Abstract
The motor cortex of eight patients with amyotrophic lateral sclerosis (ALS) and nine control subjects was used in the study. Recent stereological tools, the disector and the rotator method, were applied to the motor cortex of patients with ALS and control subjects to obtain estimates of mean perikaryon volume, mean neuronal nuclear volume, total perikaryon volume and total neuronal nuclear volume. No significant differences were found in any of the estimates. In vivo proton magnetic resonance spectroscopy studies show a decrease in the concentration of neuronal markers. We expected to find changes in perikaryon and/or nuclei neuronal volume because the total neuron number is unchanged in ALS compared with control subjects. However, this was not the case; our results suggest that metabolic changes take place in the motor cortex of ALS patients without these concomitant anatomical changes.
Keywords: amyotrophic lateral sclerosis, mean cell volume, optical disector, stereology, total neuron volume
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive motor neuron disease (MND). The clinical manifestations are upper and lower motor neuron signs due to degeneration of motor neurons in the brain, brainstem and spinal cord (Eisen & Weber, 2001). The criteria for diagnosis of ALS include lower motor neuron (LMN) symptoms, upper motor neuron (UMN) symptoms, progression of symptoms and electromyographic changes.
ALS typically presents between the ages of 50–70 years, with 90% of the incidences being sporadic and 10% familiar (Rowland & Shneider, 2001). The disease can have either a bulbar presentation, first affecting speech, mastication and swallowing, or a spinal presentation involving the limbs. The clinical symptoms are progressive pareses of extremities, muscular atrophy and fasciculations. The median survival time is approximately 3 years. The role of the motor cortex in ALS is still not understood. Many studies have attempted to elucidate the qualitative and quantitative changes that take place in this region during disease progress, including various histological investigations of cell profile, cell volume, number of neurons and concentrations of chemical substances. Microscopically, a substantial loss of large pyramidal cells and Betz cells, and astrocytic gliosis is described in motor cortex in ALS (Hughes, 1982). Changes in the brainstem include degeneration and depletion of neuron bodies in the motor nuclei of the cranial nerves and degeneration of the corticospinal tract. In the spinal cord the observed changes are reduction of motor neurons in the anterior horns, gliosis, loss of axons, loss of myelin and fibrosis of anterior nerve roots (Hughes, 1982).
Most histopathological changes described in motor cortex of ALS patients are non-specific. In an immunocytochemical study of 15 ALS patients stained for phosphorylated neurofilaments (PNF), which have been found to accumulate in degenerating motor neurons in the spinal cord in ALS patients, they found 11 of the patients to have a more intense and widespread PNF immunoreactivity in the cortex compared with control subjects (Troost et al. 1992). A Golgi study examining degeneration of Betz cells in MND found morphological degenerations consisting of swelling of the cell body, an uneven surface of the cell body, distortion of dendrites, loss of dendrites and reactive astrocytic gliosis (Udaka et al. 1986).
Ellis et al. (2001) examined brain volumes of white and grey matter using magnetic resonance imaging. They looked at the brains of eight limb-onset and eight bulbar-onset ALS patients, and eight control subjects. No differences in the total brain volumes of grey or white matter were found in the three groups, nor did they show a difference in the grey matter volume in the precentral gyrus in the ALS group compared with control subjects.
Proton magnetic resonance spectroscopy (1H-MRS) has been applied to determine the in vivo concentrations of the metabolites N-acetyl aspartate (NAA), choline and creatine/phosphocreatine (Cr/PCr) in the brain. NAA is stored primarily in neurons and is a useful marker of neuron integrity whereas choline and Cr/PCr are derived from all cells. Several studies of the motor cortex of ALS patients have shown a decrease in the absolute in vivo concentrations of NAA and/or a reduction in the ratio of NAA compared with choline and NAA compared with Cr/PCr (Pioro et al. 1994; Giroud et al. 1996; Gredal et al. 1997; Block et al. 1998; Ellis et al. 1998; Rooney et al. 1998; Pohl et al. 2001; Rule et al. 2004). These studies repeatedly reported reduced NAA levels, which are assumed to reflect neuronal loss or dysfunction in the ALS motor cortex.
