Abstract
Remyelination, the process by which new myelin sheaths are restored to demyelinated axons, represents one of the most compelling examples of adult multipotent progenitor cells contributing to regeneration of the injured central nervous system (CNS). This process can occur with remarkable efficiency in both clinical disease, such as multiple sclerosis, and in experimental models, revealing an impressive ability of the adult CNS to repair itself. However, the inconsistency of remyelination in multiple sclerosis, and the loss of axonal integrity that results from its failure, makes enhancement of remyelination an important therapeutic objective. Identifying potential targets will depend on a detailed understanding of the cellular and molecular mechanisms of remyelination. In this article we address two important issues. First, we consider the nature of the cell or cells that respond to demyelination and generate new oligodendrocytes, identifying current areas of uncertainty and addressing the role of adult CNS stem and progenitor cells. Second, we discuss the concept of adult progenitor activation following demyelination, focusing on the increased expression of (1) olig transcription factors, (2) bone morphogenetic proteins and (3) fyn, a member of the src-family of tyrosine kinases.
Keywords: stem cells, progenitors, remyelination, multiple sclerosis, demyelination, regeneration
Introduction
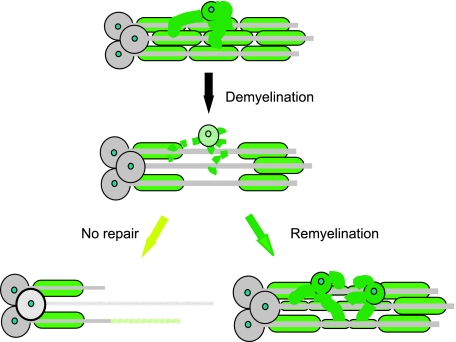
Following demyelination in the central nervous system (CNS), a hallmark event in the disease multiple sclerosis (MS), there are two possible outcomes (Fig. 1). Either the axons remain demyelinated and are vulnerable to atrophy, an event that makes a significant contribution to the progressive phase of MS (De Stefano et al. 1998; Bjartmar et al. 2003), or new myelin sheaths can be restored to the demyelinated axons in a spontaneous regenerative process called remyelination (Prineas et al. 1993; Lassmann et al. 1997). It has been known for many years that remyelination restores saltatory conduction (Smith et al. 1979). More recently, however, it has become apparent that the presence of an intact myelin sheath has a profoundly beneficial effect on axonal integrity and that remyelination may therefore provide a highly effective means of preventing axonal loss in demyelinating disease (Griffiths et al. 1998; Kornek et al. 2000; Lappe-Siefke et al. 2003; Edgar et al. 2004; Xin et al. 2005). Thus, while strategies to prevent demyelination remain a major focus of MS therapy, approaches that promote remyelination and minimize axonal loss represent an important adjunct to the therapeutic armoury. Developing such therapies is likely to depend on a detailed understanding of the mechanism of remyelination, from which will emerge a better understanding of why this repair process often fails in MS patients (Franklin, 2002; Franklin & Goldman, 2004).
Fig. 1.
Demyelination occurs when the oligodendrocyte or the myelin sheath its produces and maintains is the target of the disease process. Once axons have been demyelinated there are two possible outcomes: either the axon remains demyelinated, in which case it is vulnerable to axonal or even neuronal loss, or the axon can be remyelinated. This process involves the generation of new oligodendrocytes that reinvest the demyelinated axons with the thin, short myelin internodes characteristic of remyelination.
