Abstract
Growth/differentiation factor-5 (GDF5) is a member of the transforming growth factor-β superfamily which has potent effects on dopaminergic neurones in vitro and in vivo. GDF5 is under investigation as a potential therapeutic agent for Parkinson's disease (PD), which is caused by the progressive degeneration of dopaminergic neurones projecting from the substantia nigra (SN) to the striatum. In the rat ventral mesencephalon (VM; the developing SN), GDF5 expression peaks at embryonic day 14, the time at which dopaminergic neurones undergo terminal differentiation. Addition of GDF5 protein to cultures of embryonic rat VM increases the survival and improves the morphology of dopaminergic neurones in these cultures. GDF5 treatment also increases the number of cells which adopt a dopaminergic phenotype in cultures of VM progenitor cells. Intracerebral administration of GDF5 has potent neuroprotective and restorative effects on the nigrostriatal pathway in animal models of PD. Furthermore, addition of GDF5 protein to embryonic rat dopaminergic neuronal transplants improves their survival and function in a rat model of PD. Thus, GDF5 has potential applications to PD therapy as a dopaminergic neuroprotective agent and as a factor that may induce a dopaminergic neuronal fate in unrestricted progenitor cells.
Keywords: dopaminergic neurones, GDF5, Parkinson's disease
Parkinson's disease
Parkinson's disease (PD) is a slowly progressing neurodegenerative disorder, the pathological hallmark of which involves degeneration of dopaminergic neurones which project from the substantia nigra (SN) to the striatum (A9 group of neurones). The disease has been reported to affect ∼0.1% of the total population and ∼1% of the population over 65years of age. The progressive nature of the neurodegeneration that occurs in PD provides a window of opportunity for therapeutic intervention. Currently, two of the most promising therapies for the treatment of PD involve (1) the application of neurotrophic factors to support the remaining dopaminergic neurones and protect them against the ongoing disease process and (2) the transplantation of embryonic dopaminergic neurones to replace those that are lost. Both of these approaches require refinement and optimization.
Promising results have emerged from a clinical trial involving the application of a neurotrophic factor, glial cell-line-derived neurotrophic factor (GDNF), to the putamen of PD patients. This treatment resulted in improvements in the patients' motor symptoms for at least 2years, without any serious clinical side-effects (Gill et al. 2003; Patel et al. 2005). However, the long-term effects of such an approach remain to be determined. Transplantation of embryonic dopaminergic neurones to the striatum of PD patients has been shown to provide long-lasting relief of symptoms (for reviews see Dunnett et al. 2001; Hagell & Brundin, 2001; Bjorklund et al. 2003; Lindvall & Hagell, 2004; Sayles et al. 2004). However, two recent double-blind placebo-controlled trials funded by the National Institute of Health in the United States (Freed et al. 2001; Olanow et al. 2003) have not only caused public concern about the transplantation approach due to the appearance of disabling dyskinesias in some of the patients, but also raised ethical issues, in particular concerns about the use of sham-surgery (for a review see Dekkers & Boer, 2001). Another major problem with this approach is the poor survival of the dopaminergic neurones after transplantation. Several agents, including neurotrophic factors, are being tested for their ability to improve neuronal survival after transplantation (for a review see Brundin et al. 2000).
The development of alternative cell sources for neural transplantation in PD is crucial if this approach is to be used widely as a treatment for this disease. One such alternative source is dopaminergic neurones derived from embryonic stem (ES) cells or neural progenitor cells isolated from the embryonic or adult brain. Current research is focused on identifying the molecules involved in the normal development of nigrostriatal dopaminergic neurones, in the hope of being able to use these to direct neural stem cells to a dopaminergic fate.
This review focuses on a neurotrophic factor called growth/differentiation factor-5 (GDF5), in particular on studies which demonstrate that GDF5 may be useful for the treatment of PD. GDF5 is applicable to PD in two potential ways: (i) as a neurotrophic factor to support the remaining dopaminergic neurones and protect them against the ongoing disease process, and (ii) as a factor that may instruct neural stem cells to adopt a dopaminergic neuronal fate for use in transplantation approaches.
