chc) with adjacent intervertebral regions (iv). The chordacentrum is situated in the outer half of the notochordal sheath. In the regions where chordacentra form, the expansion of the notochord ceases and the notochordal sheath stops growing in thickness. In the intervertebral region, collagen production is maintained, resulting in a marked thickening of the sheath. The full thickness of the notochordal sheath is indicated by double arrow lines. Chordocytes (cc), chordoblasts (cb) and the external elastic membrane (arrowhead) are indicated. Scale bars: 100 µm.
In order to understand the formation of the vertebral bodies in teleosts, it is necessary to identify the cells that contribute to the development of the chordacentra. Alkaline phosphatase (ALP) is a marker of both preosteoblasts and osteoblasts, and the enzyme is thought to be essential in the mineralization of organic matrixes, although the exact mechanism is unknown (for a review see Whyte, 2002). It is thus probable that the mineralization of the notochordal sheath is associated with a segmental activity of ALP, either in the chordoblast layer of the notochord or within the sclerotomal tissue surrounding the notochordal sheath. Our objective was thus to locate ALP activity associated with the notochord, or the surrounding tissue, in the period during which the chordacentra mineralize.
Materials and methods
Maintenance of stock and collection of fish samples
Larvae and juveniles of Atlantic salmon (Salmo salar L.) were obtained from a commercial hatchery where the various stages were kept in flow-through systems. Larvae were kept at 8 °C throughout hatching, until half of the yolk sac was consumed, at which point the temperature was raised to 8.5 °C until start of feeding commenced. The temperature was then increased linearly to 13 °C over a period of 2 days. The larvae were fed commercial dry feed ad libitum. Developmental stages were classified by day°, which are defined as the sum of daily mean ambient water temperatures (°C) for each day. Hatching occurred at around 500 day°, while first-feeding commenced towards the end of the yolk-sac period at approximately 870 day°. A series of developmental stages from hatching up to 900 day°, at a sampling interval of 2 days, was analysed. Each sample consisted of 20 fish. The samples were transported to the laboratory in plastic bags containing water with gaseous oxygen above. Before preparative procedures commenced, the fish were anaesthetized by immersion in water containing 50 p.p.m. benzocain.
X-ray microcomputed tomography of whole specimens
Whole specimens were fixed by immersion in a solution of 10 mL 10% formaldehyde (freshly prepared from paraformaldehyde), 10 mL 25% glutaraldehyde, 20 mL 0.2 m cacodylate buffer and 60 mL PBS, with the pH adjusted to 7.35. They were scanned in a microcomputed tomograph (SkyScan 1072, SkyScan NV, Aartselaar, Belgium), according to the manufacturer's instructions. The X-ray source was run at 74 kV, and the data sets were acquired using a voxel side length of 5.8 µm. Stacks of tomographic images were reconstructed from primary shadow images using SkyScan software. From these image stacks, three-dimensional (3D) digital models of mineralized tissue were constructed by image thresholding and surface rendering employing Imaris 4.0.5 software (Bitplane AG, Zurich, Switzerland). Images of the 3D reconstructions were processed using Imaris and Adobe Photoshop 7.0.
Enzyme histochemistry
ALP activity was detected by means of a protocol based on Witten (1997). The specimens were fixed by immersion in 100% acetone for 15 min, and thereafter completely dehydrated by transfer to 100% acetone twice for 3 min. They were then embedded in Technovit 7100 (Heraeus Kulzer GmbH & Co. KG, Wehrheim, Germany), and 4-µm-thick sections were cut, mounted on glass slides and air dried. Neighbouring sections were stained with toluidine blue (Philpott, 1966) and processed for ALP histochemistry, respectively. Fixation, dehydration and embedding were performed at 4 °C, and the specimens were not decalcified. The sections were first pre-incubated in 50 mm Tris buffer, pH 9.5, for 1 h. ALP staining was performed by incubation for 2 h in the same buffer containing 1 mg mL−1 naphthol AS-MX phosphate (Sigma-Aldrich Co.) as substrate, and 1 mg mL−1 Fast Red TR Salt (Sigma-Aldrich Co.). All incubations were performed at 20 °C. The sections were then rinsed in distilled H2O, air dried and mounted with Mountex (Histolab Products AB, Gothenburg, Sweden). Control procedures were: (1) incubation without substrate, (2) heating at 90 °C for 10 min and (3) adding EDTA to the incubation medium (10 mg L−1).
