Abstract
Radial glial fibres provide a transient scaffold and impose constraints in the developing central nervous system (CNS) that facilitate cell migration and axon growth. Recent reports have raised doubts about the distinction between radial glia and precursor cells by demonstrating that radial glia are themselves neuronal progenitor cells in the developing cortex, indicating a dual role for radial glia in both neurogenesis and migration guidance. Radial glia shift toward exclusive generation of astrocytes after neurogenesis has ceased. Radial progenitor cell differentiation and lineage relationships in CNS development are complex processes depending on genetic programming, cell–cell interaction and microenvironmental factors. In the spinal cord, radial cells that arise directly from the neuroepithelium have been identified. At least in the spinal cord, these radial cells appear to be the precursors to radial glia. It remains unknown whether radial glial cells or their precursors, the radial cells, or both can give rise to neurons in the spinal cord. Radial glial cells are also important in regulating the axon out-growth and pathfinding processes that occur during white matter patterning of the developing spinal cord.
Keywords: radical cells, progenitor cells, stem cells, astrocytes, migration guidance
Introduction
The vertebrate nervous system originates as a flat sheet of neuroepithelial cells. Neuroepithelial cells are the earliest precursors in the central nervous system (CNS) and are present prior to the onset of neurogenesis. Radial glial cells are the first glial cell phenotype distinguishable from this neuroepithelium. Guiseppe Magini (1888) first attributed the name ‘radial cells’ to the radial processes initially identified by Golgi (1873). The Golgi impregnation technique subsequently allowed other early neuroanatomists such as Cajal, Retzius, VonLenhossek and His to further Magini's work in the CNS and reveal the morphogenic events underlying CNS development (for a review see Bentivoglio & Mazzarello, 1999). Willheim His postulated that all ventricular zone cells in the CNS arise from the neuroepithelium with separate glial and neuronal precursors soon diverging from the neuroepithelium (His, 1889). He also suggested that the ‘radial ependymal cells’ could serve to direct and orientate developing axons (His, 1888). Cajal's studies concurred with His and also suggested that the radial fibres could represent a glial phenotype, probably modified or primitive astrocytes, that functioned to maintain the shape of the cortex during development. He also observed that each radial process had one basal endfoot at the ventricular surface, whereas at the pial surface the radial fibre often branched, with each branch terminating with endfeet at the pial surface (Cajal, 1894, 1911; DeFelipe et al. 1988). Pasko Rakic finally clarified the role of these radial fibres in neuronal migration, using electron microscopy and the Golgi technique in the developing monkey cortex (Rakic, 1971, 1972). It was not until the 1980s that the glial nature of these radial fibres was identified, when primate radial glia were shown to possess several characteristics typical of astrocytes, such as glycogen granules (Choi, 1981) and, using immunocytochemistry, the intermediate filament protein glial fibrillary acidic protein (GFAP) (Levitt & Rakic, 1980; Levitt et al. 1981).
During development, radial glial cells appear early and display a bipolar shape with processes spanning along the full width of the developing CNS. Radial glial cell bodies are mainly situated in the ventricular or subventricular zones. A short process anchors the cell soma to the lumen in the ventricular zone while an elongated process reaches the pial surface (Rakic, 1995). Neuroepithelial cells have been distinguished from radial glia, based on antigenic markers (Malatesta et al. 2000). Unlike neuroepithelial cells, radial glia exhibit hallmarks of astrocytes such as the presence of glycogen granules and expression of GFAP in primates (Levitt & Rakic, 1980; Choi, 1981). Furthermore, although rodent and chick radial glia do not express GFAP, they do express brain lipid binding protein (BLBP) and the astrocyte-specific glutamate aspartate transporter (GLAST), both of which are also found in mature astrocytes (Hartfuss et al. 2001). These glial features appear around the onset of neurogenesis in ventricular zone cells and distinguish neuroepithelial cells from radial glia. Therefore, the maturation of neuroepithelial cells to radial glia and the onset of neurogenesis appear to be concurrent (Anthony et al. 2004). Radial glia continue to share certain molecular characteristics with neuroepithelial cells such as the neural precursor cell marker nestin (Frederiksen & McKay, 1988). Thus, radial glia express molecules characteristic of both CNS precursors and astrocytes, suggesting their intermediary state. Whereas radial glia persist in many lower vertebrates into adulthood, they transform into astrocytes in most CNS regions of adult mammals (Chanas-Sacre et al. 2000). GLAST- and BLBP-positive radial glia have been shown to express nestin until their morphological transformation into astrocytes when GLAST and BLBP are maintained, but nestin is down-regulated (Hartfuss et al. 2001; Barry & McDermott, 2005). The cellular and molecular events that underlie the transformation of radial glia into astrocytes are not known but the morphological transformation appears to coincide with a change in expression of intermediate filament protein in radial glia, i.e. loss of vimentin protein and acquisition of GFAP (Voigt, 1989). From studies of vimentin/GFAP expression, the transition of radial glia into astrocytes appears to occur once neurogenesis is complete (Schnitzer et al. 1981; Parnavelas et al. 1983). In addition to the appearance of astroglial features, further morphological changes occur, such as loss of tight junctional coupling and up-regulation of several adhesion and extracellular matrix molecules (Aaku-Saraste et al. 1997; Stoykova et al. 1997).