The transforming growth factor-β (TGFβ) superfamily
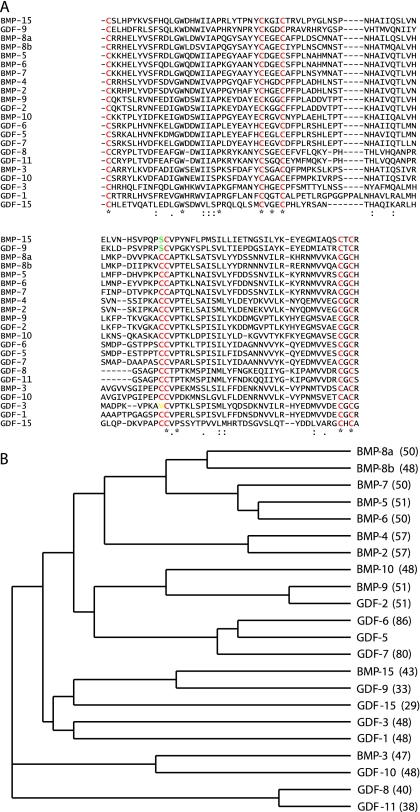
The TGFβ superfamily is a group of multifunctional cytokines that play diverse roles in many different tissues during development and in adulthood (for reviews see Sporn & Roberts, 1990; Kingsley, 1994; Herpin et al. 2004). This superfamily is made up of a number of structurally related molecules that are grouped into subfamilies based on sequence similarites. Some examples include the GDNF, TGFβ, activin and Dpp-Vgr1-related (DVR) subfamilies. The DVR subgroup is further divided into the GDF5 group, the bone morphogenetic factor (BMP) 2 group and the 60-A group, which contains BMP5, 6, 7 and 8 (see Fig.1). Members of the TGFβ superfamily are synthesized as large precursor proteins that contain a hydrophobic N-terminal signal sequence, a prodomain of varying size and a C-terminal peptide. The precursor proteins are cleaved at an (RK)-X-X-(RK) site to release a mature C-terminal segment of 110–140 amino acids. The active signalling molecules are made up of hetero- or homodimers of this C-terminal region. All members of the TGFβ superfamily contain seven highly conserved cysteine residues in their C-terminal (see Fig.1); all but one of these residues are involved in intramolecular disulphide bonds which result in a characteristic three-dimensional structure known as a cysteine knot motif (Venkataraman et al. 1995). The remaining cysteine residue is involved in intermolecular disulphide bond formation, allowing the formation of active dimers (Daopin et al. 1992; Schlunegger et al. 1992). However, some members, such as GDF3 and GDF9 (McPherron & Lee, 1993), lack this cysteine residue and are thought to function as active monomers.
Fig. 1.
(A) Alignment of known protein sequences of human BMP and GDFs beginning at the first conserved cysteine residue in the mature C-terminal protein. Alignment shows the classical seven conserved cysteine (C) residues that form the structure known as the ‘cysteine-knot motif’. Protein sequences were obtained from www.ncbi.nih.gov and the alignment was performed using a web-based version of Clustal W™ from EMBL (http://www.ebi.ac.uk/clustalw/). (B) Phylogeny tree showing evolutionary relationship between human BMPs and GDFs, generated from data obtained from Clustal W alignment of protein sequences in (A). Line lengths represent evolutionary distance. Numbers in parenthese represent percentage similiarity of the sequence to the GDF5 sequence. GDF5, GDF6 and GDF7 have a high sequence similarity with GDF5 and are grouped into their own subfamily.
TGFβ family members signal by binding to two types of serine–threonine kinase receptors, type I and type II receptors (for reviews see Massague, 1996; Yamashita et al. 1996). Upon ligand binding, a tetrameric complex is formed, consisting of two type I and two type II receptors; this induces phosphorylation of the cytoplasmic domain of the type I receptor by the type II receptor. This activates an intracellular signalling cascade which culminates in the activation of Smad proteins, causing them to translocate into the nucleus and affect transcription (for a review see Miyazono, 1999). BMPs can signal through any of several type II receptors and either of two type I receptors, BMPR1a or BMPR1b (for a review see Mehler et al. 1997). GDF5 signals predominantly through the type I receptor BMPR1b and either of the type II receptors BMPR2 or ActR2 (Nishitoh et al. 1996).
