Abstract
The red deer is an important study species because of its value in the national economy and because it provides a wealth of genetic material. To date, there has been little research into the prenatal development of the stomach of ruminants, and none of the red deer. We therefore performed a histological evaluation of the ontogenesis of the omasum in the red deer. Histomorphometric and immunohistochemical analyses were carried out on 50 embryos and fetuses of deer from the initial stages of prenatal life until birth. For test purposes, the animals were divided into five experimental groups: Group I (1.4–3.6 cm crown–rump length, CRL; 30–60 days, 1–25% of gestation); Group II (4.5–7.2 cm CRL; 67–90 days, 25–35% of gestation); Group III (8–19 cm CRL; 97–135 days, 35–50% of gestation); Group IV (21–33 cm CRL; 142–191 days, 50–70% of gestation); and Group V (36–40 cm CRL; 205–235 days, 75–100% of gestation). At 67 embryonic days, the omasum wall was differentiated, and comprised three layers: the epithelial layer, pluripotential blastemic tissue and serosa. The stratification of the epithelial layer was accompanied by changes in its structure, with the appearance of four laminae of different sizes; in order of appearance these were: primary at 67 days, secondary at 90 days, tertiary at 97 days and quaternary at 135 days. At around mid-gestation, lateral evaginations were formed from the stratum basale of the primary and secondary smaller laminae. These were the primitive corneum papillae. From 205 days, the corneum papillae were present in all four sizes of laminae. The histodifferentiation of the lamina propia-submucosa, tunica muscularis and serosa showed patterns of development similar to those reported for the rumen and reticulum of red deer. The omasum of red deer during prenatal life, especially from 67 days of gestation, was shown to be an active structure with full secretory capacity. Its histological development, its secretory capacity (detected by the presence of neutral mucopolysaccharides) and its neuroendocrine nature (detected by the presence of positive non-neuronal enolase cells and the neuropeptides vasoactive intestinal peptide and neuropeptide Y) were parallel to the development of the rumen and the reticulum. However, its prenatal development was later than that of the omasum in sheep, goat and cow.
Keywords: immunohistochemistry, omasum, prenatal development, red deer
Introduction
The genetic significance of red deer and its importance to the Spanish national economy as a species that is hunted for sport (Carranza, 1999) are both aspects which have been mentioned in published studies on the embryonic development of the rumen and reticulum of this species (Franco et al. 2004a,b). The present study follows research into the development of the stomach of ruminants (Franco et al. 1989, 1992, 1993a,b,c; Regodón et al. 1996), and within this line of research we aim to deal with the forestomachs and stomach of the red deer during prenatal life.
The omasum plays a important role in the digestion of ruminants, and serves mainly as a dehydration area and as a sieve. As food passes through the omasum it is squeezed and compressed by contractions. The leaves of the omasum absorb a large portion of the volatile fatty acids that were not absorbed through the rumen wall, water and electrolytes such as potassium and sodium. As a continuation of work carried out on the development of the rumen and reticulum of red deer during intrauterine life (Franco et al. 2004a,b), we present here data on the histological organization of the omasum of deer during prenatal development.
The objectives of this study were (1) to describe sequentially the histology of the omasum, from the differentiation of the primitive stomach to the gastric compartmentation in perinatal stages; (2) to describe the histochemical reaction towards the neutral and acidic mucopolysaccharides of the omasal epithelial layer during prenatal development; (3) to perform a morphometric analysis of the evolution of the integral layers of the omasal wall during embryogenesis; and (4) to determine the reaction of the omasal wall in embryonic development towards neuroendocrine cell markers [non-neuron enolase (NNE)], glial cell markers (glial fibrillary acidic protein (GFAP) and vimentin (VIM)] and markers of peptidergic innervation [neuropeptide Y (NPY) and vasoactive intestinal peptide (VIP)].
Materials and methods
Animals
Red deer embryos and fetuses (n = 25) from the initial prenatal stages until birth were studied. The specimens were divided into five groups of five animals each, with reference to the most relevant histomorphogenic characteristics (Table 1). These histomorphometric characteristics, which were used in the formation of the groups, were as defined in the intrauterine development of the reticulum of red deer (Franco et al. 2004b). To obtain embryos and fetuses at various stages of development, 125 laparotomies on the same number of females were performed. The females were taken from ten hunting grounds from extensive and non-enclosed estates from the Sierra of San Pedro (north-east of the province of Cáceres, Spain).
Table 1.
Neuropeptides present in the omasum of red deer during prenatal development
| Group I CRL (cm) (1.4–3.6) 30–60 days | Group II CRL (cm) (4.5–7.2) 67–90 days | Group III CRL (cm) (8–19) 97–135 days | Group IV CRL (cm) (21–33) 142–191 days | Group V CRL (cm) (36–40) 205–235 days | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | |
| NNE | − | − | − | − | − | + | + | − | − | ++ | ++ | − | − | +++ | +++ | − | − | +++ | +++ | − |
| GFAP | − | − | − | − | − | + | + | + | − | + | + | + | − | ++ | ++ | + | − | +++ | +++ | ++ |
| VIM | − | − | − | − | − | + | + | + | − | + | + | + | − | ++ | ++ | + | − | +++ | +++ | ++ |
| VIP | − | − | − | − | − | − | − | − | − | − | − | − | − | ++ | ++ | + | − | ++ | ++ | ++ |
| NPY | − | − | − | − | − | − | − | − | − | − | − | − | ++ | ++ | + | − | ++ | ++ | ++ | |
−, Non immunoreactivity; +, low immunoreactivity; ++, moderate immunoreactivity; +++, high immunoreactivity.
E, epithelium; Lp-S, lamina propia-submucosa; T, tunica muscularis; S, serosa.
Sampling and processing
Once the omasum was separated, it was analysed by visual and stereomicroscopic inspection. The colour and consistency of the omasal mucosa were determined. Square specimens measuring 1.5 × 0.5 cm were taken from the medial region of the omasum of each animal. Tissue for histological study was fixed in 4% buffered formaldehyde for 24 h, processed by conventional paraffin embedding methods, and 5-µm sections were cut in transversal direction and treated with haematoxylin and eosin (H-E), Periodic Acid-Schiff (PAS, pH 7.2) and PAS-alcian blue (pH 7.2) for specific differentiation of neutral and acid mucopolysaccharides, Von Giesson (VG), Masson's trichrome (MT) and Reticuline of Gomori (RG).
