Abstract
Uterine sympathetic innervation undergoes profound remodelling in response to physiological and experimental changes in the circulating levels of sex hormones. It is not known, however, whether this plasticity results from changes in the innervating neurons, the neuritogenic properties of the target tissue or both. Using densitometric immunohistochemistry, we analysed the effects of prepubertal chronic oestrogen treatment (three subcutaneous injections of 20 µg of β-oestradiol 17-cypionate on days 25, 27 and 29 after birth), natural peripubertal transition and late pregnancy (19–20 days post coitum) on the levels of TrkA and p75 nerve growth factor receptors in uterine-projecting sympathetic neurons of the thoraco-lumbar paravertebral sympathetic chain (T7–L2) identified using the retrograde tracer Fluorogold. For comparative purposes, levels of TrkA and p75 were assessed in the superior cervical ganglion (SCG) following prepubertal chronic oestrogen treatment. These studies showed that the vast majority of uterine-projecting neurons expressed both TrkA and p75. Both prepubertal chronic oestrogen treatment and the peripubertal transition increased the ratio p75 to TrkA in uterine-projecting neurons, whereas pregnancy elicited the opposite effect. Prepubertal chronic oestrogen treatment had no effects on levels of TrkA or p75 in sympathetic neurons of the SCG. Taken together, our data suggest that neurotrophin receptor-mediated events may contribute to regulate sex hormone-induced plasticity in uterine sympathetic nerves, and are in line with the idea that, in vivo, plasticity in uterine nerves involves changes in both the target and the innervating neurons.
Keywords: neurotrophins, oestrogen, pregnancy, puberty
Introduction
Dynamic responses of uterine sympathetic nerves to changes in the circulating levels of sex hormones represent one of the most remarkable examples of physiological plasticity in the adult autonomic nervous system. Studies performed on different mammalian species, including the rat, have shown that uterine sympathetic nerves degenerate at late pregnancy and regenerate following delivery (Sporrong et al. 1981; Owman & Stjernquist, 1988; Yamada, 1988; Haase et al. 1997). In addition to pregnancy-induced changes, the density of uterine sympathetic nerves fluctuates across the natural oestrous cycle (Melo & Machado, 1993; Zoubina et al. 1998), and reductions in noradrenaline levels and in the density of sympathetic nerves have been shown during the oestrogen-dominant phases of the cycle. Electron microscopic studies carried out on rats confirm that these changes involve cyclic phases of growth and degeneration of intrauterine sympathetic nerve terminals (Zoubina & Smith, 2000). Moreover, noradrenaline levels and the density of rat uterine sympathetic nerves are markedly and permanently reduced following the first ovulation at puberty (Brauer et al. 1992). These changes would not involve an actual degeneration of nerve fibres but a ‘spatial dilution’ of sympathetic nerves that fail to grow in parallel with puberty-induced changes in the size of the uterus. Puberty-induced changes are mimicked by acute administration of one dose of 40 µg of oestradiol at the end of the prepubertal period (25 days of age) (Brauer et al. 1995). Considering that high pharmacological doses of oestradiol block ovulation and the ovarian production of progesterone (Brauer et al. 2002), oestrogen rather than progesterone seems the more likely candidate to cause the reduction of uterine sympathetic nerves following puberty. In spite of these advances, the mechanisms underlying oestrogen-induced plasticity in uterine sympathetic nerves are not fully understood. In particular, does such plasticity reflect changes in the innervating neurons, the neuritogenic properties of the target tissue or both?
Recent studies have shown that the target uterine tissue plays a crucial role in the regulation of plasticity of uterine innervation, and that both pregnancy and oestrogen reduce the ability of uterine tissue to support sympathetic neurite outgrowth (Brauer et al. 1998, 2000a,2002; Krizsan-Agbas & Smith, 2002). In previous studies, the contribution of target levels of nerve growth factor (NGF) to oestrogen-induced plasticity in uterine sympathetic nerves was analysed. In contrast to what might be expected from the neurotrophic hypothesis (Korsching & Thoenen, 1983; Levi-Montalcini, 1987; Alberts et al. 1994), reduced levels of NGF in the uterus were found not to be the primary cause of puberty- and oestrogen-induced uterine sympathetic denervation. On the contrary, oestrogen increases the synthesis of NGF and other neurotrophins in the rodent uterus (Björling et al. 2002; Chávez-Genaro et al. 2002; Chalar et al. 2003; Krizsan-Agbas et al. 2003). The contribution of NGF deprivation to pregnancy-induced degeneration of uterine sympathetic nerves still remains controversial (Brauer et al. 2000b; Varol et al. 2000). Our studies in guinea-pigs failed to show significant reductions in NGF concentration at term pregnancy (Brauer et al. 2000b). Conversely, pregnancy has been shown to reduce the concentration of NGF in the rat uterus (Varol et al. 2000), and this change might be relevant to pregnancy-induced degeneration of uterine sympathetic nerves in this species.
The recent finding that uterine-projecting sympathetic neurons express oestrogen receptors (Zoubina & Smith, 2002) raises the possibility that, in addition to changes in neurotrophic factor availability, sex hormones could regulate plasticity in uterine sympathetic nerves by altering their receptivity to neurotrophins. Studies in other systems have shown the presence of putative oestrogen response elements in some neurotrophin receptor genes (Miranda et al. 1993; Sohrabji et al. 1995). Moreover, co-expression and reciprocal regulation of oestrogen receptor mRNA with the mRNAs for neurotrophins and their receptors has been shown in various neurotrophin-dependent cell types (Sohrabji et al. 1994a,b; Gibbs, 1998; Toran-Allerand, 2000; Jezierski & Sohrabji, 2001).
