Abstract
Ligaments are composed of two major components: cells and extracellular matrix. The cells express gap junction proteins and are arranged into a series of rows that traverse the tissue, suggesting that all the cells of the tissue are functionally interconnected. The results of our study demonstrate that medial collateral ligament (MCL) cells do not have a uniform fusiform morphology or placement along a row of cells as previously suggested, but rather display a complex placement and form that weaves within the collagen matrix in a manner that is far more extensive and complex than previously appreciated. Within this morphological context, we find that MCL cells in vivo contain functional gap junctions (verified using fluorescence recovery after photobleaching) that are localized to sites of close cell–cell contact, and this pattern imparts or reflects a bipolarity inherent to each cell. When we studied ligament cells in conventional tissue culture we found that this bipolarity is lost, and the placement of gap junctions and their related proteins, as well as general cell morphology, is also altered. Finally, our study demonstrates, for the first time, that in addition to gap junctions, adherens junctions and desmosomes are also expressed by MCL cells both in vivo and in vitro and map to sites of cell–cell contact.
Keywords: adherens junctions, connexin, desmosomes, gap junctions, ligament
Introduction
Ligaments are anatomically discrete, dense connective tissue structures that connect bones across joints and serve kinematic, biomechanical and possibly neurosensory roles in guiding and protecting joint movements in mammals. These structures are composed of two major components: (1) an abundant extracellular matrix (ECM) and (2) ligament cells embedded in the ECM. Early studies of ligament structure and function have focused primarily on the ECM; however, recently the importance of ligament cells and their cell biology has become apparent. Several studies have established that cells in tensile-bearing connective tissues are arranged into a series of widely spaced rows with cytoplasmic extensions that extend from cells in one row to those in another (Benjamin & Ralphs, 1997; Bruehlmann et al. 2002; Lo et al. 2002a,b). It has been suggested that this arrangement forms a ‘cellular matrix’ that co-mingles with the ECM (Lo et al. 2002a). Although cells from tensile-bearing tissues appear to be physically connected in vivo, it is currently not known whether they are functionally connected and have the ability to exchange material from one cell to another, although this possibility has been raised by the detection of the gap junction proteins connexin 32 and 43 in these tissues (McNeilly et al. 1996; Yamaoka et al. 2000).
Gap junctions are unique membrane structures that allow the direct transfer of small molecules (< 1 kDa in size) between adjacent, contacting cells. These structures are composed of two hexameric connexons, each provided by one of two neighbouring cells. A ‘gap’ is left between the adjacent cell membranes but the two connexons interact and dock in the extracellular space to form tightly sealed, double-membrane intercellular channels. These channels have been implicated in cellular communication essential for development, tissue function and cell homeostasis. Gap junction formation is dependent on a family of proteins known as connexins (Cxs) that are encoded by a large gene family and are predicted to comprise at least 20 isoforms in humans (Herve et al. 2004; Segretain & Falk, 2004; Sohl & Willecke, 2004). In addition, recent studies have also shown that in addition to fully formed channels, hemi-channels by themselves may function in intra/extracellular signalling and this type of structure may be present in connective tissues (Li et al. 1996; Chi et al. 2004). Studies of gap junction structure and function have established that these structures are characterized by a complex cycle of connexin biosynthesis, followed by gap junction assembly, formation and removal (Herve et al. 2004; Segretain & Falk, 2004).
The current study was undertaken to document and map the position of gap junction structures within the medial collateral ligament (MCL) and to determine their functionality within the tissue. This study was undertaken in concert with a re-examination of the cellular organization of the MCL using the techniques of transmission electron microscopy (TEM) and scanning electron microscopy (SEM). In addition, in order to demonstrate that gap junctions are present and functional, the movement of molecules between cells was tracked using the technique of fluorescence recovery after photobleaching (FRAP). We show: (1) that MCL cells do not have a uniform fusiform morphology or placement along a row of cells as previously suggested (Frank & Hart, 1990; Woo et al. 2000), but rather display a complex distribution and form that weaves within the collagen matrix in a manner that is far more extensive and complex than previously appreciated (Frank & Hart, 1990; Benjamin & Ralphs, 2000; Woo et al. 2000); (2) that MCL cells in vivo contain functional gap junctions that are preferentially localized to sites of close cell–cell contact along the long axis of a cell row. This pattern imparts or reflects a bipolarity inherent to each cell. (3) Bipolarity as reflected by the placement of gap junctions is lost when MCL cells are placed in conventional tissue culture and the placement of gap junctions and their related proteins, as well as general cell morphology, is also altered. (4) In addition to gap junctions, adherens junctions and desmosomes are also expressed by MCL cells both in vivo and in vitro and are present near sites of gap junctions.