Morphometric analysis
Specimens for morphometric analysis were embedded in paraffin, stained with H-E and viewed through a microscope (Optiphot, Nikon Inc, Tokio, Japan) equipped with a video camera. The image was reflected onto the screen of a semi-automatic image analyser (Vid IV, Rego and Cía, Madrid, Spain). Variables studied were height of various tissue strata (epithelium, lamina propria and submucosa, tunica muscularis and serosa) and total wall thickness. Eight specimens (sections) were selected for each group, and 50 measurements were made for each tissue stratum and specimen.
The results are repoerted as the mean ± SE. The data were analysed using analysis of the variance. In cases for which a significant anova result was obtained a post-hoc (Tukey) analysis was carried out in order to study the significant differences among the distinct groups. A value of P = 0.05 was considered significant.
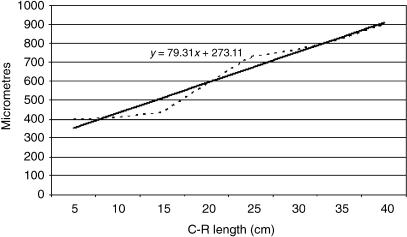
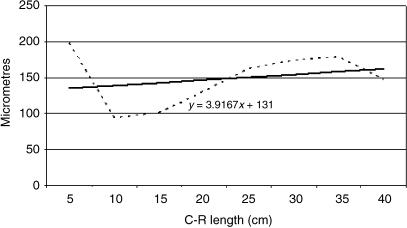
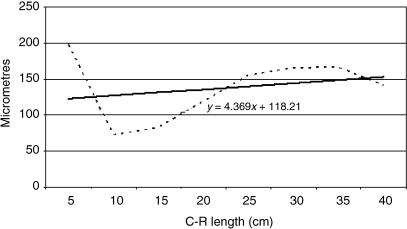
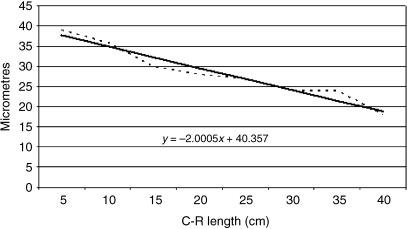
Tissue growth models were created using a personal computer and statistics program (Statgraphics v.2.1 (1986)). The graphs in Figs 25–29 represent the averages of the real growth values next to the adjusted line of regression. The goodness of fit of this adjustment was measured using the rate of determination, r2. In all cases, embryo body length (crown–rump length, CRL, in cm) was used as the independent variable; the thickness of each tissue stratum served as the dependent variable.
Immunocytochemical analysis
Extravidin peroxidase staining (EAS) was performed on deparaffinized sections from the omasum to detect the neuroendocrine cells markers (NNE), glial cells markers (GFAP and VIM) and markers of peptidergic innervation (NPY and VIP).
Tissue was deparaffinized, hydrated and treated sequentially with 0.5% hydrogen peroxide in methanol for 30 min in order to block endogenous peroxidase activity. Non-specific tissue-binding sites were blocked by incubation in 1% normal goat serum for 30 min. Samples were incubated with the following dilutions of primary antisera in PBS: 1 : 200 monoclonal anti-human NNE (no. S5768); 1 : 400 monoclonal anti-human GFAP (no. G-3893); 1 : 20 monoclonal anti-human VIM (no. V-5255); 1 : 200 monoclonal anti-human NPY (no. N9528); and 1 : 20 monoclonal anti-human VIP (no. V3508; all from Sigma/Aldrich Química, Madrid, Spain) for 3 h at 20 °C. Biotinylated goat anti-mouse IgG (1: 200 dilution) (Sigma/Aldrich Química, no. B7151) was then added to the sections for 30 min. Sections were finally incubated with diluted (1 : 50) extravidin–horseradish peroxidase (Sigma/Aldrich Química, no. E2886) for 1 h. After diaminobenzidine reaction, nuclear counterstaining with Mayer's haematoxylin was applied. Finally, the sections were mounted with Entellan (Merck 7961).
The specificity of the staining reaction was determined in control experiments. These comprised substitution of the primary antibody by PBS or normal mouse serum 1 : 100, omission of both primary and secondary antibodies, and prior absorption of the primary antibody (overnight pre-incubation of the primary antisera with the respective peptide at 50–100 µm). The antibody/peptide mixture was then applied to sections in the identical manner and concentrations as for the primary antibody.
Results
Macroscopic findings
Differentiation of the omasum as an separate compartment from the primitive stomach took place at 67 days of gestation. From this point, it displayed an irregular surface with clear lamination. From 67 days of prenatal development and throughout intrauterine life, omasal lamination was more evident, these layers exhibiting varying sizes in terms of height and width of the interlaminar space. At 97 days of intrauterine life, lateral projections from the omasal layers (corneum papillae) could be seen on these diverse laminae, increasing in size until they reached their maximum in perinatal stages. Around birth, the surface of the omasum exhibited its maximum irregularity, with laminae, interlaminar spaces and corneum papillae of varying sizes.
Omasal histomorphogenesis
Group I (1.4–3.6 cm CRL, 30–60 days, 1–25% of gestation)
All findings described for the rumen and reticulum (Franco et al. 2004a,b) are valid for the omasum og group I, as differentiation of the omasal individualized compartment from the primitive gastric tube had not taken place (Fig. 1a).
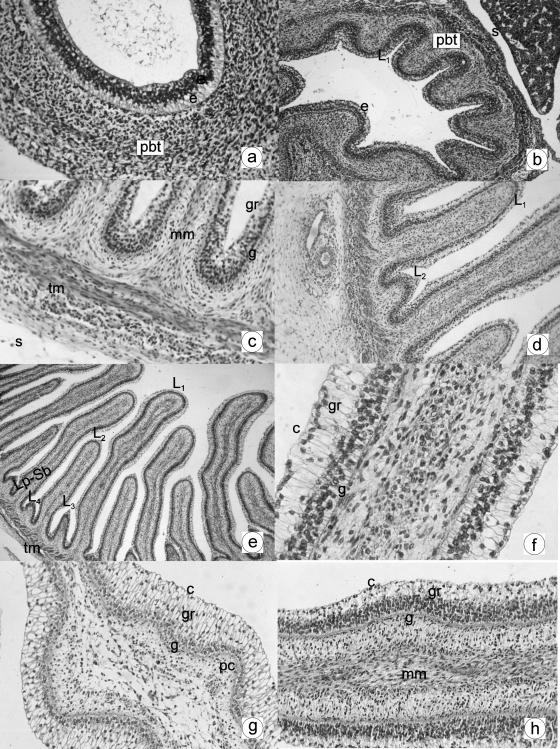
Fig. 1.
