Abstract
To characterize further non-vascular smooth muscle cells (NVSMC) in the choroid of the human eye, extensive morphological studies were performed including a three-dimensional distribution of NVSMC in the adult human eye and their appearance during development. Whole mounts and sections through the choroid and sclera of eyes of 42 human donors (between the 13th week of gestation and 89 years of age) were stained with antibodies against smooth muscle actin and other markers for smooth muscle cells. On the basis of their morphological localization, three groups of NVSMC could be distinguished in the adult eyes: (a) a semicircular arrangement of NVSMC in the suprachoroid and inner sclera, around the entry of posterior ciliary arteries and nerves; (b) NVSMC parallel to the vessels in the posterior eye segment between the point of entry of the posterior ciliary arteries and the point of exit of the vortex veins; and (c) a dense plaque-like arrangement of NVSMC in the suprachoroid, overlying the foveal region. The last of these groups showed most pronounced interindividual differences. During development, the first NVSMC to be observed at the 20th week of gestation belonged to group b. A complete NVSMC network was first observed in a 6-year-old donor eye. All three groups stained positive for smoothelin, caldesmon and calponin in all localizations. The NVSMC show a distinct distribution that might reflect different aspects of their function in the choroid and suprachoroid. All cells could be histochemically characterized as truly contractile.
Keywords: eye, human, immunohistochemistry, smooth muscle marker
Introduction
In the human choroid and inner sclera, a network of non-vascular smooth muscle α-actin-positive cells (NVSMC) has recently been described (Flügel-Koch et al. 1996; Poukens et al. 1998). These cells have a spindle- or star-shaped appearance and are most numerous in the outer choroid at the posterior pole of the temporal quadrant. In addition, NVSMC are distributed throughout the entire choroidal layers, approaching vessel walls in places and reaching Bruch's membrane. In a recent comparative study it was shown that higher primates also have NVSMC, but much fewer in number and mainly located in the suprachoroidal layer (May, 2003). As the presence of NVSMC is in parallel with the evolution of the ciliary muscle, a possible function of NVSMC might, among others, be the protection of the choroidal architecture during accommodation (Flügel-Koch et al. 1996; May, 2003).
Obtaining physiological data from NVSMC in vivo is not straightforward. The number and distribution of NVSMC in higher primates are not sufficiently comparable with those from the human eye (May, 2003). Only a few birds show similar amounts of NVSMC (Guglielmone & Cantino, 1982), but the choroid in birds is distinctly different from the human choroid in forming large lymphatic-like vessels (Meriney & Pilar, 1987; De Stefano & Mugnaini, 1997).
Owing to their location, NVSMC cannot directly be studied in the living human eye. Therefore, extended morphological investigations were performed to answer the following questions:
What is the precise three-dimensional mapping of NVSMC in the adult human eye?
When do NVSMC appear during development? In a previous study, large numbers of NVSMC were first described in human individuals older than 3 years (Poukens et al. 1998). In the present work the number of eyes examined was extended and included also immunohistochemically stained whole mounts.
Can the NVSMC further be characterized? Because double-staining revealed that about 15% of the NVSMC stain for smooth muscle myosin, but only 2% for desmin (Flügel-Koch et al. 1996), the question of whether all NVSMC are functionally contractile also arose. This question was addressed by staining for smoothelin, a novel protein first described by van der Loop et al. (1996), which is considered to be present in contractile smooth muscle cells only (van der Loop et al. 1996, 1997; Bar et al. 2002; Niessen et al. 2004). In addition, staining for caldesmon and calponin was performed, both markers for differentiated smooth muscle cells (Sobue et al. 1999; Meyer-Rochow & Royuela, 2002; Szymanski et al. 2003).
Materials and methods
Material and fixation
The eyes of 45 human donors, ranging in age between the 13th week of gestation and 89 years, without known ocular diseases were obtained at autopsy 2–42 h after death. The donors or their relatives had given permission to use the tissue for research. The research followed the tenets of the Declaration of Helsinki and local regulations.
There were nine fetal donors dated by obstetric clinical history (13, 17, 18, 18, 18, 19, 20, 25 and 25 weeks of gestation). The eyes were enucleated and immersion-fixed in 4% paraformaldehyde (PFA) for various time periods. From eyes of the first three and last three donors listed above, choroidal and scleral whole mount preparations of the temporal quadrant were prepared. From the other quadrants of the circumference and from the eyes of the other three donors, sections through the choroid and sclera of all regions of the circumference were investigated.
The early postnatal stages and childhood were represented by eyes from donors 2 days, 4 weeks, 6 years and 12 years after birth. The eyes were dissected equatorially and immersion-fixed in 4% PFA for 4–6 h. One eye of each donor was used for choroidal and scleral whole mount preparation, and the other eye was used for tangential sections through the choroid and sclera.
