Abstract
The mammalian skull vault is constructed principally from five bones: the paired frontals and parietals, and the unpaired interparietal. These bones abut at sutures, where most growth of the skull vault takes place. Sutural growth involves maintenance of a population of proliferating osteoprogenitor cells which differentiate into bone matrix-secreting osteoblasts. Sustained function of the sutures as growth centres is essential for continuous expansion of the skull vault to accommodate the growing brain. Craniosynostosis, the premature fusion of the cranial sutures, occurs in 1 in 2500 children and often presents challenging clinical problems. Until a dozen years ago, little was known about the causes of craniosynostosis but the discovery of mutations in the MSX2, FGFR1, FGFR2, FGFR3, TWIST1 and EFNB1 genes in both syndromic and non-syndromic cases has led to considerable insights into the aetiology, classification and developmental pathology of these disorders. Investigations of the biological roles of these genes in cranial development and growth have been carried out in normal and mutant mice, elucidating their individual and interdependent roles in normal sutures and in sutures undergoing synostosis. Mouse studies have also revealed a significant correspondence between the neural crest–mesoderm boundary in the early embryonic head and the position of cranial sutures, suggesting roles for tissue interaction in suture formation, including initiation of the signalling system that characterizes the functionally active suture.
Keywords: cranial sutures, Efnbl, fibroblast growth factor receptors, Msx2, neural crest, osteoprogenitor cells, Twist1
Introduction
The skull of all bony vertebrates is a composite structure made up of the neurocranium, which surrounds and protects the brain, and the viscerocranium, which supports the functions of feeding and breathing, and forms the face in mammals. The neurocranium is the principal subject of this review. Its base, underlying the brain, is formed by endochondral ossification whereas the vault (calvaria) is formed from membrane bones that are evolutionarily derived from the protective dermal plates of early jawless fishes. The cartilaginous precursors of the endochondral bones form around the pre-existing cranial nerves and blood vessels, so that in the mature skull the foramina for these connections between the brain and the rest of the body lie within the endochondral skull base. By contrast, no channels of communication access the brain through or between the membrane bones.
Construction of the skull from a number of separate bones enables growth to take place at the margins of the bones for as long as the skull is required to expand around the growing brain. The adjacent margins of membrane bones form the sutures, in which growth of the skull vault takes place; the growth regions between the bones of the skull base are cartilaginous and are referred to as synchondroses. In many fishes, amphibians and reptiles, skull growth continues throughout the life of the animal, whereas in mammals the growth period ends around the time of sexual maturity.
The evolution of human intelligence was made possible by the ability of the brain to expand within its protective casing; similarly, the development of full mental capacities in the growing child depends on long-term expansion of the skull to allow free growth of the brain. In some individuals (approximately 1 in 2500 live births) this mechanism fails due to craniosynostosis, the premature loss and ossification of sutural growth centres. Without surgery to reopen the fused sutures, pressure on the growing cerebral cortex may have a seriously detrimental effect on the child's intelligence, although unequivocally raised intracranial pressure is only present in about 50% of patients, even with multiple suture fusions (Renier et al. 1982).
Advances in molecular genetics over the past 12 years have revealed some of the mutations underlying craniosynostosis syndromes; experimental study of the genes involved is now elucidating the normal mechanisms of skull growth. More recently, genetic engineering has been used to construct mice that lack these genes, and mice with mutations equivalent to those of some of the human conditions. These mouse models are highly instructive for understanding the developmental mechanisms that lead to craniosynostosis, and may eventually help in the development of new therapeutic strategies that will normalize growth of the skull after birth, minimizing the requirement for repeated surgery.
Here we review current information on normal skull growth, the genetic basis of craniosynostosis, and the information that is being acquired from normal and mutant mice. Throughout the text, gene names are given in italics, upper case for human, lower case with initial upper case for mouse, and entirely lower case for Drosophila; protein names are given in regular type, upper case, for all species.
Structure and growth of the mammalian skull
The mammalian skull vault consists mainly of five ‘flat’ bones, the paired frontals and parietals, and the unpaired interparietal; the lateral walls have contributions from the squamous part of the temporal bone (squamosal) and the greater wing of the sphenoid bone (alisphenoid). The arrangement of the bones and sutures in newborn human and late fetal mouse skulls is shown in Fig. 1. All of these bones are formed by intramembranous ossification within a layer of mesenchyme, the skeletogenic membrane, between the dermal mesenchyme and the meninges surrounding the brain. Between the interparietal bone and the foramen magnum (outlet for the spinal cord), cartilage derived from the sclerotomal components of the occipital somites ossifies to form the supraoccipital bone, which fuses with the membranous interparietal to complete the skull vault posteriorly. In the mouse, some cartilage is transiently present in the gaps between membrane bones during late fetal stages (Fig. 1C).
Fig. 1.
Bones and sutures of the newborn human skull (A,B) and late fetal mouse skulls (stained with Alizarin red for bone and Alcian blue for cartilage) (C,D). (A,B) Lateral and vertex views; some bone has been removed to show the teeth. (C) E17.5 skull, lateral view; (D) E18.5 skull, vertex view (skull base removed for clarity). Membrane bones: al, alisphenoid; f, frontal; ip, interparietal; n, nasal; p, parietal; sq, squamosal. The membrane bones of the upper and lower jaws (maxilla and mandible) are unlabelled. Endochondral bones: eo, exoccipital; pt, petrous temporal; so, supraoccipital. Sutures: cs, coronal; ls, lambdoid; ms, metopic (interfrontal); fm, foramen magnum; ss, sagittal; af, anterior fontanelle.
Growth in the sutures is perpendicular to the orientation of the suture, and is normally maintained throughout the period of growth of the brain. Synostosis of one or more sutures is accompanied by compensatory growth, both in other sutures and by remodelling (appositional growth) of other parts of the skull. The three-dimensional computer tomography images of infants with craniosynostosis illustrated in Fig. 2 show how sagittal (A and B) and coronal (C and D) synostosis result in inhibition of growth perpendicular to the closed suture, with compensatory growth in length (A, B) or breadth (C, D) of the skull. Bony fusion does not normally occur until an advanced age in the human skull, except for the metopic suture (Fig. 2A), which begins to fuse at around 18 months of age, after the major part of growth in breadth of the forehead has taken place. In the mouse, only the posterior frontal suture (equivalent to the posterior part of the human metopic suture) undergoes fusion; the other sutures remain open throughout the short life (2–3 years) of the animal (Bradley et al. 1996).
Fig. 2.
Three-dimensional computer tomography scans showing sagittal synostosis in a child of approximately 4 years of age (A,B) and coronal synostosis in a young baby (C,D). Decreased growth in the plane of growth of the sagittal suture is compensated by increased growth in the fronto-occipital plane (A,B); decreased growth in the plane of growth of the coronal suture (C) is compensated for by increased growth in breadth, and the metopic suture is widely open (D). Arrows: open (functional) sutures; asterisk: position of fused sagittal suture (A) and coronal suture (C).
Appositional growth (remodelling) involves osteoclast-induced bone breakdown on the inner surface of the skull and osteoblast-mediated thickening on the outer surface. In the normal human skull this mechanism is important for adapting the degree of curvature of the calvarial bones to the changing circumference of the brain; in craniosynostosis it is an important compensatory mechanism for the premature loss of sutural growth centres (Fig. 2).
Developmental anatomy of the mammalian skull vault
The vertebrate skull is formed from two tissues, neural crest and mesoderm. The distinct contributions of each tissue to the skull and other craniofacial structures have only recently been elucidated in mammalian embryos, by combining mice with a Wnt1-Cre construct and a conditional reporter gene, R26R (Chai et al. 2000; Jiang et al. 2000, 2002). Neural crest cells and their descendants permanently express the LacZ reporter gene, whose product, β-galactosidase, can be stained with X-gal. These studies have defined the pattern of cranial neural crest cell migration in mouse embryos (Fig. 3) and demonstrated a relationship between the neural crest–mesoderm tissue boundaries and the position of sutures in the skull vault (Fig. 4) This information is fundamental to understanding the origin of the cranial bones and sutures. Previous studies, using cell transplantation and labelling techniques, were restricted to neural crest migration stages, when rodent embryos can be studied in vitro (Tan & Morriss-Kay, 1986; Serbedzija et al. 1992; Osumi-Yamashita et al. 1994, 1997).
