Abstract
The objective of this study was to clarify the development of the stapes in humans and its relationship with the cartilage of the second branchial arch. The study was carried out in 25 human embryos between 6 and 28 mm crown–rump length. The stapes develops at the cranial end of the second branchial arch through an independent anlage of the cartilage of this arch. Between the stapedial anlage and the cranial end of the Reichert's cartilage there is a formation called the interhyale, the internal segment of which gives rise to the tendon of the stapedial muscle. The stapedial anlage is a unique formation with two distinct parts: the superior part that will comprise the base and the inferior part that will be crossed by the stapedial artery during embryonic development and will constitute the limbs and the head of the stapes. According to the results, the otic capsule is not involved in formation of the base of the stapes.
Keywords: auditory ossicles, embryology, embryonic stage, pharyngeal archs, Reichert's cartilage
Introduction
The origin and development of the ossicles of the middle ear in humans are not totally clear and are especially unclear for the stapes.
Different theories have been proposed to explain stapedial development in the middle ear: a unique source in the cartilage of the second branchial arch or Reichert's cartilage is the classical most accepted theory and is the theory appearing in embryology textbooks (Hamilton & Mossman, 1975; Corliss, 1979; Abramovich, 1997; Moore & Persaud, 1999; Cochard, 2002; Sadler, 2004); a double source, the limbs of Reichert's cartilage and the base of the otic capsule (Cauldwell & Anson, 1942; Masuda et al. 1978; Sperber, 1989); and an origin of the second branchial arch, independent of Reichert's cartilage (Hanson et al. 1962; Mugnier, 1964) – in this case a structure has been described connecting the stapes anlage with the second visceral arch, called the interhyale or interhyal formation, that gives rise to the stapedial muscle (Hanson et al. 1962; Louryan, 1993). There is therefore no unanimous opinion regarding the origin of the stapes in humans.
The objective of this study was to clarify development of the stapes in human embryos, the relationship it has with Reichert's cartilage and the role played by the interhyal formation.
Materials and methods
Twenty human embryos were studied from 32 to 56 days of development. All the specimens studied belong to the collection of the Embryology Institute of the University Complutense of Madrid. The specimens were obtained from miscarriages and ectopic pregnancies from the department of obstetrics. Approval for the study was granted by the Ethical Committee of the Faculty of Medicine of the University Complutense of Madrid. Absence of external and internal congenital malformations was verified. Specimens were fixed in 10% formaldehyde and transferred to the laboratory. The parameters used to determine age of gestation were crown–rump length (CR), weight and cranial perimetry (O'Rahilly & Müller, 1996) (Table 1). Specimens were then dehydrated in graded ethanol and finally embedded in paraffin wax. The usual laboratory procedures were used to prepared 8–10-µm-thick transverse, frontal and sagittal serial sections, which were stained with haematoxylin–eosin and azan (McManus & Mowry, 1968) for light microscopy study.
Table 1.
Details of the embryonic period
| O'Rahilly stage | Section plane | CR length (mm) | Embryo | Results |
|---|---|---|---|---|
| 13 | T | 6 | OY-4 | Presumptive stapedial area |
| 14 | T | 7 | GV-4 | Appearance of the stapedial anlage |
| 15 | T | 8 | PT-11 | |
| 16 | T | 9 | BOT-9 | Relationship between the stapedial artery and the stapedial anlage. Appearance of the interhyale |
| 16 | T | 10.75 | CIV-3 | |
| 17 | T | 12 | MAR-12 | Delimitation of the parts of the stapedial anlage |
| 17 | T | 13 | GV-6 | |
| 17 | T | 13.6 | FAUS-6 | |
| 18 | T | 14.5 | GV-8 | |
| 18 | T | 15 | NO | |
| 18 | T | 16 | CIV-2 | Chondrogenesis phase. Start of involution of the stapedial artery |
| 19 | F | 17 | ESC-14 | |
| 19 | T | 17 | PA | |
| 19 | T | 18 | MAR-2 | |
| 20 | F | 18.5 | PT-5 | Delimitation of the ossicular anlages. Cartilaginous phase. Disappearance of the stapedial artery |
| 20 | S | 19 | PR | |
| 20 | S | 20 | BR-3 | |
| 20 | T | 20 | Be-1 | |
| 20 | F | 21.5 | AR | |
| 21 | F | 22 | GV-6 | |
| 21 | S | 22 | GV-7 | |
| 22 | F | 26 | PT-10 | Delimitation of the interhyale |
| 22 | F | 26.5 | GI-4 | |
| 23 | F | 28 | BR-4 | Anlage of the stapedial muscle tendon |
| 23 | T | 28.5 | BR-2 |
The nomenclature used corresponds to the English version of the Terminología Anatómica of the Federative Committee on Anatomical Terminology (FCAT, 2001).