The total number of neurons in the neocortex and motor cortex from eight patients suffering from ALS and nine control subjects has previously been estimated. No significant difference was found in the average number of neurons between ALS patients and control subjects in the neocortex or the motor cortex (Gredal et al. 2000). In spite of these stereological results, the in vivo1H-MRS studies indicate neuron loss, which in the absence of cell loss could be due to neuronal metabolic dysfunction and/or alteration in the size or volume fraction of the neurons. We applied recently developed stereological tools, the disector and the rotator method, to the motor cortex of patients with ALS and control subjects to obtain estimates of mean perikaryon volume, mean neuronal nuclear volume, total perikaryon volume and total neuronal nuclear volume in this selected region of the brain.
Materials and methods
All brains were collected from 1990 to 1996 in accordance with the Danish laws of autopsied human tissue. The material comprised brains from a previously reported study by Gredal et al. (2000), consisting of eight ALS cases (five males and three females, age range 51–76 years, mean 65.9, SD 9.1 years) and nine control subjects (six males and three females, age range 55–77 years, mean 65.3, SD 7.2 years). Table 1 shows data from ALS patients and control subjects including sex, age, duration of disease, cause of death, somatic or post-mortality information including post-mortem findings, concomitant neurological symptoms, and post-mortem interval.
Table 1.
Data from ALS patients and control subjects
| Sex | Age | Duration of ALS (months) | Cause of death | Somatic diseases | Autopsy finding | Concomitant neurological symptoms | PMI (h) | Hemisphere |
|---|---|---|---|---|---|---|---|---|
| ALS | ||||||||
| F | 76 | 21 | Respiratory failure bronchopneumonia | 27 | Right | |||
| M | 69 | 22 | Respiratory failure bronchopneumonia | 30 | Right | |||
| M | 72 | 20 | Respiratory failure bronchopneumonia | 27 | Right | |||
| F | 67 | 33 | Respiratory failure | Thyroid gland adenoma | 6 | Left | ||
| F | 75 | 9 | Respiratory failure | Endometrial cancer without recurrence | Dementia | 6 | Left | |
| M | 51 | 37 | Respiratory failure | 22 | Right | |||
| M | 56 | 38 | Respiratory failure bronchopneumonia | 30 | Left | |||
| M | 61 | 20 | Respiratory failure | 28 | Left | |||
| Controls | ||||||||
| M | 60 | Myocardial infarction | Oesophagus cancer | 12 | Left | |||
| M | 66 | Cardiac failure | Colon cancer with liver-metatases | 16 | Left | |||
| F | 71 | Oesophageal varices haemorrhage | Cirrhosis | 22 | Left | |||
| F | 73 | Myocardial infarction | 26 | Right | ||||
| M | 55 | Cardiac failure | Uraemia, haemodialysis (6 months) | 21 | Right | |||
| M | 61 | Myocardial infarction | NIDDM, hypertension | 22 | Right | |||
| M | 60 | Cardiac failure pulmonary infarction | 27 | Right | ||||
| M | 65 | Myocardial infarction | COPD | 32 | Left | |||
| F | 77 | Cardiac failure | Mamma cancer with metastases | 33 | Right | |||
COPD, chronic obstructive pulmonary disease; NIDDM, non-insulin-dependent diabetes mellitus; PMI, post-mortem interval.
In all ALS patients the ALS diagnosis was established by clinical criteria (signs of both upper and lower motor neuron symptoms) and electromyographical criteria (evidence of acute and chronic denervation involving limb and axial musculature). In all cases the course of the disease was typical for ALS, showing bulbar and limb progression for at least 9 months from first appearance of symptoms until death. At the time of death, all patients had moderate to complete upper and lower limb paralysis. One of the ALS patients showed concomitant signs of dementia whereas the rest showed no other disease in the central nervous system. In the ALS group, all individuals died from respiratory failure, in four cases precipitated by bronchopneumonia (Table 1). In the control group, patients who died from diseases that might affect the central nervous system (e.g. cerebrovascular incidents and metastases) were excluded. Also excluded were cancer patients who died emaciated, alcoholics, drug addicts and patients with mental disturbances or senile dementia. In the control group, four individuals died from myocardial infarction, three from congestive heart failure, one from cancer (ductogenic mamma carcinoma) and one from oesophageal varices not due to alcohol misuse. By chance, malignancy was found at autopsy in two other control cases.
All brains were fixed in 0.1 m sodium phosphate-buffered (pH 7.2) 4% formaldehyde for at least 5 months. The meninges were removed, and the cerebellum and brainstem were detached at the level of the third cranial nerve.