Fig. 3.
Organization of the neural folds and migration of the neural crest cells in mouse embryos. (A,H) Scanning electron micrographs; (B–G) X-gal-stained Wnt1-Cre/R26R embryos. A, four-somite (s)-stage scanning electron micrograph, showing preotic (arrow) and otic (arrowhead) sulci, prorhombomeres (A–C) (lettered) the occipital region (oc) and the primitive streak region with Henson's node (hn); B, 5s embryo, frontal view, showing emigrating neural crest cells (nc); (C–G) embryos of somite stages as indicated, showing migration of neural crest cells from the neural folds anterior to the preotic sulcus (arrowed) into the frontonasal and first arch regions; in the 23s embryo, the neural crest–mesoderm boundary is clearly defined (arrowheads). (H) 18s-stage embryo, bisected to show rhombomeres (numbered) and position of former preotic and otic sulci (arrow and arrowhead). A–C, Prorhombomeres; d, diencephalon; e, eye; fb, forebrain; fn, frontonasal mesenchyme; ht, heart; h, hyoid neural crest cell population; m, mandibular part of first branchial arch; mb, midbrain; n, notochord; p, pharynx; t, telencephalon; V, trigeminal ganglion crest cells; va, vagal crest cells. Images B, C, E and F were previously published in Jiang et al. (2002). Scale bars: 200 µm.
Fig. 4.
Neural crest and mesodermal contributions to the mouse head at E17.5. (A)Lateral view of a whole Wnt1-Cre/R26R head stained with Alizarin red to show mineralized bone and X-gal (blue–green) to reveal neural crest-derived tissues, including the meningeal covering of the cerebral hemispheres (arrowheads); neural crest-derived tissue extends into the sagittal suture (black arrow) and there is a separate hindbrain-derived patch in the interparietal region (white arrows). (B) Section of the coronal suture stained with X-gal and fast red, showing the neural crest-derived frontal bone (f) and the mesoderm-derived parietal bone (dashed outline) overlying the meninges (m) of the cerebral hemisphere (ch). (C) Diagram showing the neural crest-derived (blue) and mesodermal (red) contributions to the skull vault at E17.5. Modified from images in Jiang et al. (2002). bo, basioccipital; e, eye; m, meninges; pn, pinna of ear; s, skin. Other labels as Fig. 1. Scale bars: A, 1 mm; B, 100 µm.
The cranial (cephalic) region of mammalian embryos includes the first four pairs of somites and all the structures level with and rostral to them. In the early embryo this includes the heart, which receives contributions from neural crest cells originating from the level of the occipital somites in avian and mammalian embryos (Kirby et al. 1983; Fukiishi & Morriss-Kay, 1992). In the region rostral to the somites, neural crest cells emigrate as three distinct populations: (1) the trigeminal crest, which migrates to the frontonasal and first branchial arch regions, and contributes the neural crest component of the trigeminal ganglion; (2) the hyoid crest, which migrates to the second branchial arch; and (3) the vagal crest, which migrates to the third and subsequent arches and also undergoes extensive migration within the trunk. Only the first of these populations, the trigeminal, contributes to the skull. The origin and migration of this population, in the context of segmentation of the cranial neural folds and neural tube, is shown in Fig. 3. Wnt1 is also expressed in the neural plate region from which the trigeminal population originates, so is detected in the derived region of the neural tube at later stages.
The trigeminal neural crest originates from the neural folds rostral to the preotic sulcus (see below) including the future diencephalic region of the forebrain, the whole midbrain and the most rostral part of the hindbrain (Fig. 3A,C). Neural crest emigration begins at the 4–5-somite stage, when the neural folds are convex in transverse section, and the hindbrain region is divided into three prorhombomeres, A, B and C. Prorhombomeres A and B are separated by the preotic sulcus, a deep groove that is later transformed into the gyrus between rhombomeres 2 and 3; the otic sulcus, between prorhombomeres B and C, becomes rhombomere 5 (Ruberte et al. 1997). Neural crest cells are not produced from the cells of the preotic sulcus itself, forming a small gap between the trigeminal and hyoid populations. These two adjacent populations do not mix during their migration, which may in part be due to timing, but the fact that the hyoid population does not migrate rostrally to mix with the trigeminal neural crest cells suggests the deployment of a mechanism to inhibit cell mixing. Repulsive interaction between Eph receptors and their cognate ephrin ligands may be the basis of this tissue segregation: this mechanism has been demonstrated to maintain separation of migrating neural crest cell populations in the amphibian embryonic head, as well as playing roles in cell sorting and axon guidance (Wilkinson, 2001; Poliakov et al. 2004).
The trigeminal crest cells also maintain separation from the adjacent mesodermal cranial mesenchyme cells, which have migrated to the cranial region of the embryo from the primitive streak (Lawson & Pedersen, 1992). The crest cells emigrate immediately under the surface ectoderm, forming a pathway between this tissue and the mesodermal mesenchyme (Chan & Tam, 1988; Fig. 4B). In chick embryos, this subectodermal pathway is associated with secretion of hyaluronan to form a hydrated space beneath the surface ectoderm (Pratt et al. 1975), but enzymatic removal of hyaluronan from mammalian embryos at this stage does not inhibit crest cell migration (Morriss-Kay et al. 1986). Trigeminal crest cells migrate between surface ectoderm and mesoderm towards and into the first branchial arch, which consequentially undergoes a great expansion (Fig. 3D–F). They also migrate between the surface ectoderm and the most rostral part of the forebrain, which is at the same time expanding rostrally to form the telencephalic region, and has no associated mesodermal mesenchyme. This part of the trigeminal crest provides the frontonasal mesenchyme. At the 23-somite stage, migration is complete and a clear boundary forms between the neural crest-derived and mesoderm-derived tissue (Fig. 3G). Formation of this boundary similarly suggests that a repulsive interaction is acting to prevent mixing of the two cell populations.
During the period of trigeminal neural crest migration, cranial neurulation is completed and the hindbrain undergoes segmentation to form seven rhombomeres (Fig. 4H). Hox gene expression in the rhombomeres and their precursors plays a major role in craniofacial patterning (Krumlauf, 1993; Trainor & Krumlauf, 2000; Santagati & Rijli, 2003), so it is interesting that (with the exception of transitory expression of Hoxa2 in rhombomere 2) there is no Hox gene expression rostral to the preotic sulcus. In fact, Hox gene expression is inhibitory to development of the neural crest-derived craniofacial skeleton (Creuzet et al. 2002), so its absence from the trigeminal crest is functionally essential.
The mesoderm–neural crest boundary and the origin of sutures
Following the X-gal-staining pattern in successive stages of Wnt1-Cre/R26R embryos has enabled the later fate of the trigeminal neural crest cells to be traced (Jiang et al. 2002). This revealed that by E17.5, when the skull vault bones have differentiated and extended upwards towards the vertex of the skull, the boundary between the frontonasal population and the adjacent mesodermal mesenchyme defines the coronal and sagittal sutures. The caudal boundary of the neural crest forms the caudal border of the frontal bone (but not the undifferentiated mesenchyme between the frontal and parietal bones) and a small tongue of tissue between the two parietal bones. Hence both the coronal suture and the first-formed part of the sagittal suture are neural crest–mesoderm interfaces. The same may be true for the lambdoid suture, as a patch of previously undetected neural crest cells that emerge from the rostral hindbrain at E9.5 insert into the dermis and form the central part of the interparietal bone and the dermal tissue between this bone and the caudal aspect of the parietal bones (Jiang et al. 2002). This late-emigrating population of hindbrain neural crest cells does not form the whole of the lambdoid suture, nor does the neural crest-derived tissue between the parietal bones at E17.5 form the whole of the later sagittal suture (our unpublished observations). Their presence at the time of formation of these two sutures suggests that they may play a role in ‘kick-starting’ the signalling system that is required for sutural growth, and that this system, once started, can spread along the suture as a greater length of the adjacent bones becomes closely juxtaposed.