Results
O'Rahilly's stage 13: presumptive stapedial area: phase of undifferentiated mesenchyme
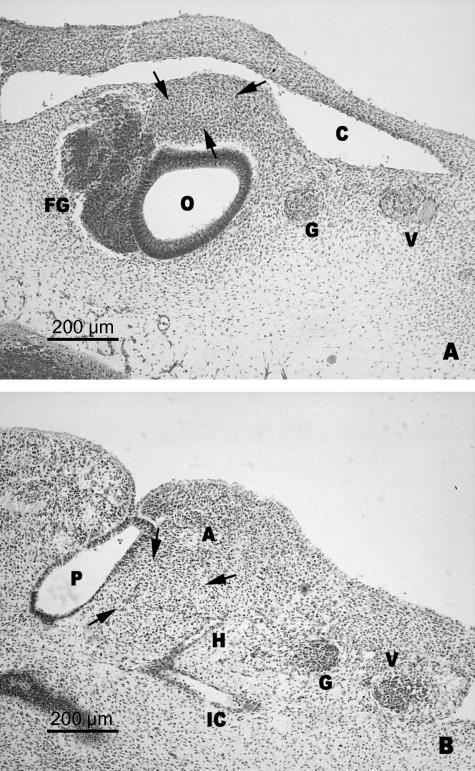
In this stage there is still no sign of the stapedial anlage. The area where the stapedial anlage is supposed to develop comprises undifferentiated mesenchyme, located at the upper end of the second branchial arch. Cranially it is delimited by the cephalic portion of the anterior cardinal vein and the anlage of the most inferior portion of the otic vesicle (Fig. 1A). Caudally, in the mesenchyme of the second arch the hyoid artery was observed arising from the primitive internal carotid artery (Fig. 1B).
Fig. 1.
(A,B) Human embryo OY-4 (6 mm CR; O'Rahilly stage 13). Transverse sections. Delimitation of the presumptive stapedial area (arrows). Anterior cardinal vein (C). Otic vesicle (O). Facio-vestibulocochlear ganglion (FG). Glosopharyngeal nerve (G). Vagus nerve (V). The hyoid artery (H) is derived from the internal carotid artery (IC). The hyoid artery penetrates the mesenchyme of the second branchial arch (A). First pharyngeal pouch (P).
O'Rahilly's stages 14 and 15: appearance of the stapedial anlage: phase of mesenchymal condensation
During these stages, the stapedial anlage consists of a mesenchymal condensation with an approximately ovoid morphology (Fig. 2B). The superior end of the stapedial anlage, located in the most cranial region of the second arch, lies between the inferior end of the cochlear pouch and the anterior cardinal vein. The inferior end of the stapedial anlage was delimited ventrally by the dorsal wall of the first pharyngeal pouch, dorsally by the second arterial arch (hyoid artery) and laterally by the nerve of the second arch (facial nerve) (Fig. 2A,B). The anlage of the stapedial artery developed from the hyoid artery (Fig. 2A,B). This arterial bud extended towards the stapedial condensed mesenchyme.
Fig. 2.
(A) Human embryo PT-11 (8 mm CR; O'Rahilly stage 15). Transverse section. (B) Diagrammatic representation. The anlage of the stapedial artery (S) originating from the hyoid artery (H) begins to enter the stapedial anlage (ST), situated between the first pharyngeal pouch (P), the hyoid artery (H) and the facial nerve (F). Otic vesicle (O). Internal carotid artery (IC).
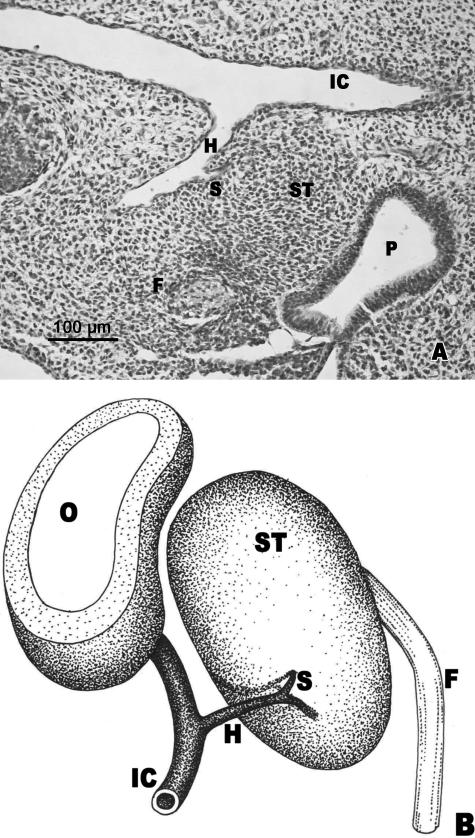
O'Rahilly's stage 16: relationship between the stapedial artery and the stapedial anlage: appearance of the interhyale
During this stage, the stapedial artery crossed the inferior end of the stapedial anlage (Fig. 3A,C). It crossed the stapedial mesenchyme following a cranial, lateral and ventral direction. After crossing the stapedial anlage it passed below the facial nerve and the anterior cardinal vein to continue as the supraorbital artery.