a Photomicrograph of a transverse section of the undifferentiated stomach at 1.4 cm CRL, 30 days. The wall was composed of two layers: epithelium (e) and pluripotential blastemic tissue (pbt). H-E, ×250. b Photomicrograph of a transverse direction section of the omasal wall at 4.5 cm CRL, 67 days. Three layers are visible: epithelium (e), pluripotential blastemic tissue (pbt) and serosa (s). In the epithelial layer the primary smaller laminae can be seen (L1). H-E, ×180. c Photomicrograph of a transverse direction section of the omasal wall at 7.2 cm CRL, 90 days. Myoblastic fibres of the tunica muscularis (tm), infiltrating into the smaller laminae and constituting the muscularis mucosae (mm), were observed. The epithelium is stratified in two zones: a basal zone or stratum germinativum (g) and another apical zone or stratum granulosum (gr). Serosa is visible (s). H-E, ×250. d Photomicrograph of a transverse direction section of the omasal wall at 7.2 cm CRL, 97 days. The secondary smaller laminae (L2) can be observed in the spaces between the primary smaller laminae (L1). H-E, ×180. e Photomicrograph of a transverse direction section of the omasal wall at 8 cm CRL, 97 days. Four layers can be detected: epithelium, lamina propia-submucosa (Lp-Sb), tunica muscularis (tm) and serosa. Presence of the primary (L1), secondary (L2), tertiary (L3) and quaternary smaller laminae (L4). H-E, ×120. f Photomicrograph of a transverse direction section of the omasal wall at 8 cm CRL, 97 days. The epithelium is stratified in three zones: a basal zone or stratum germinativum (g), stratum granulosum (gr) and another apical zone or stratum corneum (c). H-E, ×350. g Photomicrograph of a transverse direction section of the omasal wall at 19 cm CRL, 135 days. Presence of corneum papillae (pc) in the primary smaller laminae with stratified epithelium: stratum germinativum (g), granulosum (gr) and corneum (c). VG, ×250. h Photomicrograph of a transverse direction section of the omasal wall at 19 cm CRL, 135 days. Muscularis mucosae (mm), constituting the muscularis of the smaller laminae. The stratified epithelium with a stratum germinativum (g), stratum granulosum (gr) and stratum corneum (c) is visible. H-E, ×250.
Group II (4.5–7.2 cm CRL, 67–90 days, 25–35% of gestation)
At 67 days of embryonic life, omasal differentiation took place. The wall of the omasum (364 ± 13 µm thick), smooth until now, displayed an undulating surface. The parietal structure was made up of three layers: epithelial layer, pluripotential blastemic tissue and serosa.
The epithelial layer had a thickness of 119 ± 11 µm. It was stratified, composed of 5–7 layers and two zones could be distinguished within it, basal or germinal, with 4–5 layers of dark-staining cells and a large central nucleus, and another smaller apical zone made up of poorly defined globular cells and a small nucleus. At 67 days of intrauterine life, the epithelium exhibited undulations of similar height, 5–6 in number, that made up the primary primitive smaller laminae (Fig. 1b–d). At 90 days of gestation (35%) the outline of a new generation of omasal smaller laminae, smaller that the first – the secondary smaller laminae – emerged from the epithelial enlargements of the interlaminar spaces (Fig. 1d).
The pluripotential blastemic tissue (206 ± 13 µm thick) was composed of a considerable quantity of mesenchymatous cells, with a substantial quantity of ground substance. This tissue participated actively in the constitution of the omasal smaller laminae, infiltrating towards the epithelium and putting pressure on the basal zone (Fig. 1c and d). The intense vascularization of the pluripotential blastemic tissue, accompanied by a notable quantity of fibroblastic cells, marked the beginning of its differentiation in the lamina propia and submucosa, this event taking place at 90 days (35% of gestation). These structures, made up of a highly vascularized mesodermal connective tissue, took part in the formation of the primary and secondary smaller laminae (Fig. 1c and d).
Differentiation of the tunica muscularis also took place during this phase (Fig. 1c and d), consisting of the appearance of two layers of myoblasts: an internal circular layer and another external longitudinal layer. The tunica muscularis exhibited a series of undulations, coinciding with the implantation base of each layer. At 90 days of prenatal development, originating in the internal fascicule of the tunica muscularis, myoblastic fibres moved towards the interior of the smaller laminae, and constituted the muscularis mucosae (Fig. 1c and d).
The highly vascularized serosa was made up of a mesothelium of flat cells and a subserosa of a lax connective type (Fig. 1c).
Group III (8–19 cm CRL, 97–135 days, 35–50% of gestation)
The omasal wall (429 ± 30 µm thick) was formed by mucosa, lamina propia-submucosa, tunica muscularis and serosa (Fig. 1e).
The epithelial layer of the mucosa (219 ± 18 µm thick) was stratified with: a basal area of 3–4 layers of germinal cells (stratum germinativum), of a dark cytoplasm and a nucleus with pyknosis; another apical zone of 6–10 layers of globular cells and a clear cytoplasm that formed the stratum granulosum; and externally the stratum corneum, of anuclear and flat cells (Fig. 1f–h). At 97 days of gestation, the outline of a third laminar generation– the tertiary smaller laminae – appeared between the primary and secondary smaller laminae (Fig. 1e). Development of the quaternary smaller laminae also took place during this stage, at 135 days of intrauterine life, appearing as small elevations of the stratum basale of the epithelium among the other laminae, although never between the primary and secondary laminae (Fig. 1e).
Around mid-gestation, some lateral evaginations of connective tissue towards the epithelial surface were formed from the stratum basale of the primary smaller laminae. These were the primitive corneum papillae (Fig. 1g).
The lamina propia and the submucosa (99 ± 8 µm thick), without spatial separation, were formed with a highly cellular connective mesenchymatous, rich in fibroblasts and little ground substance (Fig. 1f–h).
The tunica muscularis (Fig. 1e), with its external longitudinal and internal circular fascicules, was 79 ± 10 µm in thickness. From the inner part, a strip of smooth muscular tissue (muscularis mucosae) was seen projecting towards the centre of the omasal smaller laminae (Fig. 1h).
The serosa (32 ± 7 µm thick) did not display characteristics different from Group II.
