Abstract
Antlers are the only mammalian appendages capable of epimorphic regeneration and thus provide a unique model for investigating the mechanisms that underlie mammalian regeneration. Antlers elongate by a modified endochondral ossification process while intramembranous ossification takes place concurrently around the antler shaft. In this study, sites of apoptosis in the growing antler tip were identified by TUNEL staining and related to cell proliferation, as determined by PCNA staining. Bcl-2 and bax were identified by RT-PCR and bax was also immunolocalized in tissue sections. The apoptotic index was high in perichondrium, undifferentiated mesenchymal cells and cellular periosteum but was low in skin. The proliferation index was high in mesenchyme, skin (specifically in hair follicles) and cellular periosteum; it was low in fibrous perichondrium and periosteum, and barely detectable in cartilage. Both bcl-2 and bax were found to be more highly expressed in the perichondrium/mesenchyme and non-mineralized cartilage than in skin and mineralized cartilage. Bax was immunolocalized in mesenchyme cells, chondroprogenitors, chondrocytes, osteoblasts, osteocytes and osteoclasts. In conclusion, this study shows that programmed cell death plays a necessary role in regenerating antlers, as it does during skeletal development, bone growth and bone remodelling. The high level of apoptosis and proliferation in mesenchymal progenitor cells confirms that this represents the antler ‘growth zone’. In fact, the percentage of TUNEL-positive cells in the mesenchymal growth zone (up to 64%) is higher than that recorded in any other adult tissue. This extensive cell death probably reflects the phenomenal rate of morphogenesis and tissue remodelling that takes place in a growing antler. The local and/or systemic factors that control the balance between cell growth and apoptosis in antler tissues now need to be determined.
Keywords: apoptosis, bone, cartilage, deer antler, regeneration
Introduction
In vertebrates the ability to regenerate large sections of the body plan that are lost or injured is restricted to lower organisms such as urodele amphibians (Goss, 1969; Slack, 1980; Brockes, 1997). Mammals cannot replace whole organs, with one exception, the antlers of deer, which are shed and regrown annually into large bony structures used for fighting (Goss, 1983; Price & Allen, 2005). The whole cycle of antler growth is regulated by environmental and hormonal factors, including IGF-I and testosterone (Sadighi et al. 2001). Immediately after the previous year's antlers are shed a ‘blastema-like’ structure forms and bone is regenerated by a modified form of endochondral ossification in the distal growing tip and by intramembranous bone formation around the antler shaft (Banks & Newbrey, 1983). Longitudinal growth is by a continuous process of cellular differentiation from undifferentiated, proliferating mesenchymal progenitors into chondroblasts and chondrocytes (Fig. 1), followed by extensive remodelling of cartilage into bone by osteoclasts and osteoblasts (Rolf & Enderle, 1999; Faucheaux et al. 2001). Thus, at any given time, the whole spectrum of cellular proliferation and differentiation can be observed in the growing antler tip. The mechanisms that regulate this phenomenally rapid growth rate of bone and cartilage are not well understood, but there is increasing evidence that developmental programmes are involved (Price & Allen, 2004; Faucheaux et al. 2004).
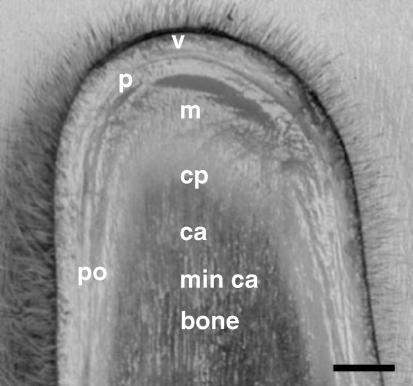
Fig. 1.
Longitudinal section through the growing tip of a deer antler at ∼35 days after the previous set of antlers had been cast. v: velvet skin, p: perichondrium, m: mesenchyme, cp: chondroprogenitors, ca. cartilage, min ca. mineralized cartilage, po: periostium. Bar, 1 cm.
Apoptosis is a necessary component of development and a characteristic of all self-renewing tissues; this led us to hypothesize that apoptosis may also play an important role in regulating antler bone regeneration. Two major intracellular apoptosis signalling cascades have been characterized, the mitochondrial pathway and the death receptor pathway (Antonsson et al. 2001). The mitochondrial pathway is regulated by members of the bcl-2 protein family, which are functionally classified as either anti-apoptotic (bcl-2, bcl-XL, bcl-w) or pro-apoptotic (bax, bcl-XS, bad). The activity of these proteins appears to be regulated by formation of homo- or hetero-complexes in the mitochondrial membrane where bax triggers the permeabilization of the outer mitochondrial membrane by either the formation of specific channels or the opening of permeability transition pores (Reed, 1997a; Antonsson & Martinou, 2001). Changes in mitochondrial permeability release mitochondrial proteins such as cytochrome c and apoptosis-inducing factor (AIF), which lead to apoptosis in a caspase-dependent or caspase-independent manner, respectively (Li et al. 1997; Susin et al. 2000).
In the skeleton maintenance of the appropriate balance between cell proliferation and apoptosis is important during limb development, bone growth, bone remodelling and in fracture repair (Wyllie et al. 1980; Noble et al. 1997; Li et al. 2002). A number of studies have shown that programmed cell death occurs in a number of skeletal cell types including chondrocytes, osteoclasts, osteoblasts and osteocytes (Bronckers et al. 1996; Silvestrini et al. 1998; Tomkinson et al. 1998; Stevens et al. 2000; Bodine & Komm, 2002; Li et al. 2002). The aim of this study was to identify sites of cellular apoptosis in the growing tip of the regenerating deer antler by TUNEL staining and to relate this process to cell proliferation, as determined by proliferating cell nuclear antigen (PCNA) staining. In addition, the mRNA expression of the products of the proto-oncogenes bcl-2 and bax was studied by RT-PCR and bax was immunolocalized in different antler tissues.
Materials and methods
Samples
Antlers were harvested at post-mortem from red deer stags (Cervus elaphus∼2 years old, weight ∼100 kg) 25–50 days after the previous set had been cast. The distal growing tip was removed aseptically and sectioned longitudinally. Tissue was fixed in 4% paraformaldehyde (pH 7.4) for 7 days then washed several times in distilled water prior to storage in 70% ethanol. In addition, velvet skin, perichondrium, mesenchyme, non-mineralized cartilage, mineralized cartilage, periosteum and bone were dissected, frozen immediately in liquid nitrogen and stored at −80 °C. These regions of the antler are illustrated in Fig. 1.