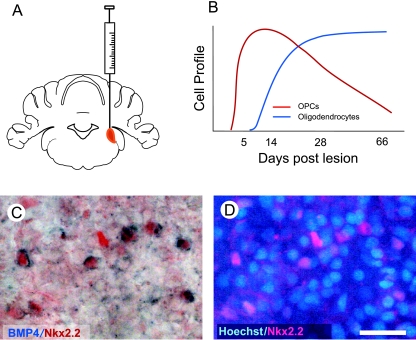
Several experimental models of CNS demyelination exist that have been used to study remyelination. Of these, the models that involve toxic death of oligodendrocytes have proved to be especially helpful because the demyelination is usually acute and focal and is followed by a defined and generally predictable regenerative response (Fig. 2A,B). From these models it has been possible to identify a sequence of events by which remyelination proceeds. The first event is the activation of a population of progenitor cells that then rapidly populate an area of demyelination by proliferating and migrating during the recruitment phase of remyelination. This phase then gives way to a second phase in which the recruited cells differentiate into oligodendrocytes, a highly specialized cell that generates new myelin sheaths around the demyelinated axons. This simple model of remyelination, which in its broad outline resembles many other regenerative processes in the body, raises a number of important questions. In this article we focus on two of these: what is the nature of the cell or cells that respond to demyelination and generate new oligodendrocytes, and how do these cells respond to injury in preparation for remyelination?
Fig. 2.
(A) The EB-CCP lesion provides a useful model for studying the biology of CNS remyelination. A focal area of primary demyelination is created by stereotaxic injection of 4 µL of 0.01% EB into the large white matter tract of the caudal cerebellar peduncle (see Woodruff & Franklin, 1999). (B) In young adult rats (2–4 months of age), the demyelination induced by EB injection undergoes a stereotypic process of remyelination in which there is first an OPC response which is then followed by the appearance of new oligodendrocytes (derived from the recruited OPCs) that remyelinate the demyelinated axons. The process is complete at around 28 days (see Sim et al. 2002a,b). (C) Nkx2.2+ OPCs express high levels of BMP4 mRNA at 7 days after lesion induction. (D) Although there are many cell types within the lesion at this time point only a proportion are Nkx2.2+ OPCs, and the BMP4+ cells illustrated in C are all Nkx2.2+. Scale bar = 16 µm.
What cells give rise to remyelinating oligodendrocytes?
In most situations these cells are a distinctive phenotype widely referred to as adult oligodendrocyte progenitor cells (OPCs). These cells are the adult descendants of an extensively studied developmental progenitor, originally called the O-2A progenitor based on its ability in vitro to give rise to a distinctive type of astrocyte (the type 2 astrocyte) as well as oligodendrocytes (Raff et al. 1983; Wren et al. 1992). Because the type 2 astrocyte is thought to occur infrequently, if at all, during normal development, these cells are now generally referred to as simply oligodendrocyte progenitor cells (Fulton et al. 1992; Levison & Goldman, 1993). In adult tissue these cells have a characteristic multipolar morphology and express several markers, of which the proteoglycan NG2 and the growth factor receptor PDGFRα are the most commonly used (Nishiyama et al. 1996; Dawson et al. 2000, 2003). Whether OPCs express both markers in all circumstances and in all regions of the adult CNS is uncertain (Hampton et al. 2004); indeed, the extent to which this is a homogeneous population of cells throughout the adult neuraxis is also unresolved. There is now clear evidence that oligodendrocytes can be generated via several distinct lineage pathways and therefore from a developmental perspective progenitor phenotypes are diverse (Mallon et al. 2002; Liu & Rao, 2004; Cai et al. 2005; Vallstedt et al. 2005). For example, two distinct populations can be described on the basis of expression of PDGFRα or DM20, an alternatively spliced isoform of the proteolipid protein gene (Spassky et al. 1998, 2000). The extent to which OPCs in the adult CNS retain an imprint of their developmental origin remains to be unequivocally determined. One possibility is that adult OPCs are a homogeneous population of cells that have a similar phenotype and responsiveness to environmental signals despite their varied ontogeny. Alternatively, distinctive types of OPC may exist, either coexisting or being specific to a particular anatomical region. There is some evidence to suggest that this may be the case; in tissue culture the markers O4 and A2B5 appear to identify distinct populations of adult forebrain OPCs that respond differently to a range and combination of growth factors (Mason & Goldman, 2002). This is clearly an important issue to resolve, especially in adult human tissue, if growth-factor-based strategies are to be used therapeutically to enhance endogenous remyelination in clinical disease. The evidence that cells other than OPCs contribute to remyelination is scant. Two studies have demonstrated that when demyelinating lesions are induced in the corpus callosum close to the subventricular zone (SVZ), then neural progenitor cells can be deflected away from their normal path towards the olfactory bulb and towards the lesion where they can contribute to the generation of new oligodendrocytes during remyelination (Nait-Oumesmar et al. 1999; Picard-Riera et al. 2002). The component of the total remyelination attributable to SVZ-derived cells is uncertain but is likely to be small given the abundance and responsiveness of locally derived OPCs. A further uncertain issue is how close an area of demyelination must be in order for SVZ progenitors to respond. Although it is clear that lesions within the adjacent corpus callosum can induce this response, it is unlikely that white matter lesions remote from the SVZ in, for example, the spinal cord or brain stem white matter will do so given that most remyelinating cells are recruited from a narrow region surrounding a lesion (Franklin et al. 1997). In white matter regions remote from the SVZ there is no clear evidence at present that cells other than OPCs contribute to remyelination.