Radial glia as guides
The long radial processes of radial glia have traditionally been thought to play a crucial role as scaffolding, guiding the migration of newborn neurons (Morest, 1970; Rakic, 1972, 2003; Edmondson & Hatten, 1987; Brittis et al. 1995; O'Rourke et al. 1995). Neurons migrate radially from the germinal layers toward the pial surface to settle in the differentiating layers (Morest, 1970; Hatten, 1999; Nadarajah et al. 2001). Two main types of cell migration exist, radial and tangential (for a review see Sobeih & Corfas, 2002), with different neuronal populations using different modes of migration depending on where the neuroblasts originate (for reviews see Parnavelas, 2000; Marin & Rubenstein, 2001). In the cortical plate as additional waves of migrating neurons arrive, they bypass earlier generated neurons to form the cortical layers in an inside-out sequence, with deeper layers being the first to form and superficial layers the last. However, patterns of neuronal migration during cortical development are now known to be more complex than once thought. Cortical interneurons take a predominantly tangential path to reach the cortex and travel relatively long distances, whereas pyramidal cells take a predominantly radial path and reach the cortex more directly.
Various molecules have been implicated in playing an important role in neuronal migration. BLBP is induced in radial glia by neurons attaching or migrating along them (Feng et al. 1994; Feng & Heintz, 1995). These migrating cortical neurons release glial growth factors that are crucial for expression of BLBP (Anton et al. 1997). As migrating neurons induce BLBP in radial glia and BLBP-positive cells are also dividing, these data suggest that radial glial cells engaged in neuronal guidance are also dividing (Gotz et al. 2002).
Among the candidate molecules involved in radial migration are neuregulin, which binds to the glial surface using ErbB2 and ErbB4 receptors (Schmid et al. 2003), and integrins, which provide the optimal level of basic neuron–glial adhesion needed to maintain neuronal migration on the radial glia (Anton et al. 1999). Reelin is another factor believed to be critical for the formation of the cortical laminae (Luque et al. 2003; Hartfuss et al. 2003). Reelin is expressed by Cajal-Retius cells and once released reelin signals directly to migrating neurons (Rice & Curran, 2001). Reelin has been implicated to be required for BLBP expression in cortical radial glia as well as radial process extension (Campbell, 2003). Reeler mutant mice, lacking reelin, display a failure of layer formation in the developing cortex and the cerebellum, with neurons dispersing across the layers and projecting dendrites in all directions (Caviness & Sidman, 1973; Caviness & Rakic, 1978; Caviness, 1982). An explanation for the malformation of the neuronal laminae is that, as reelin may regulate BLBP expression on the radial glial cell process, its absence alters this expression and thereby interferes with the migration process (Hartfuss et al. 2003). Reeler mutant mice demonstrate incorrect formation of the radial glial cell phenotype, with the cells attenuated, disordered and fewer in number (Hunter-Schaedle, 1997; Hartfuss et al. 2003).
Observation of the division of radial glia infected with a replication-incompetent retroviral vector expressing GFP in slice preparations 1 day after infection directly shows that at least some cells maintain a radial process in M phase of the cell cycle (Noctor et al. 2001). Time-lapse experiments have shown that migrating neurons extend a thin leading process in the direction of migration and that the nucleus remains in the rear of the soma during migration (Solecki et al. 2004). Studies in slice cultures have shown that neurons generated in the cortical proliferative zones pass through a series of distinct migrational stages characterized by abrupt changes in cell shape, direction of movement and speed of migration as they move to the cortical plate (Noctor et al. 2004). Transitions between these phases of migration may involve environmental signals and depend upon specific intracellular events (Kriegstein & Noctor, 2004).
Radial glia in the spinal cord
Radial glia are also found in the spinal cord. There are differences in the organization of radial glia found in the spinal cord to those found in the cortex. In the spinal cord white matter is present beneath the pial surface, whereas in the brain cortical gray matter is found in this location. In the developing cortex, neurons are organized in lamina perpendicular to the long axis of radial glia. In the developing spinal cord, such laminae are not found and neurons migrate outward from the ventricular zone to form the central gray matter of the spinal cord in an outside-in fashion.