Materials and methods
The MCL of skeletally mature New Zealand white rabbits (n = 4) were excised. In some experiments the MCL from Wistar rats were used and all data obtained from the rabbit were verified using the rat MCL (data not shown). Samples destined for immunohistochemistry were placed in Tissue-Tek® Optimal Cutting Temperature (OCT) embedding medium (Sakura, Torrance, CA, USA) and quick-frozen while samples destined for electron microscopy were fixed in Karnovsky's fixative for a minimum of 24 h at 4 °C. Portions of the ligament destined for FRAP analysis were maintained in calcium-free phosphate-buffered saline (PBS; Sigma, St. Louis, MO, USA) supplemented with 2 µM EGTA.
Cell culture
Small portions of the MCL were placed in tissue culture media in 22-mm culture dishes. The cells were allowed to grow out from the tissue sample and were maintained in culture in Dulbecco's minimal essential medium–Ham's F12 mixture (DMEM-F12; Gibco, Grand Island, NY, USA) supplemented with fetal calf serum (FCS; Gibco) and antibiotics/antimycotics (Penicillin/Streptomycin/Fungizone®, Gibco). For FRAP studies, confluent primary cultures were trypsinized and re-seeded on coverslips and cultured for 2 days to allow cells to establish cell–cell contacts.
Electron microscopy
For TEM, tissues fixed in Karnovsky's were washed twice in 0.1 M cacodylate buffer containing 5 mM calcium and post-fixed for 1 h with 2% osmium tetroxide in 0.1 M cacodylate buffer, dehydrated in ethanol, and embedded in Spurr's resin (J.B. EM Services Ltd, Dorval, Quebec, Canada). Thin cross-sections (approximately 90 nm) were cut with a diamond knife on a Reichert ultramicrotome. Sections were stained with uranyl acetate and lead citrate and examined on a Hitachi H-7000 TEM microscope. Some samples were physically torn apart along the long axis of the tissue and immediately fixed in Karnovsky's fixative, dehydrated in a graded ethanol series, critical point dried, platinum coated and examined by SEM in a Phillips XL 30 ESEM.
Immunofluorescence histochemistry
Tissue sections and cultured cells plated on to coverslips were fixed in either 100% methanol or 3% paraformaldyhyde in PBS. Fixed tissue samples were rinsed in PBS and incubated in primary antibodies to: connexin 43 (1 : 100, Zymed, San Francisco, CA, USA), as well as beta-catenin (1 : 100, Transduction Laboratories, Lexington, KY, USA), zona occludens-1/tight junction protein-1 (ZO-1, 1 : 100, Chemicon International) and desmopakin II (DSI II, 1 : 100, a gift from Dr Manijah Pasdar, University of Alberta). Following incubation in the primary antibody, all samples were rinsed three times in PBS and incubated in the appropriate Cy™-3-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 30 min at 37 °C. Samples were mounted in 90% glycerol containing the anti-fade reagent N,N-paraphenylenediamine and examined either by standard indirect immunofluorence using a Leica Dm microscope or by laser-scanning confocal microscopy (Zeiss LSM 510). Single confocal images and stacks of serial optical sections (∼30 µm high) were made using a × 25 0.8-NA multi-immersion objective (Plan-Neofluor; Zeiss). Higher magnification images were obtained using a × 63, 1.2-NA water-immersion objective (C-Apochromat; Zeiss). The fluorescence intensity of cells was measured using the calibrated measuring tool in Image J (v. 1.30, http://rsb.info.nih.gov/ij/).
Gap junction analysis by FRAP
Fresh ultrathin MCL shavings were sectioned along the long axis of the tissue using a no. 11 scalpel blade, and were immediately incubated in Ca2+-free PBS containing the fluorescent dye calcein-AM (5 µM, Molecular Probes, Eugene, OR, USA), which passes freely into the cells and is captured inside the cells after esterase cleavage. After a 30-min incubation at 37 °C, calcein-stained samples were washed three times in Ca2+-free PBS, placed on a coverslip, covered with a drop of 2% low-melting-point agarose (NuSieve®-GTG® agarose, FMC Bioproducts, Rockland, ME, USA) in Ca2+-free PBS (to immobilize the sample), and examined by confocal microscopy at room temperature. Although the configuration of our confocal microscope did not allow the control of temperature, many previous studies, including those in our laboratory (Chi et al. 2004), have demonstrated functional gap-junction activity at temperatures both lower and higher than 37 °C (Tadvalkar & Pinto da Silva, 1983; Spray & Bennett, 1985; Chen & DeHaan, 1993; Rozental et al. 1995; Stelling & Jacob, 1997; Srinivas et al. 2001). Some specimens were prepared in Ca2+-free PBS medium containing the gap-junction blocker 1-octanol at a concentration of 1.0 mM (Chi et al. 2004; Hunter et al. 2003).