For routine histology, specimens were fixed by immersion in a solution of 10 mL 10% formaldehyde (freshly prepared from paraformaldehyde), 10 mL 25% glutaraldehyde, 20 mL 0.2 m cacodylate buffer and 60 mL PBS, with the pH adjusted to 7.35. They were then dehydrated in ethanol and embedded in Technovit 7100 (Heraeus Kulzer GmbH & Co.). Semithin sections (1.5 µm) were stained with toluidine blue and mounted with Mountex (Histolab Products AB).
Digital micrographs were acquired using a Progres C14 camera (Jenoptik GmbH, Jena, Germany) on an Olympus Vanox AHBT3 microscope employing bright-field and differential interference contrast optics (Olympus, Tokyo, Japan), and the images were processed using Adobe Photoshop 7.0.
Results
Appearance of mineralized chordacentra
Micro-computed tomography showed that the chordacentra mineralized in the period between 630 and 700 day°. The first complete chordacentra appeared in the region beneath the dorsal fin, from where successive chordacentra developed in both cranial and caudal directions.
The mineralization of the chordacentra coincides with a segmental pattern of ALP activity in the chordoblast layer
The mineralization process of each chordacentrum took place in the outer half of the notochordal sheath. Simultaneously with the mineralization of the chordacentra, ALP activity was detected in the adjacent chordoblast layer. The notochord was surrounded by a morphologically non-segmented tube of mesenchymal cells, which, at this early stage (up to approximately day 700°), showed no ALP activity (Fig. 3A). Initially, ALP activity was only observed segmentally along the ventral portion of the chordoblast layer (Fig. 3B,C). From this point, ALP activity could be detected in more dorsal positions, and displayed the same spatial pattern as the mineralization of the chordacentra, which proceeded dorsad in a bilateral symmetrical manner. In addition, ALP activity was expressed in osteoblasts that formed perichondral bone on the surfaces of the neural and haemal arch cartilages, both before and after the onset of mineralization of the chordacentra (Fig. 3B). A metameric pattern of enzyme activity in the chordoblast layer was observed coincident with chordacentrum mineralization, with no staining of chordoblasts in the presumptive intervertebral regions (Fig. 3D,E). ALP activity within the chordoblast layer closely followed both the cranial and the caudal rims of the chordacentra during the progression of the mineralization process (Fig. 3F). The staining of the enzyme product was located along the cell surface of the chordoblasts (Fig. 3G).
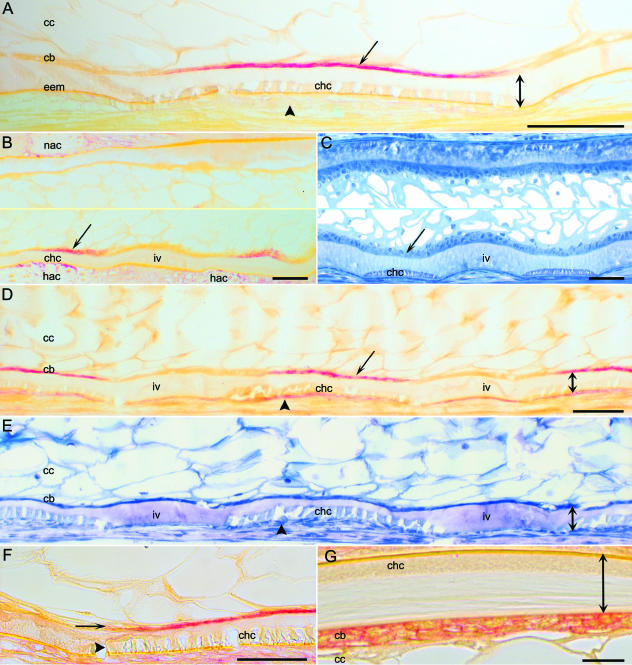
Fig. 3.