Fig. 3.
(A,B) Human embryo CIV-3 (10.75 mm CR; O'Rahilly stage 16). Transverse sections. (C) Diagrammatic representation. The stapedial artery (S) crosses the inferior part of the stapedial anlage (ST). The interhyale (I) is in front of the facial nerve (F). Otic vesicle (O). Internal carotid artery (IC). Reichert's cartilage anlage (R).
The interhyale was observed as a mesenchymal condensation located in front of the facial nerve (Fig. 3B,C). This structure established continuity between the stapedial anlage and the blastematic condensation that gives rise to Reichert's cartilage (Fig. 3C).
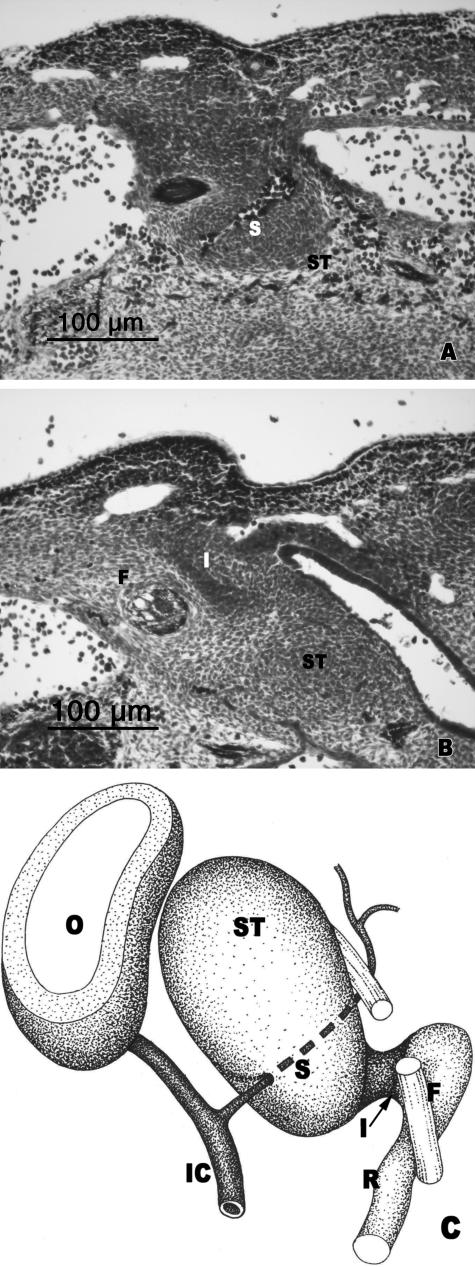
O'Rahilly's stage 17: delimitation of the parts of the stapedial anlage
During this stage it was observed how the stapedial anlage was independent of the mesenchymal condensation that will form the otic capsule. Different parts can be distinguished: the upper portion, which will constitute the base, located medially to the facial nerve, adjacent to the otic capsule but independent of it (Fig. 4A,C); and the lower portion, which will form the two limbs, appears as two condensations separated by the trajectory of the stapedial artery, which in this stage has the greatest diameter (Fig. 4B,C). The interhyale joined the dorsolateral condensation to the cranial end of the precartilaginous mesenchymal condensation that will form Reichert's cartilage (Fig. 4B,C).
Fig. 4.
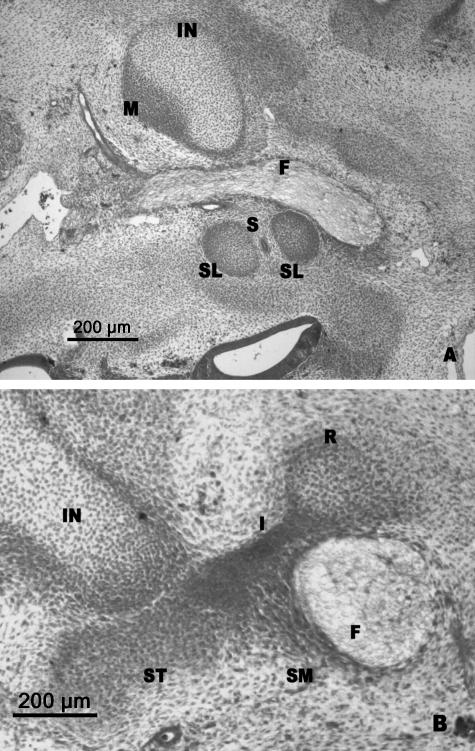
(A,B) Human embryo GV-6 (13 mm CR; O'Rahilly's stage 17). Transverse sections. (C) Diagrammatic representation of the stapedial anlage. Superior portion of the stapedial anlage (SB) medial to the facial nerve (F). Geniculate ganglion (GG). Otic vesicle (O). Anlage of the stapedial limbs (SL) separated by the stapedial artery (S). The interhyale (I) connected with the stapedial limb (SL). Facial nerve (F). Internal carotid artery (IC). Reichert's cartilage anlage (R).