The age of the adult donors ranged between 18 and 89 years. The eyes were dissected equatorially and most of the eyes were immersion-fixed in 4% PFA for 4–6 h (n = 21). Because the staining for myosin was inconsistent using PFA alone, six adult eyes were fixed for 2 days in Zamboni's fixative, containing 4% PFA and 0.01% picric acid. This fixation leads to a consistent but still incomplete staining of the NVSMC. Therefore, antibodies against myosin were also applied to unfixed specimens. The antibody against smoothelin also required choroidal tissue without prior fixation. For this purpose, five adult eyes were prepared and immediately frozen at −20 °C. Prior to staining, the choroidal and scleral whole mounts were thawed. The sections were prepared without a thawing step. Unfortunately, the post-mortem times of these eyes ranged between 10 and 36 h.
Preparation of the tissue
The fixed specimens were rinsed several times with phosphate-buffered saline (PBS, pH 7.2–7.4). The complete circumference of the eyes of 20 donors was prepared as choroidal and scleral whole mounts, divided into four quadrants. In seven temporal quadrants of these preparations, the position of the fovea centralis was marked by a needle insertion prior to removal of the retina. This allowed localization of the fovea after the staining procedure. In addition, the temporal quadrant of six eyes of donors from the prenatal stages (13, 17, 18, 20, 25 and 25 weeks of gestation) and single quadrants of an additional 12 adult donor eyes were whole-mounted. The whole mounts were processed free floating in small plastic caps.
From the other eyes and regions, 12–16 µm horizontal and tangential cryosections through the choroid and inner sclera were made using a Leitz cryostat (Leitz, Wetzlar, Germany). The sections were mounted on poly-l-lysine-coated glass slides and air-dried.
Immunohistochemistry
Incubation with the primary antibody was performed overnight at room temperature (unfixed preparations were incubated for 4 h at room temperature), the antibody diluted in PBS containing 1% bovine serum albumin and 0.1% Triton X. Control sections were performed using only the diluent solution. The primary antibodies used included mouse-anti-alpha-smooth muscle actin (dilution 1 : 200: Sigma, St. Louis, MO, USA), rabbit-anti-smooth muscle myosin (dilution 1 : 250: Biotrend, Cologne, Germany: catalogue no. 6490-0304), mouse-anti-smoothelin (dilution 1 : 50: Acris, Hiddenhausen, Germany: catalogue no. BM5081), mouse-anti-caldesmon (dilution 1 : 100: Acris: catalogue no. DM316) and mouse-anticalponin (dilution 1 : 200: Acris: catalogue no. DM345). The sections and whole mounts were then rinsed in PBS and incubated for 1 h with an appropriate fluorescent dye-conjugated secondary antibody, diluted in PBS (sheep-anti-mouse Cy3, dilution 1 : 1000: goat-anti-rabbit Cy3, dilution 1 : 1500: Dianova, Hamburg, Germany). For double staining, the steps were repeated as described above but the incubation time with the primary antibody was reduced to 4–6 h. Double staining included a Cy3 preconjugated alpha-smooth muscle actin (dilution 1 : 150, Sigma) and one of the other antibodies described (myosin, smoothelin, caldesmon, calponin), incubated with a goat-anti-mouse Cy2 or goat-anti-rabbit Cy2 antibody (dilution 1 : 1000, Dianova) in the adult eyes with appropriate fixation. In the case of myosin, caldesmon and calponin, double labelling of whole mounts revealed only insufficient results and was therefore restricted to sections. By contrast, sufficient double-immunolabelling of whole mounts was present using alpha-smooth muscle actin and smoothelin antibodies.
All sections were mounted with Kaiser's glycerol jelly (Merck, Darmstadt, Germany) and viewed with a Leica Aristoplan fluorescence microscope or with a confocal laser scanning system (Bio-Rad MRC 1000) attached to a Nikon Diaphot 300 inverted microscope.
Quantitative considerations
For the detailed description of NVSMC in the normal adult choroid and inner sclera, complete whole mount preparations of the choroid and sclera (four quadrants, from the optic nerve to the pars plana region) from 20 donors were evaluated. Due to the different distributions of NVSMC, three groups were defined and the amount of NVSMC estimated semi-quantitatively at 100 × magnification: 0 = no positive cells, (+) = single [1–3 cells per visual field], + = some [4–10 cells per visual field], ++ = many [10–30 cells per visual field], +++ = numerous positive cells [more than 30 cells per visual field].
