Abstract
Morphogenesis is underpinned by orientated cell division, motility and growth. The substratum for migrating cells in vivo comprises either extracellular matrix or the surfaces of adjacent cells and both are believed to inform the dynamic behaviour of adherent cells through contact guidance. Collisions between migrating cells in vitro can induce the phenomena of contact inhibition of locomotion and division, suggesting that their sensitivity to substratum-derived cues may also be influenced by population density. In the present study dermal fibroblasts, which are known to be motile in culture and are fundamental to the organization of the extracellular matrix, were used to examine the influence of population pressure on the ability of substratum topography to induce contact guidance. The findings suggest that sensitivity to substratum-derived morphogenetic guidance cues, as revealed by alignment of cells to microtopography, is modulated by population pressure.
Keywords: cell culture, contact guidance, development, fibroblast, morphogenesis
Introduction
By far the majority of experimental evidence supporting the notion that morphogenetic guidance cues may assist the process of embryogenesis has been derived from studies on cultured cells. One such cue, termed ‘contact guidance’ by Weiss (1934), was deduced from observations that nerve fibres regenerating from explants cultured in plasma clots tend to follow a course dictated by fibrin micelles orientated in the direction of stress within the clot. Attempts have been made to identify in vivo corollaries for such mechanisms that may operate during embryogenesis. For example, Stuart & Moscona (1967) reported that a meshwork of aligned collagen fibrils precedes the appearance of dermal papillae in developing embryonic chick skin and they suggested that the orientation of the meshwork influenced the direction of dermal cell migration. Bard & Higginson (1977) showed that fibroblasts align to an orthogonal array of fibrillar collagen in the posterior stroma of the developing chick cornea. Structures capable of guiding cell movement have been suggested to be important in chick heart (De Haan, 1963), neural crest cell migration in avian embryos (Newgreen, 1989) in teleost fin (Wood & Thorogood, 1984, 1987), in guidance of primordial germ cell migration in early embryogenesis (Wylie et al. 1979), and in the establishment of retino-tectal specificity (Horder & Martin, 1979). In the context of CNS development it has been reported that glial cell progenitors and oligodendrocytes within the optic nerve may be guided by fasciculated axonal topography within developing nerve trunks (Webb et al. 1995) and that developing neurons appear to ‘ride the glial monorail’ (Hatten, 1990).
The extent to which substratum-derived mechanisms have a role in the developing nervous system has received considerable experimental attention. Lofberg (1976) reported a conspicuous orientation of collagen fibrils on the surface of axolotyl neural tube and which would serve to guide neural crest cells. However, Dunn (1971) suggested that for neurites emerging from chick dorsal root ganglia cultured in plasma clots that contact guidance was not of prime importance for non-fasciculated outgrowths. Instead, Dunn (1971) suggested that contact inhibition of neurite extension, more specifically perhaps lateral extension, dictated the radial pattern of neurite outgrowth from ganglia explant cultures. The first observation, which would now be termed contact inhibition of locomotion in motile cells, was reported by Loeb, 1921) in studies of cultured Limulus ameobocytes. However, that cultured fibroblasts also exhibit contact inhibition of locomotion was first demonstrated by Abercrombie & Heaysman (1954), who later reported that other cells behaved similarly (Abercrombie & Ambrose, 1958). Detailed analysis of radial cell migration taking into account the number of cell–cell contacts indicated that the incidence of centrifugal migration increases with the frequency of contacts (Abercrombie & Heaysman, 1966).
It is unlikely that individual morphogenetic guidance cues would operate independently in steering cell division and migration during embryogenesis. More plausible is the notion that cell movements during development are choreographed by multiple cues acting in concert, interacting either synergistically or antagonistically and with spatio-temporal interdependency. Indeed, that this may the case has been demonstrated in several reports on cell orientation in response to the interactions between different types of guidance cue in cell culture, the conclusion from which must be that morphogenetic guidance cues acting in parallel may constitute a complex, highly integrated system for controlling aspects of development. Human skin cells in culture are known to have variable alignment and motility responses to substratum microtopography, with variation between different cell types and in their responses to variation in the dimensions of the surface features (Sutherland et al. 2000a,b,c). In the present report we present evidence to suggest that population pressure also affects the propensity for human dermal fibroblasts in cell culture to exhibit contact guidance in response to substratum microtopography.