NGF binds two distinct cell-surface receptors: the 75-kDa low-affinity neurotrophin receptor (p75), which displays rapid association and dissociation with most members of the neurotrophin family (Frade & Barde, 1998), and the 140-kDa high-affinity tyrosine kinase (TrkA) receptor, which binds NGF more specifically but with slower kinetics (Meakin & Shooter, 1991; Hartman et al. 1992). In most sympathetic neurons, p75 and TrkA form complexes to generate high-affinity binding sites for NGF (Hempstead et al. 1991; Dechant, 2001; Esposito et al. 2001). Activated p75 and TrkA mediate positive growth responses to NGF in both developing and adult sympathetic neurons (Orike et al. 2001a,b). NT3 also acts through TrkA and p75 (Brennan et al. 1999) and mediates neuritogenesis and expression of growth-associated genes in NGF-dependent sympathetic neurons (Zhou & Rush, 1996a; Belliveau et al. 1997). p75 plays an important but context-dependent role in the response of sympathetic neurons to neurotrophins, where it can either induce apoptosis or protect neurons against apoptosis following neurotrophic factor deprivation (Miller & Kaplan, 2001).
TrkA expression has been shown to fluctuate across the oestrous cycle in the rat basal forebrain (Gibbs, 1998) and in adult sensory neurons (Sohrabji et al. 1994b). TrkA is up-regulated in PC12 cells (Sohrabji et al. 1994a), basal forebrain (Gibbs, 1998), olfactory bulb neurons (Jezierski & Sohrabji, 2001) and in adult sensory neurons after 6h following administration of oestradiol (Lanlua et al. 2001). By contrast, reduced TrkA expression was demonstrated in rat adult sensory dorsal root ganglion neurons following long-term (90 days) oestrogen replacement with physiological and pharmacological doses of Premarin (Liuzzi et al. 1999). Similarly, previous studies of our group showed reductions in TrkA levels in subpopulations of rat sensory neurons supplying the uterus following prepubertal chronic oestrogen treatment (Chalar et al. 2003). Regulation of p75 by sex hormones has been shown in several neuronal types (Gibbs & Pfaff, 1992; Sohrabji et al. 1994a,b), and increased expression of p75 mRNA was seen in sensory neurons at proestrus (Sohrabji et al. 1994b).
Because none of these studies addressed a possible correlation between alterations in receptor expression and the well-known sympathetic denervation observed in the mammalian uterus at late pregnancy and in response to oestrogen, we chose to examine the effects of prepubertal chronic oestrogen treatment, peripubertal transition and pregnancy on the levels of TrkA and p75 in retrogradely labelled uterine-projecting neurons of the rat thoraco-lumbar paravertebral sympathetic chain (T7–L2). These ganglia were selected because they contain 65% of the postganglionic sympathetic neurons supplying the cephalic third of the uterine horn (Houdeau et al. 1998). Because the ganglia contain mixed populations of neurons projecting to the uterus as well as other targets, we decided to use quantitative densitometric immunohistochemistry (Cowen et al. 2003) to assess changes in receptor expression in the morphologically identified population of neurons. In order to analyse whether oestrogen-induced changes in neurotrophin receptor protein levels were selective for uterine-projecting neurons, the effects of prepubertal chronic oestrogen treatment on levels of TrkA and p75 were also assessed in the superior cervical ganglion (SCG), which supply various cephalic targets.
Materials and methods
Animals and treatments
Studies were carried out on female Wistar-derived albino rats from the breeding colony held at the Instituto de Investigaciones Biológicas Clemente Estable (Montevideo, Uruguay). Animals were sexed at birth, weaned at 3 weeks and maintained under controlled conditions of temperature (21–22 °C) and illumination (12-h light–dark cycle), with water and food available ad libitum.
Prepubertal chronic oestrogen treatment
Oestrogen treatment was performed with β-oestradiol 17-cypionate (Laboratorios König, Buenos Aires, Argentina), diluted to appropriate doses with peanut oil (Sigma, St Louis, MO, USA) and administrated subcutaneously in a final volume of 0.1mL per dose. Animals (n = 10) were given three doses of 20 µg of oestrogen on days 25, 27 and 29 after birth and killed at 30 days of age. Ten control rats from matched litters were injected with vehicle (peanut oil). This oestrogen regime was selected because it causes growth-inhibition of uterine sympathetic nerves (Brauer et al. 1995; Chávez-Genaro et al. 2002), without affecting the body growth rate of animals.
Peripubertal transition
Pubertal females (n = 6) were killed at the first oestrus following vaginal canalization (42–43 days of age). Occurrence of the first oestrus was determined by vaginal smear. Prepubertal controls (n = 6) were killed at 30 days of age and before vaginal canalization.
Pregnancy
Eight adult females (3–4 months old) were mated with fertile males and day zero of pregnancy determined by the presence of spermatozoa in the vaginal smear. Pregnant females were killed at 19–20 days of pregnancy, together with six age-matched adult controls at dioestrus I.
Retrograde tracing with Fluorogold
Rats were anaesthetized with 45 mg kg−1 of ketamine (Unimedical, Montevideo, Uruguay) plus 10mg kg−1 of xylazine (Unimedical) and the left uterine horn was exposed through a small dorsal incision. Two to 4 µL of a 4% solution of Fluorogold in PBS (FG, Fluorochrome Inc., Denver, CO, USA) was injected under aseptic conditions at one or two sites of the cephalic (tubal) third of the uterine horn using a 10-µL microsyringe. After completion of injection, the area was dried and rinsed with sterile physiological saline solution and the muscle and skin sutured. Surgical procedures were conducted in accordance with the codes for animal care approved by the Instituto de Investigaciones Biológicas Clemente Estable, which are in agreement with USA-NIH and British Home Office guidelines. Females used for studies on the effects of puberty were injected with the tracer at 34 days of age. Prepubertal rats serving as controls for peripubertal animals, and those receiving chronic oestrogen treatment or oil were injected between 21 and 23 days of age. Pregnant females were injected between 6 and 7 days postcoitum together with the age-matched adult control group. In all animal groups, total-tracing times ranged between 7 and 13 days.
