Abstract
Contemporary studies of vertebrate cranial development document the essential role played by the embryonic neural crest as both a source of adult tissues and a locus of cranial form and patterning. Yet corresponding and basic features of cranial evolution, such as the extent of conservation vs. variation among species in the contribution of the neural crest to specific structures, remain to be adequately resolved. Investigation of these features requires comparable data from species that are both phylogenetically appropriate and taxonomically diverse. One key group are amphibians, which are uniquely able to inform our understanding of the ancestral patterns of ontogeny in fishes and tetrapods as well as the evolution of presumably derived patterns reported for amniotes. Recent data support the hypothesis that a prominent contribution of the neural crest to cranial skeletal and muscular connective tissues is a fundamental property that evolved early in vertebrate history and is retained in living forms. The contribution of the neural crest to skull bones appears to be more evolutionarily labile than that of cartilages, although significance of the limited comparative data is difficult to establish at present. Results underline the importance of accurate and reliable homology assessments for evaluating the contrasting patterns of derivation reported for the three principal tetrapod models: mouse, chicken and frog.
Keywords: Bombina, bone, muscle, skull, Xenopus
Introduction
The head has occupied a central position in biological research for nearly 200years. Changes in cranial anatomy underlie virtually every major adaptive transition in the history of vertebrate animals, and discovery of the functional correlates of such changes offers unique and valuable insights into the causes and consequences of vertebrate evolution (Hanken & Hall, 1993; Schwenk, 2000). In the applied arena, the head is the locus of numerous birth defects, congenital malformations and other clinical syndromes, and detailed understanding of the causes and possible treatment of these diseases may enhance human health and longevity (Thorogood, 1997).
Among the most exciting discoveries made in recent years are those that deal with the development and evolution of the skull and the complex of other cranial tissues with which it is so intimately associated. Perhaps predominant among these is the gradual recognition, and now widespread acceptance, of the essential role played by the embryonic neural crest, both as a material source of many adult cranial tissues and as a locus of cranial form and patterning (Hall, 1999; Le Douarin & Kalcheim, 1999; Santagati & Rijli, 2003; Schneider & Helms, 2003). Whereas results of contemporary studies validate many classical observations regarding cranial development and evolution, others are forcing reassessment and re-evaluation of widely held assumptions and conventions. This is especially true with regard to the role of the neural crest, which simply did not figure in orthodox and authoritative treatments of the skull well into the twentieth century (e.g. de Beer, 1937). Indeed, the potentially predominant role of the neural crest in cranial development and evolution only began to be generally appreciated in the 1950s (e.g. Hörstadius, 1950) and it was not widely recognized for another 30 years, following the landmark publications by Northcutt & Gans (1983) and Gans & Northcutt (1983).
Comprehensive understanding of the role of the neural crest in vertebrate cranial development and evolution requires comparable data from a set of phylogenetically appropriate and taxonomically diverse species. A key group in this regard are amphibians. Reflecting their position among living vertebrates as an evolutionary ‘fulcrum’ between primitively aquatic species (fishes) and the remaining, and highly derived, tetrapods (amniotes), amphibians may potentially inform our understanding of the basal (primitive) pattern of cranial development in tetrapods, as well as the evolution of the presumably derived patterns reported for amniotes. In this report, we summarize several recent studies that help to define the basic role of the neural crest in cranial development in amphibians, and especially anurans (frogs). These data reveal certain features of the neural crest to be highly conserved among major clades, whereas others appear evolutionarily labile. They also underline the importance of accurate and reliable homology assessments for making inferences regarding the evolution of both cranial development and adult anatomy. Indeed, currently accepted homologies of cranial roofing bones in at least some major taxa may need to be reassessed in light of recent discoveries that have yielded contrasting patterns of neural crest derivation among the three principal tetrapod models: mouse, chicken and frog.