Histology, TUNEL staining and Immunohistochemistry
Paraffin sections (5 µm) were stained with haematoxylin–eosin for histology. Adjacent sections were processed for in situ detection of apoptosis by terminal dUTP nick end labelling (TUNEL) (Frag-EL Kit, Oncogene Research Products, Boston, MA, USA) and immunohistochemistry for expression of bax protein.
TUNEL assay
In this assay terminal deoxynucleotide transferase (TdT) binds to exposed 3′-OH ends of DNA fragments generated in response to apoptotic signals and catalyses the addition of biotin-labelled and unlabelled deoxynucleotides (Gavrieli et al. 1992). Briefly, paraffin sections were dewaxed, rehydrated by a graded alcohol series and washed. Sections were digested with proteinase K (20 µg mL−1) and incubated (1.5 h, 37 °C) with TdT labelling reaction mixture. After stopping the reaction by stop-wash buffer (0.5 m EDTA, pH 8, at room temperature), sections were incubated with peroxidase streptavidin-conjugated anti-digoxigenin sheep Fab′ fragments for 30 min. Sections were washed three times in Tris-buffered saline and colour development achieved with a substrate solution containing 3,3′ diaminobenzidine and H2O2/urea. For negative controls, distilled water was used instead of TdT in the reaction mixture.
Immunohistochemistry
Sections were rehydrated, and endogenous peroxidases were inhibited by immersing the sections in 3% hydrogen peroxide in distilled water for 30 min. Sections were treated with trypsin–CaCl2 (0.5%, w/v) for 15 min at 37 °C, and then incubated overnight at 4 °C with rabbit polyclonal antiserum against bax (Chemicon Int., Temecula, CA, USA) diluted 1 : 200. Slides were washed with Tris-buffered saline solution (TBS), pH 7.4, and then incubated with biotinylated goat anti-rabbit IgG diluted 1 : 100 in PBS (DAKO) for 30 min at room temperature. Sections were rinsed in TBS and then incubated with ABComplex (Vector). The stain was developed with 0.04% (w/v) diaminobenzidine tetrahydrochloride (Sigma) in TBS containing 0.04% (v/v) hydrogen peroxide for 7 min at room temperature. In control sections, PBS containing 10% (v/v) normal goat serum and 1% (w/v) bovine serum albumin (BSA) was substituted for the primary antibody.
For PCNA immunostaining, sections were rehydrated and endogenous peroxidases blocked in 3% hydrogen peroxide in methanol for 30 min. After washing in PBS, sections were incubated for 1 h at room temperature with 10% rabbit serum in PBS/0.05% Tween 20. Sections were then incubated for 1 h at room temperature with a mouse anti-PCNA antibody (Dako, Cambridge, UK) diluted 1 : 50 in 10% rabbit serum in PBS/0.05% Tween 20. Sections were then washed in PBS/0.05% Tween 20 and incubated with biotinylated rabbit anti-mouse IgG for 30 min at room temperature. Detection was carried out using the ABC method with diaminobenzidine as substrate (Vector Labs, Peterborough, UK).
Cell counting
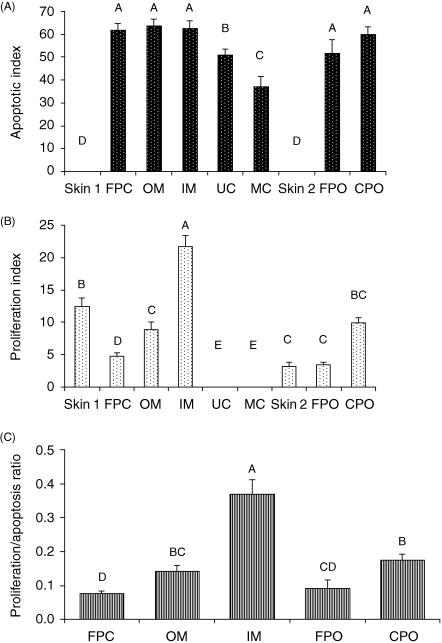
Total cell number and TUNEL-, or PCNA-, positive cells were scored in ten microscopic fields (area of 0.24 × 0.24 mm) from each of the tissue layers including velvet skin overlying the perichondrium (skin 1) and periosteum (skin 2), fibrous perichondrium, outer mesenchyme, inner mesenchyme, non-mineralized cartilage, mineralized cartilage, fibrous periosteum and cellular periosteum, at × 40 magnification. The indices of apoptotic and proliferating cells were calculated as the mean ratio of the number of positive cells to total cell number in each set of tissue sections. The ratio of proliferating to apoptotic cells was also determined.
Bcl-2 and bax sequencing and mRNA expression
RNA extraction
Total RNA was extracted from the different antler tissues using Trizol Reagent (Invitrogen, Milan, Italy), following the manufacturer's instructions. Briefly, frozen tissues were homogenized in Trizol Reagent using a Polytron mixer (Kinematica AG, Littau-Luzern, Switzerland). The homogenate was then incubated with chloroform and precipitated with isopropanol. After washing in 75% ethanol, the concentration of extracted total RNA was spectrophotometrically determined. RNA integrity was also evaluated by examination of 18S and 28S ribosomal bands using 1% agarose gel electrophoresis with ethidium bromide.
Primer design
ClustalX software was utilized to align the coding sequences for rat (GenBank accession no. NM016993), bovine (GenBank accession no. U92434) and chicken (GenBank accession no. D11382) bcl-2 cds. Similarly for bax coding, sequences for bovine (GenBank accession no. U92569), sheep (GenBank accession no. AF163774) and rat (GenBank accession no. U49729) were used. Highly conserved regions of the bcl-2 and bax cds between the examined species were assessed with Genedoc software. Primer 3 Input software was used to design the primer sequences: 5′-ATGTGTGTGGAGAGCGTCAA-3′ (bcl-2 for.) and 5′-CAGACTGAGCAGTGCCTTCA-3′ (bcl-2 rev.), and 5′-AACATGGAGCTGCAGAGGAT-3′ (bax for.) and 5′-TGGGTGTCCCAAAGTAGGAG-3′ (bax rev.). These primers amplified a fragment of 201 bp (bases 439–637) of the bovine bcl-2 cds and a fragment of 288 bp (bases 218–505) of the bovine bax cds.