The roles of GDF5 during development
Members of the BMP and GDF subfamilies play critical roles in skeletal development (for a review see Li & Cao, 2003). GDF5 (also known as cartilage-derived morphogenetic protein 1 or BMP14) is one of these molecules that has received particular interest, because naturally occurring mutations in the mouse and human gdf5 gene result in defects in the appendicular skeleton. Heterozygous mutations in the gdf5 gene cause brachydactyly type C (Polinkovsky et al. 1997), characterized by underdevelopment or absence of the phalanges and metacarpals. Homozygous mutations in gdf5 result in the more severe conditions known as brachypodism in mice (Storm et al. 1994) and the chondrodysplasias Grebe type (CGT) and Hunter-Thompson type (CHTT) in humans (Thomas et al. 1996, 1997). These conditions are characterized by pronounced shortening of the skeletal elements, with more severe effects distally and the loss of one or more joints. Interestingly, although no functional GDF5 protein is made in CGT and CHTT patients, no neurological impairments have yet been reported.
In the developing chick limb, GDF5 is expressed in the condensing mesenchyme of cartilage elements prior to joint formation and within the developing joint capsule at a later stage (Storm et al. 1994; Francis-West et al. 1999b). GDF5 has been found to be involved in the development of skeletal elements, in part by accelerating the initial steps of chondrogenesis via increases in cell adhesion, and later in the control of chondrocyte proliferation and differentiation (Hotten et al. 1996; Francis-West et al. 1999a; Nakamura et al. 1999; Buxton et al. 2001). GDF5 signals through the BMPR1b receptor to induce mesenchymal condensation, but other receptors are necessary for its role in differentiation and proliferation of chondrocytes. It has recently been reported that GDF5-induced chondrogenesis depends on the receptor ROR2, a tyrosine kinase receptor (Sammar et al. 2004).
GDF5 has also been shown to play roles in tendon and ligament formation (Wolfman et al. 1997; Aspenberg & Forslund, 1999), tooth formation (Morotome et al. 1998), morphogenesis of joints (Storm & Kingsley, 1996) and angiogenesis (Yamashita et al. 1997). One potential clinical application of GDF5 may be to improve the healing of articular cartilage, which heals poorly following damage caused by, for example, arthritis (Edwards & Francis-West, 2001).
Expression of GDF5 and its receptors in the rat brain
Almost all of the scientific literature to date has focused on the involvement of GDF5 during limb development. However, in the first paper describing the brachypodism mutation, it was noted that transcripts for GDF5 were present in the embryonic mouse brain (Storm et al. 1994). This suggests that in addition to playing roles in limb development, GDF5 may also be involved in CNS development, as has been shown for other BMPs (for a review see Mehler et al. 1997). This hypothesis is supported by the fact that GDF5 mRNA was found in the cortex, midbrain and cerebellum in the postnatal (P) day 1 rat brain (Krieglstein et al. 1995b). We have shown that GDF5 protein expression in the rat brain begins on embryonic day (E) 12, reaches a peak on E14, before decreasing with age to reach its lowest levels around the perinatal period (O'Keeffe et al. 2004a). In the postnatal period, GDF5 levels increase to reach maximal levels in the adult rat brain, being expressed in many regions, including the striatum and midbrain (O'Keeffe et al. 2004a). In the ventral mesencephalon (VM), the area of the brain that develops to become the SN, GDF5 protein levels were found to peak at E14, the day on which dopaminergic neurones undergo terminal differentiation (O'Keeffe et al. 2004a). Furthermore, we have detected, using both RT-PCR and Western blotting, the GDF5 receptors, BMPR1a, BMPR1b and BMPR2, in the rat VM at E12, E13 and E14 (G.W.O'K. et al. unpublished data).