Musculoskeletal derivatives in amphibians
Neural crest contribution to cranial cartilages
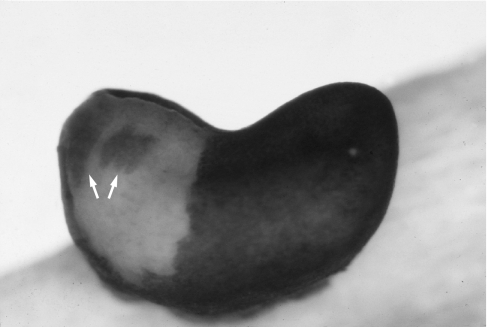
Anuran amphibians were among the first vertebrates to be assessed for neural crest derivation of the cartilaginous skull (Hörstadius, 1950). Stone (1929) produced a comprehensive fate map depicting neural crest contributions to the larval skull in the pickerel frog, Rana palustris. Stone inferred neural crest contributions in two ways. First, he followed migration of cranial neural crest cells to eventual sites of chondrogenesis in normal, untreated embryos analysed with standard histology. Such an analysis of untreated embryos is possible because in Rana, as in many amphibian species, intact neural crest cells may be distinguished from other cell types by pigmentation and gross cytological features, at least during initial stages of migration (Fig. 1). Secondly, he systematically ablated adjacent regions of the cranial neural folds and recorded the resulting deletions in the larval skull. The basic conclusion of this work is that neural crest is the chief cellular source of the cartilaginous cranium. This includes two pairs of larval-specific jaw elements, the supra- and infrarostral cartilages, which are unique to anurans among vertebrates. Indeed, only a few cranial cartilages do not receive a direct contribution from the neural crest. These include the otic capsules, the ‘basioccipital plate’ (= basal plate; de Beer, 1937) and the adjacent, caudal portions of the paired trabecular cartilages, as well as the median basihyal and second basibranchial cartilages in the hyobranchial skeleton.
Fig. 1.
Live embryo of the Oriental fire-bellied toad, Bombina orientalis, with ectoderm removed from the left rostral side. Streams of pigmented cranial neural crest cells are migrating ventrally alongside the head (arrows).
The nature and extent of neural crest contribution to the skull in larval anurans have been re-examined in recent years by using various cell-labelling techniques that avoid the potential limitations of crest ablation, which include diminished embryo survivorship and regeneration of premigratory neural crest within the neural fold (McKee & Ferguson, 1984; Langille & Hall, 1988; Scherson et al. 1993; Vaglia & Hall, 1999). Sadaghiani & Thiébaud (1987) utilized naturally occurring differences in nuclear staining pattern between two species of African clawed frogs (Xenopus) to assess neural crest derivation of various larval structures in interspecific chimaeras produced by embryonic grafting. Although their results regarding the skull were in general consistent with those of Stone (1929), cranial derivatives were not examined in similar detail, and Stone's work remained the most comprehensive account of neural crest derivation of the anuran skull well into the 1990s.
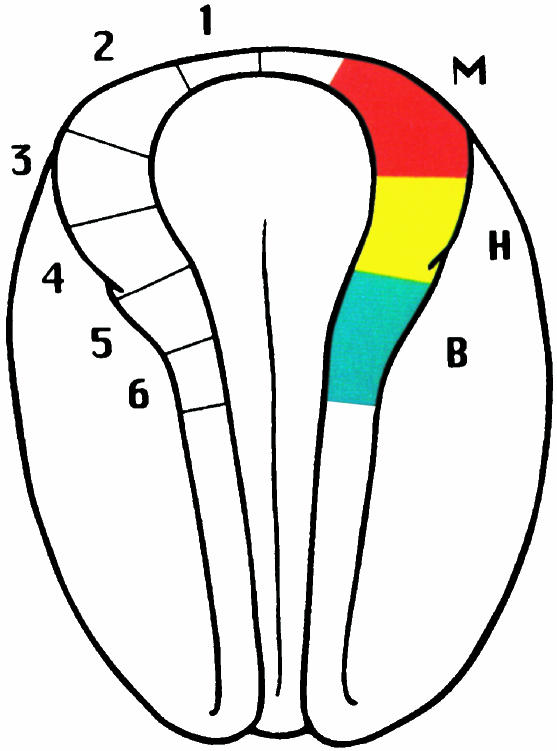
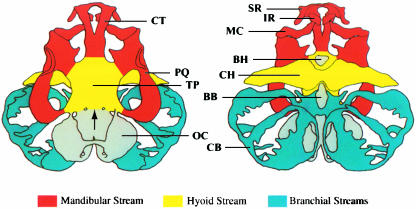
A more detailed analysis was reported by Olsson & Hanken (1996), who mapped neural crest contribution to the cartilaginous larval skull of the Oriental fire-bellied toad, Bombina orientalis, using Dil, a fluorescent vital dye. By injecting Dil into defined regions of premigratory neural crest within the cranial neural folds (Fig. 2), contributions to individual cartilages from each of the three cranial migratory crest streams could be readily documented (Fig. 3) Results from this study both validate and extend those of Stone (1929) with Rana. Overall patterns of neural crest derivation of cranial cartilages are virtually identical in the two species: cartilages derived from neural crest in one species are the same as those derived from neural crest in the other (Table 1). Indeed, this similarity even extends to the apparent absence of crest contribution to two tiny ventral midline cartilages, the basihyal and basibranchial; the rest of the hyobranchial skeleton is neural-crest-derived in both species. This identity between species suggests that the pattern of neural crest derivation of the cartilaginous skull is highly conserved during anuran evolution, including in this comparison two genera that probably shared a common ancestor no more recently than the Jurassic period, at least 144 million years ago. Moreover, partitioning neural crest contributions among different cranial migratory streams, which Stone was unable to do, revealed the existence and location of cryptic boundaries between adjacent crest-derived territories(Fig. 3).