Immunohistochemistry
Animals were killed by an overdose of ether and perfused transcardially with saline solution followed by 4% paraformaldehyde (PFA, Sigma). The sympathetic chains (T7 to L2, ipsilateral to the site of FG injection) were dissected and fixed by immersion in 4% PFA for 1.5h at 4 °C, washed in PBS and stored in 20% sucrose in PBS overnight at 4 °C. Ganglia obtained from each animal group were pooled, embedded in tissue freezing medium (Shandon, Pittsburgh, PA, USA) and immediately frozen. A similar procedure was used for SCG from oestrogen-treated animals and their controls. The tissues obtained were sectioned completely at 12µm thickness and thaw-mounted onto gelatin-coated glass slides. For each set of ganglia, 12–20 slides containing 6–8 sections each were obtained. One group of slides was immunostained with rabbit anti-TrkA (1:400, Chemicon, Temecula, USA). This antibody was raised against the extracellular domain of synthetic TrkA and it has been shown to be specific for TrkA in previous studies (Walsh et al. 1999; Xu et al. 2002). A complementary set of slides were labelled with a monoclonal antibody against p75 (anti-p75, 1:75, mouse hybridoma cell line Ig192). The specificity of this antibody was assessed previously (Cowen et al. 2003). Sections were incubated with primary antibodies overnight in a humidified chamber and at room temperature. Omission of primary antibodies resulted in complete loss of immunostaining. At the end of the incubation period, sections were washed in PBS, incubated, respectively, with goat anti-rabbit IgG or goat anti-mouse IgG conjugated with Alexa-Fluor 568 (final dilution 1:400, Molecular Probes, Eugene, OR, USA) for 1.5h at room temperature, washed in PBS and mounted in anti-fade mounting medium (Citifluor, London, UK).
Densitometric measurement of TrkA and p75
All sections were examined with a Nikon E800 microscope equipped with epifluorescence and fitted with the appropriate filters. Fluorogold-positive (FG+) uterine-projecting sympathetic neurons were recognized under UV light by the presence of yellow-gold granules in the neuronal cytoplasm. All FG+ cells showing a nuclear profile were captured using a × 20 objective lens via a CoolSNAP-Pro Monochrome Digital camera (Media Cybernetic, Silver Spring, USA). Resampling of the same neurons in adjacent sections was avoided by taking images from every fourth section (leaving a gap of 36µm between sections). Sampling of SCG neurons was performed on digital images after superimposing a 10000-µm2 square on images. All neurons falling into the square and showing nuclear profiles were used for densitometric studies.
Selected neurons were examined under green light (Nikon, filter G-2B) to assess the immunofluorescence of TrkA and p75. Digital images were captured within the first 48h after completing the immunostaining and used for densitometric studies without any contrast or brightness corrections (Cowen & Thrasivoulou, 1992; Chalar et al. 2003). Positive TrkA and p75 signal was considered to be at least three times any non-specific background. The background signal did not change with treatment or stage (data not shown). The nuclear and cytoplasmic outlines of neurons were traced interactively and used to generate a mask within which the intensity of cytoplasmic receptor labelling was measured as the mean optical density per area unit (OD) using the Image Pro Plus software by Media Cybernetics (Chalar et al. 2003). Following evidence that pregnancy alters the size of uterine-projecting sympathetic neurons, fluorescence intensity in this group was measured as both OD and integrated optical density per cell profile (IOD=OD × cytoplasmic area). Considering that experiments conducted on different days could yield different absolute values of fluorescence intensity, only intra-experimental comparisons were performed. Cell size data (total neuronal soma area and cytoplasmic area) were automatically generated. Data are presented as median (IQR) (interquartile ranges; ± 95% confidence limits). Medians were compared using the Mann–Whitney non-parametric test or the Kruskal–Wallis non-parametric anova test followed by the Dunn's multiple comparison test. Values of P < 0.05 were considered statistically significant.
Results
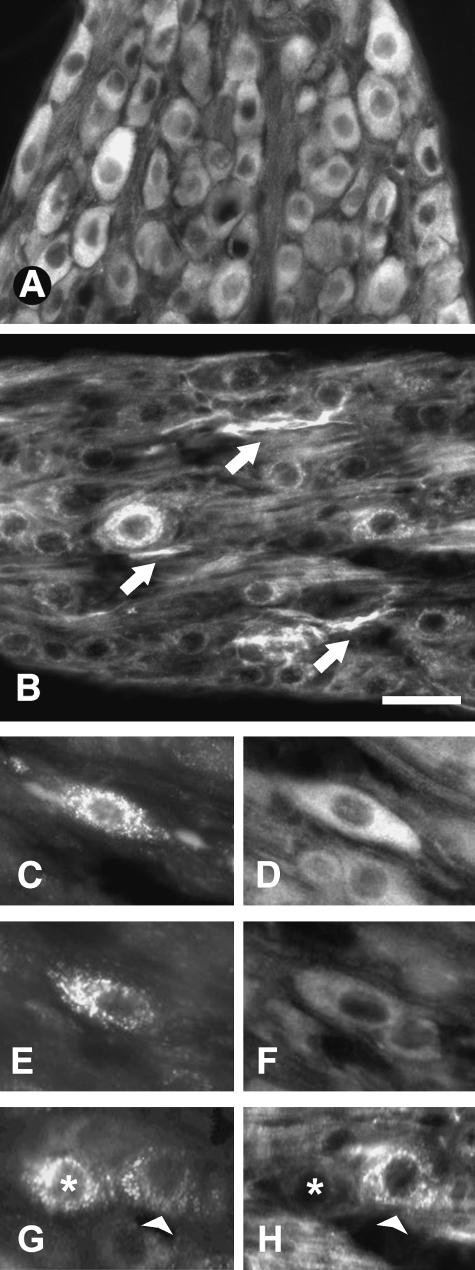
In both prepubertal and adult rats, the vast majority of sympathetic neurons of the thoraco-lumbar sympathetic chain in general (Fig. 1A,B) and uterine-projecting neurons in particular (Fig. 1C,E,G) were positive for TrkA (Fig. 1D,F) and p75 (Fig. 1H). Similarly, most sympathetic neurons of the SCG were positive for both receptors (data not shown). In all cases, levels of fluorescence intensity of TrkA and p75 showed considerable variations among neurons (Tables 1–4). Visual assessment of receptor staining in other ganglia containing uterine-projecting neurons (i.e. suprarenal ganglia) suggested a consistent pattern of change with those assessed in detail.