Group IV (21–33 cm CRL, 142–191 days, 50–70% of gestation)
The omasal wall (744 ± 38 µm thick) was similar to that of the previous stage but with some histological variations, in that the epithelial layer (390 ± 28 µm thick) had a greater number of layers in all strata (Fig. 2a–d). The globular cells of the stratum corneum appeared to be more elongated (Fig. 2b–d). At 191 days of prenatal development, between the granulosum and corneum strata, a transition zone of cells of a clear cytoplasm to others of a lightly eosinophylic colouring could be seen, corresponding to the poorly defined lucidum-spinosum stratum (Fig. 2c). Corneum papillae were abundant in the primary smaller (Fig. 2c) as well as in the secondary laminae (Fig. 2d). In these corneum papillae a greater abundance of connective tissue and smooth muscular fibres were detected than in the previous stage (Fig. 2c and d).
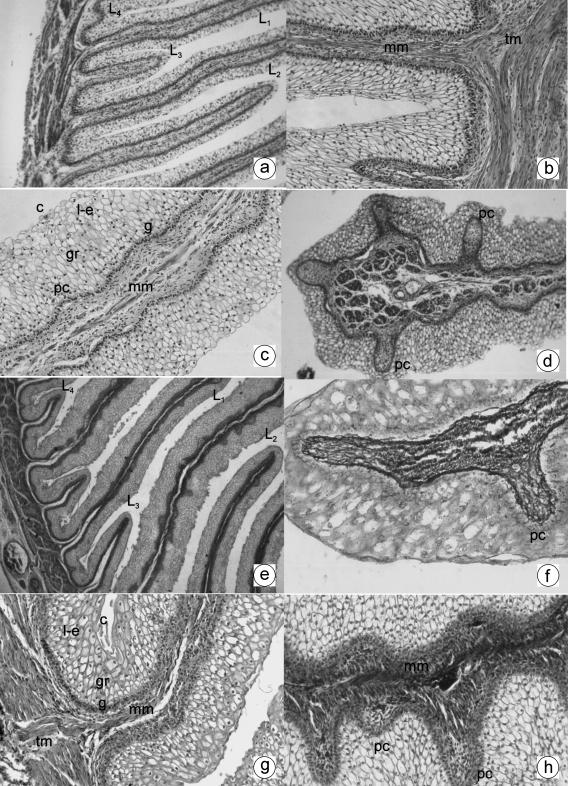
Fig. 2.
a Photomicrograph of a transverse direction section of the omasal wall at 21 cm CRL, 142 days. Omasal wall with the four laminar generations: primary (L1), secondary (L2), tertiary (L3) and quaternary (L4). H-E, ×120. b Photomicrograph of a transverse direction section of the omasal wall at 21 cm CRL, 142 days. Internal fascicule of the tunica muscularis (tm) filling the width of the smaller laminae and forming the muscularis mucosae (mm). MT, ×350. c Photomicrograph of a transverse direction section of the omasal wall at 33 cm CRL, 191 days. Primary lamina with stratified epithelium: stratum germinativum (g), granulosum (gr), lucidum-spinosum (l-e) and corneum (c). Corneum papillae (pc) and muscularis mucosae (mm) can also be seen (pc). H-E, ×250. d Photomicrograph of a transverse direction section of the omasal wall at 33 cm CRL, 191 days. Presence of corneum papillae (pc) in the secondary smaller laminae. H-E, ×180. e Photomicrograph of a transverse direction section of the omasal wall at 36 cm CRL, 205 days. Omasal mucosa with the four sizes of smaller laminae: primary (L1), secondary (L2), tertiary (L3) and quaternary (L4). H-E, ×180. f Photomicrograph of a transverse direction section of the omasal wall at 36 cm CRL, 205 days. Presence of reticulin fibres in the interior of the corneum papillae (pc) of the primary smaller laminae. RG, ×350. g Photomicrograph of a transverse direction section of the omasal wall at 40 cm CRL, 235 days. Stratified epithelial layer: stratum germinativum (g), granulosum (gr), lucidum-spinosum (l-e) and corneum (c). Muscularis mucosae (mm) coming from the internal fascicule of the tunica muscularis (tm). VG, ×350. h Photomicrograph of a transverse direction section of the omasal wall at 40 cm CRL, 235 days. Abundant presence of corneum papillae (pc) in the omasal smaller laminae. A thick muscularis mucosae can also be observed (mm). TM, ×350.
The lamina propia-submucosa (168 ± 14 µm thick), without spatial separation, as in the previous stage, displayed greater vascularization.
The tunica muscularis (160 ± 11 µm thick) exhibited considerable development of its two fascicules, especially of the internal fascicule that formed the greater part of the thickness of the smaller laminae, making up the muscularis mucosae (Fig. 2b and c).
The serosa (26 ± 4 µm thick) had a clearly defined mesothelium, a lax subserosa and intense vascularization.
Group V (36–40 cm CRL, 205–235 days, 75–100% of gestation)
From 205 days of gestation the wall of the omasum (868 ± 29 µm thick) displayed a mucosa with barely stratified epithelium (525 ± 30 µm thick) (Fig. 2e–h), where a greater number of cells in all of the formative strata could be seen. Distinctive were (1) the greater differentiation displayed by the lucidum-spinosum stratum (Fig. 2g) and (2) the presence of 2–3 layers of flat cells in the corneum stratum (Fig. 2g). In fetuses at term, we observed numerous longitudinal smaller laminae in the omasal mucosa, distributed in four distinct sizes of laminae (Fig. 2e). The corneum papillae increased in number and size in all four sizes of laminae (Fig. 2h). In the interior of the corneum papillae, reticulin fibres (Fig. 2f) and collagen fibres were seen, as well as smooth muscular fibres coming from the muscularis mucosae (Fig. 2h).
The lamina propia-submucosa (164 ± 16 µm thick), of a connective and aglandular type, the two fascicules of smooth fibre of the tunica muscularis (155 ± 13 µm thick) and the serosa (24 ± 6 µm thick) did not show significant variation from Group IV.
Histochemical behaviour of the epithelium
Just as in the rumen and reticulum (Franco et al. 2004a,b), no reaction towards the mucopolysaccharide acids, mucins and mucinous compounds was observed. Neutral mucopolysaccharides were seen, from 97 days of gestation, in the germinativum, granulosum and lucidum-spinosum strata, of the tegumentary epithelium of the omasal smaller laminae. Histochemical staining was of medium intensity, decreasing throughout prenatal development to become minimal in perinatal stages.
Immunohistochemical observation
Table 1 shows the neuropeptides present in the omasum of red deer during prenatal development. Immunohistochemical findings in the omasum of the five groups of red deer studied are summarized in Fig. 3a–h.