Stereological design
Right or left hemispheres were chosen systematically at random. The frontal, temporal, parietal and occipital regions and archicortex were delineated and painted with different colours on the pial surface, as described elsewhere (Braendgaard et al. 1990; Regeur et al. 1994; Pakkenberg & Gundersen, 1997). Each hemisphere was embedded in 6% agar, coronally sliced at 4.54-mm intervals and the neocortical volume was estimated by Cavalieri's point-counting principle. From every slice containing motor cortex, transcortical wedges were sampled uniformly and systematically. Each wedge was cut into 2-mm-wide parallel bars, resulting in 7–12 bars per specimen. Each bar was rotated randomly around its vertical axis and embedded in LKB-Historesin®. One 35-µm-thick vertical section (Baddeley et al. 1986) was cut from each bar, stained with a modified Wolbach's Giemsa stain, and used for counting and measuring in optical disectors.
The mean cortical thickness is estimated as the volume of the cortex divided by the surface area. Estimation of surface area was not based on strictly unbiased calculations, but estimated by counting intersection points between the boundary and test lines in a non-uniform design (non-rotated slices) (Gundersen, 1985; Regeur & Pakkenberg, 1989).
Counting procedure
Cell counting was performed using the optical disector. Using this method, isolated particles are sampled with a uniform probability in three-dimensional space. The optical disector equipment consisted of a BH-2 Olympus microscope with a motorized x–y stage, and an electronic Heidenhain microcator with digital readout for measuring movements to the nearest 0.5 µm in the z-direction. High image resolution and a thin focal plane can be obtained using a high numerical aperture (NA = 1.4) and a 100× oil-immersion objective for cell counting and cell volume estimation. The final magnification was approximately 3300 ×. One investigator blinded to diagnosis counted all brains on coded sections. The nucleus was used as the counting item.
The z-axis is measured in thick sections (> 35 µm netto) in which the plane of focus is moved up and down with an accuracy of 0.5 µm. The counting frame is a square that is superimposed on the image of the magnified tissue on a colour monitor used for manual counting. The volume of the disector, v(dis), with height h = 15 µm, and the area of the a(frame) = 2970 µm2, is given as v(dis) = h × a(frame).
An average of 144 (89–176) neurons in ALS patients and 137 (96–219) neurons in control subjects were counted and measured per specimen.
A number of criteria were applied to help distinguish between neurons and glia cells: a clearly defined neuronal cytoplasma, a membrane-bound nucleus with a diffuse and even chromatin pattern, and a clear nucleolus are characteristics of nerve cells. Glia cells were counted as one group, and no attempt was made to differentiate the different subgroups.
The vertical rotator
The volume of each sampled neuron was estimated by the rotator method (Jensen & Gundersen, 1993) using the CAST-GRID® software program (Olympus, Denmark). Estimation of cell volume requires some type of isotropy. The requirement of isotropic test planes can be fulfilled with the use of isotropic, uniform random sections or using a vertical design. An estimate of the volume of three-dimensional objects can be obtained when only two-dimensional profiles are available for analysis if the tissue is randomly rotated. In a so-called vertical design first described by Baddeley et al. (1986) the vertical axis can be selected arbitrarily but after random rotation all sections must be made parallel to the vertical axis and all subsequent measurements must be made with respect to the axis, which must therefore be identifiable in all sections.
The vertical axis is aligned parallel to the y-axis on the screen, with the vertical axis in the up–down direction, and the nucleolus as the centre point. The top and bottom boundary points of the cell are indicated by the investigator and the program creates systematic random test-lines perpendicular to the vertical axis (for an illustration see Fig. 1). The investigator marks all intersections between the lines and the cell boundary, and an estimate of the cell volume is calculated by the computer from the formula:
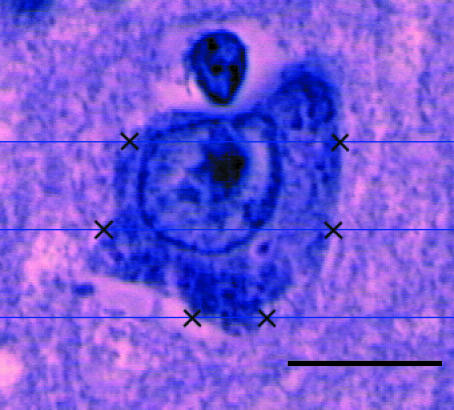
Fig. 1.