Prior to photobleaching, an initial image was obtained to establish the initial fluorescence intensity of the cells. One cell along a row of closely spaced cells was chosen randomly and repeatedly photobleached with 40–60 pulses of a 488-nm argon laser operating at 100% power, until the calcein fluorescence was barely visible (i.e. ∼30–40% of the original fluorescence). Three subsequent images of the same cells were acquired 5, 10 and 15 min after photobleaching. The fluorescence intensity of individual cells was evaluated as the mean pixel intensity within a cell using manually defined regions of interest in the Image J software.
As a small amount of photobleaching normally occurs when exposing a confocal photomicrograph, the fluorescence intensity of the cells that were repeatedly photobleached was normalized to that of a ‘non-neighbouring’ cell in the same image field for each time interval (to account for any loss due to the photographic exposure). The normalized fluorescence values were then used to calculate the percentage recovery of fluorescence 15 min after photobleaching using the following formula:
% Recovery = [(T15 − Tb)/T0] × 100
where T0 is the initial fluorescence, Tb fluorescence after photobleaching and T15 fluorescence 15 min after recovery.
Results
Ligament cells have a complex morphology and relationship to one another that is related to gap-junction placement
The proteins connexin 26, 32 and 43 have been detected in a wide variety of connective tissues derived from a number of different species using monospecific antibodies (McNeilly et al. 1996; Benjamin & Ralphs, 1997; Yamaoka et al. 2000; Bruehlmann et al. 2002; Lo et al. 2002a; Hunter et al. 2003; Chi et al. 2004). These observations have led to the assertion that gap junctions are a common feature of connective tissue cells and function in intercellular communication. Because gap junction proteins can localize to regions other than sites of fully formed gap junctions, and gap junctions have not been detected by TEM in connective tissue, it is still unclear where gap junctions are positioned in these tissues and how their placement relates to overall tissue architecture. To clarify this point we set out to map the position of gap junctions between ligament cells from the midsubstance of the MCL using electron microscopy.
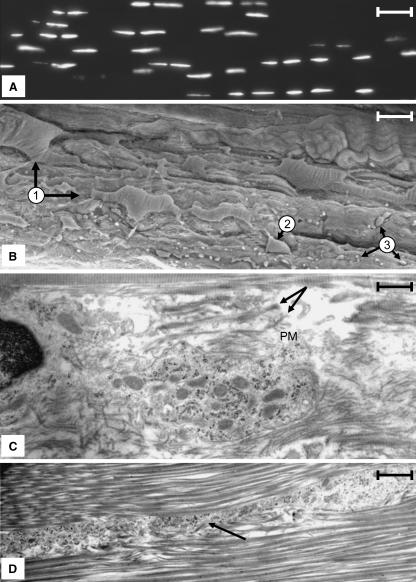
In the course of our study we found that our morphological observations of ligament cells using both light and electron microscopy did not agree with the traditional description of these cells (Frank & Hart, 1990; Woo et al. 2000). Our findings are summarized in the following section. Consistent with previous reports (Frank & Hart, 1990; Woo et al. 2000; Lo et al. 2002a,b), when the nuclei of cells were stained with DAPI and viewed by fluorescence microscopy, it was clear that ligament cells are arranged into a series of rows (Fig. 1A). However, within a single 8–15-µm section, it was difficult to follow a row of cells for an extended distance along the long axis of the tissue because the rows would extend out of the plane of the tissue section. The exact relationship between adjacent cells was clarified when rows of cells within ligament sections were examined by both SEM and TEM. SEM preparations revealed that adjacent cells within a row varied with respect to cellular morphology, alignment within the tissue and spacing between the cell bodies of adjacent cells (Fig. 1B). In addition, these images also confirmed the presence of abundant and extensive cytoplasmic projections that extended from the cell body far out into the surrounding collagen matrix (Fig. 1B).
Fig. 1.