O'Rahilly's stages 18 and 19: chondrogenesis phase: start of involution of the stapedial artery
From stage 18 onwards, the otic capsule and the anlages of the ossicles of the middle ear started to differentiate into cartilage.
The future base or footplate of the stapes was mostly enclosed by the otic capsule and clearly differentiated from it. The two limbs of the stapes were well defined and the stapedial artery ran between them (Fig. 5A). This artery started its involution during its intrastapedial trajectory, corresponding to the portion of the artery near to where this leaves the internal carotid artery.
Fig. 5.
(A,B) Human embryo CIV-2 (16 mm CR; O'Rahilly's stage 18). Transverse sections. The two stapedial limbs (SL) are differentiated and the stapedial artery (S) passes between them in involution. The interhyale (I) lies between the stapes (ST) and the cranial end of the precartilage of the second arch (R). The anlage of the stapedial muscle (SM) is medial to the facial nerve (F). Incus (IN). Malleus (M).
The head of the malleus was contiguous with the incus. The incus was connected via its crus longum with the future head of the stapes (Fig. 5B).
The interhyale did not undergo any structural modification and remained as a well-defined mesenchymal condensation. The interhyale formed a mesenchymal bridge that connected the stapes with the cranial end of the future Reichert's cartilage (Fig. 5B). Both the stapes and the precursor of Reichert's cartilage were clearly differentiated from the interhyale (Fig. 5B). The blastema was observed, which will give rise to stapedial muscle, situated medial to the facial nerve and close to the interhyale (Fig. 5B).
O'Rahilly's stages 20 and 21: delimitation of the ossicular anlages: cartilaginous phase: disappearance of the stapedial artery
During this stage, the section of the stapedial artery in its intrastapedial trajectory had almost disappeared.
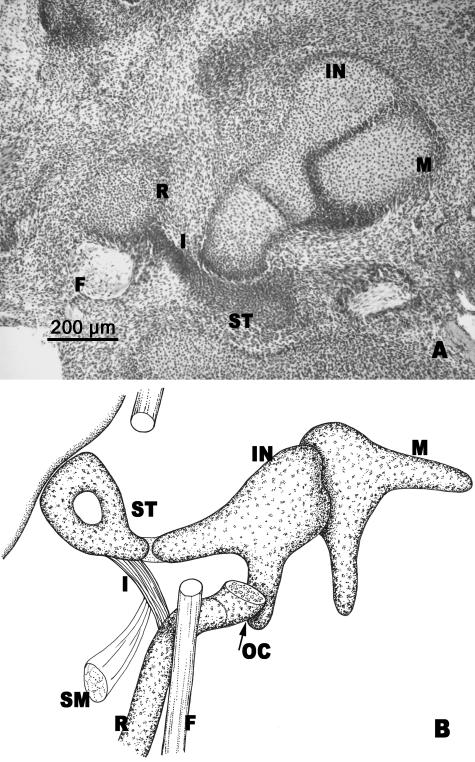
Ossicular anlages were well formed. The following arrangement was observed: the crus longum of the incus made contact with the stapes and this, in turn, with the interhyale (Fig. 6A,B). The interhyale located between the stapes and Reichert's cartilage was perfectly developed (Fig. 6A,B). Laterally, the interhyale reached the area where the cranial end of Reichert's cartilage had joined the inferior prolongation of the otic capsule (Fig. 6B).
Fig. 6.
(A) Human embryo AR (20.5 mm CR; O'Rahilly's stage 20). Frontal section. (B) Diagrammatic sketch of the ossicles of the middle ear. Malleus (M). Incus (IN). The interhyale (I) lies between the stapes (ST) and the cranial portion of Reichert's cartilage (R). Facial nerve (F). Otic capsule (OC). Stapedial muscle (SM).