Fig. 1.
Cryostat tissue sections of rat thoraco-lumbar sympathetic chain ganglia showing neurons immunostained for TrkA (A) and p75 (B). Arrows in B indicate p75-positive nerve bundles. (C–E) Fluorogold-traced (FG) uterine-projecting sympathetic neurons displaying, respectively, high (D) and low (F) levels of TrkA. Fluorogold-labelled neurons shown in G (asterisk and arrowhead) also show marked differences in their levels of p75 (H). Scale bars: A,B = 40µm; C–H = 25µm.
Table 1.
Effects of prepubertal chronic oestrogen treatment (PCOT) on the mean optical density per area unit (OD) of TrkA in Fluorogold-positive (FG+) uterine-projecting sympathetic neurons of the rat thoraco-lumbar sympathetic chain and in sympathetic neurons of the superior cervical ganglia (SCG)
| Neuron | Treatment | n | OD – Median | IQR |
|---|---|---|---|---|
| FG+ | Control 4W | 257 | 93 | 89–94 |
| PCOT | 271 | 76a | 75–79 | |
| SCG | Control 4W | 234 | 109a,b | 107–113 |
| PCOT | 225 | 111a,b | 110–117 |
Data are expressed as median (interquartile ranges, IQR) and were compared by the Kruskal–Wallis analysis of variance followed by Dunn's multiple comparison test. n = number of neurons.
Significant difference (P < 0.0001) against 4-week-old (4W) control FG+ neurons.
Significant difference (P < 0.0001) against PCOT FG+ neurons.
Table 4.
Effects of pregnancy on the mean optical density per area unit (OD) and integrated optical density (IOD = OD × cytoplasmic area) of TrkA and p75 in Fluorogold-positive uterine-projecting sympathetic neurons of the rat thoraco-lumbar sympathetic chain
| Receptor | Stage | n | OD – Median | IQR | IOD Median | IQR |
|---|---|---|---|---|---|---|
| TrkA | Control 16W – DO | 104 | 149 | 144–163 | 48049 | 49009–59031 |
| Pregnant | 71 | 187a | 164–189 | 51418 | 46022–58878 | |
| p75 | Control 16W – DO | 74 | 102 | 105–127 | 35697 | 36113–47503 |
| Pregnant | 60 | 79b | 78–98 | 25122c | 26182–38798 |
Results are expressed as median (interquartile ranges, IQR). Data pairs were compared by the Mann–Whitney non-parametric test. n = number of neurons.
Significant difference in TrkA OD (P < 0.005) against the adult control in dioestrus (DO).
Significant difference in p75 OD (P < 0.001) against the adult control in dioestrus (DO).
Significant difference in p75 IOD (P < 0.01) against the adult control in dioestrus (DO).
Prepubertal chronic oestrogen treatment (PCOT) had no significant effects on the mean size of the soma area of uterine-projecting sympathetic neurons (Control 332 ± 5 µm2 vs. PCOT 320 ± 6 µm2) or sympathetic neurons of the SCG (Control 213 ± 6 µm2 vs. PCOT 208 ± 5 µm2). Following oestrogen treatment, optical density of TrkA was reduced by 18% in uterine-projecting sympathetic neurons (Table 1), resulting in an increased ratio of p75 to TrkA. However, no changes in levels of TrkA were detected in sympathetic neurons of the SCG (Table 1). In both uterine-projecting and SCG sympathetic neurons, optical density of p75 was unchanged by chronic oestrogen treatment (Table 2).
Table 2.
Effects of prepubertal chronic oestrogen treatment (PCOT) on the mean optical density per area unit (OD) of p75 in Fluorogold-positive (FG+) uterine-projecting sympathetic neurons of the rat thoraco-lumbar sympathetic chain and in sympathetic neurons of the superior cervical ganglia (SCG)
| Neuron | Treatment | n | OD – Median | IQR |
|---|---|---|---|---|
| FG+ | Control 4W | 217 | 79 | 82–92 |
| PCOT | 217 | 84 | 87–95 | |
| SCG | Control 4W | 110 | 80 | 80–88 |
| PCOT | 114 | 82 | 80–90 |
Data are expressed as median (interquartile ranges, IQR) and were compared by the Kruskal–Wallis analysis of variance followed by Dunn's multiple comparison test. n = number of neurons.
In the course of the peripubertal transition, no significant changes in the size of the soma area of uterine-projecting sympathetic neurons were detected (Prepubertal 332 ± 5 µm2 vs. Pubertal 342 ± 8 µm2). Optical density levels of TrkA were also unaffected by natural puberty (Table 3), whereas levels of p75 were increased by 29% (Table 3). Thus, the ratio of p75 to TrkA was again increased, although via a different means compared with PCOT.
Table 3.