For the developmental stages, unfortunately no complete whole mount preparations were available in fetal preparations. Therefore, the area investigated in these eyes (temporal quadrant as a whole mount and sections through the other quadrants) comprised about 50–60% of the total choroidal and scleral area. In the postnatal stages, complete whole mount preparations could be prepared in one eye of each donor and evaluated as for the adult eyes.
Results
Distribution of NVSMC in the normal adult human choroid
Complete scleral and choroidal whole mounts of adult human donor eyes revealed a consistent distribution pattern of NVSMC within both ocular layers (Table 1, Fig. 1a).
Table 1.
Characteristics [age, sex and post-mortem time (PMT)] and semi-quantitative evaluation of non-vascular smooth muscle cells (NVSMC) in the suprachoroid-sclera (SC), parallel to the vessels and vortex veins (PVV), and as a plaque-like arrangement (PL) in the temporal quadrant of whole mounts of adult human donor eyes stained with antibodies against either smooth muscle actin (SMA) or smooth muscle myosin (smMy). 0 = no positive cells (+) = single, + = some, ++ = many, +++ = numerous positive cells
| NVSMC | ||||||
|---|---|---|---|---|---|---|
| Age (years) | Sex | PMT (h) | Antibody | SC | PVV | PL |
| 23 | M | 14 | SMA | + | ++ | + |
| 48 | F | 6 | SMA | + | ++ | +++ |
| 53 | M | 10 | SMA | + | + | 0 |
| 54 | F | 8 | SMA | + | ++ | ++ |
| 58 | F | 2 | SMA | + | ++ | +++ |
| 61 | M | 10 | SMA | (+) | + | (+) |
| 62 | F | 6 | SMA | + | + | ++ |
| 74 | M | 4 | SMA | (+) | ++ | 0 |
| 75 | F | 5 | SMA | + | ++ | +++ |
| 76 | F | 15 | SMA | + | ++ | ++ |
| 77 | F | 11 | SMA | + | + | (+) |
| 78 | F | 30 | SMA | + | + | + |
| 79 | F | 25 | SMA | + | ++ | +++ |
| 83 | F | 5 | SMA | + | ++ | +++ |
| 84 | M | 11 | SMA | + | ++ | +++ |
| 89 | F | 42 | SMA | (+) | + | + |
| 93 | M | 3 | SMA | + | ++ | + |
| 48 | F | 6 | smMy | (+) | + | ++ |
| 84 | M | 10 | smMy | (+) | (+) | (+) |
| 89 | F | 3 | smMy | (+) | (+) | 0 |
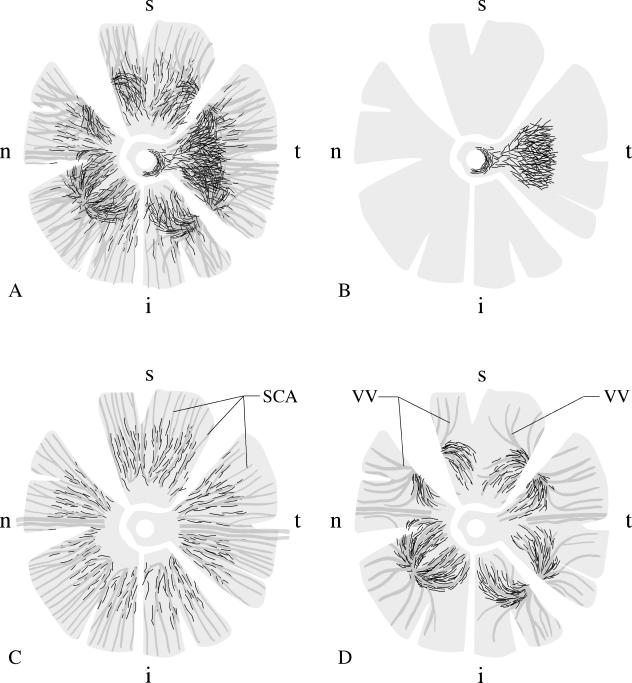
Fig. 1.
Schematic drawing of the distribution of non-vascular smooth muscle α-actin positive cells (NVSMC; black lines representing individual cells) in the different quadrants of a human choroid, using whole mount preparations. A: the complex arrangement of all NVSMC in the choroid. B: A plaque-like arrangement of NVSMC is present in the foveal region of the temporal quadrant, spreading up to the temporal rim of the optic nerve. C: In the posterior eye segment between the entry of the short posterior ciliary arteries (SCA) and the equator, the vessels are accompanied by NVSMC. NVSMC were no longer present in the peripheral choroid towards the ciliary body. D: Numerous NVSMC followed the course of the larger veins forming the vortex veins (VV) at the level of the equator bulbi. Again, the NVSMC were only present in the vessels draining the posterior choroid. The draining vessels from the ciliary body and the peripheral choroid were not paralleled by NVSMC. t = temporal, i = inferior, n = nasal, s = superior quadrant.