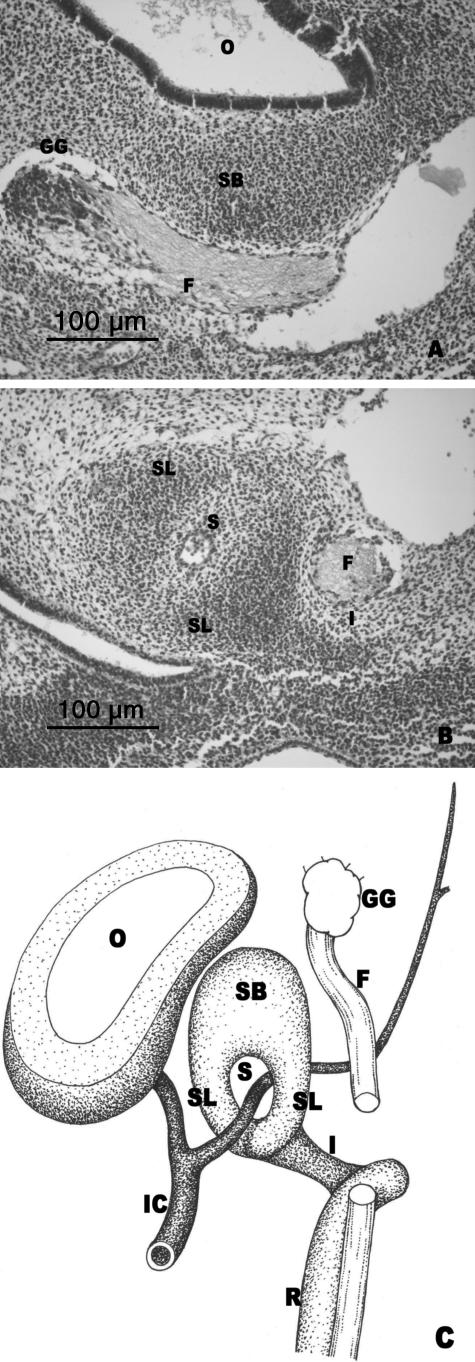
O'Rahilly's stage 22: delimitation of the interhyale
The superior segment of the stapedial anlage (anlage of the stapedial base) adopted a kidney shape surrounded by the otic capsule. Between the stapes and the membranous labyrinth there was a fine mesenchymal layer connected to the otic capsule (Fig. 7A,C).
Fig. 7.
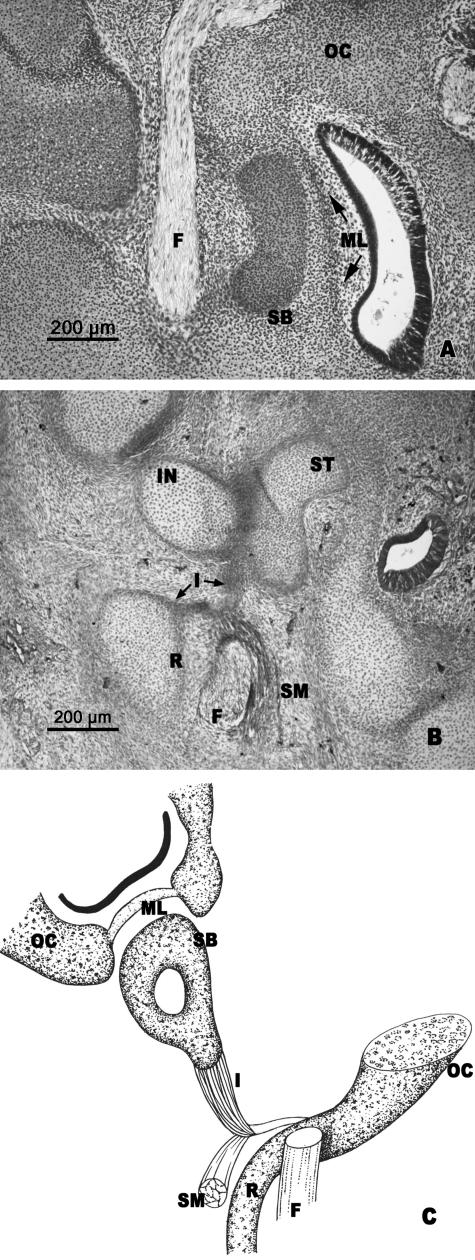
(A,B) Human embryo GI-4 (26.5 mm CR; O'Rahilly's stage 22). Frontal sections. (C) Diagrammatic sketch of the arrangement of the structures in O'Rahilly's stage 22. The anlage of the stapedial base (SB) is enclosed by the otic capsule (OC). The medial surface of the stapedial base (SB) is adjacent to the mesenchymal layer (ML), which is continuous with the otic capsule. The stapedial muscle (SM) is attached to the vertex of the angulation of the interhyale (I). The cranial end of Reichert's cartilage (R) is continuous with the otic capsule (OC). Incus (IN). Facial nerve (F). Stapes (ST).