(A) Frozen section of a rabbit MCL stained with DAPI for DNA. Nuclei are arranged in rows that pass along the long axis of the tissue (right–left). Note the rows cannot be followed continuously for long distances. Scale bar = 40 µm. (B)SEM micrograph of a rabbit MCL that has been torn along the long axis of the tissue revealing rows of cells (arrow 1). The cells are large (in many instances a portion of the cell is buried in the collagen matrix; compare the two cells denoted at 1), having a complex morphology and displaying prominent cytoplasmic extensions. Portions of these extensions permeating the collagen matrix are denoted at 3. The diversion of a row into another plane within the tissue is shown at 2. Scale bar = 25 µm. (C) A region between two adjacent cells within a row illustrating the pericellular matrix, which is bordered by large collagen fibres characteristic of the ECM. The pericellular matrix is populated, with collagen fibres of varying sizes (arrows) and distribution paths (arrow) as well as abundant vesicles. Scale bar = 11 µm. (D) A vesicle-filled seam representing an extension of the pericellular matrix that extends between collagen bundles into the ECM. These seams contain cytoplasmic extensions of the ligament cells as well as vesicular material similar that that seen in the pericellular matrix adjacent to the cell. Scale bar = 0.5 µm.
TEM micrographs revealed that the region around both the cell body and its associated cytoplasmic projections appeared to be surrounded by a structure distinct from the ECM that was characterized by a few small-diameter collagen fibres, elastin fibres (data not shown) and numerous vesicles (Fig. 1C). In this report we will refer to this zone as the pericellular matrix. The pericellular matrix encased both the cell body and the cytoplasmic extensions that extended from the cell body. In the latter case, the pericellular matrix formed vesicle-filled seams that separated bundles of collagen fibres that populated the ECM (Fig. 1D). Portions of the cell body that were positioned along the lateral margins of the rows away from points of cell–cell contact were often associated with little or no detectable pericellular matrix. In these regions, collagen fibres appeared to lie directly along the plasma membrane.
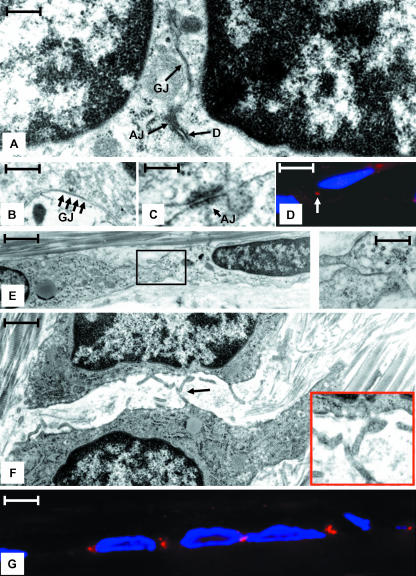
When rows of cells were examined in thin sections, it was apparent that the differential placement of the main cell body of adjacent cells resulted in the placement of adjacent nuclei either close to or far apart from one another (compare Fig. 2A,E). Mapping studies using TEM revealed the presence of gap junctions in regions of cell–cell contact between adjacent cells (Fig. 2A,E (inset)). These sites were of two types. In the first, the cells shared an extensive region of cell–cell contact and adjacent nuclei were positioned close to the paired surface. This type of surface was populated with gap junctions as well as adherens junctions, desmosomes (Fig. 2A–D) and proteins unique to these structures (Fig. 2D). In the second type, the cells were connected by an extension of the cell body and adjacent nuclei were positioned some distance from the paired surface. In this case the paired surface was smaller and structures such as gap junctions, adherens junctions and desmosomes were often found in isolation (Fig. 2E). In the regions adjacent to areas of cell–cell contact, cells displayed a series of short cytoplasmic projections. In some cases, projections from adjacent cells appeared to touch one another (Fig. 2F (arrow)).
Fig. 2.
(A) TEM micrograph illustrating two adjacent cells connected by a gap junction (GJ), an adherens junction (AJ), and a desmosome (D) in a region were two cells and their nuclei are in close appositions. Scale bar = 50 nm. (B) High-magnification image of a region similar to that shown in A demonstrating an extensive gap junction (GJ, arrows). Scale bar = 25 nm. (C) High-magnification region of a region similar to that shown in A illustrating an adherens junction (AJ). Scale bar = 30 nm. (D) Indirect immunofluorescent image of cells reacted with DSI II, a desomosome marker (red), as well as DAPI to denote nuclei (blue). Note the prominent reactivity in the region between cells (arrow) at the site of desmosomes detected by TEM. Scale bar = 8 nm. (E) Two adjacent cells connected by a gap junction (boxed regions and shown in inset) in a region where the nuclei are far apart. Scale bar = 1.5 nm, inset = 10 nm. (F) Adjacent cells in a region lateral to a region shown in A where the surfaces of the cells are not in close apposition. Note the numerous short cytoplasmic projections. These projections often come in close apposition (arrow). Scale bar = 2 µm. (G) Immunoflourescence image showing the pattern of Cx43 staining (red) in vivo. The staining pattern corresponds to the placement of gap junctions seen by TEM. Nuclei are stained with DAPI (blue). Scale bar = 10 µm.