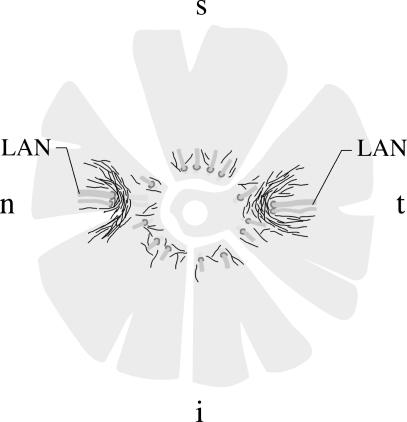
In all eyes investigated, NVSMC were located in the suprachoroid and inner sclera, arranged in a semicircular pattern around the entering posterior ciliary arteries and nerves (Figs 2 and 3a). In addition, numerous spindle-shaped NVSMC were located next to these u-like arrangements genarally in an anterior–posterior orientation. The arrangement and number were similar to that described previously in cynomolgus and rhesus monkeys (May, 2003).
Fig. 2.
Schematic drawing of the distribution of non-vascular smooth muscle α-actin positive cells (black lines representing individual cells) in the different quadrants of a human sclera, using whole mount preparations. LAN = long posterior ciliary artery and nerve, t = temporal, i = inferior, n = nasal, s = superior quadrant.
Fig. 3.
Micrographs of adult human sclera (a) and choroid (b–d) whole mounts stained for smooth muscle α-actin. a. Numerous positive cells (arrows) are arranged in a semicircular pattern around the point of entery of the long posterior ciliary artery (LCA). b. Non-vascular smooth muscle cells (NVSMC, arrows) are located almost parallel to the arteries (a) in the choroid. c. A substantial number of NVSMC (arrows) can be seen between the collecting veins (V) forming a vortex vein. d. Plaque-like arrangement of NVSMC in the foveal region of the temporal quadrant.
A second, constant group of NVSMC was located in the outer choroid between the larger vessels. This group of NVSMC was present in the posterior eye segment between the entering posterior ciliary arteries and the exiting vortex veins (Figs 1c and 3b). They paralleled the course of the larger arteries and veins in the choroid. A substantial number of NVSMC were always located in the confluence of vessels forming the vortex veins (Figs 1d and 3c). Interestingly, the vessels draining from the anterior eye segment and from the most peripheral choroid towards the vortex veins were not accompanied by NVSMC. Branches of this group of vessel-accompanying NVSMC extended through the choroidal layers towards Bruch's membrane, forming a complex three-dimensional network within the choroidal stroma.
Most interindividual differences were seen in the third group of NVSMC, predominantly located in the suprachoroid of the foveal region of the temporal quadrant. These cells formed a dense plaque-like structure about 5–10 mm in diameter (Figs 1b and 3d). The orientation of these cells included numerous transverse directions to the choroidal vessels. When numerous NVSMC of this group were present, the cells extended towards the optic nerve head region and formed a semicircular ring of NVSMC in the temporal quadrant (Fig. 4). About half of the adult donors showed a well-developed plaque-like NVSMC network (Fig. 3d) while others had nearly no additional NVSMC in the foveal region of the temporal quadrant (Table 1). This was independent of the general parameters tested (age, sex, post-mortem time).
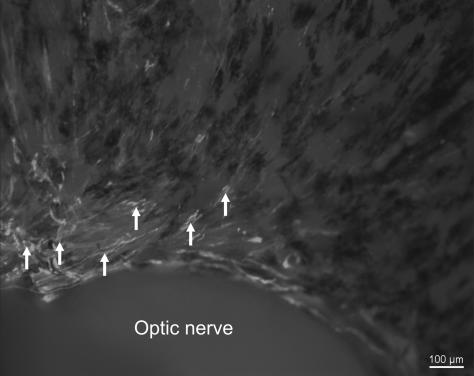
Fig. 4.
Micrograph of an adult human scleral whole mount stained for smooth muscle α-actin. Towards the temporal side of the eye (left side of the micrograph), numerous non-vascular smooth muscle cells (NVSMC, arrows) are present next to the optic nerve showing a semicircular arrangement. Towards the upper side of the eye (right side of the micrograph) the NVSMC decrease rapidly in number.
NVSMC during development of the eye (Table 2)
Table 2.