Materials and methods
Skin samples were obtained with informed consent from patients undergoing elective surgery at Bradford Royal Infirmary and Airedale District General Hospital after gaining local research ethics committee approval. Given the paucity in supply and the intrinsic value of the material, all available specimens were included in this study, the majority being derived from patients aged 18 years and over undergoing auriculoplasty, abdominoplasty and mammoplasty.
All reagents were purchased from Sigma Chemical Company (Poole, UK) unless otherwise specified. After rinsing briefly to sterilize in 70% ethanol/30% MilliQ water, the skin samples were washed three times in Ca2+/Mg2+-free Hanks balanced salt solution (HBSS) containing 100 units mL−1 penicillin and 100 µg mL−1 streptomycin. Using a sterile technique, the epidermis was raised and small pieces of tissue trimmed off, leaving behind as much connective tissue as possible. For fibroblast culture, after washing thoroughly in HBSS the dermis was macerated using a scalpel blade. The macerate was transferred into 75-cm3 tissue culture flasks (Dow Corning) with 8 mL of serum-containing media and inverted. The flasks were incubated at 37 °C for 48 h before being turned the correct way up and cultured as normal for sufficient time to allow cells to migrate from the macerate onto the tissue culture plate and to achieve confluence. Occasionally this required periods of up to 6 weeks to complete. Normal growth media for dermal macerate and isolated fibroblasts was 25 mm Hepes-buffered Hams F10 supplemented with 20% fetal calf serum, 100 units mL−1 penicillin and 100 µg mL−1 streptomycin. Serum concentration was reduced to 5% after passage 1. Experiments were carried out in triplicate and analysis of motility was done after 24 h.
Several culture substrates were produced to test cell–surface interactions. These were either planar substrata or topographic substrata fabricated in fused silica using photolithographic and electron-beam lithographic methods, followed by reactive ion etching as described previously (Britland & McCaig, 1996; Rajnicek et al. 1998). The range of dimensions of the electron-beam multiple-grooved substrates were 1, 2 and 4 µm pitch at depths of 27, 110, 460 and 1000 nm. Sets of gratings were produced using standard photolithographic methods with dimensions of 5, 10, 25, 50 and 100 µm pitch and with depths of 44, 380, 780, 1300 and 3400 nm. All substrates were derived by adsorption of serum proteins from culture medium for 1 h prior to cell plating. Cells from stock were obtained by enzymatic detachment from culture flasks and reseeded onto test surfaces at 1 × 104 mL−1 in normal culture media for low-density cultures or 2.5 × 105 mL−1 to obtain confluent cultures. Analysis of cell alignment was carried out after a further 24 h to allow cell adhesion and spreading. Cultures were viewed using inverted phase-contrast optics (Nikon PSM-2120 and Olympus CK40) and photographed using CCD cameras (Nikon CB-230 H and Kodak DC290). Analysis of cell alignment was carried out on living cell populations using phase-contrast optics using an eyepiece 360° protractor graticule taking in six fields of view per device. Individual cells were scored as aligned if the long axis of the cells deviated less than 10° from parallel with the orientation of the underlying topography. Statistical evaluation of alignment data was carried out using a χ2 test (Wardlaw, 2000).