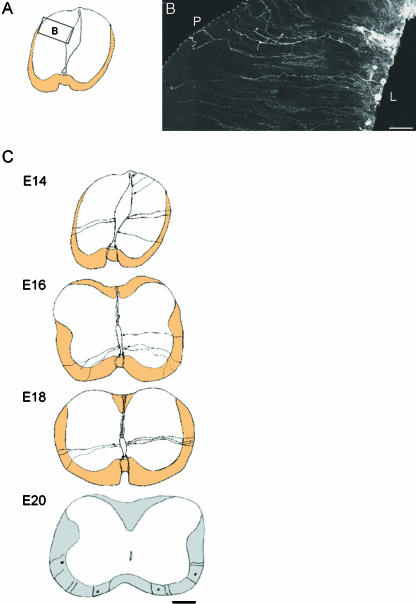
Radial glia in the spinal cord are the first glial cell phenotype distinguishable from the spinal neuroepithelium. In the spinal cord radial glia have an oval-shaped cell body located in the ventricular zone and exhibit a bipolar morphology. They extend a process, from the ventricular zone to the pial surface terminating with a conical pial endfoot (the presumptive glia limitans) and a short process to the central canal terminating with a basal endfoot. They are generally present in a plane perpendicular to the long axis of the central canal. Using DiI labelling, the distribution of radial glia from embryonic day (E)14 to E20 in the developing rat spinal cord has been investigated extensively (McMahon & McDermott, 2002). This study revealed cells with the classic morphology of radial glia at E14 (Fig. 1A,B) and loss of full radial processes with increasing developmental age (Fig. 1C). The cytoarchitecture of the spinal cord combined with the spatial and temporal appearance of spinal cord radial glia indicate that these cells may have functions different to those of radial glia found in the cortex.
Fig. 1.
(A) line drawing with inset box indicates the positions in the spinal cord of the region shown in B. (B) Montage showing the distribution of DiI-labelled radial glia in the dorsal region of the spinal cord. Most cell bodies are closer to the lumenal than to the pial surface. Dotted line corresponds to the pial surface. L, luminal surface; P, pial surface. Bars, 40 µm. (C) Schematic representation of how the distribution of representative DiI-labelled radial glia changes from E14 to E20. Bar, 100 µm.
Radial glial cell–axonal interactions in the spinal cord
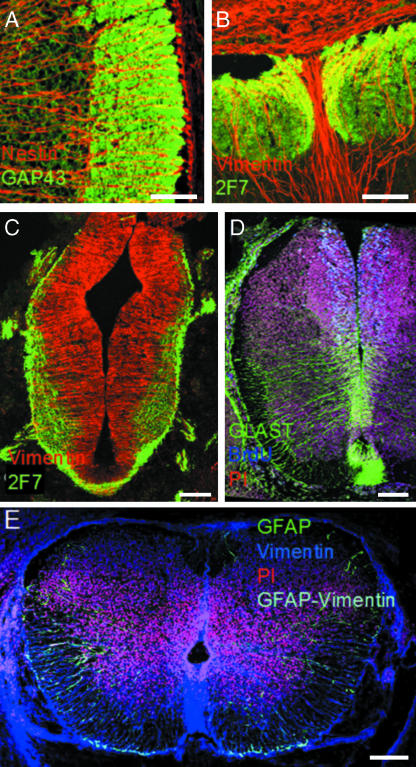
Radial glia are important in regulating the axon out-growth and pathfinding processes that occur during white matter patterning of many regions of the developing CNS, including the spinal cord (Brusco et al. 1995; Honig et al. 1996; Puche & Shipley, 2001). It has been reported that radial glial cells form cephalocaudal plates that ensheath developing axonal tracts beginning early in development in the ventral white matter and which extend laterally as the axonal tracts increase in size and number (Brusco et al. 1995). The authors suggest that these transient cephalocaudal plates are necessary for the structural organization of adult spinal cord white matter. It has also been shown that vimentin-immunoreactive radial glia are found in close apposition to the leading corticospinal tract axons in the spinal cord (Joosten & Gribnau, 1989). These radial glia are orientated perpendicularly to the axons of the corticospinal tract and appear to be arranged in tiers along the rostrocaudal axis of the spinal cord (Joosten & Gribnau, 1989). Electron microscopy revealed an adhesive form of contact between the surrounding radial glia and the axons of the fibre tract, suggesting that the glia are not only providing a physical substrate to guide pioneer axons but may also trophically influence their path of migration. This radial glial cell axon interface is absent in the decussating area of the corticospinal tract axons. Similarily, commissural axons arising in the dorsal spinal cord seem to be guided through a matrix of glial cells to the ventral floor plate (Shu et al. 2003) via diffusible factor(s) secreted by radial glia that influence the patterning and orientation of commissural axons (Tessier-Lavigne et al. 1988). In addition, we have found a strong conservation of radial glial cell process periodicity throughout the white matter during the periods of neurogenesis and axogenesis (unpublished observation) (Fig. 2A,B). This suggests a general role for radial glia in guiding the growth of all axon tracts, possibly by forming compartmentalizing sleeves through which they may travel.