In parallel with the electron microscopy study, immunofluorescence microscopy revealed that a majority of the connexin 43 (Cx43) found in the body of ligament cells was localized to the region where gap junctions were found (Fig. 2D). This distribution of Cx43 highlights the periodic nature of gap junction placement along a row of ligament cells as detected by TEM, and indicates that gap junction placement either determines or reflects the bipolar nature of each ligament cell.
Gap junctions have a different distribution pattern in ligament cells in vivo
As demonstrated in the previous section, gap junctions associated with the body of ligament cells in vivo are confined to points of cell–cell contact that are positioned at intervals along a row of cells. As cultured ligament cells are frequently used to study ligament cell biology, and are often used particularly in the fields of tissue repair and tissue engineering, we carried out a study to compare the relationship between gap junction organization in vivo and in vitro.
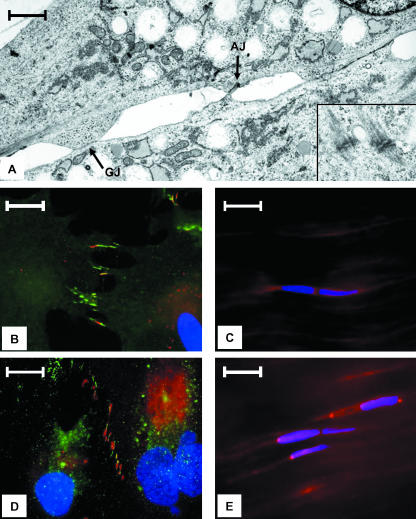
TEM studies indicated that gap junctions, adherens junctions and desmosomes are also expressed in cultured ligament cells at points of cell–cell contact (Fig. 3A and inset). When viewed using indirect immunofluoresence microscopy, these sites were found to be associated with a series of pronounced cytoplasmic extensions that extended from all the lateral surfaces of these cells (Fig. 3B). Unlike cells in vivo, sites of Cx43 staining were not confined to small foci but were distributed throughout the length of the extension (Fig. 3B). Thus, the in vivo bipolarity of cells, as reflected by gap junction placement, was not observed for cells maintained in monolayer culture in vitro.
Fig. 3.
(A) TEM micrograph taken from a region of contact between two MCL cells in culture. Note the presence of gap junctions (arrow GJ) and an adherens junction (arrow AJ). Desmosomes from a similar region of another set of cells is shown in the inset. Scale bar = 15 µm. (B) MCL cell in culture stained with Cx43 (red), beta-catenin (green) and DAPI (blue). Note that these proteins are concentrated in cytoplasmic projections and frequently co-localize. Scale bar = 20 µm. (C) Beta-catenin (red) distribution pattern and DAPI (blue) seen in MCL cells in vivo. Scale bar = 20 µm. (D) L cell in culture illustrating that Cx43 (red) and ZO-1 (green) are concentrated in cytoplasmic extensions and often co-localize. Nuclei are stained with DAPI (blue). Scale bar = 20 µm. (E) In vivo ZO-1 distribution pattern (red). Nuclei are stained with DAPI (blue). Scale bar = 20 µm.
Because gap junction formation in some cell types is known to be dependent on the proteins ZO-1 and beta-catenin (Wu et al. 2003; Giepmans, 2004), we investigated whether MCL cells in vivo and in vitro express these two proteins, and if these proteins co-localize with gap-junction sites. In monolayer culture, both beta-catenin and ZO-1 are preferentially localized to cytoplasmic extensions (Fig. 3B,D). When superimposed over Cx43 staining patterns, it was clear that beta-catenin and Cx43 co-localized in cytoplasmic extensions; however, some extensions only contained beta-catenin (Fig. 3D), consistent with the notion that the presence of beta-catenin may precipitate the aggregation of Cx43 (Wu et al. 2003). Parallel experiments in tissue indicated that both beta-catenin and ZO-1 co-localized with sites containing gap junctions, but both proteins had a more generalized distribution pattern compared with cells grown in monolayer culture (Fig. 3E and data not shown). Thus, the distribution pattern of proteins associated with specific junctional complexes changes when ligament cells are placed in culture.