The stained sections are orientated in the microscope so that the vertical axis is parallel to the y-axis on the monitor. The nucleolus is used as a reference point. Top and bottom boundary points are defined by the operator, and the computer then generates three lines with a constant distance in a uniform position perpendicular to the vertical axis. The distance between the lines is t = h/3, where h is the height of the profile projected on the vertical axis. The operator defines intersections between the lines and the cell boundary, and the computer automatically generates an estimate of the cell volumes. Scale bar = 7 µm.
where li is the distance between the intersections and the vertical axis and t the distance between the test-lines.
Total number of neurons
The estimate of the total number of neurons in motor cortex, N(neu, motor cortex), was calculated by multiplying the Cavalieri estimate of the regional neocortical reference volume, V(motor cortex), by the regional numerical density NV(neu/motor cortex): N(neu, motor cortex) = NV(neu/motor cortex) × V(motor cortex) = (Σ Q−/Σ v(dis)) × V(motor cortex),
ΣQ− being the total number of neurons counted in all disectors, and Σ v(dis) the total volume of these disectors equal to the area of the test frame multiplied by the height of the disector and by the total number of sampled disectors.
Mean particle volume
Due to the right-skew of the distribution, we logarithmically transformed all values before our calculations and statistical test on size distribution and reported geometric mean values of neuronal nuclear volume and perikaryon volume. The geometric volume of all sampled neurons was estimated using the vertical rotator method previously described. Because the sampling is random, the simple sample mean of log[vi(par)] is an estimate of the geometric mean particle volume: Vn(par) = antilog(1/m × Σ log[vi(par)])
par representing either perikaryon or nucleus volume and m the sample size.
Total perikaryon volume in motor cortex
To obtain an estimate of the total perikaryon volume, the total number of neurons in motor cortex was multiplied by the mean perikaryon volume: Vtotal perikaryon vol. = Vn(per) × N(neu, motor cortex).
Statistical analysis
Differences in total number between the two groups were tested using an unpaired Student's t-test with a significance limit of 0.05. The coefficient of variation (CV = SD/mean) is given in parentheses after the result throughout.
The coefficient of error (CE) is estimated according to the formulas in Gundersen et al. (1999) and showed the final estimate of the total neuron number to have a CE of 0.096. In the estimation of total neuron number we consider the ‘ideal’ CE to be about half the observed coefficient of variation (CV) because of the fundamental relationship: observed CV2 = biological CV2 + estimator CE2. Ideal here means that considerable more work spent on reducing the CE would have very few practical consequences and that a higher CE, if less work was undertaken, might reduce the power of our experimental design. Because the methodological variance was as low as CE2/CV2 = 0.012/0.08 = 0.15 of the biological variance, the precision we accepted, e.g. the variance of the estimate on the neuron number in one brain is less than half the biological variance between the brain neocortices.
Results
The mean neuronal perikaryon volume was not statistically significantly different, 1064 µm3 (0.17) in the ALS patients compared with 1041 µm3 (0.18) in the control subjects (P = 0.80) (Fig. 2). The mean neuronal nuclear volume was almost identical in ALS [262.2 µm3 (0.28)] and in control subjects [262.0 µm3 (0.14)] (P = 0.99) (Fig. 2).
Fig. 2.
Mean perikaryon volume and mean neuronal nuclear volume from the eight ALS patients and nine control subjects. No significant difference was found. Horizontal bars indicate the group means.
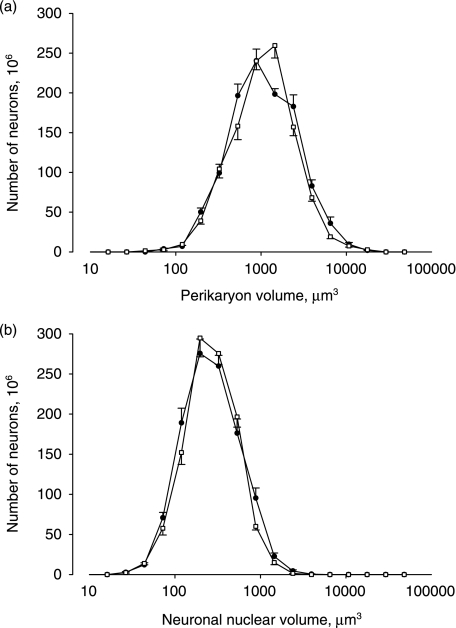
The absolute size distributions of the cell perikaryon and cell nuclei are shown in Fig. 3. The distribution of the volume of the perikaryon and the cell nuclei is seen to be the same in the ALS patients and in the control subjects.