Results
In healthy cultures fibroblastic cells normally adhere and spread on suitable substrata in less that 6 h (Fig. 1A). Although cells retain mitotic capability in culture, the actual rate of cell division is influenced by many factors such as the age of the donor and the level of passage. Typically these parameters in the present experiment were such that variation in the rate of cell division between the time of plating and the point at which analysis of alignment was commenced was of minimal consequence. In high-density cultures cells can become confluent and progress to become stratified (Fig. 1C), becoming mutually aligned but not in relation to any obvious substratum features. This is similar to cells that migrate from the dermal explants during the initial culture protocol, but here there is alignment that has directional persistence away from the explant itself (Fig. 1B). Interestingly, different layers in stratified fibroblast cultures often have a mutually opposed pattern of alignment (Fig. 1C). The degree of cell alignment in low-density cultures was dependent on both groove width and groove depth – the proportion of aligned cells generally increased with groove depth for all widths (Fig. 2, χ2 = P < 0.01). However, for these cells, an increase in alignment for all groove widths was most conspicuous for submicrometre depth devices. Cells within confluent cultures were consistently more responsive to substratum topography, particularly for devices less that 1 µm in depth. Unless there was already near complete alignment, cells in confluent populations and therefore under greater population pressure were guided more effectively by shallower topography than cells comprising lower density cultures (Fig. 3). For structures 1 µm deep and greater the majority of cells were aligned at all widths in both low-density and confluent cultures.
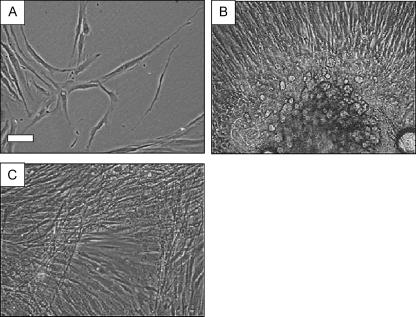
Fig. 1.
Phase contrast photomicrographs illustrating human dermal fibroblast morphology in P1 primary culture. In low-density culture (A) cells are not orientated in relation to each other, do not tend to cluster and most often adopt a multipolar morphology. Cells emanating from explants of dermis are crowded together and migrate radially outwards across the culture substratum (B). Fibroblasts in confluent monolayers are similarly crowded and tend to exhibit a parallel alignment, often becoming stratified into separate cellular layers whose orientation is often mutually perpendicular (C). Scale bar on A = 50 µm, and B and C are at the same magnification.
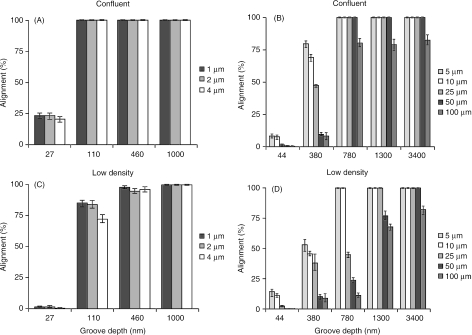
Fig. 2.
Line graphs illustrating the proportion of cells aligned to the underlying linear substratum microtopography within low-density and confluent cultures. Graphs A and B represent confluent cultures, C and D low-density cultures, and each displays alignment data for cells cultured on sets of grooves of increasing pitch and also increasing depth. Population pressure can be seen to exert maximum influence on alignment of cells on narrower grooves with depths between 380 and 1300 nm. On the narrowest grooves most cells in low-density cultures were insensitive to gratings 27 nm depth, but at confluence 25% of cells were aligned to the substratum.
Fig. 3.
Phase contrast micrographs illustrating the effect of population density on alignment responses of fibroblasts. The micrographs are in pairs illustrating the same substratum but with analysis of alignment carried out on low-density and confluent cell cultures. A and B = 110 nm depth × 2 µm pitch, C and D = 780 nm depth × 25 µm pitch, E and F = 780 nm depth × 50 µm pitch. A, C, E, confluent cultures; B, D, F, low-density cultures. Scale bar on A = 100 µm, and the other images are at the same magnification.
Discussion
The present study has demonstrated that cell–surface interaction, of a type that is mainly referred to as contact guidance, is modulated by the extent of physical contact between cells. Whether this pattern of behaviour reflects a fundamental difference in cellular reactions to their surroundings, for example differential gene expression between the low-density and confluent cultures, or whether these trends could be resolved on the basis of statistical probability is worth further consideration. Elimination of either possibility is beyond the scope of the data presented but it is reasonable to conclude that cells are capable of different behaviours in response to what amounts to only subtle variations in microenvironment conditions.