Gap junctions form a functional link between adjacent ligament cells in vivo
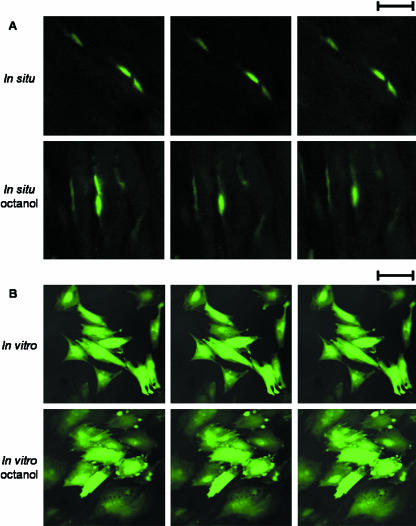
To verify that the presence of gap junctions at sites of cell–cell contact serves as a functional link between these cells in vivo, we stained MCL tissue with calcein-AM and performed FRAP using the confocal microscope. Such an approach has previously been used to verify the functional link between cells derived from other connective tissues in vitro and in vivo (Hunter et al. 2003; Chi et al. 2004), but this technique has not been employed for functional studies of cells within the ligament. To carry out these studies, nuclei from two closely apposed cells were identified in situ, the calcein-AM from the nuclei in one of these cells was photobleached, and the recovery was monitored over a 15-min interval (Fig. 4A). A similar photobleaching experiment was undertaken with MCL cells grown in monolayer culture (Fig. 4B). Fluorescence recovery was observed consistently for MCL cells in monolayer culture (18.2 ± 2.4% recovery) and in situ (49.9 ± 4.4%). When MCL cells in situ or in vitro were exposed to octanol, an agent that disrupts gap-junction activity, fluorescence recovery was minimal for MCL cells in monolayer culture (5.6 ± 2.2%) and in situ (12.2 ± 3.7%) (Fig. 4A,B). Taken together, these observations suggest that gap junctions could function as a conduit of intercellular communication between neighbouring cells in rows within the rabbit MCL. In addition, as gap junctions allow for transfer of small molecules between neighbouring cells, it is possible for molecules to be passed serially along a row of cells via transfer between neighbouring cells.
Fig. 4.
Fluorescence recovery after photobleaching (FRAP) experiments. (A) Confocal photomicrographs of MCL cells in situ. Time series showing calcein-AM fluorescence just before, just after and 15 min after photobleaching for untreated cells (top half) and for cells treated with the gap-junction disrupting agent octanol (bottom half). Scale bar = 25 µm. (B) Confocal photomicrographs of MCL cells in vitro. Time series showing calcein-AM fluorescence just before, just after and 15 min after photobleaching for untreated cells (top half) and for cells treated with the gap-junction disrupting agent octanol (bottom half). Scale bar = 25 µm.
Discussion
The preceding study illustrates the following points: (1) MCL cells do not have a uniform fusiform morphology or spacing along a row, but rather display a complex form and distribution pattern that weaves in a twisted rather than a steady linear pattern within the collagen matrix. This arrangement is far more extensive and complex than previously reported (Frank & Hart, 1990; Woo et al. 2000). (2) In MCL cells in vivo, gap junctions are preferentially localized to sites of close cell–cell apposition along a row of cells, and this placement reflects or establishes a structural bipolarity to each cell. (3) Adherens junctions and desmosomes are expressed by ligament cells both in vivo and in vitro and these structures are often positioned near sites of gap junctions. (4) Bipolarity as indicated by the placement of gap junctions is lost when ligament cells are grown in monolayer culture. In addition, the distributions of junctional proteins as well as the general architecture of the cells are also altered in vitro.
Our study adds to the current understanding of the general architecture of the MCL. Conventional staining of longitudinal sections of ligaments from a variety of sources has led to the common description of the ligament as being hypocellular with rows of fusiform cells possessing lateral cytoplasmic extensions. Our data indicate that this description is inaccurate and that individual cells have a far more complex morphology in terms of shape and the degree to which they interact with the surrounding collagen matrix, as well as the manner in which they are positioned within and aligned along the longitudinal axis of the ligament. One consequence of this arrangement is that although the number of cells in the tissue may be limited, their size and shape, as well as the presence of extensive cellular processes allows them to permeate the tissue and interact with one another and the ECM over larger distances. These observations underline the complex relationship between the linked ligament cells (cellular matrix) and the surrounding ECM, and also suggests that the accommodation of the cellular matrix within the ligament may impact the biomechanical properties of the tissue – a contribution that has to date been largely ignored. Our studies also reveal the presence of an extensive pericellular matrix surrounding the cells within the tissue, and document the complex relationship between the cell and both the pericelluar matrix and the ECM. Elaboration of the plasma membrane, as evidenced by the detection of short membrane extensions (Fig. 2F), maximizes the surface area that interfaces with the pericellular matrix. This elaboration may maximize and facilitate the interchange of information and material between the cell and the matrix, facilitating the maintenance of the ECM as well as impacting tissue biomechanics.