Segmental alkaline phosphatase (ALP) activity in the chordoblast layer of the notochord. Cranial to the left, and dorsal side up. (A,B,D,F,G) ALP histochemistry. (A) Developmental stage: 680 day°. The ventral part of the notochord from a longitudinal section. During initial chordacentrum (chc) mineralization along the ventral midline of the notochord, ALP activity (arrow) only occurs within the chordoblast layer (cb). There is no ALP activity in mesenchymal cells (arrowhead) external to the external elastic membrane (eem) of the notochord. The full thickness of the notochordal sheath (double arrow) and chordocytes (cc) are indicated. (B,C) Developmental stage: 680 day°. The notochord from longitudinal sections. The middle parts of the images have been omitted. (B) Sagittal section through the notochord and the neural (nac) and haemal arch cartilages (hac). At the onset of chordacentrum mineralization, which starts at the ventral midline, ALP activity (arrow) is present segmentally in the ventral portion of the chordoblast layer. Note that osteoblasts, which form the periochondral bone on the neural and haemal arch cartilages, express ALP activity. The location of the chordacentrum (chc) and the intervertebral region (iv) is indicated. (C) Section similar to B, stained with toluidine blue (figure from Grotmol et al. 2003). The chordacentra (chc) first appear at the ventral midline, in the outer part of the notochordal sheath. The location of a chordoblast subpopulation (arrow), equivalent to the subpopulation that expresses ALP activity, and an intervertebral region (iv) is indicated. (D,E) Developmental stage: 720 day°. The ventral half of the notochord from a longitudinal section. At this stage of development the chordacentra (chc) are complete, and they form full rings (not shown). (D) The ALP activity (arrow) shows a segmental pattern within the chordoblast layer (cb), and, in addition, mesenchymeal cells (preosteoblasts/osteoblasts) that express ALP activity (arrowhead) are present on the external surface of the notochord∖chordacentra (chc). The full thickness of the notochordal sheath (double arrow), the intervertebral regions (iv) and chordocytes (cc) are indicated. (E) Neighbouring section to D, stained with toluidine blue. The chordacentra (chc) appear as mineralized structures within the sheath (double arrow) with mesenchymal cells (preosteoblasts/osteoblasts) on the outer surface (arrowhead). Chordoblasts (cb), chordocytes (cc) and intervertebral region (iv) are indicated. (F) Developmental stage: 790 day°. Sagittal section of the ventral region of the notochord. The distribution of ALP staining (arrow) within the chordoblast layer coincides with the mineralization of the chordacentrum (chc) within the sheath. The arrowhead indicates the rim of the chordacentrum. (G) Developmental stage: 705 day°. Transverse section from the dorsal part of the notochord showing the notochordal sheath (double arrow) with a chordacentrum (chc), the chordoblast layer (cb) and chordocytes (cc). The ALP stain is localized in the area of the plasma membrane of the chordoblasts. Scale bars: (A–F) 50 µm; (G) 10 µm.
Subsequent to the mineralization of the chordacentrum, ALP activity was observed in mesenchymal cells surrounding the notochordal sheath
After the initial mineralization of chordacentra, from approximately 700 day°, ALP staining gradually increased in the mesenchyme around the notochord (Fig. 3D), and these cells eventually acquired a typical osteoblast morphology. Deposition of osteoid on the external surface of the notochordal sheath was evident from approximately 800 day° (Fig. 4A,B). In addition, ALP staining was detected, for instance, in the brush borders of the intestine and the kidney tubules. The negative controls for ALP histochemistry showed no staining.
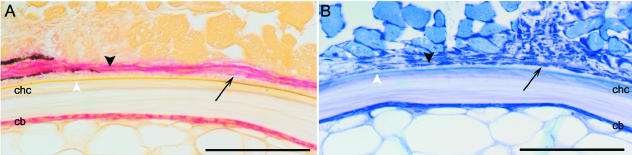
Fig. 4.
(A,B) Developmental stage: 820 day°. Neighbouring transverse sections of the lateral part of the notochord with adjacent musculature (lateral side up). (A) Alkaline phosphatase histochemistry. A thin layer of osteoid (arrow) has been deposited on the surface of the notochord, external to the elastic membrane (white arrowhead) and the chordacentrum (chc). The osteoblasts external to the notochord express ALP activity (black arrowhead). ALP staining is also present within the chordoblast layer (cb). (B) Neighbouring section stained with toluidine blue. A layer of osteoid (arrow) is present between the osteoblasts (black arrowhead) and the external elastic membrane of the notochord (white arrowhead). The chordoblast layer (cb) and the chordacentrum (chc) are indicated. Scale bars: 50 µm.