Fig. 3.
The absolute size distribution of the perikaryon volumes (a) and neuronal nuclear volumes (b) shown for the total number of neurons in the motor cortex. ALS (•), control subjects (□). Bars indicate SEM. The abscissa is logarithmic.
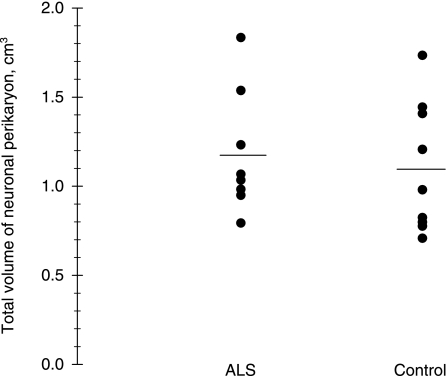
The total volume of neuronal perikaryon in motor cortex was not significantly different in ALS patients [1.18 cm3 (0.29)] compared with control subjects [1.10 cm3 (0.33)] (P = 0.65) (Fig. 4). The total nuclear volume in motor cortex of ALS patients was 0.29 cm3 (0.34), which was not significantly different from that of the control subjects, 0.27 cm3 (0.27) (P = 0.68) (Table 2).
Fig. 4.
The total neuronal perikaryon volume from ALS patients and control subjects, showing no difference between the two groups. Horizontal bars indicate the group means.
Table 2.
Results from motor cortex
| ALS | Controls | P | |
|---|---|---|---|
| Total volume of nuclei (cm3) | 0.29 (0.34) | 0.27 (0.27) | 0.68 |
| Total number of neurons (109) | 1.11 (0.22) | 1.06 (0.32) | 0.75 |
| Neuronal density (106 cm−3) | 31.8 (0.09) | 32.4 (0.13) | 0.09 |
| Glia total (109) | 2.31 (0.29) | 2.26 (0.33) | 0.88 |
| Glia density (106 cm−3) | 66.1 (0.17) | 61.2 (0.17) | 0.36 |
| Glia/neuron ratio | 1.79 (0.12) | 2.16 (0.20) | 0.53 |
| *Surface area (cm2) | 129 (0.27) | 135 (0.30) | 0.74 |
| Motor cortex volume (cm3) | 35.2 (0.25) | 36.9 (0.26) | 0.70 |
| *Cortical thickness (mm) | 2.79 (0.22) | 2.77 (0.14) | 0.92 |
The coefficient of variation (CV = SD/mean) is given in parentheses.
Estimates not based on unbiased methods.
The mean number of neurons in motor cortex in ALS patients [1.11 × 109 (0.22)] and in control subjects [1.06 × 109 (0.32)] was clearly not significantly different (P = 0.75) (Table 2).
The numerical density of neurons was not significantly different in ALS patients [NV = 31.8 × 106 cm−3 (0.09)] and control subjects [NV = 32.4 × 106 cm−3 (0.13)] (P = 0.09). No difference was found between the two groups with respect to motor cortex surface area, or volume or thickness of motor cortex (Table 2).
The bilateral total number of glia cells was 2.31 × 109 (0.29) in ALS and 2.26 × 109 (0.33) in control subjects, which was not significantly different (P = 0.88). The mean glia density in ALS patients was 66.1 × 106 cm−3 (0.17) compared with 61.2 × 106 cm−3 (0.17) in control subjects, which again was not statistically different (P = 0.36). The neuron/glia ratio in motor cortex was not statistically significantly different, 1.79 (0.12) in ALS and 2.17 (0.20) in control subjects (P = 0.53) (Table 2).
Discussion
We used stereological methods to estimate the mean perikaryon volume, mean nuclei volume, total number of motor cortex neurons, and total neuronal perikaryon and neuronal nuclear volume in the motor cortex of ALS patients and control subjects; no differences were found between the two groups.