The effects of GDF5 on embryonic dopaminergic neurones in vitro
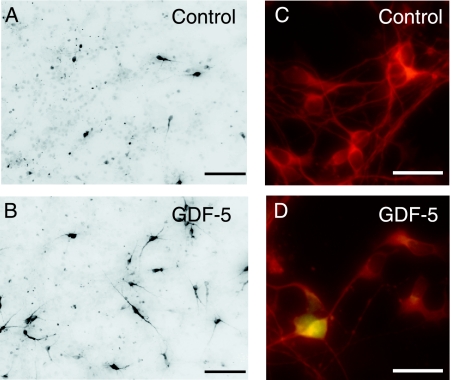
The first study that examined the effects of GDF5 on neural cells showed an increase in the numbers of dopaminergic neurones [detected by immunocytochemistry for tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis] in cultures of E14 rat VM after treatment with recombinant human (rh)GDF5 (Krieglstein et al. 1995b). In this study, GDF5 was found to be almost as effective in improving dopaminergic neuronal survival as TGFβ3 and as effective as GDNF, two established dopaminergic neurotrophins (Lin et al. 1993; Krieglstein et al. 1995a,b). Our studies agreed with these findings (O'Keeffe et al. 2004b; Wood et al. 2005; see Fig.2) and further showed that GDF5 did not induce an increase in the total number of neurones in E14 rat VM cultures, suggesting a selective effect on dopaminergic neurones (O'Keeffe et al. 2004b). GDF5 was also found to attenuate dopaminergic neuronal death induced by the selective neurotoxins N-methyl-4-phenylpyridinium ion (Krieglstein et al. 1995b) and 6-hydroxydopamine (6-OHDA) (F. M. Hurley & A. M. Sullivan, unpublished data), or by free radical donors (Lingor et al. 1999). A dramatic increase in the numbers of astrocytes was also observed in GDF5-treated E14 rat VM cultures (Krieglstein et al. 1995b; O'Keeffe et al. 2004b; Wood et al. 2005). This suggests that its neurotrophic action may be indirect, possibly by stimulating the production of glial-derived growth factor(s) that may be involved in the neurotrophic response. However, our recent studies have found that inhibition of GDF5-induced increases in astrocyte numbers does not decrease the survival-promoting effect of GDF5 on dopaminergic neurones, implying a direct neuronal action (Wood et al. 2005). In support of this, GDF5 treatment induces the phosphorylation (and thus activation) of Smad proteins in neurones within E14 VM cultures (Fig.2). The finding that BMPR2 is expressed on dopaminergic neurones in the E13.5 mouse VM (G.W.O'K. et al. unpublished data) strengthens the theory that GDF5 can exert a direct action on this neuronal population.
Fig. 2.
Photomicrographs showing representative (A) control and (B) GDF5-treated (10ngmL−1 at time of plating) cultures of E14 rat VM, immunocytochemically stained for TH for 6DIV. Scale bar = 100µm. Photomicrographs showing representative (C) control and (D) GDF5-treated (10ngmL−1 for 2h at 24h after plating) cultures of E14 rat VM immunocytochemically stained for β-III tubulin (red; a neuronal marker) and phosphorylated Smad-1/-5/-8 (green) at 1 DIV. There was no Smad activation in (C) control cultures, whereas in (D) GDF5-treated cultures, nuclear accumulation of phosphorylated Smad protein was present in a subset of neurones. Scale bar=25µm.
Although each of the receptors, BMPR1a, BMPR1b and BMPR2, is expressed in freshly dissected E14 VM tissue, the expression of BMPR1b becomes down-regulated over time in culture (O'Keeffe et al. 2004b). BMPR1b is necessary for the GDF5-induced increase in the number of E14 rat VM dopaminergic neurones in vitro, as addition of GDF5 after 6 days in vitro (DIV), when the expression of BMPR1b is down-regulated, does not result in an increase in dopaminergic neurones (O'Keeffe et al. 2004b). Our findings are substantiated by those of Brederlau et al. (2002), who reported that GDF5 did not significantly elevate the number of dopaminergic neurones in E14 VM cultures when added after 7DIV. Each of our studies which found an increase in dopaminergic neurones in response to GDF5 involved application of the factor at the time of plating of the cultures (O'Keeffe et al. 2004b; Wood et al. 2005). Together, these observations suggest that the responsiveness of dopaminergic neurones to GDF5 decreases with time in culture, due to a down-regulation of BMPR1b expression.
In vivo, the expression of GDF5 peaks on E14 in the developing VM (O'Keeffe et al. 2004a). Nigral dopaminergic neurones begin to extend their axons to the striatum from E14 onwards (Unsicker et al. 1996). Thus GDF5 may act on dopaminergic neurones not only in a survival-promoting manner, but also to influence their morphological development. In agreement with this hypothesis, we have found that GDF5 has potent effects on the morphology of E14 rat VM dopaminergic neurones in vitro. Total neurite length, number of branch points and average somal area per dopaminergic neurone were all significantly increased after GDF5 treatment (O'Keeffe et al. 2004b). Comparison of these data with those of a previous study on the effects of GDNF on cultured dopaminergic neurones (Widmer et al. 2000) showed that GDF5 was as effective as GDNF in all of these parameters, and more effective at increasing total neurite length per dopaminergic neurone.