Fig. 2.
(A) At E17, nestin-immunoreactive radial glial processes are present in a highly ordered pattern in the GAP43-immunoreactive longitudinally orientated axon fascicles in the lateral white matter. (B) At E17, 2F7-immunoreactive axons of the dorsal columns form in close association with specialized vimentin-immunoreactive radial glial fibres. Note the vertically orientated column of radial glial fascicles that separate clearly the right and left halves of the dorsal columns. (C) At E12/E13, nestin-immunoreactive neuroepithelial cells have transformed into a nestin-immunoreactive radial cell phenotype with nestin-immunoreactive processes radiating from the central canal to the pial surface. 2F7 (a neuronal marker) expression is now extensive and is clearly evident in the emerging ventral, lateral and dorsal white matter. (D) From E14, GLAST is present in radial glial cells running from the ventral half of the central canal to the ventrolateral pial surface, and in the ventral commissure. Cell proliferation, as shown by BrdU immunoreactivity, is located mostly dorsally. (E) By E17, GFAP co-localizes with vimentin in radial glial cells in ventral, lateral and dorsal white matter, and in radial glial processes in the grey matter, demonstrating the transition of radial glial cell to astrocyte. Bars, 50 µm (A,B); 100 µm (C–E).
Progenitor and stem cell roles of radial glia
Radial glia as neuronal progenitors
Cell lineage analysis has shown that precursor pools in the cerebral cortex generate different sets of descendants in vivo and in vitro (Luskin et al. 1993; Williams & Price, 1995). Early studies implied that radial glia in the mammalian cortex might be potential precursors of other cell types because they were found to be immunopositive for nestin and vimentin, proteins expressed by CNS precursor cells (Pixley & DeVellis, 1984; Frederiksen & McKay, 1988; Lendahl et al. 1990; Chanas-Sacre et al. 2000; Yamaguchi et al. 2000). Radial glia have been shown to divide rapidly and undergo interkinetic nuclear migration, a feature unique to precursor cells in the germinal ventricular zone (Misson et al. 1988). Radial glia, but not astrocytes, in the mouse express RC2 (Misson et al. 1988; Chanas-Sacre et al. 2000; Hartfuss et al. 2001), which has also recently been shown to label neuronal precursor cells. Furthermore, radial glia are known to remain in the cell cycle throughout neurogenesis (Hartfuss et al. 2001) and 3[H]-thymidine studies have shown that radial glia in the adult avian ventricular zone are capable of dividing (Alvarez-Buylla et al. 1990). Newborn neurons were shown to arise in the regions rich in mitotically active radial glia, suggesting that radial glia are not only necessary for the migration of neurons but may also be their progenitors. Furthermore, retroviral labelling lineage analysis of radial glia in the chick optic tectum confirmed the presence of radial glia, astrocytes and neurons within the same clone (Gray & Sanes, 1992). Radial glial cell marker expression also varies between vertebrates and between brain regions. For example, radial glia in the ventral telencephalon are morphologically distinct from radial glia in the dorsal telencephalon. They also exhibit different cell cycle characteristics than their dorsal counterparts (Hartfuss et al. 2001). These differences correspond to the restricted expression patterns of developmentally related molecules, such as the paired homeodomain transcription factor Pax6 in the cortex (Gõtz et al. 1998) and cellular retinal-binding protein (RBP-1) in the ventral lateral telencephalon (Toresson et al. 2000). Moreover, during neurogenesis there is heterogeneous expression of BLBP, GLAST and RC2 on radial glia in the cortex and ganglionic eminence, providing further evidence that the appearance and differentiation of these cells is developmentally regulated (Hartfuss et al. 2001).
Recent evidence has shown radial glia as both neuronal progenitors (Malatesta et al. 2000, 2003; Hartfuss et al. 2001; Miyata et al. 2001; Tamamaki et al. 2001; Noctor et al. 2002; Anthony et al. 2004) and glial progenitors/precursors (Voigt, 1989; Misson et al. 1991; Yang et al. 1993; Barry & McDermott, 2005). This new evidence poses a challenge to the prevailing view of separate neuroepithelial lineages for neurons and glia. However, as much of this evidence has been obtained using different experimental techniques, in different regions and across different species, it is probably premature to assume that all radial glial cells generate neurons in all regions of the CNS.