Discussion
The key finding of this study is of segmental expression of ALP activity by the notochord, which coincides with mineralization of the chordacentra, and precedes expression by presumed somite-derived cells external to the notochordal sheath. As we discuss below, this may indicate that the notochord plays an instructive role in the segmental patterning of the vertebral column by forming the core of the vertebral body.
Subpopulations of chordoblasts display functional specificity
Although their exact physiological roles are not fully known, ALPs are ubiquitous, with isoenzymes being abundant in specific cells of certain tissues. Studies addressing these enzymes in teleosts are few, but in mammals (Whyte, 2002), as well as in teleosts (Witten, 1997), ALP is a widely used marker for preosteoblasts and osteoblasts. ALP activity within the notochord has not previously been demonstrated. However, because the enzyme activity is expressed at the same time as the mineralization of the chordacentra, this may reflect that a subpopulation of chordoblasts shows an osteoblast-like property, which is related to the mineralization process. Although the exact mechanism of ALP in the mineralization of osteoid of bone is not firmly established, it plays an essential role in the precipitation of hydroxyapatite (for a review see Whyte, 2002). As shown in the present study, the subpopulations of chordoblasts that show ALP activity have the same location as the morphologically distinct bands of chordoblasts that develop during formation of the chordacentra (Grotmol et al. 2003). Subpopulations of chordoblasts thus seem to initiate a differentiation of the notochordal sheath into vertebral and intervertebral segments. In the regions where chordacentra form, the expansion of the notochord ceases and the notochordal sheath stops growing in thickness, while in the intervertebral regions there is a marked thickening of the sheath (Nordvik et al. 2005). This probably reflects the situation that the expression of ALP is linked to a down-regulation of collagen production. In the intervertebral regions, however, collagen production is maintained or possibly up-regulated. The regional differences in matrix secretion, linked to the pattern of ALP activity, support the notion of alternating compartments of chordoblasts displaying specific genetic signalling pathways.
Morphogenesis of the chordacentrum
Several interpretations regarding the initial formation of the vertebral bodies in teleosts have been put forward. This and previous studies (François, 1966; Laerm, 1976; Arratia et al. 2001; Grotmol et al. 2003) indicate that the vertebral bodies initially form through mineralization of the notochordal sheath as chordacentra, rather than by ossification involving deposition of osteoid by osteoblasts. Further growth of the vertebral body takes place via the formation of an autocentrum through direct ossification by presumed sclerotome-derived osteoblasts external to the notochordal sheath (Laerm, 1976; Arratia et al. 2001; Grotmol et al. 2003; Nordvik et al. 2005). A study performed on zebrafish has indicated that the notochord forms the vertebral bodies through the production and secretion of bone matrix (Fleming et al. 2004). This study, however, does not address the question of whether the formation of ‘bone matrix’ reflects mineralization within the preformed notochordal sheath (chordacentra) or true ossification with deposition of osteoid de novo by the notochord. Our observations from zebrafish indicate, however, that the whole notochordal sheath is mineralized in the regions where the vertebral bodies form, thus constituting typical chordacentra (unpublished results). By contrast, transmission electron microscopical studies performed on the advanced teleost medaka (Acanthopterygii) have claimed that the vertebral bodies are initially formed through direct ossification by osteoblasts surrounding the notochord, which results in compression of the sheath (Ekanayake & Hall, 1988, 1991). It is interesting that in their 1988 study, the authors describe a thin, heavily mineralized layer, which may be interpreted as the chordacentrum, between the notochordal sheath and the vertebral bone. Moreover, in most advanced teleosts the notochordal sheath degenerates early, and the chordacentrum may be transitory, with only vestiges appearing after the formation of perinotochordal vertebral bone (Laerm, 1976). In the Atlantic salmon, the notochordal sheath constitutes a morphologically homogeneous layer with functions related to turgor and elastic recoil of the hydroskeleton, until it finally differentiates into chordacentra and intervertebral regions (Grotmol et al. 2003). The sheath consists mainly of collagen type II (Sandell, 1994; Koob & Long, 2000), and is gradually produced by the chordoblasts from the single-cell-diameter stage of notochord development and onwards. In some aspects, the morphogenesis of the chordacentra thus differs from that of direct ossification by osteoblasts, where the main component of the osteoid is collagen type I that mineralizes shortly after it is secreted. By contrast, both bone and chordacentra have collagenous matrixes that mineralize in close association with cells that express ALP activity. Furthermore, the formation of chordacentra in the outer half of the notochordal sheath may be a parallel to that of direct ossification where the mineralization of the osteoid occurs at some distance from the osteoblasts.