Semi-quantitative evaluation of nonvascular smooth muscle cells (NVSMC) in the suprachoroid-sclera (SC), parallel to the vessels and vortex veins (PVV), and as a plaque-like arrangement (PL) in the temporal quadrant of whole mount preparations of prenatal and young human donor eyes, stained with antibodies against smooth muscle actin. 0 = no positive cells, (+) = single, + = some, ++ = many positive cells. wog = week of gestation
| Age | SC | PVV | PL |
|---|---|---|---|
| 13 wog | 0 | 0 | 0 |
| 17 wog | 0 | 0 | 0 |
| 18 wog | 0 | 0 | 0 |
| 20 wog | 0 | (+) | 0 |
| 25 wog | 0 | + | 0 |
| 25 wog | 0 | + | 0 |
| 2 days | 0 | ++ | 0 |
| 4 weeks | (+) | ++ | 0 |
| 6 years | + | ++ | + |
In fetal eyes from the 13th and 18th weeks of gestation, smooth muscle α-actin-positive NVSMC were not seen in the choroid or in the sclera. Only the vascular walls of the choroid showed bright staining of their smooth muscle cells.
From the 20th week of gestation but more clearly at the 25th week of gestation, single cells appeared in the choroid that stained positively for smooth muscle α-actin and that were not directly related to the vessel walls (Fig. 5a). Based on their morphological appearance they could be determined as NVSMC. Most of these cells were in contact with the vessel walls. Morphological re-examination of these regions showed no signs of degenerating vessels that might show a similar appearance.
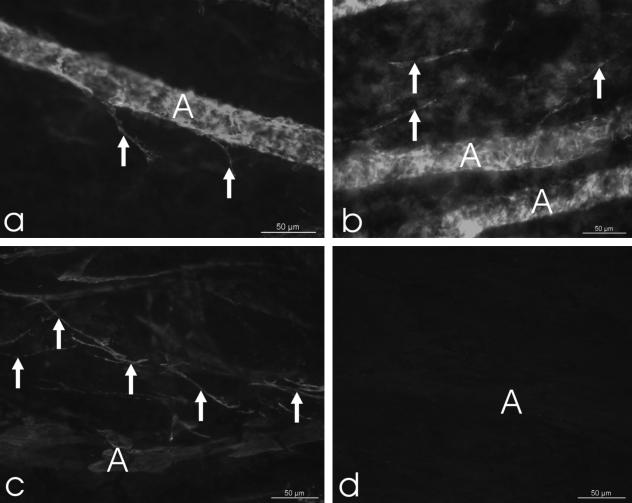
Fig. 5.
Micrographs of a fetal (a: 20th week of gestation) and a young postnatal (b: 2 days after birth) human choroidal whole mount stained for smooth muscle α-actin. Note the well-stained smooth-muscle cells of the arterial vascular wall (A). In places, single positive cells are located inbetween the vessels (arrows). c. Tangential section through the choroid of a 6-year old donor stained for smooth muscle α-actin (d = control section). Note the numerous non-vascular smooth muscle cells (arrows) next to an artery (A).
At 2 days postnatally and at 4 weeks of age, a substantial number of NVSMC were found in the choroid, mainly running parallel with the vessels and presumably representing the group of vessel-accompanying NVSMC (Fig. 5b). The NVSMC in the suprachoroid and inner sclera with a semicircular arrangement around the entering posterior ciliary arteries and nerves were not developed in these eyes. There were also no NVSMC forming a plaque-like structure in the temporal quadrant.
In the eye of the 6-year-old donor (Fig. 5c,d), all three groups of smooth muscle α-actin-positive NVSMC were well developed as described for the adult human eyes.
Immunohistochemical classification of the NVSMC
Staining of PFA- or Zamboni-fixed scleral and choroidal whole mounts and sections with antibodies against smooth muscle myosin revealed that NVSMC in all three groups distinguished above contained this smooth muscle-specific filament (Table 1). In these fixed whole mounts, the proportion of smooth muscle myosin-positive NVSMC was approximately 15–20% of all SMA-positive NVSMC in each group, similar to the general estimated proportion reported previously (Flügel-Koch et al. 1996). As an internal positive control, smooth muscle myosin stained all vessel walls in these preparations. By contrast, double-staining of unfixed choroidal whole mounts with smooth muscle myosin and smooth muscle α-actin revealed that all NVSMC (100%) were positive for both markers. However, the staining for myosin using these specimens revealed positive staining only in two eyes with a post-mortem time of 10 and 12 h. The eyes with a post-mortem time of 18, 30 and 36 h showed no myosin staining but bright smoothelin staining.