The metopic (interfrontal) suture is the only human calvarial suture that is not initiated at a line of juxtaposition between neural crest and mesoderm, being entirely within the neural crest domain. This may be relevant to the early fusion of this suture in the human skull. The mouse posterior frontal suture is similarly formed entirely within the neural crest domain, and fuses early (although the anterior part of the frontal suture does not). By contrast, sagittal suture-dependent growth in breadth of the parietal region of the human skull, and fronto-occipital growth derived from activity of the coronal and lambdoid sutures, continues throughout childhood.
Suture formation
Three of the calvarial sutures, the sagittal, metopic and lambdoid, are formed by the narrowing of membranous gaps between bones that are initially widely separate. Their positions overlie areas in which brain tissue does not lie close to the surface, i.e. the midline between the cerebral hemispheres and olfactory lobes (sagittal and metopic) and the area between the cerebral hemispheres and the cerebellum (lambdoid). The coronal suture does not form in the same way, and the parietal bone can be seen to overlap the frontal bone from the outset. This is a flexible joint and in newborn babies with very little hair, the overlapping bones can be seen to slide over each other to widen the suture when the baby cries and intracranial pressure rises. In the mouse, this overlap can be seen to originate as oblique apposition of the neural crest-derived and mesodermal components of the dermal mesenchyme at the time of boundary formation at E9, with the mesodermal (parietal) side lying external to the neural crest side of the boundary. The consistency between this oblique tissue boundary and the orientation of the overlapping bones by E16.5 is remarkable, because bone formation is initiated from tissue close to the skull base, which extends upwards without recruiting new cells (Iseki et al. 2005). The coronal suture does not form over an anatomical landmark of the brain: the original position of the neural crest–mesoderm boundary over the telencephalon–diencephalon border is maintained as each cerebral hemisphere expands, extending caudally beneath the suture to attain the final anatomical relationship in which the suture lies over the cerebral hemisphere (Jiang et al. 2002). The expanding cerebral hemispheres carry with them a covering of neural crest cells that form the meninges, which can be seen beneath the mesodermal parietal bone at E17.5 (Fig. 4A,B).
Insights into the molecular basis of suture formation and function have largely come from identification of the mutations underlying craniosynostosis syndromes. This knowledge of the genes involved in abnormal human development has been applied to experimental investigations in the mouse, so that we now have a growing understanding of the mechanisms of both normal skull growth and the biology of craniosynostosis. These topics will now be discussed.
Craniosynostosis: genes and syndromes
Until just over a decade ago, little was known about the causes of craniosynostosis. Since then, the identification of mutations in both syndromic and non-syndromic cases has led to considerable insights into the aetiology, classification and developmental pathology of these disorders. The first mutation to be identified was a heterozygous missense mutation within MSX2, in patients with Boston-type craniosynostosis (Jabs et al. 1993). This is a rare syndrome, being confined to a single large family. MSX2 encodes a homeobox-containing transcription factor, and the mutation, which is within the homeodomain, acts by stabilizing DNA binding (Ma et al. 1996). The majority of known genetic causes of craniosynostosis are mutations in the genes encoding fibroblast growth factor receptor types 1–3 (FGFR1, 2 and 3); other significant genes are TWIST1 and EFNB1.
The most common mutations in FGFR1, 2 and 3 that cause craniosynostosis and other skeletal growth disorders are dominantly acting and affect specific regions of the proteins (Fig. 5). Most of the known mutations in FGFR3 are associated with growth disorders of the long bones (dwarfism), ranging from the relatively mild hypochondroplasia through the most common form, achondroplasia, to the perinatal lethal thanatophoric dysplasia (see Ornitz, 2001 and Wilkie, 2005 for recent reviews). FGFR3 also harbours the mutation underlying Muenke syndrome, the most common syndromic form of craniosynostosis, and a rare variant of Crouzon syndrome associated with the skin disorder acanthosis nigricans.
Fig. 5.
Structure of fibroblast growth factor receptor proteins (types 1–3), showing the position of some of the common mutations causing craniosynostosis, and (FGFR3 only) some of the mutations affecting long-bone growth. Each receptor has three immunoglobulin-like domains (Ig) whose structure is maintained by disulphide bonds (s–s); TM, transmembrane domain; TK1,2, tyrosine kinase domain.
An important category of craniosynostosis is caused by mutations occuring in FGFR2, and giving rise to several related syndromes. For example, in Crouzon and Pfeiffer syndromes craniosynostosis is combined with prominent eyes, ‘beaked’ nose and hypertelorism. The midface is underdeveloped, reflecting growth defects of the facial bones and skull base, and there are variable mild limb defects (Wilkie, 2005, and references therein). These mutations are overwhelmingly grouped in two exons of FGFR2, which encode the IgIIIa/c domain of the protein (see below). Apert syndrome, which is characterized by severe bony syndactyly of the hands and feet as well as craniosynostosis, is caused mainly by mutations affecting the IgII–IgIII linker region (Wilkie et al. 1995).
Differences in the phenotypes of equivalent mutations on each of the three genes are illustrated by the IgII–IgIII linker region (Fig. 5 and Bellus et al. 1996): the P252R FGFR1 mutation was identified in a mild form of Pfeiffer syndrome (Muenke et al. 1994); the P253R FGFR2 mutation causes Apert syndrome (Wilkie et al. 1995), and the P250R mutation of FGFR3 causes Muenke syndrome (Bellus et al. 1996). The phenotypic differences resulting from the three equivalent mutations do not relate directly to different functions of the three genes; evidence is accumulating from mouse studies that they function interactively, and loss- or gain-of-function mutations in one gene that affect the function of the protein may have secondary effects on one or both of the other FGFRs. Interactions between FGFR1 and FGFR2 will be considered in more detail in the experimental section below.
Like Muenke syndrome, Saethre–Chotzen syndrome predominantly affects the coronal suture; the two are distinguishable by the characteristic low frontal hairline, ptosis and digital anomalies of Saethre–Chotzen patients, but definitive separation of the two syndromes only became possible when the mutated genes were identified. Saethre–Chotzen syndrome is caused by heterozygous loss-of-function (missense, duplication, deletion and nonsense) mutations in TWIST1 (El Ghouzzi et al. 1997; Howard et al. 1997). The gene twist, which encodes a basic helix–loop–helix (bHLH) transcription factor, was first discovered in Drosophila as essential for mesoderm formation (Thisse et al. 1987).
Identification of human FGFR1, FGFR2, FGFR3, TWIST1 and MSX2 mutations causing craniosynostosis has led to experimental analysis of the role of these genes in skull growth. Craniofrontonasal syndrome (CFNS), in which coronal synostosis is combined with hypertelorism (wide-spaced eyes) (Cohen, 1979; Gorlin et al. 2001; Fig. 6A,B), has recently been found to be associated with loss-of-function mutations in EFNB1 (Twigg et al. 2004; Wieland et al. 2004). CFNS is X-linked, but has the unusual feature that hemizygous males are very mildly affected compared with heterozygous females. The EFNB1 gene encodes ephrin-B1, a ligand for EphB receptors. Eph–ephrin interactions are mainly mutually repulsive (anti-adhesive), and play major roles in preventing cell mixing across boundaries in the embryo (Poliakov et al. 2004).
Fig. 6.
Craniofrontonasal dysplasia due to EFNB1 mutation (A,B) and wild-type mouse embryos (C–F) showing (arrowed) the correspondence between the frontonasal neural crest–mesoderm boundary in Wnt1-Cre/R26R embryos (C,D) and Efnb1 expression (E,F) in whole heads (C,E) and sections (D,F). (C,E,F) E10.5; (D) E12. ch, cerebral hemisphere; other labels as Fig. 1. C, modified from Jiang et al. (2002); A, E and F were previously published in Twigg et al. (2004). Scale bars: C, E: 1 mm; D, F: 200 µm.