Using fluorescent-activated cell sorting, Malatesta et al. (2000) described the neurogenic properties of cortical radial glia. Radial glia were isolated using a transgenic mouse line expressing the GFP under the human GFAP promoter. Radial glia isolated from E14–E16 transgenic mice predominantly generated neurons, whereas radial glia isolated from E18 mice were more restricted to an astrocyte lineage. To investigate whether radial glial cells are neuronal progenitors in vivo Noctor et al. (2001) injected a retroviral vector encoding EGFP into the lateral ventricles of E15–E16 embryonic rats to label precursor cells in the rat ventricular zone during neurogenesis. Cells labelled immediately after the injection were primarily radial glial cells. Time-lapse imaging demonstrated that radial glia undergo interkinetic nuclear movements and divide asymmetrically to give rise to mitotic radial glia and postmitotic neurons. These neurons were often observed to migrate along their own parent radial glial fibre, revealing a dual role for radial glia in both neurogenesis and migration. Similarly, neuronal origins were investigated by injecting a recombinant adenovirus expressing a membrane-targeted GFP into the lateral ventricle of the E14–E15 mouse embryo (Tamamaki et al. 2001). Labelled radial glia divided asymmetrically and gave rise either to neuronal precursors or to spherical cells, which elongated to become radial cells. Four days after the viral injection 97% of the GFP-positive cells were neocortical neurons, strongly suggesting that radial glial cells are the progenitors of neocortical neurons.
The migratory role of the parent radial glial cell fibre was studied further by Miyata et al. (2001). DiI was placed on the pial surface of slice cultures extracted from E14 mouse cortex to labelled fully a selection of radial glial cell fibres. This, combined with BrdU labelling in vivo, allowed the analysis of the cell cycle characteristics of individual radial glial cells. This showed that radial glial cells divide asymmetrically, resulting in the production of another progenitor cell and a radial neuron, which usually inherits the parent radial glial cell process. In these cases, the inherited process is used by the newborn neuron to translocate its soma away from the ventricular zone. The radial glial cell progenitors that did not inherit the parent radial glial cell process extended new radial processes.
To investigate whether all neurons in the cortical ventricular zone are generated by radial glia, Noctor et al. (2002) performed quantitative analysis on the number of radial glial cells in the ventricular zone and revealed that most precursor cells in the embryonic ventricular zone are radial glia. Electrophysiological recordings and dye filling experiments of coronal slices taken from E12–E19 rat cortex showed that cells with the membrane properties of precursor cells are radial glial cells. Pulse labelling with BrdU and subsequent immunostaining experiments revealed that the majority of proliferating cells in the ventricular zone express a variety of radial glial cell markers, including RC2, BLBP and GLAST. Application of fluorescent microspheres to the pial surface led to labelling of 90% of ventricular zone cells in S-phase, indicating that most proliferating ventricular zone cells have processes that reach the pial surface. Taken together, these findings provide compelling evidence that radial glia are the most prominent neuronal precursors present during embryonic neocortical development.
More recently, Anthony et al. (2004) demonstrated, using Cre/loxP fate mapping to mark the progeny of BLBP-positive radial glia, that radial glia throughout the mouse CNS pass through a neurogenic phase at different stages of development. This study also showed that both GLAST and BLBP are not expressed in neuroepithelial cells and that the onset of BLBP and GLAST expression coincides with the differentiation of radial glia. Fate mapping showed that radial glial cells from both the dorsal and the ventral telencephalon pass through a neurogenic stage during development and that radial glia give rise to the majority of neurons. The expression profile of BLBP in vivo showed that all radial glia differentiate in a pattern which parallels that of the neurogenic gradient.
Taken together, these findings provide compelling evidence that radial glia are the most prominent neuronal precursors present during embryonic neocortical development and perhaps in other CNS regions also.
Radial glia as astrocyte precursors
It has been known for many years that after completion of neuronal migration some radial glia undergo a dramatic alteration in phenotype and function, which sees them transform into protoplasmic and fibrous astrocytes, during the perinatal period. Postnatal radial glia share many characteristics with astrocytes, such as the expression of glycogen granules and the intermediate filament GFAP (at least in primates) (Levitt & Rakic, 1980; Choi, 1981). GLAST (Shibata et al. 1997), BLBP (Feng et al. 1994) and the intermediate filaments vimentin (Dahl, 1981) and nestin (Lendahl, 1997) are also expressed on radial glia and later on in transitional and mature astrocytes. It was Cajal who originally suggested that radial glia in most regions of the mammalian brain disappear shortly after birth, and appear to become transitional forms of astrocytes (Cajal, 1894, 1911). Many studies have since demonstrated this transformation across different brain regions and in different species.
The antigen RC2 has been used in combination with electron microscopy and immunohistochemistry to analyse the origins and lineages of radial glia in the mouse spinal cord and cerebellum (Misson et al. 1988). From the neural tube stage of development RC2 identifies neuroepithelial cells and radial glia. Subsequently, these RC2-immunoreactive radial glia undergo marked changes in morphology consistent with the radial glial–astrocyte transformation.