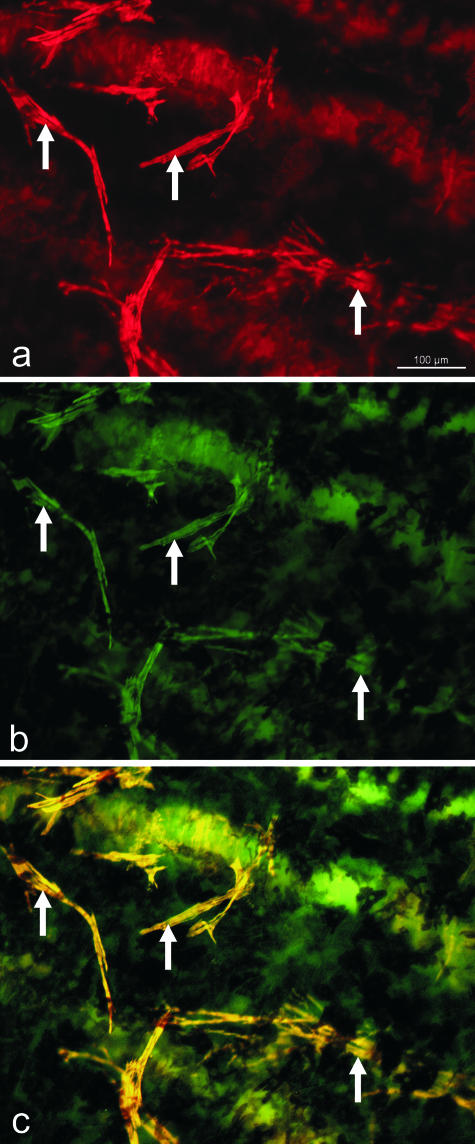
Staining of choroidal whole mounts and sections with antibodies against smoothelin, caldesmon and calponin revealed an identical staining as with antibodies against smooth muscle α-actin (Fig. 6). All NVSMC were positive with these markers for differentiated, contractile smooth muscle cells.
Fig. 6.
Micrograph of an adult human choroidal whole mount stained for smooth muscle α-actin (red, a) and smoothelin (green, b). Note the complete co-localization (c) in the vascular wall and in the non-vascular smooth muscle cells (arrows).
Discussion
Further morphological data on NVSMC in the human choroid, suprachoroid and inner sclera were collected with the aim of answering the three questions posed in the Introduction.
Distribution of NVSMC in the adult human eye
Detailed investigation of the distribution of NVSMC in the human eye revealed several ‘groups’ of NVSMC that could be differentiated due to their localization and presence. Similar to the findings in monkey eyes (May, 2003), NVSMC were located in the suprachoroid and inner sclera, parallel to the entering vessels. In addition, human eyes regularly showed numerous NVSMC in the posterior globe parallel to the large choroidal arteries and veins. The location of the ‘plaque’-like aggregation of NVSMC overlying the fovea centralis region suggested some functional influence on their formation. Unfortunately, no clinical or ophthalmological data relating to the donor eyes were available to correlate the observations with, for example, myopia. In two case studies, a large number of these NVSMC were reported as malformations (Daicker, 1983; Pe'er & BenEzra, 1986). The presence of these cells in larger numbers may be a normal finding. However, further studies with more clinically defined tissue are needed to address this question.
The present observations may provide the basis for further investigations of eyes with defined pathology such as glaucoma or age-related macular degeneration.
Appearance of NVSMC during development
It is interesting to note that the first NVSMC to be observed were in close contact with the vessel walls. Although it was not possible to identify the origin of these NVSMC in the present study, their distribution might indicate that they separate from the same source as the smooth muscle cells of the vascular wall. The NVSMC start to appear only after complete formation of the choroidal vasculature (O'Rahilly, 1975; Sellheyer & Spitznas, 1988), and also after the establishment of the autonomic innervation (May & Lütjen-Drecoll, 2005). In contrast to the observation of Poukens et al. (1998), who first noted large numbers of NVSMC in human individuals older than 3 years, a substantial number of NVSMC were seen to be present shortly after birth (using whole-mount preparations). These NVSMC were, however, only from the vascular-associated group of NVSMC, whereas the NVSMC in the suprachoroid and the plaque-like formation in the fovea centralis region were first seen in a 6-year-old donor choroid.
Specific smooth-muscle marker expression of the NVSMC
With the antibodies used in the present study it can be concluded that the NVSMC contain not only smooth muscle α-actin but also other components that are necessary for contractility. Thus, these cells can be differentiated from myofibroblasts, which in general do not express smoothelin (Chue et al. 2004) and caldesmon (Lazard et al. 1993; Nakayama et al. 2002). This might be of importance when discussing the presence of NVSMC in the choroid as a possible adaptive reaction. In contrast to the stress-induced formation of myofibroblasts, the NVSMC of the choroid develop as true contractile smooth muscle cells. In addition, the NVSMC are also innervated (Flügel-Koch et al. 1996; Poukens et al. 1998; Schrödl et al. 2003; May et al. 2004).