The epidemiology of craniosynostosis
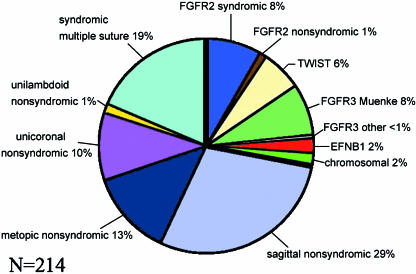
Given the different processes of formation of the various calvarial sutures, it is not surprising that the prevalence of human craniosynostosis detected at or soon after birth is different for each suture. Sagittal synostosis is most common (40–55% of all craniosynostoses), followed by coronal (unicoronal or bicoronal) at 20–25%; the prevalence of metopic synostosis is 5–15% and lambdoid is rare at 0–5% (Cohen, 2000). In 5–15% of cases, more than one suture is affected. Considering only craniosynostosis in which a molecular diagnosis has been made, the most common form is Muenke syndrome, followed by Saethre–Chotzen syndrome, the prevalence of each of these being only slightly less than that of the combined FGFR2 mutation-derived craniosynostosis syndromes (Fig. 7).
Fig. 7.
Genetic epidemiology of craniosynostosis, based on a prospective series of 214 patients born between 1993 and 2005 inclusive, and screened for mutations in the entire coding region of FGFR1, FGFR2, FGFR3, TWIST1 and EFNB1. A specific molecular diagnosis was made in 60 (28%) of cases: this is represented as the segment between the thick black lines.
Since the Oxford Craniofacial Unit opened in 1979, 79 children with apparently non-syndromic unicoronal or bicoronal synostosis and negative for FGFR2 and TWIST1 mutations have undergone operation to reopen the closed suture(s). For some of these a single operation has been sufficient, but others have required a second operation, usually due to raised intracranial pressure associated with reclosure of the sutures. In an analysis of factors that might be recognizable prior to operation as increasing the risk of reclosure, Thomas et al. (2005) found that those with the P250R FGFR3 mutation (Muenke syndrome) were five times more likely to require a second operation than those who tested negative for this mutation.
Craniosynostosis and suture formation
Craniosynostosis involves failure of the signalling system that governs the processes of growth and differentiation at the sutural margins. In most cases this appears to occur after suture formation, although sutural growth may be affected very early in fetal life. Mathijssen et al. (1999) estimated the time of fusion of the coronal suture in two Apert syndrome skulls to be at 15 weeks of gestation, based on measurement of the distance between the mineralization centres of the frontal and parietal bones in comparison with the progressive separation of these centres in a series of fetal skulls. Mineralization of both bones is detectable before 10 weeks (Corliss, 1976), and clear centres with radiating lines of mineralized matrix are visible soon afterwards, indicating that the signalling process in the Apert skulls had failed at an early stage of skull growth.
It is theoretically possible that failure of formation and maintenance of a normal functional suture could be due to events at the time of formation of the neural crest–mesoderm boundary. We suggested above that repulsive interactions between Eph receptors and ephrin ligands may be required for boundary formation. The identification of mutations in the EFNB1 gene in the families of 23 unrelated female patients with craniofrontonasal dysplasia (Twigg et al. 2004; Wieland et al. 2004) prompted us to compare the expression domain of mouse Efnb1 with the frontonasal neural crest–mesoderm boundary (Twigg et al. 2004 and Fig. 6C–F). The two boundaries coincide over the telencephalon–diencephalon part of the boundary, supporting the idea that Efnb1 plays a role in formation of the neural crest–mesoderm tissue boundary that forms the coronal suture.
We are now investigating this possible role of ephrin-B1 at early stages of craniofacial development by means of an Efnb1 knockout mouse that appears to be an excellent model for this variable, X-linked human syndrome (Compagni et al. 2003). These investigations may also elucidate the mechanism by which establishment of the neural crest–mesoderm boundary leads to initiation of the FGF–FGFR signalling system that is associated with osteogenic growth and differentiation at the sutural margins. Interestingly, there is evidence for a direct interaction between ephrin-B1 and FGF signalling pathways in other developmental systems, both in vitro (Chong et al. 2000) and in compartmentation of the eye field within the embryonic neural plate (Moore et al. 2004).
The possible role of TWIST1 in suture formation is also interesting. TWIST1 activity is essential for cranial neural tube formation, and for maintaining clearly defined crest cell migration pathways: in Twist1–/– embryos, trigeminal crest cells stray from the subectodermal pathway and fill in the gaps between trigeminal and hyoid populations (Soo et al. 2002). Twist1–/– embryos die at E11.5 (Chen & Behringer, 1995); this is too early to study suture formation but is 2 days after formation of the neural crest–mesoderm boundary at E9.5.
Roles for Twist1 in sutural growth
In the early coronal suture, Twist is expressed in the sutural mesenchyme between the proliferating osteoblasts of the frontal and parietal bone edges, and overlapping with these two populations (Johnson et al. 2000), consistent with roles in separating the two bone-forming tissues and with initiating transcription of Fgfr2. The Twist1+/– mouse is a good model for Saethre–Chotzen syndrome, showing postnatal fusion of the coronal suture (Bourgeois et al. 1998; Carver et al. 2002). Interbreeding Twist+/– and Msx2+/– mice (Ishii et al. 2003) indicated that Msx2 and Twist1 act co-operatively, but in parallel pathways, to control the proliferation and differentiation of the neural crest-derived mesenchyme that forms the frontal bones.
The molecular relationship between TWIST1 activity and FGFR signalling in skull development is a fundamental component of the initiation and maintenance of sutural growth. In Drosophila, twist is required for mesoderm formation but also affects mesodermal migration through regulating expression of the Fgfr homologue heartless (htl) (Shishido et al. 1993; Beiman et al. 1996). Twist1 is expressed in the mouse coronal suture region prior to establishment of the suture (Rice et al. 2000); the expression domain of Twist1 is in Fgfr-free intrasutural mesenchyme, extending into the Fgfr2 expression domain in osteoprogenitor cells (Johnson et al. 2000), suggestive of a functional relationship between Twist1 and Fgfr2. Exogenous FGF can up-regulate Twist1 expression in mice (Rice et al. 2000), and it would be interesting to know whether a feedback loop between Twist1 and Fgfr2 maintains the expression of both genes and hence sutural function. Further evidence that TWIST1 plays a role in controlling the onset of differentiation comes from the identification of a novel domain near the C-terminus of the TWIST1 protein which interacts with the Runx2 binding domain to inhibit its function (Bialek et al. 2004). The relevance of this ‘Twist box’ to the cranial sutures is unclear, however, because TWIST1 missense mutations in Saethre–Chotzen syndrome cluster in the bHLH region of the protein only.
Guenou et al. (2005) recently demonstrated a functional relationship between TWIST1, FGFR2 and RUNX2 in Saethre–Chotzen syndrome and in normal skull growth. They found that cranial osteoblasts from a patient heterozygous for a nonsense mutation deleting the Twist1 bHLH domain showed decreased FGFR2 mRNA levels associated with decreased expression of RUNX2 and downstream bone differentiation markers. They further found that TWIST1 protein binds to a specific region of the FGFR2 promoter in vivo, and that in mutant TWIST1 cells, RUNX2 binding to the FGFR2 promoter is reduced. This study begins to establish the nature of the molecular relationship between these three important genes in the cranial sutures.
Fibroblast growth factor signalling roles in skull vault growth
The four fibroblast growth factor receptors, together with their 18 or more known FGF ligands, play a multiplicity of roles in development from the earliest embryonic stages (reviewed by Itoh & Ornitz, 2004). Their functional diversity is further increased by alternative splicing of the genes encoding FGFR1, -2 and -3, using either exon IIIb or exon IIIc to form ‘b’ or ‘c’ sequences in the third immunoglobulin-like domain (Miki et al. 1992; Johnson & Williams, 1993; and Fig. 8). The two splice variants for each gene show different ligand affinities: in general the ‘b’ isoforms are expressed in epithelia and bind to receptors secreted from adjacent mesenchyme, whereas ‘c’ isoforms are mesenchymal and preferentially bind to epithelial ligands (Ornitz et al. 1996). The potential FGF–FGFR pairings in the skull vault are complex: RT-PCR analysis indicates that all FGFs except FGF3, -4, -5, -6 and -8 are expressed in mouse cranial sutures (Hajihosseini & Heath, 2002). FGFRc isoforms are activated by a large number of FGF ligands, including FGF2, which is present in abundance (Iseki et al. 1997). FGFRb isoforms are much more selective, with a high affinity for FGF7 and FGF10 (Ornitz, 2001).