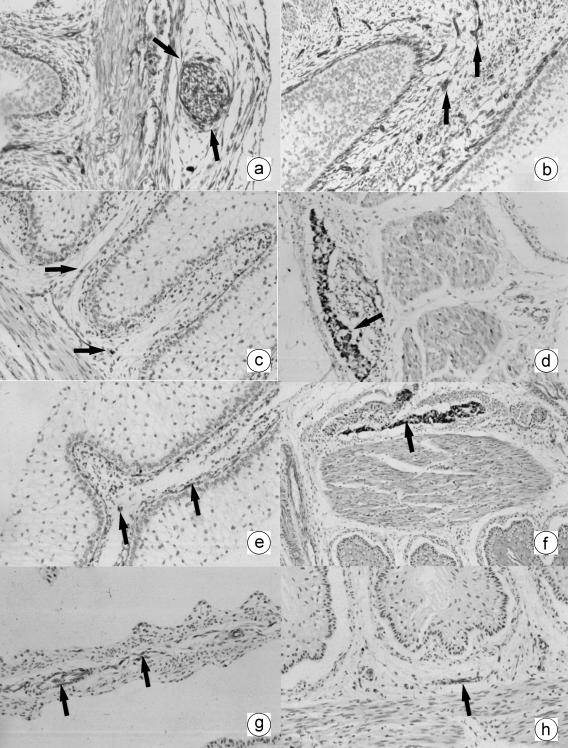
Fig. 3.
a Photomicrograph of a transverse direction section of the omasal wall at 4.5 cm CRL, 67 days. Presence of neuroendocrine cells (NNE) in the tunica muscularis (arrows). EAS, ×250. b Photomicrograph of a transverse direction section of the omasal wall at. 40 cm CRL, 235 days. Presence of neuroendocrine cells (NNE) in the lamina propia-submucosa (arrows). EAS, ×250. c Photomicrograph of a transverse direction section of the omasal wall at 8 cm CRL, 97 days. Presence of GFAP-positive cells (arrows) in lamina propia and submucosa. EAS, ×180. d Photomicrograph of a transverse direction section of the omasal wall at 40 cm CRL, 235 days. Presence of GFAP-positive cells (arrow) in tunica muscularis. EAS, ×350. e Photomicrograph of a transverse direction section of the omasal wall at 19 cm CRL, 135 days. Presence of VIM-positive cells (arrows) in lamina propia and submucosa. EAS, ×250. f Photomicrograph of a transverse direction section of the omasal wall at 40 cm CRL, 235 days. Presence of VIM-positive cells (arrow) in tunica muscularis. EAS, ×350. g Photomicrograph of a transverse direction section of the omasal wall at 36 cm CRL, 205 days. Positive immunodetection of VIP (arrows) in the lamina propia-submucosa (submucosus plexus). EAS, ×250. h Photomicrograph of a transverse direction section of the omasal wall at 36 cm CRL, 205 days. Positive immunoreaction for NPY (arrow) in the submucosus ganglion. EAS, ×250.
The presence of NNE+ neuroendocrine cells was observed from 67 days of gestation, located in the lamina propia-submucosa (Fig. 3a) and in the tunica muscularis. Immunoreactivity toward these cells increased throughout omasal histogenesis, being very high in perinatal stages (Fig. 3b).
The immunodetection of GFAP- (Fig. 3c and d) and VIM-positive glial cells (Fig. 3e and f) began at 67 days of intrauterine life in the lamina propia-submucosa (Fig. 3c and e), tunica muscularis and serosa. In stages around birth, immunoreactivity was very intense, especially in the tunica muscularis (Fig. 3d and f).
Detection of the neuropeptides VIP (Fig. 3g) and NPY (Fig. 3h) in nerve fibres and in nerve cell bodies began at 142 days of prenatal development, with a moderate immunopositivity in the lamina propia-submucosa, tunica muscularis and serosa. This immunoreactivity was similar in perinatal stages.
Histomorphometric observations
Table 2 shows the tissue layer thickness in the omasum of red deer during prenatal development. Each tissue stratum was fitted to mathematical growth models (Figs 4–8), using the corresponding growth equation.
Table 2.
Morphometrical and statistical findings of the tissue layer thickness in the omasum of red deer during prenatal development (µm)
| Group I CRL (cm) (1.4–3.6) 30–60 days | Group II CRL (cm) (4.5–7.2) 67–90 days | Group III CRL (cm) (8–19) 97–135 days | Group IV CRL (cm) (21–33) 142–191 days | Group V CRL (cm) (36–40) 205–235 days | |
|---|---|---|---|---|---|
| Epithelium | 62 ± 5 | 119 ± 11a | 219 ± 18a | 390 ± 28a | 525 ± 30a |
| Lp + Sb | pbt 195 ± 10* | pbt 206 ± 13* | 99 ± 8b | 168 ± 14b | 164 ± 16b |
| Tm | 79 ± 10b | 160 ± 11b | 155 ± 13b | ||
| Serosa | 56 ± 3 | 34 ± 8a | 32 ± 7a | 26 ± 4a | 24 ± 6a |
| Wall | 313 ± 16 | 364 ± 13a | 429 ± 30a | 744 ± 38a | 868 ± 29a |
Lp + Sb, lamina propia and submucosa; Tm, tunica muscularis; pbt, pluripotential blastemic tissue.
The pluripotential blastic tissue of Groups I and II, which will later give rise to the lamina propria and submucosa, were not statistically compared because one structure will give rise to a variety of others.
P < 0.05 vs. Group I.
P < 0.05 vs. Group III.
Fig 4.
Mathematical model of omasum growth (epithelium).
Fig. 8.
Mathematical model of omasum wall growth.
The epithelial layer increased progressively in thickness throughout omasal histogenesis, in such a way that the thickness of the epithelium of Group I was significantly less than that of Groups II, III, IV and V (F = 18.54, Tukey test P = 0.0003).
After initially increasing, the pluripotential blastemic tissue decreased in thickness as a result of differentiation of laminapropria-submucosa and tunica muscularis. Both layers increased in thickness throughout omasal development.
As indicated by main factor analysis in factorial anova, the lamina propria and submucosa of Group III was significantly different than these layers of Groups IV and V (F = 10.80, Tukey test P = 0.001). There were no significant differences between Groups IV and V.
The histomorphometric behaviour of the tunica muscularis was similar to that of the lamina propia-submucosa: Group III was significantly different from Groups IV and V (F = 16.90, Tukey test P = 0.0002), but no significant differences were found between Groups IV and V.
By contrast, serosa decreased in thickness throughout prenatal life. The mean thickness of serosa of Group I was significantly greater than in Groups II–V (F = 8.30, Tukey test P = 0.001). No significant differences between the thickness of the serosa of Groups II, III, IV and V were found.