Using antibodies directed against vimentin and GFAP the development of the rat spinal cord astroglial system has been studied (Oudega & Marani, 1991). Initially vimentin was present in a radial profile in the matrix layer of the spinal cord. GFAP first appeared at E18 in the ventral mantle layer and gradually replaced vimentin in radial glial cells during the first three postnatal weeks, leading the authors to suggest that the orderly arrangement of GFAP and vimentin in the matrix layer during development indicates their involvement in neuronal migration and regionalization. To test whether radial glia directly transformed to astrocytes, Voigt (1989) injected fluorescent dye to the subpial surface of newborn ferrets when no GFAP-immunoreactive astrocytes are present. The dye was taken up in the upper portion of the cortical plate and stained radial glial somata. As the radial glia disappeared in postnatal life, the dye was ultimately found in GFAP-immunoreactive astrocytes in the cortex (Voigt, 1989). This, for the first time, provided direct evidence that radial glia were an immature form of astrocytes, and that this transformation was accompanied by a change in the intermediate filament protein expression.
GLAST was shown to be a marker of cells of the radial glial cell–astrocyte lineage in the developing mouse spinal cord (Shibata et al. 1997). GLAST was expressed on pre-existing radial cells during the period that neurons migrate from the ventricular zone to the grey matter regions, as demonstrated by the increasing size of the dorsal and ventral horns. This occurs between E11 and E13 in the ventral cord and between E13 and E15 in the dorsal cord. GLAST mRNA was first expressed outside the ventricular zone at E15, at a time when labelled nuclei displaying glial-like features were detected in the mantle and marginal zones. Thus, migration of radial glial cell bodies to the grey and white matter appears to occur after neuronal migration has ceased. Furthermore, the cytological and morphological changes indicative of the early stages of the transformation of radial glia to astrocytes seem to occur concomitantly with the migration of radial glia, between E13 and E15 (Shibata et al. 1997).
Distinct populations of precursor cells have been described in the embryonic rat spinal cord based on their antigenic phenotype using lineage markers: neuroepithelial cells (NEPs), neuronal restricted precursors (NRPs) and glial restricted cells (GRPs) (Liu et al. 2002). NEPs of the E10.5 neural tube do not express any early astrocyte or oligodendrocyte markers, but they do express nestin, and the potential oligodendrocyte precursor markers Nkx2.2 and transiently Olig 2. GFAP was first detected at E16 in the rostral spinal cord and was not co-expressed with other glial precursors or oligodendrocyte markers. As such, GFAP-positive cells are likely to represent the last glial cell population generated in the spinal cord. These authors show that GRPs are generated by NEPs, which do not express any glial markers and further suggest that two domains of GRP appear to exist, which generate oligodendrocytes and astrocytes and are antigenically and morphologically distinct from radial glia (Liu et al. 2002).
Recently, we identified a population of self-renewing radial cells in the embryonic spinal cord, distinct from the neuroepithelium, that resemble radial glial cells in morphology, yet do not express any glial cell markers along their processes (Fig. 2C) (Barry & McDermott, 2005). These radial cells arise from the spinal cord neuroepithelium and are the precursors of radial glial cells, which first appear at E14 expressing GLAST in the ventral spinal cord (Fig. 2D). Radial glial cells were found in all regions of the cord from E16 expressing GLAST, BLBP and 3CB2. As neurogenesis, as indicated by the presence of neuronal marker 2F7, coincides with the appearance of such radial cells it is likely these cells are the primary neuronal progenitor in the spinal cord. GLAST-, BLBP- and 3CB2-positive radial glia were shown to transform gradually into differentiated astrocytes, by the gradual acquisition of GFAP and down-regulation of vimentin and nestin following a ventro-dorsal gradient (Fig. 2E). Based on these findings it is proposed that radial glia are the major source of astrocytes, while the neuroepithelial and radial neuroepithelial cells are the major neuronal progenitors, in the developing spinal cord.