Kiernan & Hudson (1991) measured the two-dimensional cross-sectional areas through the nucleus of pyramidal cells in layer V of the foot and tongue regions of precentral cortex in 12 ALS patients and ten control subjects. They found the areas of the cortical neurons to be significantly smaller in the ALS patients than in control subjects, the foot mean area of neurons being 383 µm2 in ALS patients and 516 µm2 in control subjects. When looking at only the largest 10% of the neurons, the difference between the two groups was even more pronounced, 1021 µm2 in ALS patients and 1825 µm2 in control subjects (P < 0.001) (Kiernan & Hudson, 1991). Pamphlett et al. (1995) studied the density of motor neurons in the motor cortex and the spine of ALS patients and control subjects. Neuronal cell bodies larger than 40 µm in diameter were considered by them to be motor neurons; these were found to be decreased to a mean of 6.15 cells per 100 mm2 in MND cases compared with control subjects (28.5 cells per 100 mm2), which was highly statistically significant.
In motor cortex a significantly reduced number and degeneration of Betz cells has been reported (Brownell et al. 1970; Hammer et al. 1979; Hughes, 1982; Brion & Plas, 1986; Kiernan & Hudson, 1991; Murayama et al. 1991; Troost et al. 1992; Nihei et al. 1993; Sasaki & Iwata, 2000; Tsuchiya et al. 2002). A histochemical study found a significant decrease in the density of large pyramidal neurons of layer V, including Betz cells, in ALS patients (3.9 mm−2) compared with control subjects (8.8 mm−2). A significantly reduced diameter was also found in the remaining Betz cells, 46 µm in ALS patients compared with 60 µm in control subjects, and the area of the cells was reduced in ALS patients (1026 µm2) compared with control subjects (1776 µm2) (Nihei et al. 1993). However, our study was not designed to quantify the Betz cells separately, as these cells account for only small percentage of the total neurons in the Vb layer, where Betz cells comprise 10% of the pyramidal cells (Rivara et al. 2003). Quantification of Betz cells demands a much more extensive sampling procedure than that used to obtain an estimate of the total perikaryon volume distribution and total neuronal volumes.
Our lack of changes in density, cell size and cell ratio in contrast to the papers cited above may be due to the small sample number and differences in the measuring techniques. All of the quoted studies used one- or two-dimensional measurements, hampering interpretation of results due to the three-dimensional nature of cortical cells. Furthermore, there are differences in the regions selected; we included the entire motor cortex whereas those cited above selected particular layers, regions or cell types.
The limitations of our study include the general bias that can occur in histological handling of biological tissue, which influences cell size. This bias is difficult to avoid, but we attempted to limit it by using a plastic embedding medium with a small shrinkage factor (≈ 10%) as opposed to, for example, paraffin, which has a shrinkage factor of the order of > 50%. Furthermore, we applied a standardized protocol to reduce variation in shrinkage to a minimum. However, even when the estimation is based on unbiased principles, it is obtained after formalin fixation and histological handling, including embedding, cutting and staining procedures, all of which may potentially bias the results. Size estimation in fixed tissue must therefore always be interpreted with these caveats. Furthermore, we did not use a specific staining for neurons, which could have reduced bias related to differentiating cells during the counting procedure; however, the two groups were highly comparable and did not support the suggestion that NAA is related to a decrease in perikaryon volume or neuronal nuclear volume.
The glial cell population has previously been studied. One immunocytochemical study not find astrocytosis specific for ALS patients in the precentral cortex compared with control subjects (Troost et al. 1992), whereas another immunocytochemical study performed on 23 brains from ALS patients reported astrocytosis in the primary motor area in 22 of 23 brains (Murayama et al. 1991). Gliosis in the brains of ALS patients has also been described (Brownell et al. 1970; Hammer et al. 1979; Hughes, 1982; Brion & Plas, 1986; Udaka et al. 1986; Sasaki & Iwata, 2000; Tsuchiya et al. 2002). In contrast to these previous reports we did not find any change in the total number of glia cells in motor cortex of ALS patients compared with control subjects, perhaps due to different quantitative techniques used (conventional vs. stereology) or because of a lack of specific astrocytic staining in our study. Nor did we find the cortical thickness to be significantly different in the ALS patients compared with control subjects. Our results on cortical thickness were obtained from estimation of the surface area and cortical volume, the estimate of surface area being biased, and therefore the estimates of cortical thickness also being biased. However, we assumed that the possibility of a statistically significant bias in the two groups was negligible, and therefore we included the result; but the cortical surface and cortical thickness eastimates should be interpreted with these limitations in mind. Our results are not in accordance with Nihei et al. (1993), who studied 13 ALS and ten age-matched control subjects and observed a significant decrease in cortical thickness in ALS brains (3.1 mm) compared with control subjects (3.9 mm) (P < 0.01). Such a large reduction of the cortical thickness in ALS is unexpected considering the comparatively minor extent of histopathological findings in the motor cortex. The non-uniform sampling design could have contributed to their results.