The thickness of the omasal wall increased gradually throughout prenatal development, until it reached its maximum in the perinatal stages. The thickness of the wall of Group I was significantly less than that of Groups II–V (F = 7.80, Tukey test P = 0.003).
Discussion
In the organogenesis of the primitive gastric tube of red deer, compartmental differentiation, with individualized compartment and differentiation of the omasum, took place (as for the rumen and reticulum; Franco et al. 2004a,b) at 67 days (25% of gestation). In sheep the omasal differentiation was calculated at 33 days of embryonic life (22% of gestation). Mutoh & Wakuri (1989), studying the organogenesis of goat, specified that the primordial omasum appears at 28 days (19% of gestation). Vivo et al. (1990) and Vivo & Robina (1991) placed omasal differentiation at 30 days of prenatal development (11% of gestation) in the cow.
The omasal wall, with an undulated surface, corresponding to the primitive primary smaller laminae, was structured into three layers: an epithelial layer, blastemic pluripotential tissue and serosa. The epithelial layer was stratified into two zones: a basal zone or stratum germinativum, and a smaller apical zone or stratum granulosum. At 97 days of prenatal development (35% of gestation) the corneum stratum appeared. The stratification of the epithelial layer of the omasum was completed at 142 days (50% of gestation) with the appearance of the lucidum-spinosum stratum. There was temporal agreement in terms of complete stratification of the omasal epithelial layer of red deer compared with that referenced for the reticulum of this species (Franco et al. 2004b), although note that the corneum stratum appeared earlier in the omasal epithelial layer than it did in the reticulum. Our observations indicated a stratified omasal mucosa from 67 days of gestation until birth, a fact that has also been observed in perinatal sheep (Groenewald, 1993) and in goats (Molinari & Jorquera, 1988). A light keratinization was also evident, clearly in agreement with that described by Yamamoto et al. (1998) in the omasal mucosa of goat.
In red deer, progressive stratification of the omasal epithelial layer throughout ontogenesis was accompanied by an increase in the thickness of this layer, until it reached its maximum in perinatal stages. This finding was similar to that described by Totzauer & Sinowatz (1990) in cattle. However, Ramkrishna & Tiwari (1979) specified that its thickness in goat remained unchanging throughout prenatal development in the omasum. Even Lubis & O'Shea (1978), in a study of sheep, reported a reduction in perinatal stages.
The appearance of the primary smaller laminae in the omasum of red deer (67 days, 25% of gestation) was later than that described for the omasum of sheep, where it was placed at around 30–35 days, or 20–22% of gestation (Del Rio Ortega, 1973; Lubis & O'Shea, 1978).
The temporal order of appearance of the four sizes of laminae of the omasum of red deer was: primary at 67 days (25% of gestation), secondary at 90 days (30%), tertiary at 97 days (35%) and quaternary lamina at 135 (50% of gestation). In sheep, there was a significant discrepancy in terms of the timing of the appearance of the smaller laminae. Thus Franco et al. (1993a) described the following sequence: at 21, 26, 33 and 40% of gestation, respectively. Del Rio Ortega (1973) placed them at 24, 28, 32 and 33% of gestation, respectively. Lubis & O'Shea (1978) placed them at 21, 25, 30 and 40%, and Fath-El Bab et al. (1983) at 21, 26, 33 and 52%. In cattle (Vivo et al. 1990; Vivo & Robina, 1991) and in buffalo (Osman & Berg, 1982) the process was earlier, the presence of the four sizes of laminae being calculated at around 20% of gestation.
The majority of the literature investigated described the laminae of four different sizes in the omasal mucosa: in sheep at around 33% (Del Rio Ortega, 1973), 40% (Franco et al. 1993a; Lubis & O'Shea, 1978) or 52% of gestation (Fath-El Bab et al. 1983); in goat at 33% of gestation (Ramkrishna & Tiwari, 1979); and in cattle at about 33% of gestation (Totzauer & Sinowatz 1990). However, there have been exceptions, such as that noted by Wardrop (1961) in sheep, by Ramkrishna & Tiwari (1979) in goat and by Osman & Berg (1982) in buffalo, who established a fifth laminar size in perinatal stages. Where there was unanimity, however, was that the appearance of the laminar outlines was a sequential process, as the growth of a laminar generation involved the birth of the next generation until the appearance of the four sizes of laminae. In this process, all architectural components were involved: mucosa, lamina propia-submucosa and tunica muscularis. The exception was the serosa, which had a different function, a fact previously noted by Franco et al. (1993a) in the prenatal development of sheep.
At around mid-gestation (135 days), lateral evaginations of the connective tissue were formed from the basal stratum of the primary and secondary smaller laminae towards the epithelial surface. These were the primitive corneum papillae. From 205 days of prenatal life (75% of gestation) the corneum papillae could be observed in the four different sizes of laminae. In the prenatal development of sheep, Franco et al. (1993a) observed these corneum papillae in the stratum basale of the primary smaller laminae at 69 days (45% of gestation). At 79 days (53% of gestation) they began to become apparent in the second laminar generation, but it was not until 75% of gestation (133 days) that they appeared in the two remaining generations. A significant discrepancy exists in the timing of the appearance of the corneum papillae, given that in this species Del Rio Ortega (1973) situated its development at 64 days (42% of gestation), Lubis & O'Shea (1978) at 69 days (46% of gestation) and Fath-El Bab et al. (1983) at 123 days (82% of gestation). In goat, Ramkrishna & Tiwari (1979) placed the development of the corneum papillae at 67 days (45% of gestation). Totzauer & Sinowatz (1990) referenced it at 90 days (32% of gestation) in cattle. However, there was unanimity in the arrangement of the corneum papillae in agreement with histophysiology; ie, in such a way that the movement of the folds acts on the food and introduces it towards the interlaminar spaces (Franco et al. 1993a).
In the red deer, at 90 days of prenatal development (35% of gestation), myoblastic fibres moved from the internal fascicule of the tunica muscularis towards the interior of the smaller laminae, functioning as a support element and modulating the position and shape of the laminae and constituting the muscularis mucosae. This was reported also by Franco et al. (1993a) during prenatal life in sheep, chronologically placing it at 79 days (53% of gestation). Ramkrishna & Tiwari (1979) described it at 67 days (45% of gestation) in goat. In cattle, Totzauer & Sinowatz (1990) placed it at 120 days (43% of gestation). In the perinatal stages, Kitamura et al. (2003) detected muscularis mucosae in omasal smaller laminae in goat, sheep, cow and buffalo (in decreasing order of abundance of muscular fibres).