With regard to smooth muscle myosin, the statement published earlier (Flügel-Koch et al. 1996) needs to be revised. In the current study we confirmed the presence of smooth muscle myosin in all the smooth muscle α-actin-positive NVSMC. However, staining with this antibody was very sensitive to fixation and post-mortem time.
Functional considerations
Functional considerations regarding the NVSMC described are mainly speculative. The fact that NVSMC are most numerous in the posterior pole of the eye, especially in the foveal region, and only sparse in the periphery of the choroid towards the ciliary body leads to the suggestion that they support vision rather than pure vascular regulation. The elastic fibres of the choroid become stretched during accommodation (Friberg & Lace, 1988; Wyatt, 1988; Korte & D’Aversa, 1989; Beers & van der Heijde, 1994). This could lead to a displacement of the fovea centralis and thus negatively influence the focus. NVSMC could regulate this process because they are (similar to the ciliary muscle) innervated by cholinergic nerve fibres (May et al. 2004). In addition, NVSMC receive neuronal information from intrinsic neurons (Schrödl et al. 2003). These neurons were shown to have morphological characteristics that make them capable of acting as mechanosensors (May et al. 2004).
Other forces in the choroid arise from the vessels themselves. NVSMC in the inner sclera could help the entering arteries to be kept in place even during peaks of high blood pressure. On the venous side, the NVSMC near the confluence of the vortex veins might support the thin vessel walls against the intraocular pressure and thus prevent collapsing. Both vascular considerations lack evidence, as substantial numbers of NVSMC are only present in foveates. If these processes do play a major role one would expect to find NVSMC in other species also. There is one unconfirmed report on the rabbit choroid (Haddad et al. 2001).
In summary, the findings suggest the presence of three distinct but contiguous groups of NVSMC which show the same immunohistochemical characteristics, but individual differences in their development and expression. Because physiological studies are not feasible in the living human eye, further studies investigating pathological conditions might elucidate the role of these cells.
Acknowledgments
This study was supported by Deutsche Forschungsgemeinschaft DFG SFB 539, BII, 2. I thank Jörg Pekarsky for the schematic drawings and Hong Nguyen for excellent technical help.
References
- Bar H, Wende P, Watson L, et al. Smoothelin is an indicator of reversible phenotype modulation of smooth muscle cells in balloon-injured rat carotid arteries. Basic Res Cardiol. 2002;97:9–16. doi: 10.1007/s395-002-8382-z. [DOI] [PubMed] [Google Scholar]
- Beers AP, Van Der Heijde GL. In vivo determination of the biomechanical properties of the component elements of the accommodation mechanism. Vision Res. 1994;34:2897–2905. doi: 10.1016/0042-6989(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Chue WL, Campbell GR, Caplice N, et al. Dog peritoneal and pleural cavities as bioreactors to grow autologous vascular grafts. J Vasc Surg. 2004;39:859–867. doi: 10.1016/j.jvs.2003.03.003. [DOI] [PubMed] [Google Scholar]
- Daicker B. Heterotopic smooth muscle in the choroid. Klin Monatsbl Augenheilkd. 1983;183:208–210. doi: 10.1055/s-2008-1054905. [DOI] [PubMed] [Google Scholar]
- De Stefano ME, Mugnaini E. Fine structure of the choroidal coat of the avian eye. Vascularization, supporting tissue and innervation. Anat Embryol (Berl) 1997;195:393–418. doi: 10.1007/s004290050060. [DOI] [PubMed] [Google Scholar]
- Flügel-Koch C, May CA, Lütjen-Drecoll E. Presence of a contractile cell network in the human choroid. Ophthalmologica. 1996;210:296–302. doi: 10.1159/000310728. [DOI] [PubMed] [Google Scholar]
- Friberg TR, Lace JW. A comparison of the elastic properties of human choroid and sclera. Exp Eye Res. 1988;47:429–436. doi: 10.1016/0014-4835(88)90053-x. [DOI] [PubMed] [Google Scholar]
- Guglielmone R, Cantino D. Autonomic innervation of the ocular choroid membrane in the chicken. A fluorescence-histochemical and electron-microscopic study. Cell Tissue Res. 1982;222:417–431. doi: 10.1007/BF00213222. [DOI] [PubMed] [Google Scholar]