The interhyale had an angular shape. The stapedial muscle was attached to the vertex of interhyale angulation (Fig. 7B,C). This interhyale morphology revealed the presence of two segments, a thicker one from the vertex of the angulation to the stapes and another lateral one from the attachment of the stapedial muscle to the area where the cranial end of Reichert's cartilage joined the otic capsule (Fig. 7B,C).
O'Rahilly's stage 23: anlage of the stapedial muscle tendon
At the end of the embryonic period, the cranial end of Reichert's cartilage was clearly curved. This portion was joined to the inferolateral extension of the otic capsule (Fig. 8). The interhyale was located between the stapes and the cranial end of Reichert's cartilage, with two well-defined segments, one thick internal one, the future tendon of the stapedial muscle, and another fine one in clear regression (Fig. 8).
Fig. 8.
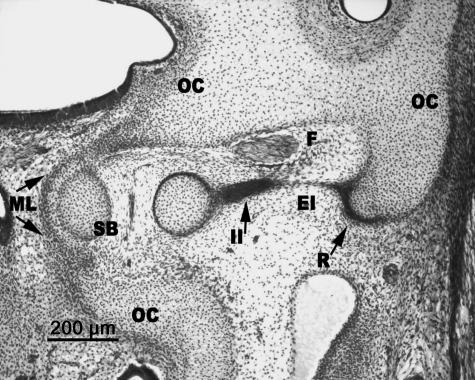
Human embryo BR-4 (28 mm CR; O'Rahilly's stage 23). Frontal section. The stapedial base (SB) is adjacent to the mesenchymal lamina (ML) that closes the anlage of the oval window in the otic capsule (OC). The internal segment of the interhyale (II) that forms the tendon of the stapedial muscle is clearly differentiated from the very thin external segment (EI) that reaches Reichert's cartilage (R) and, in turn, is joined to the otic capsule (OC).
The medial surface of the stapedial footplate was adjacent to the fine mesenchymal lamina, which was continuous with the cartilaginous otic capsule (Fig. 8).
Discussion
The stapedial anlage was first identified in an embryo of 7 mm CR length, as also described by Cauldwell & Anson (1942), Altmann (1950) and Hanson et al. (1962). According to my findings, this anlage was distinguished by its ovoid shape and its location in the cranial end of the second branchial arch with a superior pole that reached the lower portion of the otic vesicle, and another inferior one delimited by the dorsal wall of the first pharyngeal pouch, the second arterial arch (hyoid artery) and the facial nerve. The relationships presented by the stapedial anlage confirm that their origin is in the mesenchyme of the second branchial arch as Hanson et al. (1962) and Mugnier (1964) have described. However, some histochemical investigations have revealed pre-cartilaginous blastema before any morphological signs (Louryan & Glineur, 1991; Milaire, 1991).
In this same period of persistence of the segment of the second arterial arch, the stapedial artery emerges and its bud first penetrates the stapedial blastema through its inferior pole. I consider that growth and expansion of the end of the first pharyngeal pouch results in the mesenchyme of the second arch lying between this and the facial nerve being narrower, and this is where the so-called interhyale described by Hanson et al. (1962) and Louryan (1993) will be formed.
In my opinion, and in agreement with the results obtained during O'Rahilly's stage 16, the following formations can be described in the mesenchyme of the second branchial arch:
The stapedial anlage, situated in the cranial end of the second arch, characterized by its ovoid shape in which two parts or continuous areas can be distinguished not previously described by any author: (a) one cranial one, situated medial to the trajectory of the facial nerve and the anterior cardinal vein and lateral to the otic vesicle. In my opinion, this forms the anlage of the stapedial base or footplate. (b) Another larger caudal one, situated between the first pharyngeal pouch, the facial nerve and the primitive internal carotid artery. I consider this to correspond to the anlage of the stapedial limbs and head.
The stapedial mesenchyme is continuous laterally with another, very evident condensation, located ventrally to the facial nerve, which I consider to be the interhyale. I observed this at a stage earlier than those reported by Louryan (1993), who described this formation at 13.5 mm CR length.
Finally, the interhyale was continuous with the mesenchymal formation corresponding to the future Reichert's cartilage, the cranial end of which is situated lateral to the facial nerve; this portion corresponds to the laterohyale described by Hanson et al. (1962).
After these stages, the caudal portion of the stapedial anlage will be crossed by the stapedial artery and two condensed areas can be observed corresponding to the anlages of the stapedial limbs. Therefore, only the lower part of the stapedial anlage is crossed by the artery, an organization not described previously. On the other hand, I agree with Hanson et al. (1962) in that the anular form of the stapes is due to the presence of this artery. This was supported experimentally by Louryan (1991), who confirmed how the stapedial anlages of the hyoidal arches extirpated from mice were columellar shaped and were not arched. Although Masuda et al. (1987) and Tamura et al. (1993) observed experimentally that the stapes acquired a ring shape without the presence of the stapedial artery, I consider that the mouse ear cultures were made in stages that were too late to demonstrate acurately the influence of the artery on stapedial morphology.