Segmental chordoblast ALP activity substantiates a duality in vertebral column patterning in teleosts
Coincident with the segmental expression of ALP activity as shown herein, metameric morphologically distinguishable bands of chordoblasts are formed (Grotmol et al. 2003). This, in addition to the absence of ALP activity in the mesenchyme surrounding the notochord during initial formation of the chordacentra, further substantiates that the chordacentra are derived solely from the notochord, and that the chordoblasts play an instructive role in vertebral patterning. Moreover, a number of studies on zebrafish lend support to this concept. Chordacentrum development, for instance, is dependent on notochord integrity, and the notochord may also be a source of bone matrix (Fleming et al. 2004). In zebrafish fused somites (fss) mutants that display irregular somite boundaries, the metameric pattern of the vertebral bodies is normal, which may indicate that the segments are derived from the notochord, and not from the somite rows (Grotmol et al. 2003; Fleming et al. 2004). Furthermore, cell lineage analysis within the zebrafish sclerotome indicates that one half-sclerotome contributes to two adjacent vertebrae (‘leaky resegmentation’) (Morin-Kensicki et al. 2002). On the basis of these observations, the development of the vertebral column in teleosts, such as salmon and zebrafish, may be conceptualized in terms of a dual segmentation model (Grotmol et al. 2003; Fleming et al. 2004). This model implies that the segmental pattern of the vertebral bodies arises as a primary metameric process within the chordoblast layer of the notochord, while the segmental appearance of the neural and haemal arches originates from the somites. Thus, apart from being a structural element that embeds the vertebra within the notochordal sheath (Nordvik et al. 2005), the chordacentra may pattern the surrounding mesenchyme, possibly through cell–matrix interactions, which may induce osteoblast differentiation.
Does notochord morphogenesis reveal additional metameric features?
Evidence of segmental activity within the notochord has been obtained through studies focusing on the segmentation of the vertebral column, which becomes evident at a relatively late stage in ontogeny. Interesting questions are thus whether the notochord has segmental properties in earlier stages, and how this information is transmitted to the vertebral column.
The differentiated notochord varies significantly in structure across the major chordate clades, but early morphogenesis appears to be conserved (Glickman et al. 2003; Tada, 2005). After medio-lateral intercalation across a field of cells, the notochord forms into a single-cell-diameter column of discoid cells. This stage has been identified in Urochordata (Burighel & Cloney, 1997), Cephalochordata (Flood, 1975), Hyperotreta (Myxinoidea) (Pasteels, 1958), Hyperoartia (Petromyzonoidea) (Pasteels, 1958), Chondrichthyes (Boeke, 1908), Teleostei (Boeke, 1908 (Salmo); Kimmel et al. (1995) (zebrafish)) and Amphibia (Hausen & Riebesell, 1991), but not in Amniota (Starck, 1979). In urochordate larvae, each cell within the stack has a distinct cranio-caudal functional polarization shown by specific localization of planar cell polarity gene products (Jiang et al. 2005; Tada, 2005).
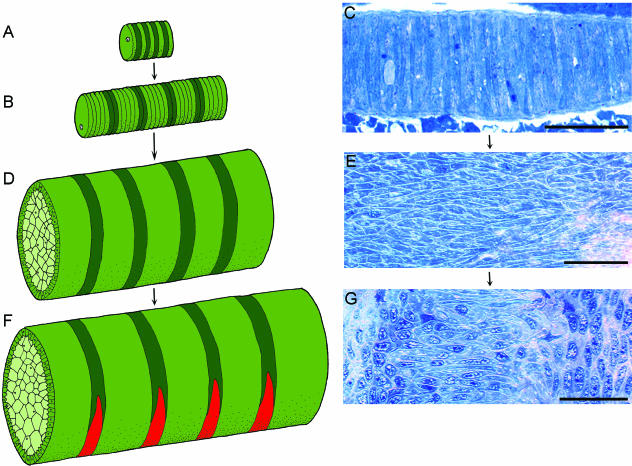
Although it has not been elucidated, it is tempting to speculate upon whether the single-cell-diameter stage reflects metameric properties of the notochord. Within this structure, a single cell or a group of cells may constitute a compartment with a distinct position relative to the cranio-caudal axis (Fig. 5A–C). From this stage, the notochord of the Atlantic salmon develops into a stratified hydroskeleton that lacks overt morphological metamerism, and it subsequently displays segmental features related to the morphogenesis of the vertebral column (Grotmol et al. 2003). However, the presumed compartments within the single-cell stack may be transformed into defined band-like segments within the chordoblast layer (Fig. 5D). Underlying mechanisms may include the formation of cell clonal compartments, or alternating domains expressing different genetic signalling pathways. Nevertheless, at this stage, the chordoblasts retain a defined cranio-caudal polarity through the alignment of an ellipsoid ‘foot-like’ cell basis with the axis (Fig. 5E). Coincident with the mineralization of the chordacentra, the chordoblast layer expresses a metameric pattern of ALP activity (Fig. 5F), and concurrently morphologically distinct patterns develop within the chordoblast layer (Fig. 5G) (Grotmol et al. 2003).