Variation in alignment responses of cells to substratum topography has been reported previously in cells derived from immortalized lines. MDCK epithelial cells grown on similar substrata to that used here only aligned to the underlying topography when solitary, i.e. when physically isolated from other cells (Clark et al. 1990). This tendency for isolated cells to align to substratum topography was diminished to a large extent when the cells congregated in clusters (Clark et al. 1991), with the cells also assuming a different morphology irrespective of whether they resided on micrometre-scale surface features or finer. This is opposite to the present findings, where sensitivity to substratum shape was heightened by the proximity of other cells. Although there are differences between the two studies in terms of the precise dimensions of the substratum features and differences in culture conditions, this dissimilarity nevertheless points to the fact that epithelial and fibroblastic cells are programmed to respond differently to similar circumstances. The origin of these differences in behaviour may have a molecular genetic basis in that both cells are programmed to behave in a unique way to similar circumstances. A key difference here may relate to MDCK and other epithelial cells normally existing as colonies, whereas, in the context of the present study, fibroblasts in vivo are usually solitary and are known to exhibit contact inhibition, a manifestation of avoidance of population pressure. The heightened sensitivity of the fibroblasts to substratum shape is consistent with their propensity to display alignment only in stratified cultures where substratum topography appears to have a contributory role, but is derived from the proximity of neighbouring cells. This relationship between population pressure and alignment could have functional significance, especially as it recognized that tissue regeneration during wound healing, for example, is underpinned by orientated cell division and migration (Clark, 1996).
Other studies on primary porcine epithelial cells similarly reported variable responses of single, pairs and clusters of cells to substratum topography (Oakley & Brunette, 1995). For dermal fibroblasts, alignment to the substrata serves as a predictor for orientated motility (Sutherland et al. 2000a,b,c). Interestingly, the general principles of cell migration originating from studies on tissue explants cultured in clotted plasma and lymph at the beginning the last century (Harrison, 1912; Loeb & Fleischer, 1917), suggesting that ‘cells tend to follow the path of least resistance’, are not contradicted by the results of this study.
Since Weiss (1934, 1945) first suggested the term ‘contact guidance’ to describe the migratory movement of cells, attempts to unravel the precise nature of cells’ interactions with the physicochemical properties of their substrata have continued unabated. The advent of microelectronics fabrication technology allowed the production of culture substrates incorporating guidance structures with meaningful and relevant dimensions for cells of both topographic and chemical nature (Curtis & Clark, 1990). Curtis & Clark (1990) specifically addressed the issue of density-dependent responsiveness of cells to substratum topography, concluding that it was a significant modulatory factor. The range of model culture substrata incorporating topographic cues (Flemming et al. 1999) and chemical heterogeneity (Ito, 1999) that have been employed in examining the phenomenon of contact guidance is now immense. Contact guidance represents only one of several substratum-related and other microenvironmental mechanisms that are thought to influence cell motility. Evidence is accumulating that such processes are relevant in vivo and may include chemotaxis (Zicha & Dunn, 1995) and chemokinesis (Horikawa et al. 1995), haptotaxis (Carter, 1965; Brandley & Schnaar, 1989; Koivisto et al. 1999), galvanotaxis (McCaig, 1986), and indeed population pressure (Abercrombie & Gitlin, 1965) and surface roughness, termed rugophilia (Rich & Harris, 1981; Chehroudi & Brunette, 1995). Recent reports have suggested that cells also exhibit orientation responses to variation in surface rigidity, termed durotaxis (Lo et al. 2000; Wang et al. 2001), and extraneous extracellular tensional forces (Vandenburgh, 1988; Eastwood et al. 1998).
It is noteworthy that a small number of studies have reported a hierarchical nature for the influence of separate guidance cues on cell alignment and motility when applied simultaneously (McCaig, 1986; Britland et al. 1996; Britland & McCaig, 1996), and the molecular genetic responses of cells to dual cues such as contact guidance and mechanical stimuli (Mudera et al. 2000). Furthermore, it has been shown that population pressure can affect cell–cell interaction in a manner that affects normal growth patterns by a mechanism termed ‘topoinhibition’ (Dunn & Ireland, 1984). The introduction of laminar flow of media across cells in culture, which could also be considered a morphogenetic guidance cue, reduces topoinhibition, which in turn suppresses cell growth mainly at the upstream end, probably by reducing the concentration of available growth factors.