Do CNS stem cells contribute to remyelination?
If one applies strict criteria to the definition of a stem cell (a multipotent cell, generally attached to a basal lamina, that divides slowly and that is both self-renewing and able to give rise to rapidly proliferating progenitor cells by asymmetric division), then true stem cells within the adult mammalian CNS are rare, comprising the glial fibrillary acidic protein (GFAP)-expressing B cells of the SVZ and perhaps their hippocampal equivalents (Doetsch et al. 1999a,b; Sanai et al. 2004; Seri et al. 2004). There is currently no evidence that either of these cells directly contributes to remyelination (although one could argue that the SVZ B cells do so indirectly by giving rise to SVZ neural progenitors). Thus, adult CNS stem cells make a small and anatomically restricted contribution to endogenous remyelination in the adult. This is similar to other regenerating tissues where the proliferation of the stem cell population is scarcely affected by the sudden demand for new differentiated cells following injury. Instead, this demand is taken up by the transit-amplifying population of progenitors, which, unlike the stem cells from which they are generated, have the proliferative responsiveness rapidly to generate the new cells required to repair damaged tissue. Should one regard the OPCs of the adult brain as being stem cells or progenitor cells? OPCs certainly exhibit some stem cell properties: they exhibit multipotency, giving rise to oligodendrocytes, neurons and, at least in vitro, astrocytes (ffrench-Constant & Raff, 1986; Kondo & Raff, 2000; Belachew et al. 2003; Nunes et al. 2003), and have very high levels of telomerase activity allowing them to undergo many rounds of proliferation before undergoing senescence (Tang et al. 2001). However, their rapid proliferation, symmetrical division (the daughter cells of OPC proliferation are still OPCs regardless of whether they subsequently differentiate into oligodendrocytes or not) and absence of a distinct anatomical relationship with a basal lamina are more consistent with their being a transit-amplifying population and in our view are more accurately regarded as progenitors rather than stem cells. Indeed, a pertinent question to consider is how similar OPCs are to other multipotent neural progenitor cells within the adult CNS, and whether perhaps a generic term of neural progenitor should be more widely applied (Goldman, 2003)?
Because the cells responsible for generating new oligodendrocytes are transit-amplifying progenitor cells, can the capacity of these cells to proliferate in response to injury become exhausted if repeatedly tested? This question has important implications for understanding why remyelination often fails and how easy it will be to mobilize OPCs therapeutically. The ability of adult OPCs to repopulate areas from which they are deficient appears to be very robust (Chari & Blakemore, 2002). When the same area of CNS is exposed to several rounds of demyelination/remyelination, the OPC numbers are not reduced and the efficiency of remyelination is not impaired by previous rounds of remyelination (Penderis et al. 2003). This implies that a failure of remyelination is not due to an exhaustion of OPCs available to repopulate the demyelinated area and give rise to new oligodendrocytes. However, this appears only to be the case if sufficient time is left between demyelinating episodes to allow the OPC numbers to be replenished. If an area of demyelination is exposed to a continual demyelinating insult then OPC numbers do gradually diminish (Ludwin, 1980; Mason et al. 2004). The interpretation of these long-term experiments in rodents is confounded by ageing, as this process alone can significantly impair the responsiveness of OPCs to demyelination (Sim et al. 2002b), partly due to changes in the signalling environment with ageing (Hinks & Franklin, 2000) and possibly also due to intrinsic changes in the responsiveness of aged OPCs (Decker et al. 2002; Chari et al. 2003).