References
- Aaku-Saraste E, Oback B, Hellwig A, Huttner WB. Neuroepithelial cells downregulate their plasma membrane polarity prior to neural tube closure and neurogenesis. Mech Dev. 1997;69:71–81. doi: 10.1016/s0925-4773(97)00156-1. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation ‘hot spots’ in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha (v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–197. doi: 10.1002/glia.20166. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Mazzarello P. The history of radial glia. Brain Res Bull. 1999;49:305–315. doi: 10.1016/s0361-9230(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Meiri K, Dent E, Silver J. The earliest patterns of neuronal differentiation and migration in the mammalian central nervous system. Exp Neurol. 1995;134:1–12. doi: 10.1006/exnr.1995.1031. [DOI] [PubMed] [Google Scholar]
- Brusco A, Gomez LA, Lopez EM, Tagliaferro P, Saavedra JP. Relationship between glial organization and the establishment of nerve tracts in rat spinal cord. Int J Neurosci. 1995;82:25–31. doi: 10.3109/00207459508994287. [DOI] [PubMed] [Google Scholar]
- Cajal SRy. New Ideas on the Structure of the Nervous System in Man and Vertebrates. Cambridge, UK: MIT Press; 1894. [Google Scholar]
- Cajal SRy. Histology of the Nervous System of Man and Vertebrates. New York: Oxford University Press; 1911. [Google Scholar]
- Campbell K. Signaling to and from radial glia. Glia. 2003;43:44–46. doi: 10.1002/glia.10247. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;148:141–151. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Chanas-Sacre G, Rogister B, Moonen G, Leprince P. Radial glia phenotype: origin, regulation, and transdifferentiation. J Neurosci Res. 2000;61:357–363. doi: 10.1002/1097-4547(20000815)61:4<357::AID-JNR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Choi BH. Radial glia of developing human fetal spinal cord: Golgi, immunohistochemical and electron microscopic study. Brain Res. 1981;227:249–267. doi: 10.1016/0165-3806(81)90112-7. [DOI] [PubMed] [Google Scholar]
- Dahl D. The vimentin-GFA protein transition in rat neuroglia cytoskeleton occurs at the time of myelination. J Neurosci Res. 1981;6:741–748. doi: 10.1002/jnr.490060608. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Conti F, Van Eyck SL, Manzoni T. Demonstration of glutamate-positive axon terminals forming asymmetric synapses in cat neocortex. Brain Res. 1988;455:162–165. doi: 10.1016/0006-8993(88)90127-8. [DOI] [PubMed] [Google Scholar]
- Edmondson JC, Hatten ME. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J Neurosci. 1987;7:1928–1934. doi: 10.1523/JNEUROSCI.07-06-01928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Feng L, Heintz N. Differentiating neurons activate transcription of the brain lipid-binding protein gene in radial glia through a novel regulatory element. Development. 1995;121:1719–1730. doi: 10.1242/dev.121.6.1719. [DOI] [PubMed] [Google Scholar]
- Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgi C. Sulla struttura della sostanza grigia dell cervello. Gazz Med Lombarda. 1873;33:244–246. [Google Scholar]
- Gõtz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Gõtz M, Hartfuss E, Malatesta P. Radial glial cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Res Bull. 2002;57:777–788. doi: 10.1016/s0361-9230(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Gray GE, Sanes JR. Lineage of radial glia in the chicken optic tectum. Development. 1992;114:271–283. doi: 10.1242/dev.114.1.271. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Förster E, Bock HH, et al. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development. 2003;130:4597–4609. doi: 10.1242/dev.00654. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- His W. Zur Geschichte des Gehirns sowie der centralen und peripherischen Nervenbahnen beim menschlichen Embryo. Abh Kgl Sachs Ges Wissensch Math Phys Kl. 1888;24:339–392. [Google Scholar]
- His W. Die Neuroblasten und deren Entstehung im embryonalen Mark. Abh Kgl Sachs Ges Wissensch Math Phys Kl. 1889;15:311–372. [Google Scholar]
- Honig LS, Hermann K, Shatz CJ. Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex. 1996;6:794–806. doi: 10.1093/cercor/6.6.794. [DOI] [PubMed] [Google Scholar]
- Hunter-Schaedle KE. Radial glial cell development and transformation are disturbed in reeler forebrain. J Neurobiol. 1997;33:459–472. doi: 10.1002/(sici)1097-4695(199710)33:4<459::aid-neu9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Joosten EA, Gribnau AA. Astrocytes and guidance of outgrowing corticospinal tract axons in the rat. An immunocytochemical study using anti-vimentin and anti-glial fibrillary acidic protein. Neuroscience. 1989;31:439–452. doi: 10.1016/0306-4522(89)90386-2. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lendahl U. Transgenic analysis of central nervous system development and regeneration. Acta Anaesthesiol Scand Suppl. 1997;110:116–118. doi: 10.1111/j.1399-6576.1997.tb05524.x. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- Levitt P, Cooper ML, Rakic P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Lee JC, et al. Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. Glia. 2002;40:25–43. doi: 10.1002/glia.10111. [DOI] [PubMed] [Google Scholar]