Fig. 5.
Changes in chordoblast morphology and arrangement during development of the notochord in Atlantic salmon. Cranial to the left, and dorsal side up. (A,B,D,F) Schematic view of a possible developmental sequence for notochord-derived metamerism. Presumptive compartments within the chordoblast cell population are in green and dark green. Chordocytes are in light green. (C,E,G) Chordoblast morphology. Sagittal sections stained with toluidine blue. (A,B,C) Single-cell-diameter stage. (A) At an early stage, there may be alternating properties of single cells within the stack. (B) At a later stage, groups of cells may constitute metameric compartments. (C) Developmental stage: 171 day°. The notochord at the singe-cell-diameter stage. Within this structure, a single cell or a group of cells may constitute a segment with a distinct position relative to the cranio-caudal axis. (D) The notochord develops into a stratified hydroskeleton through differentiation of chordocytes and chordoblasts. At this stage, there are no overt morphological metameric patterns within the chordoblast layer. The presumed compartments within the single-cell stack may, however, be transformed into bands within the chordoblast layer. (E) Developmental stage: 560 day°. The section passes tangentially through the basal part of the chordoblasts, which rest on the basal lamina (Fig. 1). At this stage the chordoblasts retain a defined cranio-caudal polarity through the alignment of the ellipsoid ‘foot-like’ basis with the axis. (F) During chordacentrum mineralization, the chordoblast layer expresses a metameric pattern of ALP activity, which is indicated in red. (G) Developmental stage: 730 day°. The section passes tangentially through the chordoblasts. Coincident with the expression of ALP, morphologically distinct band-like patterns develop within the chordoblast layer. Panels E and G are from Grotmol et al. (2003). Scale bars: 50 µm.
It is intriguing that the notochord of amniotes does not pass through a single-cell-diameter column stage (Starck, 1979), and this may reflect a different developmental mechanism in which the paraxial mesoderm may have evolved as the major source of segmental patterning of the vertebral column within this clade. Nevertheless, the results presented here lend support to the concept that the notochord is a synapomorph segmental structure in vertebrates, which orchestrates segmental patterning of the vertebral column within the trunk and tail (Stern, 1990; Fleming et al. 2001, 2004; Grotmol et al. 2003). Moreover, this may imply that the vertebra, in spite of diverse patterns of morphogenesis across the different clades (Arratia et al. 2001), share a deep homology.
Acknowledgments
This work was funded by the Research Council of Norway. We gratefully acknowledge the expert help of EWOS AS at Lønningdal in rearing the salmon. Expert technical assistance was provided by T. Cieplinska, N. Ellingsen and C. Krossøy.