The integration of the influences on motility extends beyond substratum-derived cues to include trophic factors. Ware et al. (1998) reported that epidermal growth factor alters the rate of fibroblast migration and directional persistence reciprocally and that this relationship is altered according to the physicochemical properties of the substratum, similar to the variable orientation responses of extending Xenopus neurites on different substrates (Rajnicek et al. 1998). However, it is worth noting that by far the majority of all research reports relating to the concept of contact guidance have involved studies on cell lines or often a single primary cell type derived from an animal model. By comparison, there has been a paucity of reports concerning even contact guidance in human-derived cells.
The weight of experimental evidence at present appears to support the notion that at the cellular level development may be influenced, in part, by the nature of the cell–substratum interaction. Given that several other putative morphogenetic guidance cues have been shown to modulate cells’ reactions to their substratum, it is possible that synergistic and hierarchical interactions within this system of guidance cues forms the basis of a highly integrated control system for cell movement and positioning during development.
Acknowledgments
We would like to express our gratitude to the patients who allowed their cells to be used in these experiments, and Professor Sharpe, Department of Plastic Surgery, Bradford Royal Infirmary, and Mr John Brash, Department of Obstetrics and Gynaecology, Airedale District Hospital, and all their support staff for supplying the skin specimens. Thanks to Professor Wilkinson, Bill Monaghan and Mary Robertson at the Department of Electronics, University of Glasgow, for fabrication support.
References
- Abercrombie M, Heaysman JEM. Observations on the social behaviour of cells in tissue culture II. ‘Monolayering’ of fibroblasts. Exp. Cell Res. 1954;6:293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- Abercrombie M, Ambrose EJ. Interference microscope studies of cell contacts in tissue culture. Exp.Cell Res. 1958;15:332–345. doi: 10.1016/0014-4827(58)90034-x. [DOI] [PubMed] [Google Scholar]
- Abercrombie M, Gitlin G. The locomotory behaviour of small groups of fibroblasts. Proc. R. Soc. Biol. 1965;162:289–302. [Google Scholar]
- Abercrombie M, Heaysman JEM. The directional movement of fibroblasts emanating from cultured explants. Ann. Med. Exp. Fenn. 1966;44:161–165. [PubMed] [Google Scholar]
- Bard JBL, Higginson K. Fibroblast collagen interactions in the formation of the secondary stroma of the chick cornea. J. Cell Biol. 1977;74:816–827. doi: 10.1083/jcb.74.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandley BK, Schnaar RL. Tumor-cell haptotaxis on convalently immobilized linear and exponential gradients of a cell-adhesion peptide. Dev. Biol. 1989;135:74–86. doi: 10.1016/0012-1606(89)90159-0. [DOI] [PubMed] [Google Scholar]
- Britland ST, McCaig CD. Embryonic Xenopus neuritis integrate and respond to simultaneous electrical and adhesive guidance cues. Exp. Cell Res. 1996;225:31–38. doi: 10.1006/excr.1996.0199. [DOI] [PubMed] [Google Scholar]
- Britland S, Morgan H, WojiakStodart B, Riehle M, Curtis A, Wilkinson C. Synergistic and hierarchical adhesive and topographic guidance of BHK cells. Exp. Cell Res. 1996;228:313–325. doi: 10.1006/excr.1996.0331. [DOI] [PubMed] [Google Scholar]
- Carter SB. Haptotaxis and the mechanism of cell motility. Nature. 1965;213:256–260. doi: 10.1038/213256a0. [DOI] [PubMed] [Google Scholar]
- Chehroudi B, Brunette DM. Nanofabrication and Biosystems. In: Wise DL, Trantolo DJ, Altobelli DE, YasZemski MJ, Gresser JD, Schwartz ER, editors. Encyclopedic Handbook of Biomaterials and Bioengineering. New York: Decker; 1995. pp. 813–842. [Google Scholar]