Effects of puberty on the mean optical density per area unit (OD) of TrkA and p75 in Fluorogold-positive uterine-projecting sympathetic neurons of the rat thoraco-lumbar sympathetic chain
| Receptor | Stage | n | OD – Median | IQR |
|---|---|---|---|---|
| TrkA | Prepubertal | 165 | 127 | 125–139 |
| Peripubertal | 162 | 121 | 125–140 | |
| p75 | Prepubertal | 150 | 78 | 82–96 |
| Peripubertal | 150 | 101a | 101–117 |
Results are expressed as median (interquartile ranges, IQR). Data pairs were compared by the Mann–Whitney non-parametric test. n = number of neurons.
Significant difference in p75 (P < 0.0005) against the prepubertal stage.
Between the prepubertal and adult stage, the size of the soma areas of uterine-projecting sympathetic neurons was increased by 42% (Prepubertal 332 ± 5 µm2 vs. Adult diestrous 470 ± 15 µm2, t-test, P < 0.0001). In late pregnancy, however, soma size was significantly reduced by 15% (400 ± 19 µm2, t-test, P < 0.002). It is important that these size changes are taken into account when considering alterations in receptor expression. Thus, the optical density (per unit area of soma) of TrkA was increased by 26% in late pregnancy (Table 4). However, because of the reduced size of the neurons at this stage, no significant changes in the integrated optical density (equivalent to the total receptor density per neuron) of TrkA were observed (Table 4). By contrast, both the optical density and the integrated optical density of p75 were significantly reduced (22.5%) at late pregnancy (Table 4), resulting in a reduced ratio of p75 to TrkA, in contrast to the changes at earlier stages.
Discussion
The results reported in this study showed that oestrogen influences the levels of TrkA and p75 in uterine-projecting sympathetic neurons, as indicated by quantitative immunohistochemistry. Prepubertal chronic oestrogen treatment decreased the levels of TrkA in uterine-related neurons, without affecting levels of p75. Considering that in both developing and adult sympathetic neurons the growth-promoting effects of NGF and NT3 are mediated by TrkA activation (Zhou & Rush, 1996a; Belliveau et al. 1997; Orike et al. 2001a,b), oestrogen-induced reductions in levels of TrkA could contribute to the inhibitory effects of oestrogen on uterine sympathetic nerves. Reductions in TrkA increased the ratio of p75 to TrkA by 30%, and this change might also be relevant in determining the response of uterine sympathetic neurons to neurotrophins, for example, by favouring the p75-mediated inhibitory actions of brain-derived neurotrophic factor (BDNF) (Kohn et al. 1999). Increased levels of BDNF have been demonstrated in the uterus of adult ovariectomized rats following oestrogen replacement (Krizsan-Agbas et al. 2003). Moreover, co-culture studies indicate that oestrogen may affect the neuritogenic properties of the uterine tissue by altering the target synthesis of BDNF (Krizsan-Agbas et al. 2003). It has been shown that under optimal survival conditions, BDNF-mediated activation of p75 antagonizes NGF-mediated growth in sympathetic neurons (Kohn et al. 1999). The mechanism by which p75 inhibits TrkA-mediated neuronal growth is still unclear, but could involve p75-mediated generation of intracellular ceramide (Dobrowsky et al. 1994, 1995; Posse de Chaves et al. 1997). In addition, it has been suggested that inhibition of sympathetic neurite outgrowth could occur if the activated p75 directly down-regulates TrkA-mediated growth signals by serine–threonine phosphorylation of TrkA receptor via ceramide-activated kinases (Kohn et al. 1999).
In contrast to uterine-projecting neurons, levels of TrkA were unaffected by prepubertal chronic oestrogen treatment in sympathetic neurons of the SCG. This difference could be explained by differences in their constitutive expression of oestrogen receptors (ER) as well as by selective oestrogen-induced changes in both oestrogen receptor levels and subcellular localization (Taleghany et al. 1999). Studies conducted in adult ovariectomized rats have shown that the vast majority of paravertebral chain sympathetic neurons express ER-beta. However, whereas 76% of uterine sympathetic neurons also express ER-alpha, only a small proportion (29%) of the SCG neurons express this receptor subtype (Zoubina & Smith, 2002). Inhibitory effects of oestrogen on uterine sympathetic nerves appear to be mediated by ER-alpha, as suggested by the uterine hyperinnervation observed in oestrogen receptor alpha knockout mice (Zoubina & Smith, 2001).
The endocrine changes that accompany the first ovulation at puberty, like prepubertal oestrogen treatment, also increased the ratio of p75 to TrkA (37%) in uterine-projecting neurons. However, this imbalance was achieved by a different pattern of changes in receptor levels than that elicited by PCOT. In contrast to PCOT, the first pubertal oestrus has no effects on the levels of TrkA, but increases levels of p75. Increases in the ratio of p75 to TrkA could favour p75-mediated inhibitory actions of BDNF on uterine sympathetic nerves. However, whether natural oestrus, like oestrogen treatment, increases BDNF in the rat uterus remains to be determined.
The differences initiated by PCOT and the first pubertal oestrus on levels of TrkA and p75 in uterine-projecting neurons are not easy to explain at present, but some interpretations could be advanced. For example, it could involve the influence of other hormones, such as progesterone, testosterone and prolactin (Ojeda & Urbanski, 1994). Progesterone levels are elevated at natural oestrus, whereas chronic oestrogen treatment to prepubertal rats inhibits ovulation and the ovarian production of progesterone (Brauer et al. 2002). Progesterone receptors have been shown in different neuronal types (Blaustein, 2003; Gruber & Huber, 2003; Lonstein & Blaustein, 2004); however, to our knowledge their presence in sympathetic neurons has not been investigated. The differential responses elicited by PCOT and natural puberty could also be related to developmental changes in the maturational state of both neurons and target, as well as to selective sex hormone-induced changes in hormone receptor levels and subcellular localization at both sites (Medlock et al. 1991; Katsuda et al. 1999; Taleghany et al. 1999; Toran-Allerand, 2004).