Fig. 8.
Diagram illustrating the alternative use of either exon IIIb or IIIc to form alternative splice forms of FGFR receptors, in this case FGFR2b and FGFR2c, respectively.
The reciprocal epithelial–mesenchymal system of FGF–FGFR signalling does not precisely apply in the sutures, where FGFs secreted by osteoblasts at the differentiating edge of the bones activate receptors involved in both osteoprogenitor cell proliferation and the conversion of these cells to differentiating osteoblasts. This receptor–ligand interaction occurs entirely within mesenchyme-derived tissues. Schematic diagrams explaining this system are shown in Fig. 9. Experimental evidence from implanting FGF2-soaked beads onto the fetal mouse coronal suture indicates that a low level of FGF signalling promotes the proliferation of osteogenitor cells through FGFR2, whereas a higher level of signalling leads to down-regulation of FGFR2, withdrawal of cells from the cell cycle and up-regulation of FGFR1, leading to osteoblastic differentiation (Iseki et al. 1999). FGF2 is also secreted by the dura, and stimulates osteoblastic differentiation in dura–osteoblast co-cultures (Warren et al. 2003a).
Fig. 9.
The relationship between Fgfr expression and the progression from proliferating osteoprogenitor cells to differentiating osteoblasts at the edge of a bone in the mouse coronal suture. (A) Osteoblasts (blue) express Fgfr1 and secrete bone matrix proteins (light blue) and FGF (pink); FGF diffuses into the region of proliferating osteoprogenitor cells (green). (B) Hypothetical scheme suggesting that a threshold of FGF concentration effects the change in gene expression from Fgfr2 to Fgfr1 and the change in cell behaviour from proliferation to differentiation.
FGF2 and gain-of-function FGFR mutations suppress expression of the bone morphogenetic protein (BMP) antagonist noggin, and noggin misexpression prevents cranial suture fusion in vitro and in vivo (Warren et al. 2003b). Dura-derived TGFβ has also been shown to influence the timing of sutural closure (Opperman, 2000). No mutations associated with craniosynostosis have been identified in TGFβ genes, but mutations in the cognate receptors TGFBR1 and TGFBR2 were recently described in a new syndrome comprising cleft palate, characteristic tortuosity of the large arteries and significant (∼40%) prevalence of craniosynostosis (Loeys et al. 2005).
Once the FGFR signalling system is established in the sutures, long-term skull growth depends on maintenance of a balance between the differentiation of new bone and proliferation of the osteoprogenitor cell population as a reservoir of potential new osteoblasts. Mutations of the receptors have been shown to destabilize this balance through more than one mechanism. Crouzon syndrome is associated with mutations that result in an unpaired cysteine residue, enabling ligand-independent cross-linking through disulphide bonds between mutant receptors; the resulting receptor activation (phosphorylation) causes excessive signalling (Neilson & Friesel, 1995; Robertson et al. 1998). Pfeiffer syndrome, which differs from Crouzon syndrome mainly in having a broad thumb/great toe, can manifest as a phenotypic variant of Crouzon syndrome, as the C278F and C342Y mutations of FGFR2 cause both syndromes (Rutland et al. 1995; Wilkie, 1997; and Fig. 5). Other Pfeiffer mutations act through Apert syndrome-like mechanisms in which there are increased and/or new FGF binding affinities (Anderson et al. 1998; Yu et al. 2000; Ibrahimi et al. 2004b) or (more rarely) ectopic expression of the FGFR2b isoform (Oldridge et al. 1999; Hajihosseini et al. 2001). Proline→arginine mutations in the IgII–IgIII linkers of FGFR1 (P252R) and FGFR3 (P250R) also exhibit increased ligand binding affinities, notably for FGF9 (Ibrahimi et al. 2004a).
Mechanical factors may also be involved in maintaining sutural patency. It is a common observation that skull growth matches brain size in infants with microcephaly and hydrocephaly as well as in infants with normal brains, and growth of the brain is generally assumed to generate tensile strains in the sutures. Henderson et al. (2004) measured the bone deposition rate and tensile strains in normal infants and found that both showed an approximately exponential decrease from 1 month to 4 years of age; however, the results suggested that tissue level strains in the sutures may be too small to influence osteoblast biology. Head position within the maternal pelvis in the final months of pregnancy and during birth may also be important, due to unequal pressures on the two sides of the head. In non-syndromic craniosynostosis, where mutation in all known craniosynostosis genes has been excluded, the right coronal suture is affected more than twice as commonly as the left, correlating with a laterality bias in head orientation prior to and during delivery (Bennett & Brown, 1999).
Mouse models for human craniosynostosis syndromes
Discovery of the human mutations in FGFR1, FGFR2, FGFR3, TWIST1 and MSX2 in patients with growth disorders of the skull and long bones stimulated the engineering of mice lacking these genes and with mutations equivalent to the human gain-of-function mutations. Loss-of-function mutations are somewhat easier to achieve, and have been very informative, as described earlier for Twist1 and Msx2. Disruption of total Fgfr2 causes defects in the trophoblast lineage, resulting in pregastrulation lethality (Arman et al. 1998) or, in a less extensive deletion, death at E10.5 with placental insufficiency and multiple organ defects including failure of limb bud growth (Xu et al. 2000). Similarly, disruption of Fgfr1 results in failure of gastrulation and embryonic lethality (Yamagouchi et al. 1994; Deng et al. 1996). The early lethality problems have been circumvented by making chimaeras, hypomorphs and conditional knockouts. An Fgfr1 hypomorph revealed that this gene is essential for mesoderm formation and patterning, mainly through activity of the IIIc isoform (Partanen et al. 1998). Fgfr1b is involved in skin development, analogous to the functions of Fgfr2b, through activation of the receptor by FGF10 (Beer et al. 2000).
Disruption of the Fgfr2c isoform has been more informative with respect to the role of this receptor in skeletogenesis. Fgfr2c–/– mice were created by insertion of a stop codon preventing translation of exon IIIc (Eswarakumar et al. 2002). They show delayed onset of ossification throughout the skeleton, followed by premature fusions of skull bones, including the coronal suture and the occipital synchondroses of the skull base, and decreased growth of the long bones. These growth defects are associated with premature loss of proliferation in osteoprogenitor cells in the coronal suture and of proliferating chondrocytes in the growth plates of endochondral bones. Similarly, conditional inactivation of Fgfr2 enabled analysis of the roles of Fgfr2 in the osteoblast and chondrocyte lineages, and showed that Fgfr2 is essential for osteoblast proliferation but not differentiation (Yu et al. 2003), consistent with the experimental findings of Iseki et al. (1999).
In contrast to the effects of disruption of total Fgfr1 and Fgfr2, Fgfr3–/– mice are viable and fertile; they show excessive growth of long bones through increased proliferation of the growth plate chondrocytes (Colvin et al. 1996; Deng et al. 1996). No skull abnormalities have been described in these mutants, and we know very little about the function of FGFR3 signalling in the cranial sutures. Fgfr3 is expressed at low levels in the mouse coronal suture, overlapping with the expression domains of Fgfr1 and Fgfr2 (Johnson et al. 2000).