In conclusion, 1H-MRS results have shown a decrease in the concentration of NAA, which could be due to a decreased number of neurons, a diminished neuron volume or metabolic changes in the brain of ALS patients. We found no differences in the number of neurons, in their size distribution or in the global total neuronal cell body volume, so a decrease in the total perikaryon volume could not explain the decrease in NAA found in 1H-MRS studies. An alternative explanation could be that ALS patients have a lower concentration of NAA in their neurons due to metabolic changes. Although NAA in ALS as first assumed is not correlated with the total perikaryon volume or cell number, mean NAA might still be effective in monitoring disease progress and evaluating treatment, and may possibly be helpful in distinguishing between pure LMN diseases and ALS in patients with only LMN symptoms (Elliott, 1998).
Acknowledgments
We wish to thank Dorte and Mick Pelvig for help with the data processing, Dr Katie Swank for proofreading, and the Lundbeck Foundation for economic support.
References
- Baddeley AJ, Gundersen HJG, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Block W, Karitzky J, Träber F, et al. Proton magnetic resonance spectroscopy of the primary motor cortex in patients with motor neuron disease. Arch Neurol. 1998;55:931–936. doi: 10.1001/archneur.55.7.931. [DOI] [PubMed] [Google Scholar]
- Braendgaard H, Evans SM, Howard CV, Gundersen HJG. The total number of neurons in the human neocortex unbiasedly estimated using optical disectors. J Microsc. 1990;157:285–304. doi: 10.1111/j.1365-2818.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Brion S, Plas J. Lesions of the motor cortex in amytophic lateral sclerosis. Encephale. 1986;12:81–87. [PubMed] [Google Scholar]
- Brownell B, Oppenheimer DR, Hughes JT. The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiat. 1970;33:338–357. doi: 10.1136/jnnp.33.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A, Weber M. The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:564–573. doi: 10.1002/mus.1042. [DOI] [PubMed] [Google Scholar]
- Elliott JL. A clearer view of upper motor neuron dysfunction in amyotrophic lateral sclerosis. Arch Neurol. 1998;55:910–912. doi: 10.1001/archneur.55.7.910. (editorial) [DOI] [PubMed] [Google Scholar]
- Ellis CM, Simmons A, Andrews C, Dawson JM, Williams SCR, Leigh PN. A proton magnetic resonance spectroscopic study in ALS. Neurology. 1998;51:1104–1109. doi: 10.1212/wnl.51.4.1104. [DOI] [PubMed] [Google Scholar]
- Ellis CM, Suckling J, Amaro E, Jr, et al. Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology. 2001;57:1571–1578. doi: 10.1212/wnl.57.9.1571. [DOI] [PubMed] [Google Scholar]
- Giroud M, Walker P, Bernard D, et al. Reduced brain N-acetyl-aspartate in frontal lobes suggests neuronal loss in patients with amyotrophic lateral sclerosis. Neurol Res. 1996;18:241–243. doi: 10.1080/01616412.1996.11740412. [DOI] [PubMed] [Google Scholar]
- Gredal O, Rosenbaum S, Toop S, Karlsborg M, Stange P, Werdelin L. Quantification of brain metabolites in amyotrophic lateral sclerosis by localized proton magnetic resonance spectroscopy. Neurology. 1997;48:878–881. doi: 10.1212/wnl.48.4.878. [DOI] [PubMed] [Google Scholar]
- Gredal O, Pakkenberg H, Karlsborg M, Pakkenberg B. Unchanged total number of neurons in motor cortex and neocortex in amyotrophic lateral sclerosis: a stereological study. J Neurosci Meth. 2000;95:171–176. doi: 10.1016/s0165-0270(99)00175-2. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG. Stereology and sampling of biological surfaces. In: Echlin P, editor. The Analysis of Organic and Biological Surfaces. New York: Wiley; 1985. pp. 477–506. [Google Scholar]
- Gundersen HJG, Jensen EBV, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology – reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hammer RP, Jr, Tomiyasu U, Scheibel AB. Degeneration of the human Betz cell due to amytrophic lateral sclerosis. Exp Neurol. 1979;63:336–346. doi: 10.1016/0014-4886(79)90129-8. [DOI] [PubMed] [Google Scholar]