Reactive OPCs – a critical phenotypic switch for remyelination?
The reactive changes that occur in astrocytes and microglia in response to CNS injury are well documented and comprise a series of phenotypic changes that distinguish the reactive state from the resting state. Similar reactive changes occur in OPCs (Levine et al. 2001). This was first described as a change in morphology and more intense staining with the OPC marker NG2 (Levine & Reynolds, 1999). More recently it has become apparent that a number of distinct changes in gene expression occur that are associated with OPC activation. In this section we consider three of these, the Olig transcription factors, the bone morphogenetic proteins and the tyrosine kinase fyn.
Olig transcription factors
In the normal adult white matter PDGFRα+ OPCs also express mRNA of the bHLH transcription factor Olig1 at levels easily detectable by in situ hybridization. By contrast, the mRNA expression levels of the related transcription factor Olig2 and the homeodomain transcription factor Nkx2.2 are below the threshold for detection by this method. However, the expression of both Olig2 and Nkx2.2 mRNA within OPCs dramatically increases following induction of focal demyelination (Fancy et al. 2004; Watanabe et al. 2004; Talbott et al. 2005). The increase in Nkx2.2 expression occurs in the absence of detectable increases in sonic hedgehog mRNA expression (Fancy et al. 2004), which is responsible for its induction during development but appears not to be required for CNS remyelination (Briscoe et al. 1999; Lu et al. 2000). However, the increased expression of Olig2 mRNA could be accounted for by the rapid increase in expression of FGF-2 that occurs in toxin-induced demyelination (Hinks & Franklin, 1999). During development, the convergence of expression of Olig2 and Nkx2.2 within the same cell population is a necessary event for these precursor cells to differentiate into oligodendrocytes (Sun et al. 2001; Zhou et al. 2001). It seems possible therefore that the increased expression of these two genes in response to demyelination is a critical event required to convert quiescent OPCs into cells able to differentiate into remyelinating oligodendrocytes. Consistent with this hypothesis, the increases in Olig2 and Nkx2.2 expression are delayed in old animals where the rate of OPC differentiation is slower than in young animals (Sim et al. 2002b; Fancy et al. 2004).
Bone morphogenetic proteins (BMPs)
BMPs, members of the transforming growth factor superfamily of growth factors, play important roles in the development of many cell types including CNS glial cells (Mehler et al. 1997). They are also implicated in repair processes of several tissues, especially bone (Reddi, 1998). Their effects on OPCs include (1) promoting differentiation of OPCs into astrocytes while inhibiting oligodendrocyte differentiation (Mabie et al. 1997; Grinspan et al. 2000; Gomes et al. 2003), an effect mediated by their induction of the helix-loop-helix transcription inhibitors Id2 and Id4 and that can be overridden by the BMP antagonist noggin (Kondo & Raff, 2004; Samanta & Kessler, 2004), and (2) the inhibition of maturation of oligodendrocytes while promoting process formation in these cells (See et al. 2004). BMPs are expressed at high levels in developmental white matter. We carried out a study to investigate the expression of BMP2, BMP4 and BMP7, members of the family that most potently affect OPCs, and their antagonist noggin during the repair of focal toxin-induced demyelination in adult rat brain. The model employed involved stereotaxic injection of the DNA-intercalating agent ethidium bromide (EB) into the large white matter tract of the caudal cerebellar peduncle (CCP) of adult rats. Provided very dilute solutions of EB are used, this procedure creates a discrete focal area of primary demyelination in which few of the axons are damaged. This