Fig. 2.
Neural plate-stage embryo of Bombina orientalis depicting Dil injection sites within the neural fold (left) and the location of neural crest cells that contribute to the three cranial migratory streams (right). Dorsal view; anterior is at the top. Abbreviations: M, mandibular; H, hyoid; B, branchial. Reproduced with permission from Olsson & Hanken (1996).
Fig. 3.
Neural crest derivation of the larval cartilaginous skull and hyobranchial skeleton of Bombina orientalis seen in dorsal (left) and ventral views. Colours correspond to the three crest migratory streams depicted in Fig. 2; noncrest-derived cartilages are shaded grey. The arrowhead points to the boundary between crest- and noncrest-derived portions of the floor of the braincase (neurocranium). Anterior is at the top. Abbreviations: BB, basibranchial; BH, basihyal; CB, ceratobranchials I–IV; CH, ceratohyal; CT, trabecular horn (cornu trabecula); IR, infrarostral; MC, Meckel's cartilage; OC, otic capsule; PQ, palatoquadrate; SR, suprarostral; TP, trabecular plate. Reproduced with permission from Olsson & Hanken (1996).
Table 1.
Neural crest contribution to the cartilaginous skull of larval anurans. A ‘plus’ (+) denotes cartilages that are derived from neural crest; the embryonic origin of remaining cartilages remains unknown (–), although it is presumed to be from cranial mesoderm. A ‘±’ denotes dual origin. Although these species belong to distantly related families, and the corresponding analyses were conducted nearly 70 years apart and by using very different analytical methods, the two patterns of derivation are nearly identical. Data for Bombina and Rana are based on Olsson & Hanken (1996) and Stone (1929), respectively. After Hanken (1999)
| Cartilage | Bombina orientalis | Rana palustris |
|---|---|---|
| Suprarostral | + | + |
| Infrarostral | + | + |
| Meckel's | + | + |
| Palatoquadrate | + | + |
| Ceratohyal | + | + |
| Ceratobranchials I–IV | + | + |
| Trabecular cartilage/plate | + | ± |
| ‘Basioccipital plate’ (= basal plate*) | − | − |
| Otic (auditory) capsule | − | − |
| Basihyal | − | − |
| Basibranchial (second basibranchium) | − | − |
Neural crest contribution to muscle
Historically, neural crest was never attributed a significant role in cranial muscle development in any vertebrate. This is especially the case for amphibians, which were favoured subjects of classical studies of both neural crest biology and cranial muscle development (reviewed in Edgeworth, 1935; Hall & Hörstadius, 1988). None of this early work claimed a direct neural crest contribution to cranial musculature.
Neural crest contribution to the connective tissue component of many cranial muscles was first reported in birds relatively recently (Le Lièvre & Le Douarin, 1975). This important discovery has since been validated in numerous studies, which also have revealed a corresponding prominent and direct role by neural crest in mediating muscle patterning during embryogenesis (e.g. Noden, 1983a,b; Couly et al. 1992; Graham et al. 1996; Köntges & Lumsden, 1996). Initially, authors were careful to restrict these important properties of cranial muscle development, and their significant implications for cranial organization, to amniote tetrapods. They were subsequently extended to zebrafish, an important fish model (Schilling & Kimmel, 1994). However, this work left unanswered the question of whether a neural crest contribution to cranial muscle development and a direct role in muscle patterning are truly absent in amphibians, as classical studies appear to suggest, or if these features are present but undetected in earlier work. These two scenarios offer contrasting implications for evolution. In the former case, an essential role of neural crest in cranial muscle development and patterning would have been either absent in the common ancestor of bony fishes and tetrapods and then acquired independently in at least some bony fishes and some amniotes, or present in the common ancestor and lost in at least some Recent amphibians. Alternatively, neural crest contribution to cranial muscle and a corresponding prominent role in muscle patterning would be fundamental characteristics of cranial development that evolved early in the history of the vertebrate lineage and are retained in descendant groups. The latter scenario would represent a further example of the evolutionary conservatism of the contribution of the neural crest to vertebrate cranial tissues and their development, whereas the former would suggest that the neural crest and its functional roles are evolutionarily labile.