- Clark P, Connolly P, Curtis ASG, Dow JA, Wilkinson CD. Topographical control of cell behaviour. II. Multiple grooved substrata. Development. 1990;108:635–644. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Cell guidance by ultrafine topography in vitro. J. Cell Sci. 1991;99:73–77. doi: 10.1242/jcs.99.1.73. [DOI] [PubMed] [Google Scholar]
- Clark RAF. Wound repair: overview and general considerations. In: Clark RAF, editor. The Molecular, Cellular Biology of Wound Repair. New York: Plenum Press; 1996. pp. 3–35. [Google Scholar]
- Curtis ASG, Clark P. The effects of topographic and mechanical properties of materials on cell behavior. Crit. Rev. Biocompat. 1990;5:343–362. [Google Scholar]
- De Haan RL. Migration patterns of the precardiac mersoderm in early chick embryo. Exp. Cell Res. 1963;29:554–560. doi: 10.1016/s0014-4827(63)80016-6. [DOI] [PubMed] [Google Scholar]
- Dunn GA. Mutual contact inhibition of extension of chick sensory nerve fibres in vitro. J. Comp. Neurol. 1971;143:491–508. doi: 10.1002/cne.901430406. [DOI] [PubMed] [Google Scholar]
- Dunn GA, Ireland GW. New evidence that growth in 3T3 cell cultures is a diffusion-limited process. Nature. 1984;31:63–65. doi: 10.1038/312063a0. [DOI] [PubMed] [Google Scholar]
- Eastwood M, Mudera VC, McGrouther DA, Brown RA. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Mot. Cytoskel. 1998;40:13–21. doi: 10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro-and nanostructured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- Harrison RG. The cultivation of tissues in extraneous media as a method of morphogenetic study. Anat. Rec. 1912;6:181–193. [Google Scholar]
- Hatten ME. Riding the glial monorail: a common mechanism for glial-guided neuronal migration in different regions of the developing mammalian brain. Trends Neuro. sci. 1990;13:170–184. doi: 10.1016/0166-2236(90)90044-b. [DOI] [PubMed] [Google Scholar]
- Horder TJ, Martin KAC. Morphogenetics as an alternative to chemospecificity in the formation of nerve projections. Symp. Soc. Exp. Biol. 1979;32:275–359. [PubMed] [Google Scholar]
- Horikawa T, Norris DA, Yohn JJ, Zekman T, Travers JB, Morelli JG. Melanocyte mitogens induce both melanocyte chemokinesis and chemotaxis. J. Invest. Dermatol. 1995;104:256–259. doi: 10.1111/1523-1747.ep12612795. [DOI] [PubMed] [Google Scholar]
- Ito Y. Surface micropatterning to regulate cell functions. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Koivisto L, Larjava K, Hakkinen L, Uitto VJ, Heino J, Larjava H. Different integrins mediate cell spreading, haptotaxis and lateral migration of HaCaT keratinocytes on fibronectin. Cell Adhesion Comm. 1999;7:245–257. doi: 10.3109/15419069909010806. [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L, Fleischer MS. On the factors which determine the movements of tissues in culture media. J.Med. Res. 1917;37:75–99. [PMC free article] [PubMed] [Google Scholar]
- Loeb L. Ameboid movement, tissue formation and consistency of protoplasm. Am. J. Physiol. 1921;56:140–167. doi: 10.1126/science.53.1368.261. [DOI] [PubMed] [Google Scholar]
- Lofberg J. Scanning and transmission electron microscopy of early neural crest cell migration and extracellular fiber systems in the amphibian embryo. J. Ultrastruct. Res. 1976;159:484A. [Google Scholar]
- McCaig CD. Electric fields, contact guidance and the direction of nerve growth. J. Embryol. Exp. Morph. 1986;94:245–255. [PubMed] [Google Scholar]