Our study also documents that within the MCL, gap junctions are functional and populate sites of cell–cell association along a row of ligament cells. This observation reinforces the concept that chemical information is transmitted between cells within the ligament and also defines where this transmission occurs. This chemical information is probably essential for cell homeostasis and the maintenance of the ECM. Unfortunately, our in vivo study was unable to verify the previous assertion based on indirect immunofluorescent staining that gap junctions exist not only at sites associated with the cell body but also at sites where cytoplasmic extensions extend from one cell row to another (McNeilly et al. 1996; Benjamin & Ralphs, 2000; Bruehlmann et al. 2002; Lo et al. 2002a,b). This failure may be due to the rarity of cytoplasmic extension-associated gap junctions, their relative instability and/or the absence of gap junctions even in the presence of connexins. Nevertheless, our study does illustrate that the pattern of gap junctions within a row reflects or establishes an organizational bipolarity to the cell. This bipolarity would ensure information exchange along a row of cells, and it seems likely that general cellular architecture may be directly related and integrated within this bipolarity. This supposition is supported by the recent finding that the actin cytoskeleton in tendon cells in vivo is orientated predominantly along the long axis of the cellular row and hence from one gap junction surface to another (Ralphs et al. 2002).
Our study indicates that the position of gap junctions, as well as the proteins that are associated with their formation and structure, is not maintained when these cells are placed in culture. This change may be related to the loss of tissue integrity when cells are prepared for culture and/or the entrance of the cells into the cell cycle. This change may have serious effects on the ability of cells to replicate and maintain the appropriate flow of information between cells in vivo, maintain cell homeostasis, as well as sense and respond to the extracellular environment. This may explain at least in part why MCL cells cannot be maintained in long-term culture and do not appear to be able to synthesize and maintain an extensive ECM. These findings suggest that these cells may have limited use in studying native MCL cell function as well as a cell source for inclusion in tissue repair strategies. It is interesting to note that the arrangement of MCL cells in culture appears morphologically similar to cells found in MCL scars. Although scar cells also express gap junctions, they may also fail to establish native bipolarity and this deficiency may in part explain the compromised architecture and biomechanics of MCL scar tissue (Lo et al. 2002b; Thornton et al. 2003). In addition, it is currently not known whether MCL cells maintained in three-dimensional culture will eventually regain the structural polarity that exists in vivo, but is subsequently lost when the cells are removed from their ECM and maintained in vitro in monolayer culture.
To our knowledge, this study is the first to detect adherens junctions and desmosomes in ligament tissue, although the presence of adherens junctions has been suggested in tendon based on the distribution pattern of actin and N-cadherin, two components associated with adherens junctions (Ralphs et al. 2002). We have detected a similar distribution pattern for these components in ligament (unpublished data). The coordinated placement of adherens junctions, gap junctions and desmosomes is strikingly similar to that found in other tissues, such as cardiac intercalated discs, where they serve to ensure the coordinated electrical and mechanical activation of the heart's constituent myocytes (Gutstein et al. 2003). In ligaments, adherens junctions and desmosomes may ensure the mechanical association of adjacent cells and prepare sites for the formation of gap junctions and the transmission of chemical information between cells. For example, adherens junctions and desmosomes are associated with members of the catenin protein family, and the docking of connexons delivered to the plasma membrane has been shown to be enabled at least in part by calcium-dependent adhesion molecules. Fujimoto et al. (1997) have suggested that adherens junctions act as foci for gap junction formation, while Wu et al. (2003) have supplied evidence to suggest that a catenin/ZO-1/Cx43 complex is present in rat cardiomyocytes and that binding of catenins to the membrane-associated ZO-1 is required for Cx43 transport to the plasma membrane during the assembly of gap junctions. Although a relationship between catenin/ZO-1/Cx43 has only been documented for rat cardiomyocytes, our observations both in vivo and in vitro suggest that a similar relationship and process probably occurs in ligament cells. In addition, although the function of these associations has yet to be established in any tissue, identifying and clarifying these functions could be the focus of future studies. In summary, we show that ligament cells have both a complex form and distribution pattern and are interconnected by a variety of junctional complexes that establish both mechanical and chemical connections.
Acknowledgments
I.K.Y.L. receives support from the Alberta Heritage for Medical Research (AHFMR), and J.B.R. receives support from the National Science and Engineering Research Council of Canada (NSERC). We gratefully acknowledge Linda Marchuk for critically reading the manuscript.