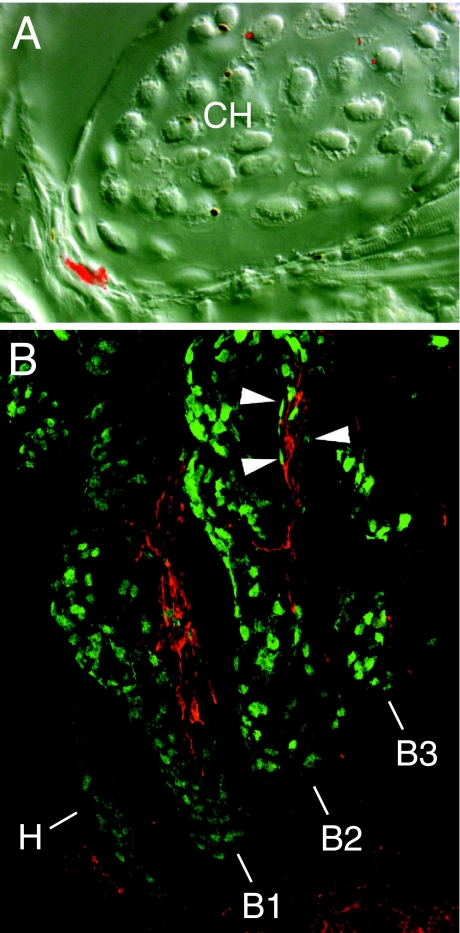
This question has been answered in the last few years in a series of studies by Olsson and colleagues (Olsson et al. 2000, 2001; Ericsson et al. 2004). Using a combination of vital labelling and ablation of premigratory neural crest in neural plate-stage embryos, they provide unequivocal and direct evidence of neural crest contribution to the connective tissue components of several cranial muscles in both frogs (B. orientalis) and salamanders (Ambystoma mexicanum; Fig. 4). In terms of comparative biology, the main conclusion from these studies is that a direct contribution by neural crest to cranial muscle and the corresponding prominent role of neural crest in the development of musculoskeletal patterning are primitive properties shared by jawed vertebrates (or at least the common ancestor of amniotes, Recent amphibians and bony fishes), which evolved early in the history of vertebrates and are retained in living descendants. The full extent of neural crest contribution to cranial muscles in amphibians remains to be assessed, however, with the possibility of more subtle differences from the pattern of derivation observed in amniotes.
Fig. 4.
Neural crest derivation of the connective-tissue components of cranial muscles in living amphibians (frogs and salamanders). (A) Cross-section through the ceratohyal cartilage (CH) and associated muscle attachments of a larval frog (Bombina orientalis) following embryonic labelling of premigratory cranial neural crest with Dil. The bright red area at lower left is within the tendinous insertion of a cranial muscle. Lateral is left; dorsal is up. Reproduced with permission from Olsson & Hanken (1996). (B) Branchial region of a stage-36 axolotl embryo (Ambystoma mexicanum). Neural crest-derived cells expressing green fluorescent protein (GFP; green) are present in the dermis of branchial arches 1–3 (B1, B2, B3). Other crest-derived cells (arrowheads) surround muscle anlage within each arch (red). Lateral view; anterior is to the left. Additional abbreviation: H, hyoid arch. Reproduced with permission from Ericsson et al. (2004).
Neural crest contribution to bone
A prominent neural crest contribution to the vertebrate osteocranium is widely accepted (e.g. Schilling, 1997). Surprisingly, empirical evidence of this developmental relationship, as provided by detailed labelling or ablation studies, has been obtained for only two species. Both species are widely used amniote models, the domestic chicken and the laboratory mouse (Le Lièvre, 1978; Noden, 1978; Couly et al. 1993; Jiang et al. 2002; Ishii et al. 2003). In one of these species (chicken), the methodology employed has allowed the relative contributions of both neural crest and cranial mesoderm to be mapped directly and the contributions of neural crest to be defined according to individual migratory streams (e.g. Köntges & Lumsden, 1996). Yet contemporary fate maps for the chicken skull derived by different laboratories differ in important respects. Depending on which of two basic maps is accepted, the overall pattern of crest-derived vs. noncrest-derived elements differs to a greater or lesser extent from that published for the mouse and inferred for other vertebrates (Morriss-Kay, 2001; Santagati & Rijli, 2003; Matsuoka et al. 2005), and in neither comparison are chicken and mouse maps identical (see below).