In O'Rahilly's stage 18, the blastema of the stapedial muscle was situated medial to the facial nerve and close to the interhyale but independent of it. I therefore reject the classical theory of Hanson et al. (1962) and the most recent one by Louryan (1993) who suggested that the interhyale was the origin of this muscle.
In stage 20, the inferior and lateral prolongation of the otic capsule, termed by Macklin (1921) the crista parotica, had become continuous with the cranial end of Reichert's precartilage and this, in turn, was connected by the interhyale with the precartilaginous mould of the stapes. This embryonic arrangement of the cranial end of Reichert's cartilage has not been described before. The interhyale has a similar structure to that presented by the insertion tendon of the malleus muscle. Although the precartilage could be demonstrated before its morphological sign (Louryan & Glineur, 1991; Milaire, 1991), I did not observe in any stage chondrification of the interhyale. The obliteration stage of the stapedial artery coincides with establishment of the precartilaginous mould of the stapes.
I observed how the muscular blastema of the stapes muscle, medial to the facial nerve, ended in the interhyale. The muscle and tendon must therefore have different origins, in contrast to the opinion given by Hanson et al. (1962) and Louryan (1993).
In O'Rahilly's stage 22, a mesenchymal area was observed between the stapedial anlage and the membranous labyrinth, belonging to the otic capsule, which I do not consider to form the base, as suggested by Cauldwell & Anson (1942), Bast et al. (1956) and Masuda et al. (1978), but only the fine perichondral lamina of its vestibular surface, as this did not undergo any cartilaginous transformation. In this stage, the interhyale had acquired an angular shape, attaching itself to the stapedial muscle at the vertex of the angulation. Owing to this interhyale morphology two segments are observed: one thicker internal one from the vertex of the angulation to the stapes and another lateral one from the vertex of the angulation to the point where the cranial end of Reichert's cartilage continued with the inferior prolongation of the otic capsule.
These descriptions, not noted previously, suggest that only the internal part of the interhyale gives rise to the tendon of the stapedial muscle, which is why I refer to it as the stapedial portion. I reject the theory that the whole of the interhyale produces the tendon and the stapedial muscle (Hanson et al. 1962; Louryan, 1993).
Therefore, at the second arch and at the end of the embryonic period, I differentiated craniocaudally the following formations: the stapedial anlage, the interhyale and Reichert's cartilage.
I conclude that the only relationship existing between the cranial portion of Reichert's cartilage and the stapedial anlage is based on an interhyale structure, part of which regresses and part – the stapedial portion or internal segment – will form the tendon of the stapedial muscle.
The cranial portion of Reichert's cartilage does not correspond to or give rise to the stapes. The craneal end of Reichert's cartilage forms a hook which was in contact with the inferior portion of the otic capsule and corresponds to the laterohyale described by Hanson et al. (1962).
According to the observations reported here, the stapes will develop from the cranial mesenchyme of the second arch, which becomes rapidly differentiated from the cranial end of Reichert's cartilage by the interhyale. I cannot therefore agree with claims that Reichert's cartilage is the source of the stapes (Cauldwell & Anson, 1942; Hamilton & Mossman, 1975; Masuda et al. 1978; Corliss, 1979; Louryan & Glineur, 1992; Abramovich, 1997; Moore & Persaud, 1999; Nandapalan & Tos, 2000; Cochard, 2002; Sadler, 2004).
My results agree with those of Louryan & Glineur (1992) and Louryan et al. (2003) that formation of the stapes is completely independent of the otic capsule. The relationship between the stapes and the otic capsule is clearly observed at the end of the embryonic period in the area of the otic capsule differentiated in a thin mesenchymal cellular layer. This mesenchymal layer bears a relationship with the stapedial base, it covers the area of the oval window and peripherally constitutes the anlage of the anular ligament.
Therefore, I did not find any evidence for the dual development for the stapes from two separate anlages, one for the base and the other for the limbs and head (Cauldwell & Anson, 1942; Masuda et al. 1978; Ars, 1989; Sperber, 1989; Mallo, 1997; Nandapalan & Tos, 2000).