Multiplex reverse transcriptase (RT) PCR
Multiplex PCR analysis was used to compare the relative levels of transcriptional activity for bcl-2 and bax in tissue samples. RT-PCR reactions were performed by using a ‘one-step’ RT-PCR (Invitrogen, Milan, Italy) kit. For each reaction, total RNA (2 µg) from antler tissue was reverse-transcribed (50 °C for 30 min) and amplified using an MJ thermal cycler (PT-100; MJ Research, Inc., Waltham, MA, USA). RT-PCR cycle conditions were as follows: cDNA synthesis: 50 °C, 30 min; SuperScript II RT inactivation: 94 °C, 2 min; cDNA amplification: [94 °C (30 s), 59.4 °C (30 s), 72 °C (1 min) 40 cycles]; 72 °C (5 min), 15 °C. β-actin (GenBank accession no. U62112) was used as internal control for RNA integrity. PCR reactions carried out without reverse transcription of the RNA samples, using these sets of primers, did not give any amplification product, ruling out the possibility that the observed bands may be due to the presence of contaminant genomic DNA. The RT-PCR products for bcl-2 and bax were purified from agarose gels using a Gel Extraction Kit (Genomed, Berlin-Mahlsdorf, Germany), cloned into a pCR II-TOPO cloning vector (Invitrogen) and sequenced (Primm, Milan, Italy). The band densities of bcl-2 and bax PCR products in each sample were determined from the ethidium bromide-stained agarose gels using Image-J software.
Statistical analysis
Significant differences in the index of apoptotic/proliferating cells between the tissues of the antler tip were evaluated by analysis of variance. Differences in the cellular expression of the bcl-2/bax mRNA between different tissues of the antler were determined using a multifactorial anova followed by the Duncan's least significant difference test (SPSS Inc., 1997).
Results
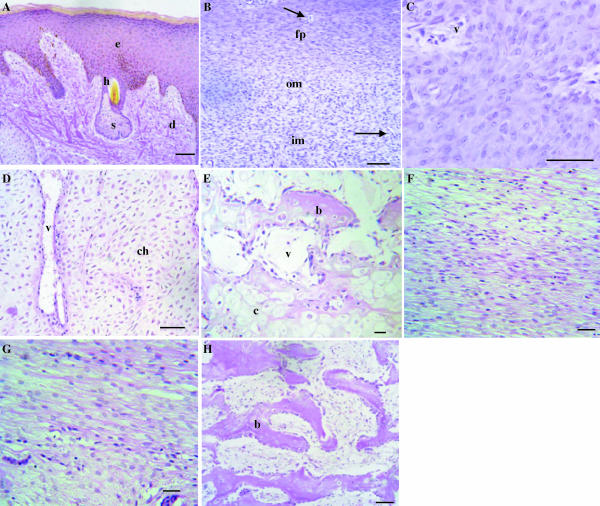
The anatomy of the growing antler tip is shown in Fig. 1, and the histology illustrated in H&E-stained sections included in Fig. 2. There is a layer of velvet skin (Figs 1 and 2A), below which is the perichondrium then a cellular zone of mesenchymal cells (outer and inner) (Fig. 2B) that proximally differentiate into chondroprogenitors arranged in columns (Fig. 2C). Lower down is an extensive zone of non-mineralized vascular cartilage consisting of chondrocytes in trabeculae, numerous vascular channels and perivascular tissue (Fig. 2D). This cartilage then becomes mineralized and is eventually replaced by bone (Fig. 2E). The shaft of the antler is surrounded by skin and the periosteum which has a fibrous (Fig. 2F) and inner cellular component (Fig. 2G); this is the site of intramebranous bone formation.
Fig. 2.
Histology of the growing antler tip. Haematoxylin and eosin (H&E) stain. (A) Velvet skin. e: epidermis, d: dermis, h: hair follicles, s: sebaceous gland. (B) Perichondrium/mesenchyme. fp: fibrous perichondrium, om: outer mesenchyme, im; inner mesenchyme. Arrows indicate vascular channels. (C) Chondroprogenitor zone. v: vascular channels. (D) Cartilage. Chondrocytes (ch) are arranged in vertical trabeculae between which are vascular spaces (v). (E) Site of endochondral bone formation. c: mineralized cartilage, b: newly formed bone, v: vascular space. (F) Fibrous periosteum. (G) Cellular periosteum. (H) Newly formed spongy bone (b). Bars: A–E, H, 200 µm; F, G, 50 µm.
Apoptosis and proliferation
Velvet skin
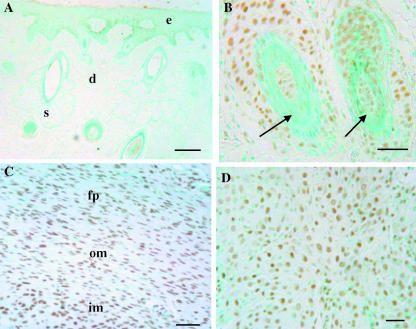
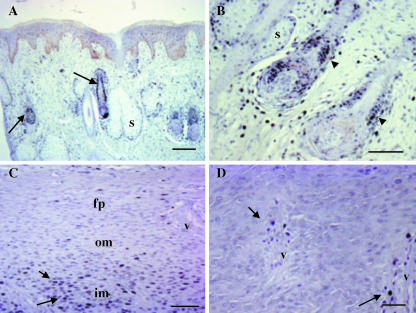
No TUNEL-positive cells were seen in the epidermis or the dermis (Fig. 3A); however, TUNEL-positive cells were observed associated with some hair follicles (Fig. 3B). PCNA-positive cells were also observed in the ‘bulge’ region and at the base of hair follicles and scattered throughout the dermis (Fig. 5A,B). The proliferation index was higher in skin covering the perichondrium (skin 1) than in the skin lower down the antler shaft adjacent to the periosteum (skin 2) (12.4 ± 4.7% vs. 3.25 ± 1.91%, P < 0.01) (Fig. 7B).
Fig. 3.
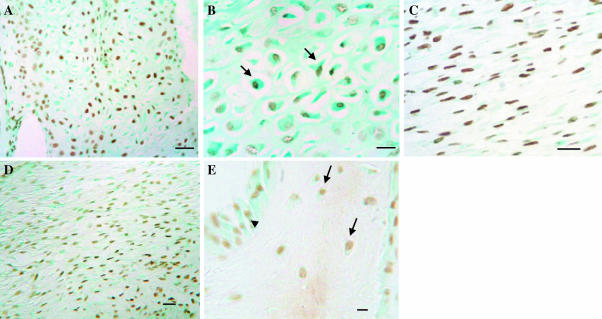
Apoptosis in velvet skin, perichondrium and mesenchyme. TUNEL staining, with methyl green counterstain. (A) Velvet skin; no apoptotic cells are detected in epidermis or dermis. (B) Higher power view to show apoptotic cells associated with hair follicles (arrows). (C) Perichondium/mesenchyme. There are large numbers of apoptotic cells in the perichondrium (fp) and subjacent outer (om) and inner (im) mesenchyme. (D) A significant proportion of chondroprogenitors are apoptotic. Bars: A, 200 µm; B, 32 µm; C, 100 µm; D, 50 µm.