Our current studies show that pregnancy decreases the mean size of uterine-projecting sympathetic neurons. This reduction could reflect a reduced neurotrophic support resulting from: (i) complete degeneration of intrauterine sympathetic peripheral branches observed in the rat and other mammalian species at late pregnancy (Owman & Stjernquist, 1988; Haase et al. 1997; Brauer et al. 2000b), (ii) reductions in the target levels of NGF observed in the rat uterus at term (Varol et al. 2000) and/or (iii) changes in the neuronal levels of neurotrophin receptors. As shown in the present study, pregnancy decreases the optical density and the integrated optical density of p75 in uterine-projecting sympathetic neurons and provokes a marked reduction in the ratio of p75 to TrkA. We previously argued that an increased ratio of p75 to TrkA might be associated with a BDNF-mediated inhibition of nerve growth in response to PCOT and pubertal oestrus. However, it has also been argued that p75 when co-expressed with TrkA, as seems to be the case in the vast majority of uterine-projecting sympathetic neurons, may exert a positive influence on the responsiveness of neurons to trophic factors. Moreover, in adult sympathetic neurons, activated p75 and TrkA mediate positive growth responses to NGF (Orike et al. 2001a) and both receptors are required for the uptake and retrograde transport of NGF (Gatzinsky et al. 2001). Although recent studies in adult sympathetic neurons (Cowen et al. 2003) failed to show the NGF-induced up-regulation of p75 demonstrated in immature sympathetic neurons (Miller et al. 1991, 1994; Ma et al. 1992; Zhou & Rush, 1996b), they do show a down-regulation of the receptor in specific populations of neurons that are vulnerable to age-related neurodegeneration. If p75 is considered to act cooperatively with TrkA in binding NGF (Hempstead et al. 1991), it is therefore likely that in uterine-projecting neurons, reductions in levels of p75 could adversely affect TrkA activation and/or NGF retrograde transport, and by so doing contribute to the degeneration of uterine sympathetic fibres at late pregnancy. An alternative explanation is that reductions in p75 levels could represent a protective response of uterine-related sympathetic neurons to the NGF deprivation that occurs at late pregnancy. Reductions of p75 have been suggested to contribute to the survival of sympathetic neurons deprived of NGF for short periods of time (Zhou & Rush, 1996b). Further studies, including examination of the impact of altered expression levels on NGF uptake in sympathetic neurons, are required to resolve the functional significance of the changes observed.
Our current results suggest that oestrogen may regulate plasticity in uterine sympathetic nerves through neurotrophin receptor-mediated events. However, the differential pattern of changes elicited by oestrogen, puberty and pregnancy indicates that neurotrophin receptor expression in sympathetic neurons supplying the uterus may be affected by several factors, including the hormonal status of the animal, the balance of sex hormone receptors at both target and neurons, the balance of target-derived neurotrophins, and probably the maturational state of sympathetic neurons. Taken together, these results are consistent with the idea that, in vivo, plasticity in uterine sympathetic nerves involves changes in both the target and the innervating neurons.
Acknowledgments
We are grateful for the support given by Aurelia Fernandez and Marcelo Fernandez (Animal Facility, Instituto de Investigaciones Biológicas Clemente Estable, Montevideo). This work was partially supported by PEDECIBA, Universidad de la República, Montevideo, Uruguay.
References
- Alberts KM, Wright DR, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of peripheral nervous system. J Neurocytol. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau DJ, Krivko I, Kohn J, et al. NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis. J Cell Biol. 1997;136:375–388. doi: 10.1083/jcb.136.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björling DE, Beckman M, Clayton MK, Wang ZY. Modulation of nerve growth factor in peripheral organs by estrogen and progesterone. Neuroscience. 2002;110:155–167. doi: 10.1016/s0306-4522(01)00568-1. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann NY Acad Sci. 2003;1007:238–250. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- Brauer MM, Lincoln J, Blundell D, Corbacho A. Postnatal development of noradrenaline-containing nerves of the rat uterus. J Autonom Nerv Syst. 1992;39:37–50. doi: 10.1016/0165-1838(92)90249-g. [DOI] [PubMed] [Google Scholar]
- Brauer MM, Corbacho A, Burnstock G. Effects of chronic and acute oestrogen treatment on the development of noradrenaline-containing nerves of the rat uterus. Int J Dev Neurosci. 1995;13:791–798. doi: 10.1016/0736-5748(95)00079-8. [DOI] [PubMed] [Google Scholar]
- Brauer MM, Burnstock G, Thrasivoulou C, Cowen T. In oculo transplants of myometrium from postpartum guinea pigs fail to support sympathetic reinnervation. J Anat. 1998;193:509–517. doi: 10.1046/j.1469-7580.1998.19340509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer MM, Chávez-Genaro R, Llodrá J, Richeri A, Scorza MC. Effects of chronic oestrogen treatment are not selective for uterine sympathetic nerves: a transplantation study. J Anat. 2000a;196:347–355. doi: 10.1046/j.1469-7580.2000.19630347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer MM, Shockley KP, Chávez R, Richeri A, Cowen T, Crutcher KA. The role of NGF in pregnancy-induced degeneration and regeneration of sympathetic nerves in the guinea pig uterus. J Autonom Nerv Syst. 2000b;79:19–27. doi: 10.1016/s0165-1838(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Brauer MM, Chávez-Genaro R, Richeri A, et al. The oestrogenized rat myometrium inhibits organotypic sympathetic reinnervation. Auton Neurosci. 2002;101:13–22. doi: 10.1016/s1566-0702(02)00173-x. [DOI] [PubMed] [Google Scholar]
- Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influence NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- Chalar C, Richeri A, Viettro L, et al. Plasticity in rat uterine sensory nerves: the role of NGF and TrkA. Cell Tissue Res. 2003;314:191–205. doi: 10.1007/s00441-003-0799-9. [DOI] [PubMed] [Google Scholar]
- Chávez-Genaro R, Crutcher K, Viettro L, et al. Differential effects of oestrogen on developing and mature uterine sympathetic nerves. Cell Tissue Res. 2002;308:61–73. doi: 10.1007/s00441-002-0521-3. [DOI] [PubMed] [Google Scholar]
- Cowen T, Thrasivoulou C. A microscopical assay using densitometric application of image analysis to quantify neurotransmitter dynamics. J Neurosci Meth. 1992;45:107–116. doi: 10.1016/0165-0270(92)90048-i. [DOI] [PubMed] [Google Scholar]
- Cowen T, Woodhoo A, Sullivan CD, et al. Reduced age-related plasticity of neurotrophin receptor expression in sympathetic neurons of the rat. Aging Cell. 2003;2:59–69. doi: 10.1046/j.1474-9728.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- Dechant G. Molecular interactions between neurotrophin receptors. Cell Tissue Res. 2001;305:229–238. doi: 10.1007/s004410100378. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis modulation by co-expressing p75 with Trk receptors. J Cell Chem. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- Esposito D, Patel P, Stephens RM, et al. The cytoplasmic and transmembrane domain of the p75 and TrkA receptors regulate high affinity binding to nerve growth factor. J Biol Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Nerve growth factor: two receptors, multiple functions. Bioessays. 1998;20:137–145. doi: 10.1002/(SICI)1521-1878(199802)20:2<137::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gatzinsky KP, Haugland RP, Thrasivoulou C, Orike N, Budi-Santoso AW, Cowen T. p75 and TrkA receptors are both required for uptake of NGF in adult sympathetic neurons: use of a novel fluorescent NGF conjugate. Brain Res. 2001;920:226–238. doi: 10.1016/s0006-8993(01)03099-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW. Effect of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Exp Neurol. 1992;116:23–39. doi: 10.1016/0014-4886(92)90173-n. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Huber JC. Differential effects of progestins on the brain. Maturitas. 2003;46S1:S71–S75. doi: 10.1016/j.maturitas.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Haase EB, Buchman J, Tietz AE, Schramm LP. Pregnancy-induced uterine neuronal degeneration in the rat. Cell Tissue Res. 1997;288:293–306. doi: 10.1007/s004410050815. [DOI] [PubMed] [Google Scholar]
- Hartman DS, McCormack M, Schnubenel R, Hertel C. Multiple trkA proteins in PC12 cells bind NGF with a slow association rate. J Biol Chem. 1992;267:24516–24522. [PubMed] [Google Scholar]
- Hempstead BM, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High affinity binding requires co-expression of trk proto-oncogen and the low affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Houdeau E, Rousseau A, Meusnier C, Prud’homme MJ, Rousseau JP. Sympathetic innervation of the upper and lower regions of the uterus and cervix in the rat have different origins and routes. J Comp Neurol. 1998;399:403–412. [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Katsuda SI, Yoshida M, Watanabe T, et al. Estrogen receptor mRNA in uteri of normal estrous cycling and ovariectomised rats by in situ hybridization. Proc Soc Exp Biol Med. 1999;221:207–214. doi: 10.1046/j.1525-1373.1999.d01-78.x. [DOI] [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haal-Frendsch M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19:5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat, correlation with density of sympathetic innervation. Proc Natl Acad Sci USA. 1983;80:3513–3616. doi: 10.1073/pnas.80.11.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Smith PG. Estrogen modulates myometrium-induced sympathetic neurite formation through actions on target and ganglion. Neuroscience. 2002;114:339–347. doi: 10.1016/s0306-4522(02)00262-2. [DOI] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Pedchenko T, Hasan W, Smith PG. Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus. Eur J Neurosci. 2003;18:2760–2768. doi: 10.1111/j.1460-9568.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Lanlua P, Decorti F, Gangula PRR, Chung Taglialatela G, Yallampalli C. Female steroid hormones modulate receptors for nerve growth factor in rat dorsal root ganglia. Biol Reprod. 2001;64:331–338. doi: 10.1095/biolreprod64.1.331. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Liuzzi FJ, Scoville SA, Bufton SM. Long-term estrogen replacement coordinately decreases trkA and beta-PPT mRNA levels in dorsal root ganglion neurons. Exp Neurol. 1999;155:260–267. doi: 10.1006/exnr.1998.6999. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Blaustein JD. Immunocytochemical investigation of nuclear progestin receptor expression with dopaminergic neurons of the female rat brain. J Neuroendocrinol. 2004;16:534–543. doi: 10.1111/j.1365-2826.2004.01198.x. [DOI] [PubMed] [Google Scholar]
- Ma Y, Campenot RB, Miller FD. Concentration-dependent regulation of neuronal gene expression by nerve growth factor. J Cell Biol. 1992;117:135–141. doi: 10.1083/jcb.117.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. Molecular investigations on the high affinity nerve growth factor receptor. Neuron. 1991;6:153–163. doi: 10.1016/0896-6273(91)90130-r. [DOI] [PubMed] [Google Scholar]
- Medlock KL, Forrester TM, Sheehan DM. Short-term effects of physiological and pharmacological doses of estradiol on estrogen receptor and uterine growth. J Recept Res. 1991;11:743–756. doi: 10.3109/10799899109064677. [DOI] [PubMed] [Google Scholar]