References
- Abramovich A. Embriología de la región maxilofacial. Buenos Aires: Editorial Médica Panamericana; 1997. [Google Scholar]
- Altmann F. Normal development of the ear and its mechanics. Arch Otolaryng. 1950;52:725–766. doi: 10.1001/archotol.1950.00700030751003. [DOI] [PubMed] [Google Scholar]
- Ars B. Organogenesis of the middle ear structures. J Laryngol Otol. 1989;103:16–21. doi: 10.1017/s0022215100107947. [DOI] [PubMed] [Google Scholar]
- Bast TH, Anson BJ, Richany SF. The development of the second branchial arch (Reichert's cartilage), facial canal and associated structures in man. Quart Bull Northw University Med Sch. 1956;30:235–249. [PMC free article] [PubMed] [Google Scholar]
- Cauldwell EW, Anson BJ. Stapes, fissula ante fenestram, and associated structures in man. Arch Otolaryng. 1942;36:891–925. doi: 10.1001/archotol.1948.00690040274001. [DOI] [PubMed] [Google Scholar]
- Cochard LR. Netter's Atlas of Human EmbryologyIcon Learning Systems. New Jersey: Teterboro; 2002. [Google Scholar]
- Terminología Anatómica. Madrid: Editorial Médica Panamericana; 2001. Comité Federal sobre Terminología Anatómica (FCAT) [Google Scholar]
- Corliss CE. Embriología humana de PattenFundamentos del desarrollo clínico. Buenos Aires: El Ateneo; 1979. [Google Scholar]
- Hamilton WI, Mossman HW. Embriología Humana. 4th. Buenos Aires: Intermédica; 1975. [Google Scholar]
- Hanson JR, Anson BJ, Strickland EM. Branchial sources of the auditory ossicles in man. Part II: Observations of embryonic stages from 7 mm to 28 mm (CR length) Arch Otolaryng. 1962;76:200–215. doi: 10.1001/archotol.1962.00740050208004. [DOI] [PubMed] [Google Scholar]
- Louryan S. In vitro development of mouse middle ear ossicles: a preliminary report. Eur Arch Biol. 1991;102:55–58. [Google Scholar]
- Louryan S, Glineur R. Lectin histochemistry in the developing oto-maxillo-facial primordial of the mouse embryo. Bull Group Int Rech Sci Stomatol Odontol. 1991;34:79–87. [PubMed] [Google Scholar]
- Louryan S, Glineur R. The mouse stapes develops mainly from the Reichert's cartilage independently of the otic capsule. Eur Arch Biol. 1992;103:211–212. [Google Scholar]
- Louryan S. Le développement des osselets de l’ouie chez l’embryon humain: corrélations avec les données recueillies chez la souris. Bull Ass Anat. 1993;77:29–32. [PubMed] [Google Scholar]
- Louryan S, Vanmuylder N, Résimont S. Ectopic stapes: a case report with embryologic correlations. Surg Radiol Anat. 2003;25:342–344. doi: 10.1007/s00276-003-0127-9. [DOI] [PubMed] [Google Scholar]
- Macklin ChC. The skull of a human fetus of a 48 millimeters greatest length. Carnegie Contrib Embryol. 1921;10:57–103. [Google Scholar]
- Mallo M. Retinoic acid disturbs mouse middle ear development in a stage specific fashion. Dev Biol. 1997;184:175–186. doi: 10.1006/dbio.1997.8519. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Saito R, Endo Y, Kondo Y, Ocura Y. Histological development of stapes footplate in human embryos. Acta Med Okayama. 1978;32:109–117. [PubMed] [Google Scholar]
- Masuda Y, So S, Naito M, Ogura Y. Bilaminar structure of the developing stapedial footplate in the mouse. A histological study using a light microscope. Auris Nasus Larynx. 1987;14:1–7. doi: 10.1016/s0385-8146(87)80002-0. [DOI] [PubMed] [Google Scholar]
- McManus JFA, Mowry RW. Técnica Histológica. Madrid: Atika; 1968. [Google Scholar]
- Milaire J. Lectin binding sites in developing mouse limb buds. Anat Embryol. 1991;184:479–488. doi: 10.1007/BF01236054. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. Embriología Clínica. 6th. México D.F.: McGraw-Hill Interamericana; 1999. [Google Scholar]
- Mugnier A. Embryologie et Développement Bucco-Facial. Paris: Masson; 1964. [Google Scholar]
- Nandapalan V, Tos M. Isolated congenital stapes ankylosis: an embryologic survey and literature review. Am J Otol. 2000;21:71–80. [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. Human Embryology and Teratology. 2nd. New York: Wiley-Liss; 1996. [Google Scholar]
- Sadler TW. Langman Embriología Médica. Con Orientación Clínica. 9th. Buenos Aires: Editorial Médica Panamericana; 2004. [Google Scholar]
- Sperber GH. Craniofacial Embryology. 4th. Cambridge: Wright; 1989. [Google Scholar]
- Tamura K, Nishizaki K, Takeda Y, Sumida S, Masuda Y. Suspension organ culture of the fetal mouse ear. Auris Nasus Larynx. 1993;20:239–246. doi: 10.1016/s0385-8146(12)80115-5. [DOI] [PubMed] [Google Scholar]