Fig. 5.
Proliferation in velvet skin, perichondrium and mesenchyme. PCNA staining (black). (A) Velvet skin. There are proliferating cells in the dermis and associated with hair follicles (arrows). (B) Higher power image of velvet skin showing large numbers of PCNA-positive cells in the bulb region (arrowhead) and at the base of a hair follicle. Some proliferating cells are also seen around sebaceous glands (s). (C) Perichondium/mesenchyme. There is very little proliferation in the fibrous perichondrium (fp) and outer mesenchyme (om), but the proliferation index increases dramatically in the inner mesenchyme (im) (arrows). (D) Chondroprogenitors do not proliferate, but there is a population of smaller, PCNA-positive cells adjacent to vascular channels (arrows). Bars: A, C, 100 µm; B, 32 µm; D, 50 µm.
Fig. 7.
Apoptotic and proliferation indexes for different tissues in the antler tip. Values were determined by counting cells in ten fields from three different deer and are expressed as a percentage of the total number of cells. Bars represent the mean ± SEM. The fields included: skin 1 (overlying the perichondrium), fibrous perichondrium (FPC), outer mesenchyme (OM), inner mesenchyme (IM), unmineralized cartilage (UC), mineralized cartilage (MC), skin 2 (overlying the periosteum), fibrous periosteum (FPO) and cellular periosteum (CPO). The apoptotic index (A) was highest in FPC, OM, IM, FPO and CPO. The proliferation index (B) was highest in the inner mesenchyme. Bars with different letters represent statistical differences between regions (P < 0.01). (C) Ratio of proliferation to apoptosis for different tissues in the antler tip. Bars with different letters represent statistical differences between regions at P < 0.01. Values are not shown for skin, UC or MC because the value for either the apoptotic index or the proliferation index was zero.
Perichondrium/mesenchyme/chondroprogenitors
A significant proportion of cells were TUNEL positive in the fibrous perichondrium and in the outer and inner mesenchyme (Fig. 3C,D). The apoptotic indices were, respectively, 61.6 ± 11.6%, 63.8 ± 9.2% and 62.4 ± 11.6% (Fig. 7A), although these values were not significantly different. Few PCNA-positive cells were observed in the fibrous perichondrium (4.76 ± 1.8%), and although there were significantly more dividing cells in the outer mesenchyme (8.88 ± 4.2%, P < 0.01), the highest number were located in the inner mesenchyme (21.67 ± 6.04%, P < 0.01) (Figs 5C and 7B). The proliferation to apoptosis ratio was also highest in the inner mesenchyme (P < 0.01) (Fig. 7C). Chondroprogenitors do not proliferate (Fig. 5D), although PCNA-positive cells were observed surrounding vascular spaces in this region.
Cartilage
The apoptotic index was higher in non-mineralized than in mineralized cartilage (51.01 ± 8.37% and 36.96 ± 15.8%, respectively, P < 0.01) (Figs 4A,B,E and 7A), although in mineralized cartilage this may reflect loss of nuclei in some sections. Interestingly, apoptotic chondrocytes were often observed adjacent to cells that appeared morphologically normal or lacunae that were empty. Apoptotic bodies were also observed in some open lacunae (Fig. 4A,B). No proliferation was observed in chondrocytes, although PCNA-positive cells were identified in the perivascular tissue, which contains osteoblast and osteoclast precursors (Fig. 6a,B).
Fig. 4.
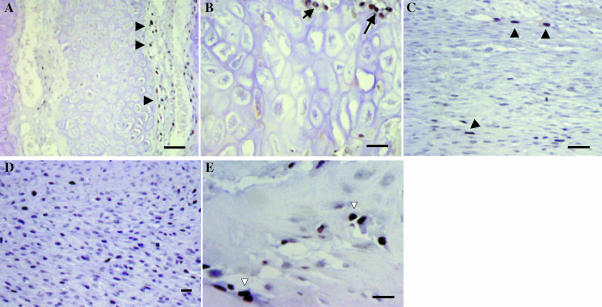
Apoptosis in antler cartilage and at sites of intramembranous bone formation. TUNEL staining, with methyl green counterstain. (A) Cartilage. There are apoptotic chondrocytes throughout the cartilage trabeculae. (B) Higher power image of cartilage showing apoptotic cells within very large lacunae (arrows). (C) Fibrous periosteum. (D) Cellular periostium. (E) Apoptotic osteoblasts (arrowhead) on the surface of bone and osteocytes (arrows) within the bone matrix. Bars: A, 100 µm; B, C, 50 µm; D, 25 µm; E, 20 µm.
Fig. 6.
Proliferation in antler cartilage and at sites of intramembranous bone formation. PCNA staining (black). (A) Cartilage. Chondrocytes do not proliferate, but there are proliferating cells in the perivascular tissue that contains osteoblast and osteoclast precursors (arrowheads). (B) Higher power image of cartilage showing PCNA-positive cells close to vascular channels (arrows). (C) Few proliferating cells are detected in fibrous periosteum (arrowheads). (D) Proliferating cells are more abundant in cellular periosteum. (E) Bone. A proportion of osteoblasts are PCNA positive (open arrows). Bars: A, C, 100 µm; B, 50 µm; D, 25 µm; E, 20 µm.
Intramembranous bone formation
In the fibrous and cellular layers of the periosteum a significant proportion of cells were TUNEL-positive (apoptotic indices were 51.6 ± 20.8% and 59.8 ± 11.9%, respectively, Figs 4C,D and 7A), significantly more than in the overlying skin (P < 0.01). By contrast, very few cells were PCNA-positive in the fibrous periosteum (3.5 ± 1.14%), although the number of PCNA-positive cells did increase significantly in cellular periosteum (9.9 ± 2.75%, P < 0.01) (Figs 6C,D and 7B). As expected, the ratio of proliferation to apoptosis was higher in cellular periosteum than fibrous periosteum (0.12 ± 0.13 vs. 0.09 ± 0.08, P < 0.01), the latter being similar to that of the fibrous perichondrium (0.09 ± 0.08 vs. 0.07 ± 0.02, respectively) (Fig. 7C). At sites of intramembranous bone formation a proportion of osteocytes and osteoblasts were TUNEL-positive (Fig. 4E); however, as expected, a cell count was not possible because the quality of sections from this region was not adequate. No apoptotic osteoclasts were observed. PCNA staining was also observed in osteoblasts (Fig. 6E), but as expected none was identified in osteocytes.