model, called the EB-CCP, has been used in many studies to examine the biology of CNS remyelination (Sim et al. 2002a; Arnett et al. 2004; Stidworthy et al. 2004) (Fig. 2). Using non-radioactive in situ hybridization, we found that neither BMP2 nor noggin were detectable in the demyelinated areas. Small numbers of weakly labelled BMP7 mRNA-expressing cells were seen at 14 days after lesion induction but not at earlier or later time points. However, many cells expressing readily detectable levels of BMP4 mRNA were detected within the lesion throughout the remyelination process. The distribution of BMP4-expressing cells resembled that of PDGFRα+ OPCs, but with a lower density and staining intensity. By combining BMP4 in situ hybridization with immunocytochemistry using antibodies to Nkx2.2 (Fancy et al. 2004), we were able to confirm that the majority of BMP4+ cells were OPCs (Fig. 2C). This co-labelling only occurred during the process of remyelination (Fig. 2B): when remyelination was complete at 28 days (Shields et al. 1999) the OPCs no longer expressed BMP4. Thus, the expression of BMP4 mRNA coincides with the stage at which OPCs are reactive and is a further indication of the complex changes in gene expression associated with this state. The functional significance of BMP4 mRNA expression by OPCs is not known at present. Based on developmental studies one would predict that high levels of BMP4 would contribute to an environment that favours astrocyte differentiation. Yet the majority of OPCs in toxin-induced demyelination appear to differentiate into oligodendrocytes. Is this because high levels of noggin counter this effect, as in the case in the myelinating optic nerve (Kondo & Raff, 2004)? The absence of detectable changes in noggin expression within the lesions does not support this hypothesis, although the expression of other BMP antagonists, such as chordin, follistatin or gremlin, has yet to be investigated. The functional role of BMP signalling during remyelination remains an intriguing and unresolved issue.
Fyn tyrosine kinase
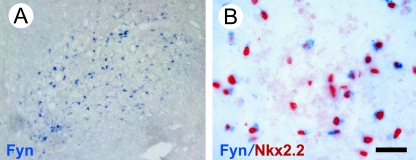
Fyn, a member of the non-receptor-type Src family of tyrosine kinases, is a critical part of the signalling pathways by which oligodendrocytes undergo the morphological changes required for myelination. In vitro, interference with fyn prevents process extension and myelin sheath formation (Klein et al. 2002; Colognato et al. 2004), while in vivo the deletion of Fyn in oligodendrocytes results in hypo-myelination (Umemori et al. 1994; Biffiger et al. 2000; Sperber et al. 2001). One would therefore predict that Fyn expression would be increased within oligodendrocyte lineage cells responding to demyelination and maturing into remyelinating oligodendrocytes. To test this we used non-radioactive in situ hybridization with Fyn-specific cRNA probes. We initially confirmed the specificity of these probes by demonstrating Fyn mRNA expression during the myelination of white matter tracts in 2–3-day-old neonatal rats. In adult white matter, however, Fyn mRNA expression was barely at the level of detection. We then looked for Fyn mRNA-expressing cells after induction of demyelination in the adult rat using the EB-CCP model (Fig. 3). Fyn mRNA-expressing cells were clearly detectable at 5 days after lesion induction and were most abundant after 7 days (Fig. 3A). Thereafter, the numbers gradually decreased. Double labelling studies revealed that a high proportion of Fyn mRNA-expressing cells also expressed Nkx2.2 (Fig. 3B), and that Fyn therefore had increased levels of message expression occurring in reactive OPCs present within the lesion at early stages of remyelination before myelin sheaths appear (Shields et al. 1999; Woodruff & Franklin, 1999; Sim et al. 2000). Fyn was exclusive to oligodendrocyte lineage cells because there was no colocalization with either astrocytes or macrophage/microglia.
Fig. 3.