The histodifferentiation of the lamina propia and submucosa displayed patterns of behaviour similar to those referenced for the rumen and reticulum of red deer (Franco et al. 2004a,b), although what stood out was that there was no clear separation of the two structures, as in the rumen (Franco et al. 2004a).
From 67 days onwards (25% of gestation), coming from the pluripotential blastemic tissue, differentiation of the tunica muscularis took place, with the appearance of two layers of myoblasts: an internal circular layer and another, external longitudinal layer. Ramkrishna & Tiwari (1979) noted the presence of the fascicule of the tunica muscularis at around 67 days (45% of gestation) in goat. In sheep, Duncan & Phillison (1955) and Franco et al. (1993a) outlined this development at 39 days (26% of gestation).
The differentiation of the serosa did not show characteristics distinct from those described in prenatal development of the rumen and reticulum of red deer (Franco et al. 2004a,b).
The omasal mucosa of red deer, from 97 days of intrauterine life, displayed full secretory capacity of neutral mucopolysaccharides, mucins and mucinous compounds, in agreement with that described by Franco et al. (2004a,b) for the rumen and reticulum of this species.
The presence of neuroendocrine cells in the omasal mucosa of deer in development was not detected until 67 days of prenatal development. These cells were located in the lamina propria-submucosa and tunica muscularis. Similar observations were reported by Kitamura et al. (1986, 1993), in rumen and reticulum of cattle, by Groenewald (1994) in the forestomach of sheep, and by Franco et al. (2004a,b) in rumen and reticulum of red deer. The omasal mucosa from 67 days of intrauterine life displayed immunoreactivity towards GFAP and VIM at the level of the lamina propia-submucosa, tunica muscularis and serosa. The presence of glial cells in the submucosal and myenteric plexi and submucosa were also reported by Yamamoto et al. (1995) in sheep, by Teixeira et al. (1998) in cow and by Franco et al. (2004a,b) in red deer.
In agreement with that noted for the prenatal development of rumen and reticulum of red deer (Franco et al. 2004a,b), the detection of the neuropeptides VIP and NPY began at 142 days with moderate immunopositivity in the lamina propia-submucosa, tunica muscularis and serosa. This immunoreactivity remained unchanged until the end of gestation. The presence of the neuropeptides VIP and NPY has previously been described in the omasum of adult sheep (Yamamoto et al. 1994) and in the omasum of adult cattle (Kitamura et al. 1987). Immunoreactivity towards VIP and NPY has also been reported in the rumen, reticulum and omasum of cattle, in the reticulum of sheep from 90 days of prenatal development (Pospieszny, 1979), in ovine perinatal and postnatal stages (Vergara-Esteras et al. 1990; Groenewald, 1994; Pfannkuche et al. 2003), in the stomach of the fetal pig (Van Ginneken et al. 1996), in the small intestine of the adult pig (Timmermans et al. 1990) and in the small intestine of guinea-pigs (Mawe & Gershon, 1989).
From the observations made here, in terms of the prenatal structure of the omasum of red deer, in the same way as in the rumen and reticulum (Franco et al. 2004a,b), we can deduce that the deer is less precocious than small and large domestic ruminants. Thus, its secretory capacity (as detected by the presence of neutral mucopolysaccharides) and its neuroendocrine nature [as determined by the presence of positive NNE cells, and of neuropeptides of nerve fibres and nerve cell bodies (VIP and NPY)] were evident in more advanced stages of prenatal development than that detected in sheep, goat and cow.
Fig. 5.
Mathematical model of omasum growth (lamina propia and submucosa).
Fig. 6.
Mathematical model of omasum growth (tunica muscularis).
Fig. 7.
Mathematical model of omasum growth (serosa).
Acknowledgments
We would like to thank Ma Mercedes Carrasco Toral and Juana Ollero Granados for their invaluable technical assistance and contributions
References
- Carranza J. Aplicaciones de la etología al manejo de las poblaciones de ciervo en el suroeste de la Península Ibérica: producción y conservación. Etología. 1999;7:5–18. [Google Scholar]
- Del Rio Ortega S. Desarrollo prenatal del estómago de la oveja. Zaragoza: Facultad de Veterinaria; 1973. Doctoral. [Google Scholar]
- Duncan DL, Phillison AT. The development of motor responses in the stomach of the foetal sheep. J. Exp. Biol. 1955;28:32–40. doi: 10.1242/jeb.28.1.32. [DOI] [PubMed] [Google Scholar]
- Fath-El Bab MR, Schwarz R, Ali AM. Micromorphological studies on the stomach of sheep during prenatal life. Anat. Histol. Embryol. 1983;12:139–153. doi: 10.1111/j.1439-0264.1983.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Franco A, Vivo JM, Guillén MT, Regodón S, Robina A. Evolución parietal del retículo ovino de raza merina desde los 68 días de gestación hasta el nacimiento. Histol. Med. 1989;5:57–58. [Google Scholar]
- Franco A, Regodón S, Robina A, Redondo E. Histomorphometric analysis of the rumen of the sheep during development. Am. J. Vet. Res. 1992;53:1209–1217. [PubMed] [Google Scholar]
- Franco A, Robina A, Regodón S, Vivo JM, Masot AJ, Redondo E. Histomorphometric analysis of the omasum of sheep during development. Am. J. Vet. Res. 1993a;54:1221–1229. [PubMed] [Google Scholar]
- Franco A, Robina A, Guillén MT, Mayoral AI, Redondo E. Histomorphometric analysis of the abomasum of sheep during development. Anat. Anz. 1993b;175:119–125. doi: 10.1016/s0940-9602(11)80164-0. [DOI] [PubMed] [Google Scholar]
- Franco A, Robina A, Regodón S, Vivo JM, Masot AJ, Redondo E. Histomorphometric analysis of the reticulum of the sheep during development. Histol. Histopathol. 1993c;8:547–556. [PubMed] [Google Scholar]
- Franco A, Masot AJ, Gómez L, Redondo E. Morphometric and immunohistochemical study of the rumen of red deer during prenatal development. J. Anat. 2004a;204:501–513. doi: 10.1111/j.0021-8782.2004.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Redondo E, Masot AJ. Morphometric and immunohistochemical study of the reticulum of red deer during prenatal development. J. Anat. 2004b;205:277–289. doi: 10.1111/j.0021-8782.2004.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewald HB. Ultrastructure of the epithelium of the rumen, reticulum and omasum of grey, white and black karakul lambs. Onderstepoort J. Vet. Res. 1993;60:197–204. [PubMed] [Google Scholar]