Bcl-2 and bax sequence analysis and mRNA expression in antler tissues
The bcl-2 and bax cDNAs amplified and sequenced from antler were highly homologous to bovine (U92434) and ovine sequences (AF163774), respectively. In particular, the cervine bcl-2 partial sequence was highly homologous (98%) with bovine between nucleotides 439 and 637. For bax, there was 98% homology between the cervine and the ovine sequence (GenBank accession no. AF163774) between nucleotides 134 and 421. The sequences for cervine bcl-2 and bax were submitted to GenBank and have been assigned accession numbers AF512029 and AF512030, respectively. The cervine and bovine amino acid (GenBank accession no. AAC48806) sequences were 100% homologous between amino acids 147 and 212. The cervine amino acid sequence for bax was 100% homologous with feline (GenBank accession no. BAB85810), bovine and ovine sequences (GenBank accession no. AAF98242) between amino acids 168 and 286.
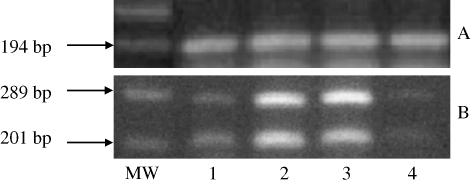
Bcl-2 and bax mRNA levels were compared by RT-PCR using specific primers that amplified bcl-2 and bax cDNAs concurrently. Tissues used for this analysis included velvet skin, perichondrium/mesenchyme, non-mineralized cartilage and mineralized cartilage (Fig. 8). Both bcl-2 and bax were more highly expressed in the perichondrium/mesenchyme and non-mineralized cartilage than in skin and mineralized cartilage.
Fig. 8.
mRNA expression of bax and bcl-2 in antler tissues. (A) β-actin and (B) bax (289 bp) and bcl-2 (201 bp) were detected by multiplex RT-PCR. RT-PCR products were resolved by agarose gel electrophoresis and stained with ethidium bromide. MW = 100-bp molecular weight; 1 = skin; 2 = perichondrium/mesenchyme; 3 = non-mineralized cartilage; 4 = mineralized cartilage. Bax expression was higher than that of bcl-2.
Immunolocalization of Bax
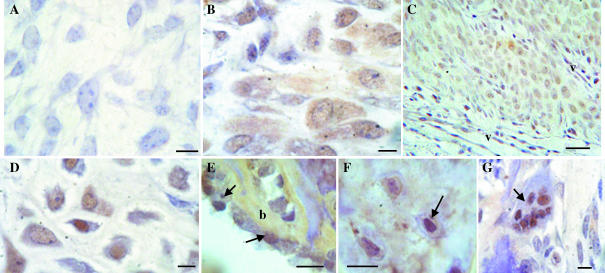
Cells staining positive for bax were present in perichondrium, staining being moderate in both the cytoplasm and the nucleus (Fig. 9B). Chondroprogenitors showed staining for bax, which was generally more cytoplasmic than nuclear (Fig. 9C). Positive immunostaining was also found in chondrocytes of non-mineralized cartilage and was mainly localized to the nucleus (Fig. 9D). Bax was also immunolocalized in the cytoplasm of osteoblasts, osteocytes and in the nuclei of some osteoclasts (Fig. 9E–G). No staining was observed when primary antibody was omitted (Fig. 9A).
Fig. 9.
Bax immunoreactivity in different cell types of the antler. (A) Negative control where the primary antibody was omitted. (B) Perichondrium. (C) Chondroprogenitors (v: vascular channels). (D) Non-mineralized cartilage. (E) Osteoblasts (arrows) on bone (b) surface. (F) Osteocytes (arrows). (G) Multinucleated osteoclast (arrow). Bars: A, 10 µm; B, D, G, 20 µm; C, 50 µm; E, F, 1.6 µm.
Discussion
It is well established that organogenesis and tissue remodelling are determined by a combination of cell proliferation, differentiation and programmed cell death (Guo & Hay, 1999). In this study we have demonstrated that apoptosis also plays a potentially important role in growing antlers, the only mammalian organs capable of repeated rounds of regeneration. Indeed, the percentage of TUNEL-positive cells in the mesenchymal growth zone of the regenerating antler (up to 64%) is higher than that recorded in any other adult tissue. This extensive cell death probably reflects the phenomenal rate of morphogenesis and tissue remodelling that takes place in an antler; for example, in red deer stags, the species studied here, antlers weighing over 20 kg will grow from an antler bud that weighs only a few grams in less than 3 months.
Longitudinal growth occurs at the distal tip of each branch of the antler and in this region the skin or velvet contains fewer hair follicles than ‘normal’ skin, but each of these has a sebaceous gland associated with it which gives it a ‘shiny’ appearance. No TUNEL-positive cells were detected in the epidermis or dermis of velvet covering the growing tip, nor in the more ‘mature’ skin that overlies periosteum and bone lower down the antler shaft. Unsurprisingly, the proliferation index was higher in skin at the growing tip (where growth can exceed 1 cm day−1) that at more proximal sites. However, TUNEL-stained cells were observed associated with hair follicles where PCNA-positive cells are also located. These observations are consistent with studies in other species that have shown there to be a cycle of keratinocyte proliferation, differentiation, cell death and quiescence that continues throughout the life of the hair follicle (Hardy, 1992; Paus et al. 1993; Stenn & Paus, 2001).
Below the velvet skin is the perichondrium and a number of TUNEL-positive cells were detected in this tissue, although few proliferating cells were identified. This suggests that if there is expansion of a progenitor cell population in the perichondrium this occurs at an early stage of regeneration, e.g. in the ‘blastema’. By contrast, in antler mesenchymal cells (Price et al. 1996), the majority of cells were PCNA positive, consistent with what has been shown previously (Matich et al. 2003; Faucheaux et al. 2004). As a number of observations suggest that signalling between proliferation and cell death machinery occurs (Guo & Hay, 1999) it was therefore not surprising that a large number of mesenchymal cells were also TUNEL positive. Bcl-2 and bax mRNAs were also found to be highly expressed in perichondrium/mesenchyme and bax protein was also localized in cells at this site. The large number of TUNEL-positive and PCNA-positive cells in this region confirms that this represents the antler's ‘growth zone’. This high rate of apoptosis may be required to prevent transformation in this rapidly growing tissue (Medh & Thompson, 2000). Undifferentiated mesoderm is also a major area of programmed cell death in the developing limb, particularly in interdigital zones thus delineating the digits (Hurle et al. 1996; Pizette & Niswander, 2001). Programmed cell death may also be the process that delineates the position and number of antler branches, a process about which very little is known.