- Melo RCN, Machado RS. Noradrenergic and acetylcholinesterase-positive nerve fibres of the uterus in sexually immature and cycling rats. Histochem J. 1993;25:213–218. doi: 10.1007/BF00163817. [DOI] [PubMed] [Google Scholar]
- Miller FD, Mathew TC, Toma JG. Regulation of nerve growth factor receptor gene expression by nerve growth factor in the developing peripheral nervous system. J Cell Biol. 1991;112:303–312. doi: 10.1083/jcb.112.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Speelman A, Mathew TC, et al. Nerve growth factor derived from terminals selectively increases the ratio p75 to trkA NGF receptors on mature sympathetic neurons. Dev Biol. 1994;161:206–217. doi: 10.1006/dbio.1994.1021. [DOI] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. Neurotrophin signaling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Estrogen target neurons co-localize the mRNAs for the neurotrophins and their receptors during development: a basis for the interactions of estrogen and the neurotrophins. Molec Cell Neurosci. 1993;4:510–525. doi: 10.1006/mcne.1993.1063. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobill E, Neil JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 363–409. [Google Scholar]
- Orike N, Thrasivoulou C, Wrigley A, Cowen T. Differential regulation of survival and growth in adult sympathetic neurons: an in vitro study of neurotrophin responsiveness. J Neurobiol. 2001a;47:295–305. doi: 10.1002/neu.1036. [DOI] [PubMed] [Google Scholar]
- Orike N, Middleton G, Borthwick E, Buchman VL, Cowen T, Davis AM. Role of PI 3-kinase, Art and BCl-2-related proteins in sustaining the survival of neurotrophic factor-independent adult sympathetic neurons. J Cell Biol. 2001b;154:995–1005. doi: 10.1083/jcb.200101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owman C, Stjernquist M. Origin, distribution and functional aspects of aminergic and peptidergic nerves in the male and female genital tracts. In: Björklund A, Hökfelt T, Owman C, editors. Handbook of Chemical Neuroanatomy Vol. 6, The Peripheral Nervous System. Amsterdam: Elsevier Science Publishers; 1988. pp. 445–544. [Google Scholar]
- Posse de Chaves EI, Bussière M, Vance DE, Campenot RB, Vance JE. Elevation of ceramide within distal neurites inhibits neurite growth in cultured rat sympathetic neurons. J Biol Chem. 1997;272:3028–3035. doi: 10.1074/jbc.272.5.3028. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Greene LA, Miranda RC, Toran-Allerand CD. Reciprocal regulation of estrogen and NGF receptors by their ligands in PC12 cells. J Neurobiol. 1994a;25:974–988. doi: 10.1002/neu.480250807. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand D. Estrogen differentially regulates estrogen and nerve growth factor receptors mRNAs in adult sensory neurons. J Neurosci. 1994b;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative oestrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporrong B, Alm P, Owman Ch, Sjöberg NO, Thorbert G. Pregnancy is associated with extensive adrenergic nerve degeneration in the uterus. An electronmicroscopic study in the guinea pig. Neuroscience. 1981;6:1119–1126. doi: 10.1016/0306-4522(81)90076-2. [DOI] [PubMed] [Google Scholar]
- Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res. 1999;57:603–615. [PubMed] [Google Scholar]
- Toran-Allerand CD. Novartis Foundation 230Neuronal and Cognitive Effects of Oestrogen. Chichester: John Wiley & Sons; 2000. Novel sites and mechanisms of oestrogen action in the brain; pp. 56–73. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Varol FG, Duchemin AM, Neff HN, Hadjiconstantinou M. Nerve growth factor (NGF) and NGF mRNA change in rat uterus during pregnancy. Neurosci Lett. 2000;294:58–62. doi: 10.1016/s0304-3940(00)01533-0. [DOI] [PubMed] [Google Scholar]
- Walsh G, Krol KM, Kawaja MD. Absence of the p75 neurotrophin receptor alters the pattern of sympathosensory sprouting in the trigeminal ganglia of mice overexpressing nerve growth factor. J Neurosci. 1999;19:258–273. doi: 10.1523/JNEUROSCI.19-01-00258.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Michalski B, Racine RJ, Fahnestock M. Continuous infusion of neurotrophin-3 triggers sprouting, decreases the levels of TrkA and inhibits epileptogenesis amd activity-dependent axonal growth in adult rats. Neuroscience. 2002;115:1295–1308. doi: 10.1016/s0306-4522(02)00384-6. [DOI] [PubMed] [Google Scholar]
- Yamada M. Changes in the innervation of the uterus during pregnancy and following parturition: histochemical and electron microscopic observations in the rat and man. Acta Obstet Gynecol Jpn. 1988;40:145–152. [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Functional roles of neurotrophin 3 in the developing and mature sympathetic nervous system. Mol Neurobiol. 1996a;13:185–197. doi: 10.1007/BF02740622. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Endogenous nerve growth factor is required for regulation of the low affinity neurotrophin receptor (p75) in sympathetic but not sensory ganglia. J Comp Neurol. 1996b;372:37–48. doi: 10.1002/(SICI)1096-9861(19960812)372:1<37::AID-CNE4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Fan Q, Smith PG. Variation in uterine innervation during the estrous cycle. J Comp Neurol. 1998;397:561–571. [PubMed] [Google Scholar]
- Zoubina EV, Smith PG. Axonal degeneration and regeneration in rat uterus during the estrous cycle. Auton Neurosci. 2000;84:176–185. doi: 10.1016/S1566-0702(00)00209-5. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Smith PG. Sympathetic hyperinnervation of the uterus in the estrogen receptor alpha knock-out mouse. Neuroscience. 2001;103:237–244. doi: 10.1016/s0306-4522(00)00549-2. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Smith PG. Distributions of estrogen receptors alpha and beta in sympathetic neurons of female rats: enriched expression by uterine innervation. J Neurobiol. 2002;52:14–23. doi: 10.1002/neu.10064. [DOI] [PubMed] [Google Scholar]