(A) Fyn mRNA+ cells detected by in situ hybridization within an EB-CCP lesion 7 days after induction. (B) The Fyn mRNA+ cells also express the OPC marker Nkx2.2. Scale bar = 100 µm (A), 20 µm (B).
Thus, in response to demyelination, OPCs switch on or increase the expression of multiple genes including Nkx2.2, Olig2, BMP4 and Fyn, converting them from the relatively quiescent state of the intact adult CNS to one where they are responsive to the complex environmental cues within demyelinating lesions and enabling them to differentiate into remyelinating oligodendrocytes. The identification of genes associated with the reactive state will be helpful not only in obtaining a clearer understanding of the molecular mechanisms of remyelination, from which therapeutic targets may emerge, but also in determining the functional status of OPCs present with MS lesions. For example, activation markers will make it possible to determine the likely receptiveness of these cells within areas of chronic demyelination to potential remyelination-enhancing therapies.
Although the prospects for devising pro-remyelination therapies are realistic and exciting there are many critical questions that need to be addressed. In this review we have raised several of these, including the diversity of progenitor cells types that can contribute to remyelination and the manner in which these cells become activated and are able to engage in the repair process. Resolving these questions will form a major part of the developing field of translational ‘stem cell’ regeneration therapies for demyelinating disease in the forthcoming years.
Acknowledgments
We acknowledge the support of The Wellcome Trust, The UK Multiple Sclerosis Society and the BBSRC.
References
- Arnett HA, Fancy SPJ, Alberta JA, et al. The bHLH transcription factor Olig1 is required for repair of demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffiger K, Bartsch S, Montag D, Aguzzi A, Schachner M, Bartsch U. Severe hypomyelination of the murine CNS in the absence of myelin-associated glycoprotein and Fyn tyrosine kinase. J Neurosci. 2000;20:7430–7437. doi: 10.1523/JNEUROSCI.20-19-07430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–171. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, et al. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, et al. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of nkx6 regulation and shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Chari DM, Blakemore WF. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia. 2002;37:307–313. [PubMed] [Google Scholar]
- Chari DM, Crang AJ, Blakemore WF. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. J Neuropathol Exp Neurol. 2003;62:908–916. doi: 10.1093/jnen/62.9.908. [DOI] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen M, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167:365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Dawson MRL, Levine JM, Reynolds R. NG-2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dawson MRL, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Fu L, et al. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121:1469–1477. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- Decker L, Picard-Riera N, Lachapelle F, Baron-Van Evercooren A. Growth factor treatment promotes mobilization of young but not aged adult subventricular zone precursors in response to demyelination. J Neurosci Res. 2002;69:763–771. doi: 10.1002/jnr.10411. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Yool D, et al. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J Cell Biol. 2004;166:121–131. doi: 10.1083/jcb.200312012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SPJ, Zhao C, Franklin RJM. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27:247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, Gilson JM, Blakemore WF. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res. 1997;50:337–344. doi: 10.1002/(SICI)1097-4547(19971015)50:2<337::AID-JNR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Franklin RJM. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, Goldman JE. Remyelination by endogenous glia. In: Lazzarini RA, editor. Myelin Biology and Disorders. San Diego: Elsevier; 2004. pp. 173–196. [Google Scholar]
- Fulton BP, Burne JF, Raff MC. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. Glia as neural progenitor cells. Trends Neurosci. 2003;26:590–596. doi: 10.1016/j.tins.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Edell E, Carpio DF, et al. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- Hampton DW, Rhodes KE, Zhao C, Franklin RJM, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJM. Distinctive patterns of PDGF-A, FGF-2, IGF-I and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Mol Cell Neurosci. 1999;14:153–168. doi: 10.1006/mcne.1999.0771. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJM. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16:542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- Klein C, Krämer EM, Cardine AM, Schraven B, Brandt R, Trotter J. Process outgrowth of oligodendrocytes is promoted by interaction of Fyn kinase with the cytoskeletal protein Tau. J Neurosci. 