- Groenewald HB. Neuropeptides in the myenteric ganglia and nerve fibres of the forestomach and abomasum of grey, white and black karakul lambs. Onderstepoort J. Vet. Res. 1994;61:207–213. [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamashita T. Immunohistochemical study on the distribution of neuron-specific enolase- and peptide-containing nerves in the reticulorumen and the reticular groove of cattle. J. Comp. Neurol. 1986;248:223–234. doi: 10.1002/cne.902480205. [DOI] [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamashita T. Immunohistochemical study on the distribution of neuron-specific enolase- and peptide-containing nerves in the omasum of cattle. J. Comp. Neurol. 1987;256:590–599. doi: 10.1002/cne.902560411. [DOI] [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamamoto Y, Yamashita T. Substance P-immunoreactive neurons of the bovine forestomach mucosa: their presumptive role in a sensory mechanism. Arch. Histol. Cytol. 1993;56:399–410. doi: 10.1679/aohc.56.399. [DOI] [PubMed] [Google Scholar]
- Kitamura N, Yoshiki A, Sasaki M, et al. Immunohistochemical evaluation of the muscularis mucosae of the ruminant forestomach. Anat. Histol. Embryol. 2003;32:175–178. doi: 10.1046/j.1439-0264.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Lubis D, O'Shea JD. Development of the omasum in sheep. Acta Anat. 1978;100:400–410. doi: 10.1159/000144924. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Gershon MD. Structure, afferent innervation, and transmitter content of ganglia of the guinea pig gallbladder: relationship to the enteric nervous system. J. Comp. Neurol. 1989;283:374–390. doi: 10.1002/cne.902830306. [DOI] [PubMed] [Google Scholar]
- Molinari E, Jorquera B. Intrauterine development stages of the gastric compartments of the goat (Capra hircus) Anat. Histol. Embryol. 1988;17:121–137. doi: 10.1111/j.1439-0264.1988.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Mutoh K, Wakuri H. Early organogenesis of the caprine stomach. Nippon Juigaku Zasshi. 1989;51:474–484. doi: 10.1292/jvms1939.51.474. [DOI] [PubMed] [Google Scholar]
- Osman AHR, Berg R. Studies on the histogenesis of the tunica mucosa of the stomach of the egyptian water buffalo (Bos bubbalis) 4. Histogenesis of the omasal mucosa. Anat. Anzeiger. 1982;151:467–471. [PubMed] [Google Scholar]
- Pfannkuche H, Schellhorn C, Schemann M, Gabel G. Reticular groove and reticulum are innervated by myenteric neurons with different neurochemical codes. Anat. Rec. 2003;274A:917–922. doi: 10.1002/ar.a.10104. [DOI] [PubMed] [Google Scholar]
- Pospieszny N. Distribution of the vagus nerve of the stomach and certain lymph nodes of the sheep in the prenatal period. Anat Anz. 1979;146:47–59. [PubMed] [Google Scholar]
- Ramkrishna V, Tiwari GP. Histological and histochemical observations on the forestomach of goat during pre-natal life. Acta Anat. (Basel) 1979;103:292–300. doi: 10.1159/000145026. [DOI] [PubMed] [Google Scholar]
- Regodón S, Franco A, Masot AJ, Redondo E. Comparative ontogenic analysis of the epithelium of the non-glandular stomach compartments of merino sheep. Anat. Histol. Embriol. 1996;25:233–241. doi: 10.1111/j.1439-0264.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Teixeira AF, Wedel T, Krammer HJ, Kuhnel W. Structural differences of the enteric nervous system in the cattle forestomach revealed by whole mount immunohistochemistry. Anat. Anz. 1998;180:393–400. doi: 10.1016/S0940-9602(98)80099-X. [DOI] [PubMed] [Google Scholar]
- Timmermans JP, Scheuermann DW, Stach W, Adriansen D, De Groot-Lasseel MHA. Distinct distribution of CGRP-, enkephalin-, galanin-, neuromedin U-, neuropeptide Y-, somastotatin-, substance P-, VIP- and serotonin-containing neurons in the two submucosal ganglionic neural netwoks of the porcine small intestine. Cell Tisue Res. 1990;260:367–379. doi: 10.1007/BF00318639. [DOI] [PubMed] [Google Scholar]
- Totzauer I, Sinowatz F. Fetal development of the omasum of cattle (Bos taurus) Tierarztl. Prax. 1990;18:577–583. [PubMed] [Google Scholar]
- Van Ginneken C, Weyns A, van Meir F, Ooms L, Verhofstad A. Intrinsic innervation of the stomach of the fetal pig: an immunohistochemical study of VIP-immunoreactive nerve fibres and cell bodies. Anat. Histol. Embryol. 1996;25:269–275. doi: 10.1111/j.1439-0264.1996.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Vergara-Esteras P, Harrison FA, Brown D. The localization of somatostatin-like immunoreactivity in the alimentary tract of the sheep with observations of the effect of an infection with the parasite Haemonchus contortus. Exp. Physiol. 1990;75:779–789. doi: 10.1113/expphysiol.1990.sp003460. [DOI] [PubMed] [Google Scholar]
- Vivo JM, Robina A, Regodón S, Guillén MT, Franco A, Mayoral AI. Histogenetic evolution of bovine gastric compartments during prenatal period. Histol. Histopathol. 1990;5:461–476. [PubMed] [Google Scholar]
- Vivo JM, Robina A. The development of the bovine stomach: morphologic and morphometric analysis. II. Observations of the morphogenesis associated with the omasum and abomasum. Anat Histol. Embryol. 1991;20:10–17. doi: 10.1111/j.1439-0264.1991.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Wardrop JD. Some preliminary observations on the histological development on the forestomach of the lamb. I. Histological changes due to age in the period from 46 days of foetal life to 77 days of posnatal life. J. Agric. Sci. 1961;57:335–341. [Google Scholar]
- Yamamoto Y, Atoji Y, Agungpriyono S, Suzuki Y. Morphological study of the forestomach of the japanese serow (Capricornis crispus) Anat. Histol. Embriol. 1998;27:73–81. doi: 10.1111/j.1439-0264.1998.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kitamura N, Yamada J, Yamashita T. Immunohistochemical study of the distributions of the peptide and catecholamine-containing nerves in the omasum of the sheep. Acta Anat. 1994;149:104–110. doi: 10.1159/000147564. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Atoji Y, Suzuki Y. Morphological study of the submucosal and mucosal plexuses of the sheep forestomach. Anat. Anz. 1995;177:405–412. doi: 10.1016/S0940-9602(11)80145-7. [DOI] [PubMed] [Google Scholar]