References
- Benjamin M, Ralphs JR. Tendons and ligaments – an overview. Histol Histopathol. 1997;12:1135–1144. [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. The cell and developmental biology of tendons and ligaments. Int Rev Cytol. 2000;196:85–130. doi: 10.1016/s0074-7696(00)96003-0. [DOI] [PubMed] [Google Scholar]
- Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat. 2002;201:159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, DeHaan RL. Temperature dependence of embryonic cardiac gap junction conductance and channel kinetics. J Membr Biol. 1993;136:125–134. doi: 10.1007/BF02505757. [DOI] [PubMed] [Google Scholar]
- Chi S, Rattner JB, Matyas JR. Communication between paired chondrocytes in the superficial zone of articular cartilage. J Anat. 2004;205:363–370. doi: 10.1111/j.0021-8782.2004.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CB, Hart DA. The biology of tendons and ligaments. In: Mow VC, Ratcliffe A, SLY Woo, editors. Biomechanics of Diarthrodial Joints. New York: Springer-Verlag; 1990. pp. 32–69. [Google Scholar]
- Fujimoto K, Nagafuchi A, Tsukita S, Kuraoka A, Ohokuma A, Shibala Y. Dynamics of connexins, E-cadherin and alpha-catenin on cell membranes during gap junction formation. J Cell Sci. 1997;110(Pt. 3):311–322. doi: 10.1242/jcs.110.3.311. [DOI] [PubMed] [Google Scholar]
- Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Gutstein DE, Liu FY, Meyers MB, Choo A, Fishman GI. The organization of adherens junctions and desmosomes at the cardiac intercalated disc is independent of gap junctions. J Cell Sci. 2003;116:875–885. doi: 10.1242/jcs.00258. [DOI] [PubMed] [Google Scholar]
- Herve JC, Bourmeyster N, Sarrouilhe D. Diversity in protein–protein interactions of connexins: emerging roles. Biochim Biophys Acta. 2004;1662:22–41. doi: 10.1016/j.bbamem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat. 2003;202:279–291. doi: 10.1046/j.1469-7580.2003.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu TF, Lazrak A, et al. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo IK, Chi S, Ivie T, Frank CB, Rattner JB. The cellular matrix: a feature of tensile bearing dense soft connective tissues. Histol Histopathol. 2002a;17:523–537. doi: 10.14670/HH-17.523. [DOI] [PubMed] [Google Scholar]
- Lo IK, Ou Y, Rattner JP, et al. The cellular networks of normal ovine medial collateral and anterior cruciate ligaments are not accurately recapitulated in scar tissue. J Anat. 2002b;200:283–296. doi: 10.1046/j.1469-7580.2002.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell–cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21:67–74. doi: 10.1016/s0945-053x(01)00179-2. [DOI] [PubMed] [Google Scholar]
- Rozental R, Mehler MF, Morales M, Andrade-Rozental AF, Kessler JA, Spray DC. Differentiation of hippocampal progenitor cells in vitro: temporal expression of intercellular coupling and voltage- and ligand-gated responses. Dev Biol. 1995;167:350–362. doi: 10.1006/dbio.1995.1029. [DOI] [PubMed] [Google Scholar]
- Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Spray DC, Bennett MV. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proc Natl Acad Sci USA. 2001;98:10942–10947. doi: 10.1073/pnas.191206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelling JW, Jacob TJ. Functional coupling in bovine ciliary epithelial cells is modulated by carbachol. Am J Physiol. 1997;273:C1876–C1881. doi: 10.1152/ajpcell.1997.273.6.C1876. [DOI] [PubMed] [Google Scholar]
- Tadvalkar G, Pinto da Silva P. In vitro, rapid assembly of gap junctions is induced by cytoskeleton disruptors. J Cell Biol. 1983;96:1279–1287. doi: 10.1083/jcb.96.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton GM, Shrive NG, Frank CB. Healing ligaments have decreased cyclic modulus compared to normal ligaments and immobilization further compromises healing ligament response to cyclic loading. J Orthop Res. 2003;21:716–722. doi: 10.1016/S0736-0266(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Woo SLY, An KN, Frank CB. Anatomy, biology, and biomechanics of tendon and ligament. In: Simon R, editor. Orthopaedic Basic Science. Rosemont: AAOS; 2000. pp. 581–616. [Google Scholar]
- Wu JC, Tsai RY, Chung TH. Role of catenins in the development of gap junctions in rat cardiomyocytes. J Cell Biochem. 2003;88:823–835. doi: 10.1002/jcb.10390. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y, Sawa Y, Ebata N, Ibuki N, Yoshida S, Kawasaki T. Double expressions of connexin 43 and 32 in human periodontal ligament fibroblasts. Tissue Cell. 2000;32:328–335. doi: 10.1054/tice.2000.0122. [DOI] [PubMed] [Google Scholar]