2002;22:698–707. doi: 10.1523/JNEUROSCI.22-03-00698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff MC. A role for Noggin in the development of oligodendrocyte precursor cells. Dev Biol. 2004;267:242–251. doi: 10.1016/j.ydbio.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti C, Rodriguez M. Remyelination in multiple sclerosis. Mult Scler. 1997;3:133–136. doi: 10.1177/135245859700300213. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rao MS. Olig genes are expressed in a heterogeneous population of precursor cells in the developing spinal cord. Glia. 2004;45:67–74. doi: 10.1002/glia.10303. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, et al. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Ludwin SK. Chronic demyelination inhibits remyelination in the central nervous system. Lab Invest. 1980;43:382–387. [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, Kessler JA. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Goldman JE, et al. A2B5(+) and O4(+) cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- Mason JL, Toews A, Hostettler JD. Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am J Pathol. 2004;164:1673–1682. doi: 10.1016/S0002-9440(10)63726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–317. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin X-H, Giese N, Heldin C-H, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Penderis J, Shields SA, Franklin RJM. Impaired remyelination and depletion of oligodendrocyte progenitors does not occur following repeated episodes of focal demyelination in the rat CNS. Brain. 2003;126:1382–1391. doi: 10.1093/brain/awg126. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci USA. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho E-S. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- See J, Zhang X, Eraydin N, et al. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–492. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Shields SA, Gilson JM, Blakemore WF, Franklin RJM. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28:77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Hinks GL, Franklin RJM. The re-expression of the homeodomain transcription factor Gtx during remyelination of experimentally-induced demyelinating lesions in young and old rat brain. Neuroscience. 2000;100:131–139. doi: 10.1016/s0306-4522(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Li W-W, Lakatos A, Franklin RJM. Expression of the POU domain transcription factors SCIP/Oct-6 and Brn-2 is associated with Schwann cell but not oligodendrocyte remyelination of the CNS. Mol Cell Neurosci. 2002a;20:669–682. doi: 10.1006/mcne.2002.1145. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJM. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002b;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–396. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- Spassky N, Goujet-Zalc C, Parmantier E, et al. Multiple restricted origin of oligodendrocytes. J Neurosci. 1998;18:8331–8343. doi: 10.1523/JNEUROSCI.18-20-08331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Olivier C, Perez-Villegas E, et al. Single or multiple oligodendroglial lineages: a controversy. Glia. 2000;29:143–148. [PubMed] [Google Scholar]
- Sperber BR, Boyle-Walsh EA, Engleka MJ, et al. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21:2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidworthy MF, Genoud S, Li W-W, et al. Notch1 and Jagged1 are expressed after CNS demyelination but are not a major rate-determining factor during remyelination. Brain. 2004;127:1928–1941. doi: 10.1093/brain/awh217. [DOI] [PubMed] [Google Scholar]
- Sun T, Echelard Y, Lu R, et al. Olig bHLH proteins interact with homeodomain proteins to regulate cell fate acquisition in progenitors of the ventral neural tube. Curr Biol. 2001;11:1413–1420. doi: 10.1016/s0960-9822(01)00441-9. [DOI] [PubMed] [Google Scholar]
- Talbott JF, Loy DN, Liu Y, et al. Endogenous Nkx2.2(+)/Olig2(+) oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Apperly JA, Lloyd AC, Raff MC. Lack of replicative senescence in cultured rat oligodendrocyte precursor cells. Science. 2001;291:868–871. doi: 10.1126/science.1056780. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sato S, Yagl T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hadzic T, Nishiyama A. Transient upregulation of Nkx2.2 expression in oligodendrocyte lineage cells during remyelination. Glia. 2004;46:311–322. doi: 10.1002/glia.20006. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJM. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide and complement/anti-galactocerebroside – a comparative study. Glia. 1999;25:216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wren D, Wolswijk G, Noble M. In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. J Cell Biol. 1992;116:167–176. doi: 10.1083/jcb.116.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–1365. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]