- Hughes JT. Pathology of amyotrophic lateral sclerosis. Adv Neurol. 1982;36:61–74. [PubMed] [Google Scholar]
- Jensen EBV, Gundersen HJG. The rotator. J Microsc. 1993;170:35–44. [Google Scholar]
- Kiernan JA, Hudson AJ. Changes in sizes of cortical and lower motor neurons in amyotrophic lateral sclerosis. Brain. 1991;114:843–853. doi: 10.1093/brain/114.2.843. [DOI] [PubMed] [Google Scholar]
- Murayama S, Inoue K, Kawakami H, Bouldin TW, Suzuki K. A unique pattern of astrocytosis in the primary motor area in amyotrophic lateral sclerosis. Acta Neuropathol. 1991;82:456–461. doi: 10.1007/BF00293379. [DOI] [PubMed] [Google Scholar]
- Nihei K, McKee AC, Kowall NW. Pattern of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathol. 1993;86:55–64. doi: 10.1007/BF00454899. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Pamphlett R, Kril J, Hng TM. Motor neuron disease: a primary disorder of corticomotoneurons? Muscle Nerve. 1995;18:314–318. doi: 10.1002/mus.880180308. [DOI] [PubMed] [Google Scholar]
- Pioro EP, Antel JP, Cashman NR, Arnold DL. Detection of cortical neuron loss in motor neuron disease by proton magnetic resonance spectroscopic imaging in vivo. Neurology. 1994;44:1933–1938. doi: 10.1212/wnl.44.10.1933. [DOI] [PubMed] [Google Scholar]
- Pohl C, Block W, Karitzky J, et al. Proton magnetic resonance spectroscopy of the motor cortex in 70 patients with amyotrophic lateral sclerosis. Arch Neurol. 2001;58:729–735. doi: 10.1001/archneur.58.5.729. [DOI] [PubMed] [Google Scholar]
- Regeur L, Pakkenberg B. Optimimiziong sampling designs for volume measurements of components of human brain using a sterological method. J Microsc. 1989;155:113–121. doi: 10.1111/j.1365-2818.1989.tb04300.x. [DOI] [PubMed] [Google Scholar]
- Regeur L, Jensen GB, Pakkenberg H, Evans SM, Pakkenberg B. No global neocortical nerve cell loss in brains from patients with senile dementia of Alzheimer's type. Neurobiol Aging. 1994;15:347–352. doi: 10.1016/0197-4580(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Rivara CB, Sherwood CC, Bouras C, Hof PR. Stereological characterization and spatial distribution patterns of Betz cells in the human primary motor cortex. Anat Rec. 2003;270A:137–151. doi: 10.1002/ar.a.10015. [DOI] [PubMed] [Google Scholar]
- Rooney WD, Miller RG, Gelinas D, Schuff N, Maudsley AA, Weiner MW. Decreased N-acetylaspartate in motor cortex and corticospinal tract in ALS. Neurology. 1998;50:1800–1805. doi: 10.1212/wnl.50.6.1800. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Rule RR, Suhy J, Schuff N, Gelinas DF, Miller RG, Weiner MW. Reduced NAA in motor and non-motor brain regions in amyotrophic lateral sclerosis: a cross-sectional and longitudinal study. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:141–149. doi: 10.1080/14660820410017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Immunocytochemical and ultrastructural study of the motor cortex in patients with lower motor neuron disease. Neurosci Lett. 2000;281:45–48. doi: 10.1016/s0304-3940(00)00789-8. [DOI] [PubMed] [Google Scholar]
- Troost D, Smitt PAES, de Jong JMBV, Swaab DF. Neurofilament and glial alterations in cerebral cortex in amyotrophic lateral sclerosis. Acta Neuropathol. 1992;84:664–673. doi: 10.1007/BF00227744. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Ikeda K, Mimura M, et al. Constant involvement of the Betz cells and pyramidal tract in amyotrophic lateral sclerosis with dementia: a clinicopathological study of eight autopsy cases. Acta Neuropathol. 2002;104:249–259. doi: 10.1007/s00401-002-0543-7. [DOI] [PubMed] [Google Scholar]
- Udaka F, Kameyama M, Tomonaga M. Degeneration of Betz cells in motor neuron disease. A golgi study. Acta Neuropathol. 1986;70:289–295. doi: 10.1007/BF00686086. [DOI] [PubMed] [Google Scholar]