These mouse loss-of-function Fgfr mutations are informative for elucidating the roles of FGFR signalling in normal skeletogenesis, but gain-of-function Fgfr mutants are required for developmental studies that will provide clues to the links between genotype and phenotype of the human craniosynostosis syndromes. Zhou et al. (2000) created a mouse model of Pfeiffer syndrome by introducing a P250R mutation (equivalent to the human P252R mutation) into Fgfr1. The phenotype includes craniosynostosis involving the sagittal and coronal sutures, associated with accelerated osteoblast differentiation; premature expression of the bone differentiation gene Runx2 suggests that this gene is downstream of FGFR1 signalling, consistent with the experimental evidence that FGFR1 is associated with differentiation (Iseki et al. 1999). Chen et al. (2003) introduced an S250W mutation into Fgfr2 (equivalent to the human Apert mutation S252W) but observed only coronal synostosis, without syndactyly. The most significant phenotypic observations in the Fgfr2S250W/+ mouse were decreased bone formation and increased apoptosis in the coronal suture, suggesting a possible role for apoptosis as a cellular mechanism underlying some forms of craniosynostosis. Yu et al. (2000) and Ibrahimi et al. (2004b) showed by biochemical analysis that the human S252W FGFR2 mutant receptor shows increased binding affinity for natural FGF ligands and that the mutant FGFR2c splice form shows novel binding to the FGFR2b-specific ligand FGF10. It is therefore possible that the limbs of the Fgfr2S250W/+ mutant mouse escape morphogenetic defects because only the first of these binding alterations takes place. Alternatively, there may be differences in timing or feedback regulation that mitigate the effect of the mutation on mouse limbs.
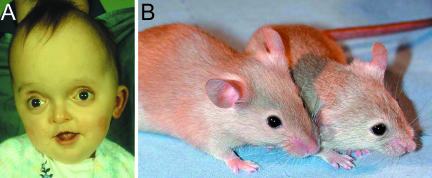
The most common mutation of Crouzon syndrome, which sometimes also manifests a Pfeiffer syndrome phenotype (Reardon et al. 1994), is the C342Y mutation of FGFR2 (Figs 2C,D and 10A). Eswarakumar et al. (2004) introduced a C342Y mutation into exon IIIc of mouse Fgfr2; the heterozygous mutant phenotype has all the hallmarks of Crouzon syndrome, including coronal synostosis, shortened face, domed skull vault, protruding eyes, premature osteogenic differentiation, increased bone density and occasional cleft palate. Some of these features can be seen in the living mice (Fig. 10B). Homozygotes show additional features that have been reported in some human cases, including replacement of the tracheal rings by a continuous sleeve of cartilage and synostosis of the knee joint. Further study of this mutant has revealed that most pups show craniosynostosis involving sagittal and lambdoid as well as coronal sutures; some pups also have broad first digits, i.e. a Pfeiffer-like phenotype, reflecting the phenotypic variability of this mutation in humans (C. Perlyn, C. Babbs and G. M. Morriss-Kay, unpublished observations).
Fig. 10.
Crouzon syndrome. (A) Characteristic flattened midface and proptosis due to shallow orbits; the domed skull shape compensates for premature loss of the coronal suture (see Fig. 2). (B) Fgfr2C342Y mouse (right) showing shortened face, proptosis and domed skull vault compared with the wild-type head.
Ectopic expression of the FGFR2 IIIb isoform, detected in Pfeiffer syndrome patients with exon IIIc acceptor splice site mutations and an Apert patient with Alu insertions (Oldridge et al. 1999), is mimicked by a mouse mutant with heterozygous abrogation of Fgfr2 exon IIIc (Hajihosseini et al. 2001). The mouse phenotype shows the coronal synostosis and ocular proptosis characteristics of the human phenotypes but not the limb anomalies; there are also soft tissue abnormalities, neonatal growth retardation and death.
A mouse model for the P250R FGFR3 mutation, which causes Muenke syndrome, has now been constructed (S. R. F. Twigg and A. O. M. Wilkie, unpublished). The equivalent mouse mutation, P244R, was created by a knock-in technique. The homozygotes have the domed skull characteristic of impaired coronal suture function but the suture does not show synostosis, and heterozygotes appear normal. These mice are currently being bred onto several different strain backgrounds in order to maximize the possibility of creating a more faithful model of Muenke syndrome for detailed analysis.
FGF receptor interactions
The mouse mutant studies are now revealing increasingly strong evidence of functional interactions between receptors. The phenotype of the Fgfr2c–/– mice is much less severe than expected for this important gene, and the possibility that some functional redundancy with Fgfr3 may be involved is currently being tested by interbreeding a conditional Fgfr2–/– with an Fgfr3–/– mutant (D. M. Ornitz, personal communication). FGF18 is a prime candidate for the integration of FGFR2c and FGFR3c activity, as it activates both receptors and is known to co-ordinate chondrocyte and osteoblast differentiation in endochondral ossification (Liu et al. 2002; Ohbayashi et al. 2002).
It is also necessary to invoke receptor interaction to understand why coronal synostosis results from both loss- and gain-of-function Fgfr2c mutations (Eswarakumar et al. 2002, 2004): as illustrated in Fig. 9, FGFR2 signalling is required to maintain osteoprogenitor cell proliferation, but above a certain threshold Fgfr2 is down-regulated and the cells switch to Fgfr1 expression and the differentiation pathway (Iseki et al. 1999). Hence, both excessive FGFR2 signalling and the absence of FGFR2 signalling will enhance the rate at which osteogenic stem cells are transformed into Fgfr1-expressing osteoblasts. A dosage effect of Fgfr1 expression on osteogenic differentiation in the cranial sutures (and sternum) has also been demonstrated directly, by introducing variable copy numbers of a hypermorphic Fgfr1 mutation carried by a bacterial artificial chromosome (BAC) (Hajihosseini et al. 2004): increasing the BAC copy number was correlated with an increased severity of the sutural fusions.
As discussed in this review, recent research has advanced our understanding of the molecular nature and biological significance of interactions between FGF receptors, TWIST1, RUNX2 and downstream osteogenic differentiation genes in normal and abnormal skull growth. Future work will provide similar insights into how these components interact with ephrin-B1, MSX2 and other genes, providing a more complete understanding of the network of gene and protein interactions that control development and growth of the skull vault in the normal fetus and in craniosynostosis.
Acknowledgments
The authors’ work described in this review was supported by grants from Action Research to G.M.M-K. and the Wellcome Trust to A.O.M.W. We thank Dr Philip Anslow for providing the images used in Fig. 2, Dr Rachel Locklin for Fig. 10(B), Mr Steven Wall (Director of the Oxford Craniofacial Unit) for his ongoing collaborations, and Dr Chad Perlyn for helpful comments on the manuscript.