- Mudera VC, Pleass R, Eastwood M, et al. Molecular responses of human dermal fibroblasts to dual cues: contact guidance and mechanical load. Cell Motil. Cytoskel. 2000;45:1–9. doi: 10.1002/(SICI)1097-0169(200001)45:1<1::AID-CM1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Newgreen DF. Physical influences on neural crest cell migration in avian embryos: contact guidance and spatial restriction. Dev. Biol. 1989;131:136–148. doi: 10.1016/s0012-1606(89)80045-4. [DOI] [PubMed] [Google Scholar]
- Oakley C, Brunette DM. Responses of single, pairs and clusters of epithelial cells to substratum topography. Bio. chem. Cell Biol. 1995;73:473–489. doi: 10.1139/o95-053. [DOI] [PubMed] [Google Scholar]
- Rajnicek AM, Robinson KR, McCaig CD. The direction of neurite growth in a weak DC electric field depends on the substratum: contributions of adhesivity and net surface charge. Dev. Biol. 1998;203:412–423. doi: 10.1006/dbio.1998.9039. [DOI] [PubMed] [Google Scholar]
- Rich AM, Harris AK. Anomolous preferences on cultured macrophages for hydrophobic and roughened substrata. J. Cell Sci. 1981;50:1–7. doi: 10.1242/jcs.50.1.1. [DOI] [PubMed] [Google Scholar]
- Stuart ES, Moscona AA. Embryionic morphogenesis: role of fibrous lattice in the development of feathers and feather patterns. Science. 1967;157:947–948. doi: 10.1126/science.157.3791.947-a. [DOI] [PubMed] [Google Scholar]
- Sutherland J, Robertson M, Monaghan W, Riehle M, Britland ST. Human skin cells exhibit variable alignment responses to substratum topography. J. Anat. 2000a;198:P89. [Google Scholar]
- Sutherland J, Robertson M, Monaghan W, Riehle M, Britland ST. Cellular components of human skin are differentially motile in primary culture. J. Anat. 2000b;198:P91. [Google Scholar]
- Sutherland J, Robertson M, Monaghan W, Riehle M, Britland ST. Facilitated migration of human epidermal keratinocytes and dermal fibroblasts in primary culture. J. Anat. 2000c;198:A65. [Google Scholar]
- Vandenburgh HH. A computerised mechanical cell stimulator for tissue culture – effects on skeletal muscle organogenesis. In Vitro Cellular Dev. Biol. 1988;24:609–619. doi: 10.1007/BF02623597. [DOI] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang YL. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl Acad. Sci. USA. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw AC. Practical Statistics for Experimental Biologists. 2. Chichester: Wiley and Sons; 2000. [Google Scholar]
- Ware MF, Wells A, Lauffenburger D. Epidermal growth factor laters fibroblasts migration speed and dierectional persistence reciprocally and in a matrix dependent manner. J. Cell Sci. 1998;111:2423–2432. doi: 10.1242/jcs.111.16.2423. [DOI] [PubMed] [Google Scholar]
- Webb A, Clark P, Skepper J, Compton A, Wood A. Guidance of oligodendrocytes and their progenitors by substratum topography. J. Cell Sci. 1995;108:2747–2760. doi: 10.1242/jcs.108.8.2747. [DOI] [PubMed] [Google Scholar]
- Weiss P. In vitro experiments on the factors determining the course of the outgrowing nerve fiber. J. Exp. Zool. 1934;69:393–448. [Google Scholar]
- Weiss P. Experiments on cell and axon orientation in vitro: the role of colloidal exudates in tissue organisation. J. Exp. Zool. 1945;100:253–386. doi: 10.1002/jez.1401000305. [DOI] [PubMed] [Google Scholar]
- Wood AT, Thorogood P. An analysis of in vivo cell migration during teleost fin morphogenesis. J. Cell Sci. 1984;66:205–222. doi: 10.1242/jcs.66.1.205. [DOI] [PubMed] [Google Scholar]
- Wood AT, Thorogood P. An ultrastructural and morphometric analysis of an in vivo contact guidance system. Development. 1987;101:363–381. [Google Scholar]
- Wylie CC, Heasman J, Swan AP, Anderton BH. Evidence for substrate guidance of primordial germ cells. Exp. Cell Res. 1979;121:315–324. doi: 10.1016/0014-4827(79)90010-7. [DOI] [PubMed] [Google Scholar]
- Zicha D, Dunn GA. Are growth factors chemotactic agents? Exp. Cell Res. 1995;221:526–529. doi: 10.1006/excr.1995.1404. [DOI] [PubMed] [Google Scholar]