The effects of GDF5 on dopaminergic progenitor cells in vitro
The increase in dopaminergic cell numbers observed in the above-mentioned in vitro studies could have been due to (i) induction of a dopaminergic phenotype (i.e. TH expression) in uncommitted precursor cells, (ii) proliferation of dopaminergic neurones which were already in the tissue at the time of harvesting and/or (iii) increased survival of existing dopaminergic neurones. We carried out a set of experiments on E12 rat VM cultures, which contain early progenitors of dopaminergic neurones, to explore these possibilities (G.W.O'K. et al. unpublished data). GDF5 treatment induced an increase in the numbers of dopaminergic neurones in these cultures. A direct action for GDF5 in these cultures was substantiated by immunocytochemical analysis showing that each of the receptors BMPR1a, BMPR1b and BMPR2 was present. There was no significant difference in the number of apoptotic cells or in the total number of cells in control cultures compared with GDF5-treated cultures, suggesting that GDF5 did not influence cell survival. No cells that were double-labelled with proliferating cell nuclear antigen (PCNA) and TH were observed in any of the cultures, suggesting that GDF5 did not stimulate the proliferation of dopaminergic neurones. Furthermore, there was no significant difference in the numbers of BrdU-positive cells in control cultures than in GDF5-treated cultures, confirming that GDF5 did not have an effect on cell proliferation. This set of experiments thus suggests that the increase in TH-positive cell numbers induced by GDF5 treatment in E12 rat VM cultures was a result of induction of TH expression in progenitor cells (G.W.O'K. et al. unpublished data). In agreement with this theory, we have found, using RT-PCR studies, that GDF5 treatment up-regulates the expression of TH in proliferating neural progenitor cell cultures prepared from E12 rat VM (G.W.O'K. et al. unpublished data). Although GDF5 expression peaks on E14 during the development of the rat VM, it is also expressed at E12 (O'Keeffe et al. 2004a), which is the day on which TH expression begins in the VM (Hanaway et al. 1971; Lauder & Bloom, 1974; Altman & Bayer, 1981; Solberg et al. 1993). Thus, these data imply a role for GDF5 in the induction of a dopaminergic phenotype in cells in the developing VM.
The effects of GDF5 on dopaminergic neurones in vivo
The studies described above demonstrate that GDF5 is expressed in the developing rat VM and that it promotes both survival and morphological differentiation of embryonic dopaminergic neurones in vitro. In vivo studies have shown that GDF5 can also protect adult rat nigrostriatal dopaminergic neurones from death induced by 6-OHDA, a selective dopaminergic neuronal toxin (Sullivan et al. 1997, 1999; Hurley et al. 2004). In the initial in vivo studies, rhGDF5 was administered intracerebrally at the same time as a 6-OHDA lesion of the adult rat medial forebrain bundle (MFB), which induces a complete lesion of the nigrostriatal pathway (Sullivan et al. 1997, 1999). GDF5 prevented the development of amphetamine-induced rotational asymmetry due to the lesion and preserved the integrity of striatal dopaminergic neuronal terminals against damage induced by 6-OHDA, as measured by positron emission tomography (PET). Immunocytochemistry for TH showed significant sparing of dopaminergic neuronal cell bodies in the SN against 6-OHDA-induced death (Sullivan et al. 1997, 1999). The second study showed that administration of GDF5 into either the SN or the striatum had significant neuroprotective effects on the nigrostriatal dopaminergic pathway (Sullivan et al. 1999). These neuroprotective effects are similar to those induced by GDNF in the same in vivo model (F. M. Hurley & A. M. Sullivan, unpublished data).
A later study was aimed at examining whether GDF5 could prevent the progressive death of the lesioned nigrostriatal dopaminergic system, when administered after the lesion, i.e. whether it could induce restorative effects on this pathway (Hurley et al. 2004). GDF5 was administered at either 1 or 2 weeks after an intrastriatal 6-OHDA lesion, which induces a progressive degeneration of the nigrostriatal pathway over several weeks (Ichitani et al. 1991; Sauer & Oertel, 1994).GDF5 was injected into either the striatum or the SN, to examine whether delivery at the level of dopaminergic cell bodies or of axon terminals could affect the ongoing degenerative process. Injection of GDF5 into either the SN or striatum at 1week after lesion surgery led to a significant behavioural improvement, measured using amphetamine-stimulated rotational testing, and sparing of dopaminergic cell bodies in the ipsilateral SN, measured using TH immunocytochemistry. However, GDF5 injection into either site at 2 weeks after the lesion did not confer significant neuroprotection, suggesting that the degree of neurodegeneration at this time was too great to be reversed. In contrast to its effects on the nigral cell bodies, GDF5 treatment did not induce significant effects on the striatal dopaminergic denervation induced by 6-OHDA. Thus, administration of GDF5 after 1 or 2 weeks is probably too late to induce protective effects on striatal dopaminergic terminals. The lack of striatal re-innervation suggests that the observed motor improvements were due to an up-regulation of dopaminergic function, rather than axonal sprouting, in the remaining nigrostriatal neurones. It is possible that GDF5 acted by increasing dopamine levels in the remaining striatal neurones, which would explain the observed improvements in motor behaviour.