References
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Brombrugghe B. Transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H, Asamoto K. Determination of somite cells: independence of cell differentiation and morphogenesis. Development. 2000;104:482–494. doi: 10.1242/dev.104.1.15. [DOI] [PubMed] [Google Scholar]
- Arratia G, Schultze H-P, Casciotta J. Vertebral column and associated elements in dipnoans and comparison with other fishes: development of homology. J. Morph. 2001;250:101–172. doi: 10.1002/jmor.1062. [DOI] [PubMed] [Google Scholar]
- Bagnall KM, Higgins SJ, Sanders EJ. The contribution made by a single somite to the vertebral column: experimental evidence in support of resegmentation using the chick-quail chimaera model. Development. 1988;103:69–85. doi: 10.1242/dev.103.1.69. [DOI] [PubMed] [Google Scholar]
- Balling R, Neubuser A, Christ B. Pax genes and sclerotome development. SeminCell Dev. 1996;7:129–136. [Google Scholar]
- Boeke J. Das Geldrollenstadium der Vertebraten-Chorda und des Skelettes der Mundcirren von Branchiostoma lanceolatum, und seine cytomechanische Bedeutung. Anat. Anz. 1908;33:541–556. 574–580. [Google Scholar]
- Brand-Saberi B, Christ B. Evolution and development of distinct cell lineages derived from somites. Curr. Top. Dev. Biol. 2000;48:1–42. doi: 10.1016/s0070-2153(08)60753-x. [DOI] [PubMed] [Google Scholar]
- Burighel P, Cloney RA. Urochordata: Ascidiacea. In: Harrison FW, Ruppert EE, editors. Microscopic Anatomy of Invertebrates. Vol. 15. New York: Wiley-Liss; 1997. pp. 221–347. [Google Scholar]
- Christ B, Huang R, Wilting J. The development of the avian vertebral column. Anat. Embryol. 2000;202:179–194. doi: 10.1007/s004290000114. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, et al. Mutations affecting somite formation and patterning in the zebrafish Danio rerio. Development. 1996;123:153–164. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- Ekanayake S, Hall BK. Ultrastructure of the osteogenesis of acellular vertebral bone in the japanese medaka, Oryzias latipes (Teleostei, Cyprinidontidae) Am. J. Anat. 1988;182:241–249. doi: 10.1002/aja.1001820305. [DOI] [PubMed] [Google Scholar]
- Ekanayake S, Hall BK. Development of the notochord in the japanese medaka, Oryzias latipes (Teleostei, Cyprinidontidae), with special emphasis on the desmosomal connections and functional integration with adjacent tissue. Can. J. Zool. 1991;69:1171–1177. [Google Scholar]
- Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Fan CM, Porter JA, Chiang C, Chiang DT, Beachy PA, Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signalling pathway. Cell. 1995;81:457–465. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- Fleming A, Keynes RJ, Tannahill D. The role of the notochord in vertebral column formation. J. Anat. 2001;199:177–180. doi: 10.1046/j.1469-7580.2001.19910177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Keynes RJ, Tannahill D. A central role for the notochord in vertebral patterning. Development. 2004;131:873–880. doi: 10.1242/dev.00952. [DOI] [PubMed] [Google Scholar]
- Flood PR. Fine structure of the notochord of amphioxus. Symp Zool. Soc. Lond. 1975;36:81–104. [Google Scholar]
- François Y. Structure et développement de la vertèbre de Salmo et des téléostéens. Arch. Zool. ExpGén. 1966;107:287–328. [Google Scholar]
- Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Development. 2003;130:873–887. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- Goldstain RS, Kalcheim C. Determination of epithelial half-somites in skeletal morphogenesis. Development. 1992;116:441–445. doi: 10.1242/dev.116.2.441. [DOI] [PubMed] [Google Scholar]
- Grotmol S, Kryvi H, Nordvik K, Totland GK. Notochord segmentation may lay the pathway for the development of the vertebral bodies of the Atlantic salmon Salmo salar. Anat. Embryol. 2003;207:263–272. doi: 10.1007/s00429-003-0349-y. [DOI] [PubMed] [Google Scholar]
- Hall BK. Chondrogenesis of the somatic mesoderm. Adv. Anat. Embryol. 1977;53:1–50. [PubMed] [Google Scholar]
- Hausen P, Riebesell M. The Early Development of Xenopus laevis. An Atlas of the Histology. Berlin: Springer; 1991. [Google Scholar]
- Holtzer H. An experimental analysis of the development of the spinal column. II. The dispensability of the notochord. J. Exp. Zool. 1952;121:573–591. [Google Scholar]