- Haddad A, Laicine EM, Tripathi BJ, Tripathi RC. An extensive system of extravascular smooth muscle cells exists in the choroid of the rabbit eye. Exp Eye Res. 2001;73:345–353. doi: 10.1006/exer.2001.1042. [DOI] [PubMed] [Google Scholar]
- Korte GE, D'Aversa G. The elastic tissue of Bruch's membrane. Connections to choroidal elastic tissue and the ciliary epithelium of the rabbit and human eyes. Arch Ophthalmol. 1989;107:1654–1658. doi: 10.1001/archopht.1989.01070020732037. [DOI] [PubMed] [Google Scholar]
- Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery JP, Koteliansky VE. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA. 1993;90:999–1003. doi: 10.1073/pnas.90.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loop FT, Schaart G, Timmer ED, Ramaekers FC, van Eys GJ. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol. 1996;134:401–411. doi: 10.1083/jcb.134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loop FT, Gabbiani G, Kohnen G, Ramaekers FC, van Eys GJ. Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vasc Biol. 1997;17:665–671. doi: 10.1161/01.atv.17.4.665. [DOI] [PubMed] [Google Scholar]
- May CA. Nonvascular smooth muscle α-actin positive cells in the choroid of higher primates. Curr Eye Res. 2003;27:1–6. doi: 10.1076/ceyr.27.2.1.15459. [DOI] [PubMed] [Google Scholar]
- May CA, Neuhuber WL, Lütjen-Drecoll E. Immunohistochemical classification and functional morphology of human choroidal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:361–367. doi: 10.1167/iovs.03-0624. [DOI] [PubMed] [Google Scholar]
- May CA, Lütjen-Drecoll E. Choroidal ganglion cells in prenatal, young and middle-aged human donor eyes. Curr Eye Res. 2005;30:1–6. doi: 10.1080/02713680590968231. [DOI] [PubMed] [Google Scholar]
- Meriney SD, Pilar G. Cholinergic innervation of the smooth muscle cells in the choroid coat of the eye and its development. J Neurosci. 1987;7:3827–3839. doi: 10.1523/JNEUROSCI.07-12-03827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Rochow VB, Royuela M. Calponin, caldesmon, and chromatophores: the smooth muscle connection. Microsc Res Techn. 2002;58:504–513. doi: 10.1002/jemt.10169. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Enzan H, Miyazaki E, Toi M. Alpha smooth muscle actin positive stromal cells in gastric carcinoma. J Clin Pathol. 2002;55:741–744. doi: 10.1136/jcp.55.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen P, Clement S, Fontao L, et al. Biochemical evidence for interaction between smoothelin and filamentous actin. Exp Cell Res. 2004;292:170–178. doi: 10.1016/j.yexcr.2003.09.005. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R. The prenatal development of the human eye. Exp Eye Res. 1975;21:93–112. doi: 10.1016/0014-4835(75)90075-5. [DOI] [PubMed] [Google Scholar]
- Pe'er J, BenEzra D. Heterotopic smooth muscle in the choroid of two patients with cryptophthalmos. Arch Ophthalmol. 1986;104:1665–1670. doi: 10.1001/archopht.1986.01050230103042. [DOI] [PubMed] [Google Scholar]
- Poukens V, Glasgow BJ, Demer JL. Nonvascular contractile cells in sclera and choroid of humans and monkeys. Invest Ophthalmol Vis Sci. 1998;39:1765–1774. [PubMed] [Google Scholar]
- Schrödl F, De Laet A, Tassignon MJ, et al. Intrinsic choroidal neurons in the human eye: projections, targets, and basic electrophysiological data. Invest Ophthalmol Vis Sci. 2003;44:3705–3712. doi: 10.1167/iovs.03-0232. [DOI] [PubMed] [Google Scholar]
- Sellheyer K, Spitznas M. Morphology of the developing choroidal vasculature in the human fetus. Graefes Arch Clin Exp Ophthalmol. 1988;226:461–467. doi: 10.1007/BF02170009. [DOI] [PubMed] [Google Scholar]
- Sobue K, Hayashi K, Nishida W. Expressional regulation of smooth muscle cell–specific genes in association with phenotypic modulation. Mol Cell Biochem. 1999;190:105–118. [PubMed] [Google Scholar]
- Szymanski PT, Dickie R, Rogers R, Fredberg JJ. Extraction and reconstitution of calponin and consequent contractile ability in permeabilized smooth muscle fibers. Anal Biochem. 2003;321:8–21. doi: 10.1016/s0003-2697(03)00395-6. [DOI] [PubMed] [Google Scholar]
- Wyatt HJ. Some aspects of the mechanics of accommodation. Vision Res. 1988;28:75–86. [PubMed] [Google Scholar]