Antler mesenchymal cells eventually differentiate into chondrocytes and the large number of TUNEL-positive cells in the growth zone suggests that apoptosis may also play a role in the formation and patterning of the cartilaginous templates in the antler, as it does in the developing skeleton (Shum & Nuckolls, 2002). A large number of TUNEL-positive cells were detected in the extensive zone of non-mineralized cartilage as well as in mineralized cartilage, although there was no cell division in chondrocytes. However, the TUNEL technique is known to generate false positives in cartilage (Aigner et al. 2001) and therefore the level of apoptosis should be confirmed using another technique in a future study, e.g. by immunodetection of caspase 3. Programmed cell death has not been shown to be a widespread phenomenon in cartilage during skeletal development, although it does occur in the proximal growth plate where it is confined to the interface with invading vasculature (Roach et al. 1995). However, unlike growth plate cartilage, antler cartilage is extensively vascularized and so most chondrocytes have a close association with a vascular channel (Banks & Newbrey, 1983). This may explain the apparently high level of apoptosis we observed in cartilage. There is also an association between the hypertrophic chondrocyte phenotype and apoptosis in the growth plate (Silvestrini et al. 1998; Aigner et al. 2001; Vortkamp, 2001; Provot & Schipani, 2005) and it is noteworthy that type X collagen, a marker of the hypertrophic chondrocyte, is expressed in the majority of antler chondrocytes (Price et al. 1996). The extensive blood supply of antlers not only supplies the high metabolic needs of a rapidly growing tissue but is also a source of progenitors that subsequently differentiate into mature osteoclasts which are required for the extensive remodelling of cartilage (Faucheaux et al. 2001). Programmed cell death may help to facilitate the migration of blood vessels through cartilage and/or the migration of osteoclasts. In fact, our findings are consistent with the presence of impressively high numbers of TUNEL-positive cells in tissues that exhibit enhanced cellular proliferation and remodelling during fracture healing (Li et al. 2002).
In the antler, bone forms by both endochondral (in the distal tip) and intramembranous (around the circumference of the antler shaft) ossification. The periosteum is extremely thick, a reflection of the high rate of bone formation, and although TUNEL-positive cells were detected in both fibrous and cellular layers, the ratio of proliferation to apoptosis was higher in the deeper cellular zone. This reflects rapid expansion of cells of the osteoblast lineage; previously, we have shown that cells at this site express osteocalcin, a marker of the osteoblast phenotype (Allen et al. 2002). Apoptosis in osteocytes, osteoblasts and osteoclasts is well documented under normal and pathological conditions (Noble et al. 1997; Jilka et al. 1998; Silvestrini et al. 1998), as well as following fatigue loading (Verborgt et al. 2002). It was therefore not surprising that a number of osteoblasts and osteocytes in the antler were TUNEL positive and that bax protein could be localized in these cells, as well as in osteoclasts.
The initiation of cell death involves complex interactions between a number of signalling pathways, including control by cytokines (Jilka et al. 1998). The decision to proceed to cell death is dependent upon the action of the bcl-2 family of proteins (Korsmeyer, 1999) and previously it has been shown that both pro- and anti-apoptotic bcl-2 family proteins are expressed in cartilage and bone (Stevens et al. 2000; Mocetti et al. 2001). RT-PCR showed that the cervine partial cDNA sequences we obtained for bax and bcl-2 were highly homologous with bovine sequences. In fact, the molecular machinery responsible for apoptosis shows a high degree of conservation throughout evolution (Reed, 1997b). PCR demonstrated that bax and bcl-2 appeared to be expressed at high levels in perichondrium, mesenchyme and unmineralized cartilage, tissues with a high apoptotic index. The pro-apoptotic protein, bax, was also immunolocalized in perichondrium, chondroprogenitors, chondrocytes, osteoblasts and osteocytes. However, bax and bcl-2 mRNAs were also found to be expressed in skin whereas very few cells in skin were TUNEL positive. The reason for this discrepancy is not clear and requires further investigation. Notwithstanding, these data do demonstrate the presence of bax in tissues that contain TUNEL-positive cells, which confirms the potential importance of programmed cell death as a physiological regulator of mammalian regeneration, and our results are consistent with the finding that bax is expressed in areas of cell death in the developing limb (Dupé et al. 1999). Our results are also in agreement with Wang et al. (1997), who demonstrated immunohistochemically the presence of bcl-2 and bax in epiphyseal growth plate cartilage and trabecular bone of growing rats. However, bcl-2 and bax are only components of a very complex machinery, and other regulators of apoptosis (caspases, D10-1, Gas 1, Gas 2, etc.) in antler tissues now need to be investigated. In future, RT-PCR could also be used to provide a quantitative measure of the ratio between bcl-2 and bax mRNAs and its relationship to apoptosis, as determined by TUNEL and other markers of apoptosis.
Studies undertaken in the developing limb have shown that the pathways that control programmed cell death, cell growth and cell differentiation are very similar. Because a number of developmental signalling pathways appear to function during antler regeneration (Price & Allen, 2004), it would be reasonable to suppose that similar mechanisms would control apoptosis in antlers. Bone morphogenetic proteins (BMPs) are likely candidates because they are important apoptotic triggers in the developing limb (Zou & Niswander, 1996). Both BMP-4 (Feng et al. 1995) and BMP-2 (Feng et al. 1997) have been cloned from growing antlers and we have shown that BMP-2 stimulates both the growth and the differentiation of antler mesenchymal cells in vitro (Price et al. 2005). The regulation of programmed cell death by BMPs in the developing limb involves interactions with a number of other pathways, of which the retinoic acid (RA) signalling pathway is particularly important (Dupé et al. 1999; Rodriguez-Leon et al. 1999). We have shown previously that antler tissues contain endogenous retinoids, the RA-synthesizing enzyme RALDH2, and retinoic acid receptors (Allen et al. 2002). Fibroblast growth factors (FGFs) are another family of molecules that have been identified in antlers (Barling et al. 2004) and could potentially control programmed cell death, as they are known to cooperate with BMPs in the control of mesodermal apoptosis (Montero et al. 2001). The TGF-β superfamily has also been shown to control interdigital cell death (Dunker et al. 2002) and we have recently immunolocalized TGF-β1 in antler tissues (Faucheaux et al. 2004). Furthermore, sex steroids may also play a role in the control of apoptosis in antlers because the whole cycle of antler regeneration is closely linked to the animal's reproductive cycle (Price et al. 2005) and it is known that the protective effect of sex steroids on bone are mediated, at least in part, by apoptosis (Bland, 2000). IGF-I could also act as a systemic regulator of apoptosis as circulating concentrations are high during antler growth (Suttie et al. 1995), IGF receptors are present in the antler tip (Elliott et al. 1992, 1993) and IGF-I and IGF-II promote antler cell proliferation (Price et al. 1994; Sadighi et al. 1994). There is evidence that IGF-I immunolocalizes in regions of cell death in the developing mouse and chick limb (van Kleffens et al. 1998; McQueeney & Dealy, 2001) and IGF-I has been identified in terminally differentiated hypertrophic chondrocytes which undergo apoptosis (Gibson et al. 1997).