- Holtzer H, Detwiler SR. An experimental analysis of the development of the spinal column. III. Induction of skeletogenous cells. J. Exp. Zool. 1953;123:335–366. [Google Scholar]
- Huang R, Zhi Q, Neubuser A, et al. Function of somite and somitocoele cells in the formation of the vertebral motion segment in avian embryos. Acta Anat. 1996;155:231–241. doi: 10.1159/000147811. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Brand-Saberi B, Christ B. New experimental evidence for somite resegmentation. Anat. Embryol. 2000;202:195–200. doi: 10.1007/s004290000110. [DOI] [PubMed] [Google Scholar]
- Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochordal cells. Curr. Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Stern CD. Mechanisms of vertebrate segmentation. Development. 1988;103:413–429. doi: 10.1242/dev.103.3.413. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kitchin IC. Effects of notochordectomy in Amblystoma mexicanum. J. Exp. Zool. 1949;112:393–415. doi: 10.1002/jez.1401120303. [DOI] [PubMed] [Google Scholar]
- Koob TJ, Long JH. The vertebral body axis: evolution and mechanical function. Am. Zool. 2000;40:1–18. [Google Scholar]
- Laerm J. The development, function, and design of amphicoelous vertebrae in teleost fishes. Zool. J. Linn. Soc. 1976;58:237–254. [Google Scholar]
- Lettice LA, Purdie LA, Carlson GJ, Kilanowski F, Dorin J, Hill RE. The mouse bagpipe gene controls development of axial skeleton, skull and spleen. Proc. Natl Acad. Sci. USA. 1999;96:9695–9700. doi: 10.1073/pnas.96.17.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Melancon E, Eisen JS. Segmental relationship between somites and vertebral column in zebrafish. Development. 2002;129:3851–3860. doi: 10.1242/dev.129.16.3851. [DOI] [PubMed] [Google Scholar]
- Nordvik K, Kryvi H, Totland GK, Grotmol S. The salmon vertebral body develops through mineralization of two preformed tissues that are encompassed by two layers of bone. J. Anat. 2005;206:103–114. doi: 10.1111/j.1469-7580.2005.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteels J. Développement des agnathes. In: Grassé P, editor. Traité de Zoologie. Paris: Masson et Cie; 1958. pp. 106–144. 13. [Google Scholar]
- Philpott DE. A rapid method for staining plastic-embedded tissues for light microscopy. Sci. Instrum. 1966;11:11–12. [Google Scholar]
- Remak R. Untersuchungen Über die Entwicklung der Wirbeltiere. Berlin: G Reimer; 1855. [Google Scholar]
- Sandell LJ. In situ expression of collagen and proteoglycan genes in notochord and during skeletal development and growth. Microsc. Res. Techn. 1994;28:470–482. doi: 10.1002/jemt.1070280603. [DOI] [PubMed] [Google Scholar]
- Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus and intervertebral discs. Development. 2003;130:1135–1148. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- Starck D. Allgemaines, Skelettsubstanzen, Skelett der Wirbeltiere Einschliesslich Lokomotionstypen. Berlin: Springer-Verlag; 1979. Vergleichende Anatomie der Wirbeltiere auf evolutionsbiologischer Grundlage. 2. Das Skelettsystem. [Google Scholar]
- Stern CD. Two distinct mechanisms for segmentation? Semin. Dev. Biol. 1990;1:109–116. [Google Scholar]
- Strudel G. L’action morphogène du tube nerveux et de la corde sur la différenciation des vertèbres et des muscles vertebraux chez l’embryon de poulet. Arch. Anat. Microsc. Morph. Exp. 1955;44:209–235. [PubMed] [Google Scholar]
- Tada M. Notochord morphogenesis: a prickly subject for Ascidians. Curr. Biol. 2005;15:14–16. doi: 10.1016/j.cub.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Tribioli C, Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development. 1999;126:5699–5711. doi: 10.1242/dev.126.24.5699. [DOI] [PubMed] [Google Scholar]
- Verbout AJ. A critical review of the ‘neugliederung’ concept in relation to the development of the vertebral column. Acta Biotheor. 1976;25:219–258. doi: 10.1007/BF00046818. [DOI] [PubMed] [Google Scholar]
- Whyte MP. Hypophosphatasia: Nature's window on alkaline phosphatase function in man. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2nd. Vol. 2. London: Academic Press; 2002. pp. 1220–1249. [Google Scholar]
- Witten PE. Enzyme histochemical characteristics of osteoblasts and mononucleated osteoclasts in a teleost fish with acellular bone (Oreochromis niloticus, Cichlidae) Cell Tissue Res. 1997;287:591–599. doi: 10.1007/s004410050782. [DOI] [PubMed] [Google Scholar]