Another in vivo study examined the effects of GDF5 treatment on embryonic rat VM grafts and found that this factor is at least as effective as the widely studied dopaminergic neurotrophin, GDNF, in promoting the survival and function of embryonic dopaminergic neurones after grafting in 6-OHDA-lesioned rats (Sullivan et al. 1998). Specifically, GDF5 pretreatment improved the survival of grafted dopaminergic neurones from approximately ∼11% to ∼30%. It also enhanced graft-induced compensation of amphetamine-stimulated rotations and increased graft-induced recovery of dopaminergic nerve terminals, as measured by PET.
Thus, these studies support the in vitro experiments, showing that the neurotrophic and protective effects of GDF5 are applicable to the in vivo situation and thus may be useful in therapeutic approaches to PD. In order for GDF5 to have long-term effects in vivo, however, use of the recombinant protein may not be sufficient, as it is susceptible to rapid enzymatic degradation. We are currently investigating methods of providing a long-term supply of GDF5 in vivo, using viral vectors and stably transfected cell lines.
Summary
GDF5 is a neurotrophic protein which has been shown to have potent effects to improve the survival of nigrostriatal dopaminergic neurones and to protect them from injury, both in vitro and in vivo. Our recent studies suggest that GDF5 may also play a role in inducing a dopaminergic phenotype in progenitor cells within the developing rat VM. These properties of GDF5 make it a candidate for use in studies aimed at therapeutic approaches for PD. The survival-promoting properties of GDF5 warrant its use as a neuroprotective agent; its delivery to the nigrostriatal system using encapsulated cells or viral vectors could help to repair the damaged dopaminergic neurones and slow their degeneration. GDF5 may also be applied, in combination with other factors which are known to induce a dopaminergic neuronal fate, to stem/progenitor cells for use in transplantation approaches to PD.
Acknowledgments
This work was supported by the Irish Research Council for Science Engineering and Technology (IRCSET), under the National Development Plan. We thank Dr Jens Pohl of Biopharm GmbH for the generous donations of GDF5 protein and antibodies.
References
- Altman J, Bayer S. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. J Comp Neurol. 1981;198:677–716. doi: 10.1002/cne.901980409. [DOI] [PubMed] [Google Scholar]
- Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–54. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB, Brundin P, et al. Neural transplantation for the treatment of Parkinson's disease. Lancet. 2003;2:437–445. doi: 10.1016/s1474-4422(03)00442-3. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Faigle R, Kaplan P, Odin P, Funa K. Bone morphogenetic proteins but not growth differentiation factors induce dopaminergic differentiation in mesencephalic precursors. Mol Cell Neurosci. 2002;21:367–378. doi: 10.1006/mcne.2002.1178. [DOI] [PubMed] [Google Scholar]
- Brundin P, Karlsson J, Emgard M, et al. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 2000;9:179–195. doi: 10.1177/096368970000900205. [DOI] [PubMed] [Google Scholar]
- Buxton P, Edwards C, Archer CW, Francis-West P. Growth/differentiation factor 5 (GDF-5) and skeletal development. J Bone Joint Surg. 2001;83-A:S1–23. [PubMed] [Google Scholar]
- Daopin S, Piez KA, Ogawa Y, Davies DR. Crystal structure of transforming growth factor-beta 2: an unusual fold for the superfamily. Science. 1992;257:369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- Dekkers W, Boer G. Sham neurosurgery in patients with Parkinson's disease: is it morally acceptable? J Med Ethics. 2001;27:151–156. doi: 10.1136/jme.27.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Bjorklund A, Lindvall O. Cell therapy in Parkinson's disease – stop or go? Nature Rev Neurosci. 2001;2:365–369. doi: 10.1038/35072572. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Francis-West PH. Bone morphogenetic proteins in the development and healing of synovial joints. Semin Arthritis Rheum. 2001;31:33–42. doi: 10.1053/sarh.2001.24875. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Abdelfattah A, Chen P, et al. Mechanisms of GDF-5 action during skeletal development. Development. 1999a;126:1305–1315. doi: 10.1242/dev.126.6.1305. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Parish J, Lee K, Archer CW. BMP/GDF–signalling interactions during synovial joint development. Cell Tissue Res. 1999b;296:111–119. doi: 10.1007/s004410051272. [DOI] [PubMed] [Google Scholar]