In conclusion, this study has demonstrated for the first time that programmed cell death plays a potentially important role in regenerating deer antlers and that bcl-2 family members are involved in this process. The mechanisms by which local and systemic factors interact to control the balance between cell death and cell growth in antler tissues should now be determined.
Acknowledgments
This work was supported by grants from the Medical Research Council and by Scienze Animali departmental funds.
References
- Aigner T, Hemmel MD, Gebhard PM, Zeiler G, Kirchner T, McKenna L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Allen SP, Maden M, Price JS. A role for retinoic acid in regulating the regeneration of deer antlers. Dev Biol. 2002;251:409–423. doi: 10.1006/dbio.2002.0816. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–57. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- Banks JW, Newbrey WJ. Light microscopic studies of the ossification process in developing antlers. In Antler Development in Cervidae. In: Brown RD, editor. Kingsville, TX: Caesar Kleberg Wildlife Research Institute; 1983. pp. 231–260. [Google Scholar]
- Barling PM, Liu H, Matich J, et al. Expression of PTHrP and the PTH/PTHrP receptor in growing red deer antler. Cell Biol Int. 2004;28:661–673. doi: 10.1016/j.cellbi.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Bland R. Steroid hormone receptor expression and action in bone. Clin Sci. 2000;98:217–240. [PubMed] [Google Scholar]
- Bodine PVN, Komm HS. Tissue culture models for studies oh hormone and vitamin action in bone cells Vitamins and Hormones. In: Litwack G, editor. Vol. 64. New York: Academic Press; 2002. pp. 101–151. [DOI] [PubMed] [Google Scholar]
- Brockes JP. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Goei W, Luo G, et al. DNA fragmentation during bone formation in neonatal rodents assessed by transferase-mediated end labelling. J Bone Miner Res. 1996;11:1281–1291. doi: 10.1002/jbmr.5650110913. [DOI] [PubMed] [Google Scholar]
- Dunker N, Schmitt K, Krieglstein K. TGF-beta is required for programmed cell death in interdigital webs of the developing mouse limb. Mech Dev. 2002;113:111–120. doi: 10.1016/s0925-4773(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Dupé V, Ghyselinck NB, Thomazy V, Nagy L, Davies PJ, Chambon P, Mark M. Essential roles of retinoic acid signaling in interdigital apoptosis and control of BMP-7 expression in mouse autopods. Dev Biol. 1999;208:30–43. doi: 10.1006/dbio.1998.9176. [DOI] [PubMed] [Google Scholar]
- Elliott JL, Oldham JM, Ambler GR, et al. Presence of insulin-like growth factor-I receptors and absence of growth hormone receptors in the antler tip. Endocrinology. 1992;130:2513–2520. doi: 10.1210/endo.130.5.1315246. [DOI] [PubMed] [Google Scholar]
- Elliott JL, Oldham JM, Ambler GR, et al. Receptors for insulin-like growth factor-II in the growing tip of the deer antler. J Endocrinol. 1993;138:233–242. doi: 10.1677/joe.0.1380233. [DOI] [PubMed] [Google Scholar]
- Faucheaux C, Nesbitt SA, Horton MA, Price JS. Cells in regenerating deer antler cartilage provide a microenvironment that supports osteoclast differentiation. J Exp Biol. 2001;204:443–455. doi: 10.1242/jeb.204.3.443. [DOI] [PubMed] [Google Scholar]
- Faucheaux C, Nicholls BM, Allen S, Danks JA, Horton MA, Price JS. Recapitulation of the parathyroid hormone-related peptide-Indian hedgehog pathway in the regenerating deer antler. Dev Dyn. 2004;231:88–97. doi: 10.1002/dvdy.20117. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Chen D, Esparza J, Harris MA, Mundy GR, Harris SE. Deer antler tissue contains two types of bone morphogenetic protein 4 mRNA transcripts. Biochim Biophys Acta. 1995;1263:163–168. doi: 10.1016/0167-4781(95)00106-q. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Chen D, Ghosh-Choudhury N, Esparza J, Mundy GR, Harris SE. Bone morphogenetic protein 2 transcripts in rapidly developing deer antler tissue contain an extended 5′ non-coding region arising from a distal promoter. Biochim Biophys Acta. 1997;1350:47–52. doi: 10.1016/s0167-4781(96)00178-9. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GJ, Link D-L, Roque M. Apoptosis of terminally differentiated chondrtocytes in culture. Exp Cell Res. 1997;233:372–382. doi: 10.1006/excr.1997.3576. [DOI] [PubMed] [Google Scholar]
- Goss RJ. Photoperiodic control of antler cycles in deer. II. Alteration in amplitude. J Exp Zool. 1969;171:223–234. [Google Scholar]
- Goss RJ. New York: Academic Press; 1983. Deer AntlersRegeneration, Function and Evolution. [Google Scholar]
- Guo M, Hay BA. Cell proliferation and apoptosis. Curr Opin Cell Biol. 1999;11:745–752. doi: 10.1016/s0955-0674(99)00046-0. [DOI] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Ros MA, Climent V, Garcia-Martinez V. Morphology and significance of programmed cell death in the developing limb bud of the vertebrate embryo. Microsc Res Techn. 1996;34:236–246. doi: 10.1002/(SICI)1097-0029(19960615)34:3<236::AID-JEMT6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- van Kleffens M, Groffen C, Rosato RR, et al. mRNA expression patterns of the IGF system during mouse limb bud development, determined by whole mount in situ hybridization. Mol Cell Endocrinol. 1998;138:151–161. doi: 10.1016/s0303-7207(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ. Litwack G, editor. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59(7):1693s–1700s. [PubMed] [Google Scholar]
- Li G, White G, Connolly C, Marsh D. Cell proliferation and apoptosis during fracture healing. J Bone Miner Res. 2002;17:791–799. doi: 10.1359/jbmr.2002.17.5.791. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Matich J, Basford Nicholson LF, Barling PM. Mitotic activity in the growing red deer antler. Cell Biol Int. 2003;27:625–632. doi: 10.1016/s1065-6995(03)00118-5. [DOI] [PubMed] [Google Scholar]
- McQueeney K, Dealy CN. Roles of insuline-like growth factor-I (IGF-I) and IGF-I binding protein-2 (IGFBP-2) and -5 (IGFBP-5) in developing chick limbs. Growth Horm IGF Res. 2001;11:346–363. doi: 10.1054/ghir.2001.0250. [DOI] [PubMed] [Google Scholar]