References
- Anderson J, Burns HD, Enriquez-Harris P, Wilkie AOM, Heath JK. Apert syndrome mutations in fibroblast growth factor receptor 2 exhibit increased affinity for FGF ligand. Hum Mol Genet. 1998;7:1475–1483. doi: 10.1093/hmg/7.9.1475. [DOI] [PubMed] [Google Scholar]
- Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of FGFR2 suggests a role for FGF signalling in pre-gastrulation mammalian development. Proc Natl Acad Sci USA. 1998;95:5082–5087. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer HD, Vindevoghel L, Gait MJ, et al. Fibroblast growth factor (FGF) receptor 1-IIIb is a naturally occurring functional receptor for FGfs that is a naturally occurring functional receptor for FGfs that is preferentially expressed in the skin and brain. J Biol Chem. 2000;275:16091–16097. doi: 10.1074/jbc.275.21.16091. [DOI] [PubMed] [Google Scholar]
- Beiman M, Shilo BZ, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10:2993–3002. doi: 10.1101/gad.10.23.2993. [DOI] [PubMed] [Google Scholar]
- Bellus GA, Gaudenz K, Zackai EH, et al. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nature Genet. 1996;14:174–176. doi: 10.1038/ng1096-174. [DOI] [PubMed] [Google Scholar]
- Bennett VR, Brown LK. Myles Textbook for Midwives. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- Bialek P, Kern B, Yang X, et al. A Twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–435. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- Bourgeois P, Bolcato-Bellemin AL, Danse JM, et al. The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre–Chotzen syndrome. Hum Mol Genet. 1998;7:945–957. doi: 10.1093/hmg/7.6.945. [DOI] [PubMed] [Google Scholar]
- Bradley JP, Levine JP, Roth DA, McCarthy JG, Longaker MT. Studies in cranial suture biology. IV. Temporal sequence of posterior frontal cranial suture fusion in the mouse. Plast Reconstr Surg. 1996;98:1039–1045. doi: 10.1097/00006534-199611000-00018. [DOI] [PubMed] [Google Scholar]
- Carver EA, Oram KF, Gridley T. Craniosynostosis in Twist heterozygous mice: a model for Saethre–Chotzen syndrome. Anat Rec. 2002;268:90–92. doi: 10.1002/ar.10124. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chan WY, Tam PPL. A morphological and experimental study of the mesencephalic neural crest cells in the mouse embryo using wheat germ agglutinin–gold conjugate as the cell marker. Development. 1988;102:427–442. doi: 10.1242/dev.102.2.427. [DOI] [PubMed] [Google Scholar]
- Chen L, Li D, Li C, Engl A, Deng CX. A Series 250Trp substitution in mouse fibroblast growth factor receptor 2 (Fgfr2) results in craniosynostosis. Bone. 2003;33:169–178. doi: 10.1016/s8756-3282(03)00222-9. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- Chong LD, Park EK, Latimer E, Friesel R, Daar IO. Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol Cell Biol. 2000;20:724–734. doi: 10.1128/mcb.20.2.724-734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM., Jr Craniofrontonasal dysplasia. Birth Defects. 1979;15(5B):85–89. [PubMed] [Google Scholar]
- Cohen MM., Jr . Epidemiology of craniosynostosis. In: Cohen MM Jr, MacLean RE, editors. Craniosynostosis. 2nd edition. New York: Oxford University Press; 2000. pp. 112–118. [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwan DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1–EphB interactions. Dev Cell. 2003;5:217–230. doi: 10.1016/s1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Corliss CE. Patten's Human Embryology. New York: McGraw-Hill; 1976. [Google Scholar]
- Creuzet S, Couly G, Vincent C, le Douarin NM. Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development. 2002;129:4301–4313. doi: 10.1242/dev.129.18.4301. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- El Ghouzzi V, LeMerrer M, Perrin-Schmidt F, et al. Mutations of the TWIST gene in the Saethre–Chotzen syndrome. Nat Genet. 1997;15:42–46. doi: 10.1038/ng0197-42. [DOI] [PubMed] [Google Scholar]
- El Ghouzzi V, Legeai-Mallet L, Benoist-Lasselin C, et al. Mutations in the basic domain and the loop-helix II junction of TWIST abolish DNA binding in Saethre–Chotzen syndrome. FEBS Lett. 2001;492:112–118. doi: 10.1016/s0014-5793(01)02238-4. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Monsonego-Ornan E, Pines M, Antonopoulou I, Morriss-Kay GM, Lonai P. The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development. 2002;129:3783–3793. doi: 10.1242/dev.129.16.3783. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Horowitz MC, Locklin R, Morriss-Kay GM, Lonai P. A gain-of-function mutation of Fgfr2c demonstrates the roles of this receptor variant in osteogenesis. Proc Natl Acad Sci USA. 2004;101:12555–12560. doi: 10.1073/pnas.0405031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukiishi Y, Morriss-Kay GM. Migration of cranial neural crest cells to the pharyngeal arches and heart in rat embryos. Cell Tissue Res. 1992;268:1–8. doi: 10.1007/BF00338048. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM, Hennekam RCM. Syndromes of the Head and Neck. 4th. New York: Oxford University Press; 2001. [Google Scholar]
- Guenou H, Kaabeche K, Le Mée S, Marie PJ. A role for fibroblast growth factor receptor-2 in the altered osteoblast phenotype induced by Twist haploinsufficiency in the Saethre–Chotzen syndrome. Hum Mol Genet. 2005;14:1429–1439. doi: 10.1093/hmg/ddi152. [DOI] [PubMed] [Google Scholar]
- Hajihosseini MK, Wilson S, De Moerlooze L, Dickson C. A splicing switch and gain-of-function mutation in FgfR2-IIIc hemizygotes causes Apert/Pfeiffer syndrome-like phenotypes. Proc Natl Acad Sci USA. 2001;98:3641–3643. doi: 10.1073/pnas.071586898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajihosseini MK, Heath JK. Expression patterns of fibroblast growth factors-18 and -20 in mouse embryos is suggestive of novel roles in calvarial and limb development. Mech Dev. 2002;113:79–83. doi: 10.1016/s0925-4773(01)00656-6. [DOI] [PubMed] [Google Scholar]
- Hajihosseini MK, Lalioti MD, Arthaud S, Burgar HR, Brown JM, Twigg SR, Wilkie AO, Heath JK. Skeletal development is regulated by fibroblast growth factor receptor 1 signalling dynamics. Development. 2004;131:325–335. doi: 10.1242/dev.00940. [DOI] [PubMed] [Google Scholar]
- Henderson JH, Longaker MT, Carter DR. Sutural bone deposition rate and strain magnitude during cranial development. Bone. 2004;34:271–280. doi: 10.1016/j.bone.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Howard TD, Paznekas WA, Green ED, et al. Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre–Chotzen syndrome. Nat Genet. 1997;15:36–41. doi: 10.1038/ng0197-36. [DOI] [PubMed] [Google Scholar]
- Ibrahimi OA, Zhang F, Eliseenkova AV, Linhardt RJ, Mohammadi M. Proline to arginine mutations in FGF receptors 1 and 3 result in Pfeiffer and Muenke craniosynostosis syndromes through enhancement of FGF binding affinity. Hum Mol Genet. 2004a;13:69–78. doi: 10.1093/hmg/ddh011. [DOI] [PubMed] [Google Scholar]
- Ibrahimi OA, Zhang F, Eliseenkova AV, Itoh N, Linhardt RJ, Mohammadi M. Biochemical analysis of pathogenic ligand-dependent FGFR2 mutations suggests distinct pathophysiological mechanisms for craniofacial and limb abnormalities. Hum Mol Genet. 2004b;13:2313–2324. doi: 10.1093/hmg/ddh235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki S, Wilkie AOM, Heath JK, Ishimaru T, Eto K, Morriss-Kay GM. Fgfr2 and osteopontin domains in the developing skull vault are mutually exclusive and can be altered by locally applied FGF2. Development. 1997;124:3375–3384. doi: 10.1242/dev.124.17.3375. [DOI] [PubMed] [Google Scholar]
- Iseki S, Wilkie AOM, Morriss-Kay GM. Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development. 1999;126:5611–5620. doi: 10.1242/dev.126.24.5611. [DOI] [PubMed] [Google Scholar]
- Iseki S, Yoshida T, Ishikawa I, Eto K. Mouse skull vault bones grow by expanding of their primordia and Bmps are involved in this process. J Anat. 2005;207 in press. [Google Scholar]