- Freed CR, Breeze RE, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. New Eng J Med. 2001;345:147. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nature Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol. 2001;60:741–752. doi: 10.1093/jnen/60.8.741. [DOI] [PubMed] [Google Scholar]
- Hanaway J, McConnell JA, Netsky MG. Histogenesis of the substantia nigra, ventral tegmental area of Tsai and interpeduncular nucleus: an autoradiographic study of the mesencephalon in the rat. J Comp Neurol. 1971;142:59–74. doi: 10.1002/cne.901420105. [DOI] [PubMed] [Google Scholar]
- Herpin A, Lelong C, Favrel P. Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol. 2004;28:461–485. doi: 10.1016/j.dci.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Hotten GC, Matsumoto T, Kimura M, et al. Recombinant human growth/differentiation factor 5 stimulates mesenchyme aggregation and chondrogenesis responsible for the skeletal development of limbs. Growth Factors. 1996;13:65–74. doi: 10.3109/08977199609034567. [DOI] [PubMed] [Google Scholar]
- Hurley FM, Costello DJ, Sullivan AM. Neuroprotective effects of delayed administration of growth/differentiation factor-5 in the partial lesion model of Parkinson's disease. Exp Neurol. 2004;185:281–289. doi: 10.1016/j.expneurol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ichitani Y, Okamura Y, Masamoto I, Nagatsu I, Ibata Y. Degeneration of nigral dopamine neurons after 6-hydroxydopamine injection into the rat striatum. Brain Res. 1991;549:350–353. doi: 10.1016/0006-8993(91)90481-a. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Suter-Crazzolara C, Fischer WH, Unsicker K. TGF-β superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 1995a;14:736–742. doi: 10.1002/j.1460-2075.1995.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Suter-Crazzolara C, Hotten G, Pohl J, Unsicker K. Trophic and protective effects of growth/differentiation factor 5, a member of the transforming growth factor-b superfamily, on midbrain dopaminergic neurons. J Neurosci Res. 1995b;42:724–732. doi: 10.1002/jnr.490420516. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra in the rat. I. Cell Differentiation. J Comp Neurol. 1974;155:469–482. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Li X, Cao X. BMP signaling and HOX transcription factors in limb development. Front Biosci. 2003;8:805–812. doi: 10.2741/1150. [DOI] [PubMed] [Google Scholar]
- Lin L-FH, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Hagell P. Clinical observations after neural transplantation in Parkinson's disease. Prog Brain Res. 2004;127:299–320. doi: 10.1016/s0079-6123(00)27014-3. [DOI] [PubMed] [Google Scholar]
- Lingor P, Unsicker K, Krieglstein K. Midbrain dopaminergic neurons are protected from radical induced damage by GDF-5 application. J Neural Transm. 1999;106:139–144. doi: 10.1007/s007020050146. [DOI] [PubMed] [Google Scholar]
- Massague J. Neurotrophic factors. Crossing receptor boundaries. Nature. 1996;382:29–30. doi: 10.1038/382029a0. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem. 1993;268:3444–3449. [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–317. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- Miyazono K. Signal transduction by bone morphogenetic protein receptors: functional roles of Smad proteins. Bone. 1999;25:91–93. doi: 10.1016/s8756-3282(99)00113-1. [DOI] [PubMed] [Google Scholar]
- Morotome Y, Goseki-Sone M, Ishikawa I, Oida S. Gene expression of growth and differentiation factors-5-6 and -7 in developing bovine tooth at the root forming stage. Biochem Biophys Res Commun. 1998;244:85–90. doi: 10.1006/bbrc.1998.8213. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F. p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiaion factor-5 in ATDC5 cells. Exp Cell Res. 1999;250:351–363. doi: 10.1006/excr.1999.4535. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, et al. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- O'Keeffe GW, Hanke M, Pohl J, Sullivan AM. Expression of growth differentiation factor-5 in the developing and adult rat brain. Dev Brain Res. 2004a;151:199–202. doi: 10.1016/j.devbrainres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- O'Keeffe GW, Dockery P, Sullivan AM. Effects of growth/differentiation factor on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro. J Neurocytol. 2004b;33:479–488. doi: 10.1007/s11068-004-0511-y. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- Polinkovsky A, Robin NH, Thomas JT, et al. Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nature Genet. 1997;17:18–19. doi: 10.1038/ng0997-18. [DOI] [PubMed] [Google Scholar]