- Medh RD, Thompson EB. Hormonal regulation of physiological cell turnover and apoptosis. Cell Tissue Res. 2000;301:101–124. doi: 10.1007/s004419900159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocetti P, Silvestrini G, Ballanti P, et al. Bcl-2 and Bax expression in cartilage and bone cells after high-dose corticosterone treatment in rats. Tissue Cell. 2001;33:1–7. doi: 10.1054/tice.2000.0144. [DOI] [PubMed] [Google Scholar]
- Montero JA, Ganan Y, Macias D, et al. Role of FGFs in the control of programmed cell death during limb development. Development. 2001;128:2075–2084. doi: 10.1242/dev.128.11.2075. [DOI] [PubMed] [Google Scholar]
- Noble BS, Stevens H, Loveridge N, Reeve J. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone. 1997;20:273–282. doi: 10.1016/s8756-3282(96)00365-1. [DOI] [PubMed] [Google Scholar]
- Paus R, Rosenbach T, Haas N, Czarnetzki BM. Patterns of cell death: the significance of apoptosis for dermatology. Exp Dermatol. 1993;2:3–11. doi: 10.1111/j.1600-0625.1993.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Pizette S, Niswander L. Early steps in limb patterning and chondrogenesis. Novartis Found Symp. 2001;232:23–36. doi: 10.1002/0470846658.ch3. [DOI] [PubMed] [Google Scholar]
- Price JS, Frazer A, Oyajobi BO, Russell RGG. Type X collagen and aggrecan expression in deer antler: an in situ hybridization and immunocytochemical study. Bone. 1994;15:230. [Google Scholar]
- Price JS, Oyajobi BO, Nalin AM, Frazer A, Russell RG, Sandell LJ. Chondrogenesis in the regenerating antler tip in red deer: expression of collagen types I, IIA, IIB, and X demonstrated by in situ nucleic acid hybridization and immunocytochemistry. Dev Dyn. 1996;205:332–347. doi: 10.1002/(SICI)1097-0177(199603)205:3<332::AID-AJA12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Price JS, Allen SP. Exploring the mechanisms regulating regeneration of deer antlers. Phil Trans R Soc Lond B Biol Sci. 2004;359:809–822. doi: 10.1098/rstb.2004.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JS, Faucheux C, Allen SP. Deer antlers as a model of mammalian regeneration. Curr Topics Dev Biol. 2005;67:1–48. doi: 10.1016/S0070-2153(05)67001-9. [DOI] [PubMed] [Google Scholar]
- Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328:658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- Reed JC. Double identity for proteins of the bcl-2 family. Nature. 1997a;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- Reed JC. Vol. 53. New York: Academic Press; 1997b. Bcl-2 family proteins and the hormonal control of cell life and death In Vitamins and Hormones; pp. 99–138. [DOI] [PubMed] [Google Scholar]
- Roach HI, Erenpreisa J, Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995;131:483–494. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Leon J, Merino R, Macias D, Ganan Y, Santesteban E, Hurle JM. Retinoic acid regulates programmed cell death through BMP signalling. Nat Cell Biol. 1999;1:125–126. doi: 10.1038/10098. [DOI] [PubMed] [Google Scholar]
- Rolf HJ, Enderle A. Hard fallow deer antler: a living bone till antler casting? Anat Rec. 1999;255:69–77. doi: 10.1002/(SICI)1097-0185(19990501)255:1<69::AID-AR8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Sadighi M, Haines SR, Skottner A, Harris AJ, Suttie JM. Effects of insulin-like growth factor-I (IGF-I) and IGF-II on the growth of antler cells in vitro. J Endocrinol. 1994;143:461–469. doi: 10.1677/joe.0.1430461. [DOI] [PubMed] [Google Scholar]
- Sadighi M, Li C, Littlejohn RP, Suttie JM. Effects of testosterone either alone or with IGF-1 on growth of cells derived from the proliferation zone of regenerating antler in vitro. Growth Horm IGF Res. 2001;11:240–246. doi: 10.1054/ghir.2001.0232. [DOI] [PubMed] [Google Scholar]
- Shum L, Nuckolls G. The life cycle of chondrocytes in the developing skeleton. Arthritis Res. 2002;4:94–106. doi: 10.1186/ar396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini G, Mocetti P, Ballanti P, Di Grezia R, Bonucci E. In vivo incidence of apoptosis evaluated with the TdT FragEL DNA fragmentation detection kit in cartilage and bone cells of the rat tibia. Tissue Cell. 1998;30:627–633. doi: 10.1016/s0040-8166(98)80081-5. [DOI] [PubMed] [Google Scholar]
- Slack MJ. Morphogenetic properties of the skin in axolotl skin regeneration. J Embyol Exp Morph. 1980;58:265–288. [PubMed] [Google Scholar]
- Chicago, IL: SPSS Inc; 1997. SPSS® Statistical Package for Social Science Advanced Statistics 75. [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Stevens HJ, Reeve J, Noble BS. Bcl-2, tissue transglutaminase and p53 protein expression in the apoptotic cascade in ribs of premature infants. J Anat. 2000;196:181–191. doi: 10.1046/j.1469-7580.2000.19620181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Daugas E, Ravagna L, et al. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000;192:571–580. doi: 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie JM, Fennessy PF, Lapwood KR, Corson ID. Role of steroids in antler growth of red deer stags. J Exp Zool. 1995;271:120–130. doi: 10.1002/jez.1402710207. [DOI] [PubMed] [Google Scholar]
- Verborgt O, Tatton NA, Majeska RJ, Schaffler MB. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res. 2002;17:907–914. doi: 10.1359/jbmr.2002.17.5.907. [DOI] [PubMed] [Google Scholar]
- Vortkamp A. Interaction of growth factors regulating chondrocyte differentiation in the developing embryo. Osteoarthritis Cartilage. 2001;9(Suppl. A):S109–S117. [PubMed] [Google Scholar]
- Wang Y, Toury R, Hauchecorne M, Balmain N. Expression of Bcl-2 protein in the epiphyseal plate cartilage and trabecular bone of growing rats. Histochem Cell Biol. 1997;108:45–55. doi: 10.1007/s004180050145. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Zou H, Niswander L. Requirement for BMP signalling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]