- Ishii M, Merrill AE, Chan YS, et al. Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development. 2003;130:6131–6142. doi: 10.1242/dev.00793. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Jabs EW, Muller U, Li X, et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell. 1993;75:443–450. doi: 10.1016/0092-8674(93)90379-5. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Williams LT. Structural and functional diversity of the FGF receptor multigene family. Ad Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Johnson D, Iseki I, Wilkie AOM, Morriss-Kay GM. Expression patterns of Twist and Fgfr1-2 and -3 in the developing mouse coronal suture suggest a key role for Twist in suture initiation and biogenesis. Mech Dev. 2000;91:341–345. doi: 10.1016/s0925-4773(99)00278-6. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes and pattern formation in the branchial region of the vertebrate head. Trends Genet. 1993;9:106–112. doi: 10.1016/0168-9525(93)90203-t. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA. Clonal analysis of cell fate during gastrulation and early neurulation in the mouse. Ciba Fnd Symp. 1992;165:3–21. doi: 10.1002/9780470514221.ch2. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu J, Colvin JS, Ornitz DJ. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or Tgfbr2. Nat Genet. 2005;37:275–279. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- Ma L, Golden S, Wu L, Maxson R. The molecular basis of Boston-type craniosynostosis: the Pro148-His mutation in the N-terminal arm of the MSX2 homeodomain stabilises DNA binding without altering nucleotide sequence preferences. Hum Mol Genet. 1996;5:1915–1920. doi: 10.1093/hmg/5.12.1915. [DOI] [PubMed] [Google Scholar]
- Mathijssen IMJ, van Splunder J, Vermeij-Keers C, et al. Tracing craniosynostosis to its developmental stage through bone center displacement. J Craniofac Genet Dev Biol. 1999;19:57–63. [PubMed] [Google Scholar]
- Miki T, Bottaro DP, Fleming TP, et al. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci USA. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KB, Mood K, Daar IO, Moody SA. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signalling pathways. Dev Cell. 2004;6:55–67. doi: 10.1016/s1534-5807(03)00395-2. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM, Tuckett F, Solursh M. The effects of Streptomyces hyaluronidase on tissue organization and cell cycle time in rat embryos. J Embryol Exp Morph. 1986;98:57–70. [PubMed] [Google Scholar]
- Muenke M, Schell U, Hehr A, et al. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat Genet. 1994;8:269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- Neilson KM, Friesel RE. Constitutive activation of fibroblast growth factor receptor-2 by a point mutation associated with Crouzon syndrome. J Biol Chem. 1995;270:26037–26040. doi: 10.1074/jbc.270.44.26037. [DOI] [PubMed] [Google Scholar]
- Ohbayashi N, Shibayama M, Kurotaki Y, et al. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldridge M, McDonald-McGinn DM, Iseki S, et al. De novo Alu element insertions in FGFR2 identify a distinct pathological basis for Apert syndrome. Am J Hum Genet. 1999;64:446–461. doi: 10.1086/302245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman LA, Adab K, Gakunga PT. Transforming growth factor-beta 2 and TGF-beta 3 regulate fetal rat cranial suture morphogenesis by regulating rates of cell proliferation and apoptosis. Dev Dyn. 2000;219:237–247. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1044>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. Regulation of chondrocyte growth and differentiation by fibroblast growth factor receptor 3. Novartis Found Symp. 2001;232:63–76. doi: 10.1002/0470846658.ch6. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Osumi-Yamashita N, Ninomiya Y, Doi H, Eto K. The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of mouse embryos. Dev Biol. 1994;164:409–419. doi: 10.1006/dbio.1994.1211. [DOI] [PubMed] [Google Scholar]
- Osumi-Yamashita N, Ninomiya Y, Eto K. Mammalian craniofacial embryology in vitro. Int J Dev Biol. 1997;41:187–194. [PubMed] [Google Scholar]
- Partanen J, Schwartz L, Rossant J. Opposite phenotypes of hypomorphic and Y766 phosphorylation site mutations reveal a function for Fgfr1 in anteroposterior patterning of mouse embryos. Genes Dev. 1998;12:2332–2344. doi: 10.1101/gad.12.15.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of EPH receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Pratt RM, Larsen MA, Johnston MC. Migration of cranial neural crest cells in a cell-free hyaluronate-rich matrix. Dev Biol. 1975;44:298–305. doi: 10.1016/0012-1606(75)90400-5. [DOI] [PubMed] [Google Scholar]
- Reardon W, Winter RM, Rutland P, Pulleyn LJ, Jones BM, Malcolm S. Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nat Genet. 1994;14:174–176. doi: 10.1038/ng0994-98. [DOI] [PubMed] [Google Scholar]
- Renier D, Sainte-Rose C, Marchac D, Hirsch JF. Intracranial pressure in craniostenosis. J Neurosurg. 1982;57:370–377. doi: 10.3171/jns.1982.57.3.0370. [DOI] [PubMed] [Google Scholar]
- Rice DPC, Åberg T, Chan Y-S, et al. Integration of FGF and TWIST in calvarial bone and suture development. Development. 2000;127:1845–1855. doi: 10.1242/dev.127.9.1845. [DOI] [PubMed] [Google Scholar]
- Robertson SC, Meyer AN, Hart KC, Galvin BD, Webster MK, Donoghue DJ. Activating mutations in the extracellular domain of the fibroblast growth factor receptor 2 function by disruption of the disulphide bond in the third immunoglobulin-like domain. Proc Natl Acad Sci USA. 1998;95:4567–4572. doi: 10.1073/pnas.95.8.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberte E, Wood HB, Morriss-Kay GM. Prorhombomeric subdivision of the mammalian embryonic hindbrain: is it functionally meaningful? Int J Dev Biol. 1997;41:213–222. [PubMed] [Google Scholar]
- Rutland P, Pulleyn LJ, Reardon W, et al. Identical mutations in the FGFR2 gene cause both Pfeiffer and Crouzon syndrome phenotypes. Nat Genet. 1995;9:173–176. doi: 10.1038/ng0295-173. [DOI] [PubMed] [Google Scholar]
- Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806–818. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Shishido E, Higashijima S-I, Emory Y, Saigo K. Two FGF-receptor homologues of Drosophila: one is expressed in mesodermal primordium in early embryos. Development. 1993;117:751–761. doi: 10.1242/dev.117.2.751. [DOI] [PubMed] [Google Scholar]
- Soo K, O'Rourke MP, et al. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev Biol. 2002;247:251–270. doi: 10.1006/dbio.2002.0699. [DOI] [PubMed] [Google Scholar]
- Tan S-S, Morriss-Kay GM. Analysis of cranial neural crest cell migration and early fates in postimplantation rat chimaeras. J Embryol Exp Morph. 1986;98:21–58. [PubMed] [Google Scholar]
- Thisse B, el Messal M, Perrin-Schmitt F. The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987;15:3439–3453. doi: 10.1093/nar/15.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GPL, Wilkie AOMW, Richards PG, Wall SA. FGFR3 P250R mutation increases the risk of reoperation in apparent ‘non-syndromic’ coronal craniosynostosis. J Craniofac Surg. 2005;16:347–352. doi: 10.1097/01.scs.0000157024.56055.f2. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Krumlauf R. Patterning the cranial neural crest: hindbrain segmentation and Hox gene plasticity. Nat Rev Neurosci. 2000;1:116–124. doi: 10.1038/35039056. [DOI] [PubMed] [Google Scholar]
- Twigg S, Kan R, Babbs C, et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci USA. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SM, Greenwald JA, Nacamuli RP, et al. Regional dura mater differentially regulates osteoblast gene expression. J Craniofac Surg. 2003a;14:363–370. doi: 10.1097/00001665-200305000-00015. [DOI] [PubMed] [Google Scholar]
- Warren SM, Brunet LJ, Harland RM, Economides AN, Longaker MT. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003b;422:625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- Wieland I, Jacubiczka S, Muschke P, et al. Mutations of the ephrin-B1 gene cause craniofrontonasal syndrome. Am J Hum Genet. 2004;74:1209–1215. doi: 10.1086/421532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie AOM, Slaney SF, Oldridge M, et al. Apert syndrome results from localised mutations of FGFR2 and is allelic with Crouzon syndrome. Nat Genet. 1995;9:165–172. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]
- Wilkie AOM. Craniosynostosis: genes and mechanisms. Hum Mol Genet. 1997;6:1647–1656. doi: 10.1093/hmg/6.10.1647. [DOI] [PubMed] [Google Scholar]
- Wilkie AOM. Bad bones, absent smell, selfish testes: the pleiotropic consequences of human FGF receptor mutations. Cytokine Growth Factor Rev. 2005;16:187–203. doi: 10.1016/j.cytogfr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Xu Q, Mellitzer G, Wilkinson DG. Roles of Eph receptors and ephrins in segmental patterning. Philos Trans R Soc Lond B Biol Sci. 2000;355:993–1002. doi: 10.1098/rstb.2000.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Herr AB, Waksman G, Ornitz DM. Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc Natl Acad Sci USA. 2000;97:14536–14541. doi: 10.1073/pnas.97.26.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Yamagouchi TP, Harpal K, Henkemeyer M, Rossant J. Fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- Zhou YX, Xu X, Chen L, Li C, Brodie SG, Deng CX. A Pro250Arg substitution in mouse Fgfr1 causes increased expression of Cbfa1 and premature fusion of calvarial sutures. Hum Mol Genet. 2000;9:2001–2008. doi: 10.1093/hmg/9.13.2001. [DOI] [PubMed] [Google Scholar]