- Sammar M, Stricker S, Schwabe GC, et al. Modulation of GDF5/BRI–b signalling through interaction with the tyrosine kinase receptor Ror2. Genes Cells. 2004;9:1227–1238. doi: 10.1111/j.1365-2443.2004.00799.x. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Sayles M, Jain M, Barker RA. The cellular repair of the brain in Parkinson's disease – past, present and future. Transplant Immunol. 2004;12:321–342. doi: 10.1016/j.trim.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Schlunegger MP, Cerletti N, Cox DA, McMaster GK, Schmitz A, Grutter MG. Crystallization and preliminary X-ray analysis of recombinant human transforming growth factor beta 2. FEBS Lett. 1992;303:91–93. doi: 10.1016/0014-5793(92)80484-x. [DOI] [PubMed] [Google Scholar]
- Solberg Y, Silverman WF, Pollack Y. Prenatal ontogeny of tyrosine hydroxylase gene expression in the rat ventral mesencephalon. Dev Brain Res. 1993;73:91–97. doi: 10.1016/0165-3806(93)90050-k. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB. TGF-beta: problems and prospects. Cell Regul. 1990;1:875–882. doi: 10.1091/mbc.1.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkin NA, Kingsley DM, Lee S-J. Limb alterations in bradypodism mice due to mutations in a new member of the TGFβ-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122:3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- Sullivan AM, Opacka-Juffry J, Hotten G, Pohl J, Blunt SB. Growth/differentiation factor 5 protects nigrostriatal dopaminergic neurones in a rat model of Parkinson's disease. Neurosci Lett. 1997;233:73–76. doi: 10.1016/s0304-3940(97)00623-x. [DOI] [PubMed] [Google Scholar]
- Sullivan AM, Pohl J, Blunt SB. Growth/differentiation factor 5 and glial cell line-derived neurotrophic factor enhance survival and function of dopaminergic grafts in a rat model of Parkinson's disease. Eur J Neurosci. 1998;10:3681–3688. doi: 10.1046/j.1460-9568.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- Sullivan AM, Opacka-Juffry J, Pohl J, Blunt SB. Neuroprotective effects of growth/differentiation factor 5 depend on the site of administration. Brain Res. 1999;818:176–179. doi: 10.1016/s0006-8993(98)01275-x. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, Luyten FP. A human chondrodysplasia due to a mutation in a TGF-β superfamily member. Nature Genet. 1996;12:315–317. doi: 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Kilpatric MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat. Genetics. 1997;17:58–64. doi: 10.1038/ng0997-58. [DOI] [PubMed] [Google Scholar]
- Unsicker K, Suter-Crazzolara C, Krieglstein K. Growth factor function in the development and maintenance of midbrain dopaminergic neurons: concepts, facts and prospects for TGFβ. Ciba Found Symp. 1996;196:70–84. doi: 10.1002/9780470514863.ch6. [DOI] [PubMed] [Google Scholar]
- Venkataraman G, Sasisekharan V, Cooney CL, Langer R, Sasisekharan R. Complex flexibility of the transforming growth factor b superfamily. Proc Natl Acad Sci USA. 1995;92:5406–5410. doi: 10.1073/pnas.92.12.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer HR, Schaller B, Meyer M, Seiler RW. Glial cell line-derived neurotrophic factor stimulates the morphological differentiation of cultured ventral mesencephalic calbindin- and calretinin-expressing neurons. Exp Neurol. 2000;164:71–81. doi: 10.1006/exnr.2000.7418. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-β gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TK, McDermott KW, Sullivan AM. Differential effects of GDF5 and GDNF on dopaminergic neurones and astroglia in cultures of embryonic rat midbrain. J Neurosci Res. 2005 doi: 10.1002/jnr.20507. in press. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Heldin C-H, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Shimizu A, Kato M, et al. Growth/differentiation factor-5 induces angiogenesis in vivo. Exp Cell Res. 1997;235:218–226. doi: 10.1006/excr.1997.3664. [DOI] [PubMed] [Google Scholar]