- Luque JM, Morante-Oria J, Fairen A. Localization of ApoER2, VLDLR and Dab1 in radial glia: groundwork for a new model of reelin action during cortical development. Brain Res Dev Brain Res. 2003;140:195–203. doi: 10.1016/s0165-3806(02)00604-1. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Parnavelas JG, Barfield JA. Neurons, astrocytes, and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–1750. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magini G. Sur la neuroglie et les cellules nerveuses cerebrales chez les foetus. Arch Ital Biol. 1888;9:59–60. [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, et al. Neuronal or glial progeny: Regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- McMahon SS, McDermott KW. Morphology and differentiation of radial glia in the developing rat spinal cord. J Comp Neurol. 2002;454:263–271. doi: 10.1002/cne.10427. [DOI] [PubMed] [Google Scholar]
- Misson JP, Edwards MA, Yamamoto M, Caviness VS. Identification of radial glial-cells within the developing murine central nervous-system – studies based upon a new immunohistochemical marker. Dev Brain Res. 1988;44:95–108. doi: 10.1016/0165-3806(88)90121-6. [DOI] [PubMed] [Google Scholar]
- Misson JP, Takahashi T, Caviness VS. Ontogeny of radial and other astroglial cells in murine cerebral-cortex. Glia. 1991;4:138–148. doi: 10.1002/glia.440040205. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Morest DK. Pattern of neurogenesis in retina of rat. Z Fur Anat Entwicklungsgeschichte. 1970;131:45–67. doi: 10.1007/BF00518815. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong ROL, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- O'Rourke NA, Sullivan DP, Kaznowski CE, Jacobs AA, McConnell SK. Tangential migration of neurons in the developing cerebral-cortex. Development. 1995;121:2165–2176. doi: 10.1242/dev.121.7.2165. [DOI] [PubMed] [Google Scholar]
- Oudega M, Marani E. Expression of Vimentin and Glial Fibrillary Acidic protein in the developing rat spinal-cord – an immunocytochemical study of the spinal-cord glial system. J Anat. 1991;179:97–114. [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG, Luder R, Pollard SG, Sullivan K, Lieberman AR. A qualitative and quantitative ultrastructural study of glial cells in the developing visual cortex of the rat. Philos Trans R Soc Lond B Biol Sci. 1983;301:55–84. doi: 10.1098/rstb.1983.0022. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG. The origin and migration of cortical neurones: new vistas. Trends Neurosci. 2000;23:126–131. doi: 10.1016/s0166-2236(00)01553-8. [DOI] [PubMed] [Google Scholar]
- Pixley SKR, Devellis J. Transition between immature radial glia and mature astrocytes studied with a monoclonal-antibody to vimentin. Dev Brain Res. 1984;15:201–209. doi: 10.1016/0165-3806(84)90097-x. [DOI] [PubMed] [Google Scholar]
- Puche AC, Shipley MT. Radial glia development in the mouse olfactory bulb. J Comp Neurol. 2001;434:1–12. doi: 10.1002/cne.1160. [DOI] [PubMed] [Google Scholar]
- Rakic P. Guidance of neurons migrating to fetal monkey neocortex. Brain Res. 1971;33:471–&. doi: 10.1016/0006-8993(71)90119-3. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–&. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Radial versus tangential migration of neuronal clones in the developing cerebral-cortex. Proc Natl Acad Sci USA. 1995;92:11323–11327. doi: 10.1073/pnas.92.25.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Elusive radial glial cells: historical and evolutionary perspective. Glia. 2003;43:19–32. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the Reelin signaling pathway in central nervous system development. Annu Rev Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Schmid RS, McGrath B, Berechid BE, et al. Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci USA. 2003;100:4251–4256. doi: 10.1073/pnas.0630496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J, Franke WW, Schachner M. Immuno-cytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult-mouse nervous-system. J Cell Biol. 1981;90:435–447. doi: 10.1083/jcb.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Yamada K, Watanabe M, et al. Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci. 1997;17:9212–9219. doi: 10.1523/JNEUROSCI.17-23-09212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu TZ, Sundaresan V, McCarthy MM, Richards LJ. Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. J Neurosci. 2003;23:8176–8184. doi: 10.1523/JNEUROSCI.23-22-08176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeih MM, Corfas G. Extracellular factors that regulate neuronal migration in the central nervous system. Int J Dev Neurosci. 2002;20:349–357. doi: 10.1016/s0736-5748(02)00040-0. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6 alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Gotz M, Gruss P, Price J. Pax6-dependent regulation of adhesive patterning, R–cadherin expression and boundary formation in developing forebrain. Development. 1997;124:3765–3777. doi: 10.1242/dev.124.19.3765. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Placzek M, Lumsden AGS, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous-system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- Voigt T. Development of glial-cells in the cerebral wall of ferrets – direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol. 1989;289:74–88. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- Williams BP, Price J. Evidence for multiple precursor cell-types in the embryonic rat cerebral-cortex. Neuron. 1995;14:1181–1188. doi: 10.1016/0896-6273(95)90265-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- Yang HY, Lieska N, Shao D, Kriho V, Pappas GD. Immunotyping of radial glia and their glial derivatives during development of the rat spinal-cord. J Neurocytol. 1993;22:558–571. doi: 10.1007/BF01189043. [DOI] [PubMed] [Google Scholar]