Comparable data from other vertebrate groups, but especially amphibians, are highly desirable because of their value in interpreting the patterns of cranial derivation obtained for chicken and mouse and for inferring the likely ancestral condition(s) of both early vertebrates and tetrapods. Yet there have been few attempts to derive such data. In part this can be attributed to the much greater attention devoted to amniote models in craniofacial research. However, this can also be attributed to the difficulties posed by those vertebrates, including most living amphibians, that possess a complex (biphasic) life history in which the definitive adult form does not develop until after metamorphosis. In many species of fishes and amphibians, metamorphosis may not commence for weeks, months or even years after hatching.
These difficulties are perhaps most extreme in metamorphosing anurans, in which bone differentiation is an entirely post-embryonic event (Hanken & Hall, 1988): embryonic and larval skulls are entirely cartilaginous, cranial bones do not begin to appear until metamorphosis and the full (adult) complement of skull bones is not present until after metamorphosis is complete. This delay in cranial ossification, relative to that of most other vertebrates in which skull bone formation commences during embryogenesis, has long posed a technical challenge: it has proven difficult, if not impossible, to label embryonic neural crest cells in such a way that the label remains stable and visible over the prolonged interval between crest migration and bone differentiation. Moreover, because cranial ossification in metamorphosing frogs commences well after the onset of feeding, neural crest ablation methods, which have been used effectively to map neural crest contribution to the cartilaginous larval skull in these same species (see above), are impractical. Ablated embryos typically survive only a few days following surgery and never attain developmental stages when bones would normally form.
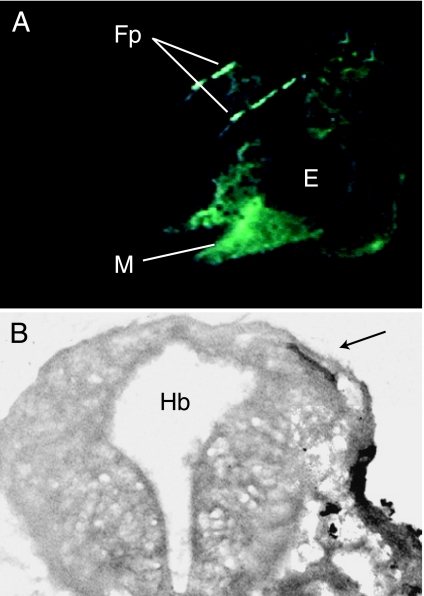
Our laboratory is employing new techniques for labelling neural crest in amphibians that largely circumvent the technical limitations of earlier methods. These techniques have enabled us to begin to assess reliably the full extent of neural crest contribution to cranial bones in frogs. Initial efforts utilized chimeric Xenopus embryos produced by grafting cranial neural folds from donors labelled with green fluorescent protein (GFP) into unlabelled hosts (Carl et al. 2000). Labelled donor embryos were produced by injecting zygotes with GFP mRNA shortly after fertilization. The GFP remained visible in donor-derived cells throughout the larval period and successfully labelled early ossification centres of the adult frontoparietal bone, which is among the first skull bones to form during anuran metamorphosis (Hanken & Hall, 1988; Fig. 5). This method, however, proved difficult to apply to the large number of embryos that are required to conduct a comprehensive assessment of the contribution of individual neural crest streams to the entire complement of skull bones.
Fig. 5.
Neural crest derivation of the frontoparietal bone in Xenopus laevis, assessed by using green fluorescent protein (GFP). (A) Head of a living tadpole (larva) that as an embryo received bilateral grafts of GFP-labelled cranial neural crest. Paired, splint-like frontoparietal bones (Fp) are brightly labelled in the cranial roof when viewed with fluorescent illumination. Other neural crest derivatives, such as the cartilaginous mandible (M), are also prominently labelled. Lateral view; anterior is to the left. Modified from Carl et al. (2000). (B) Cross-section through the head of a tadpole that as an embryo received a unilateral graft of GFP-labelled cranial neural crest. The frontoparietal bone on the grafted side is brightly labelled (arrow). Additional abbreviations: E, eye; Hb, hindbrain.
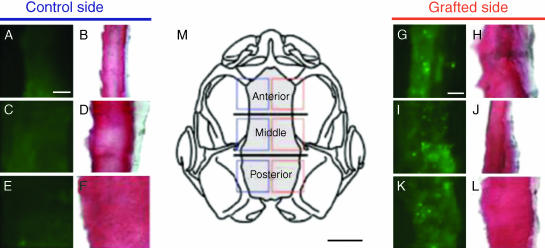
We next chose to label donor Xenopus embryos with fluorescein dextran, an indelible, fixative-stable cell marker that is both suitable for use over long developmental periods and readily applied to large numbers of embryos (Gross & Hanken, 2004, 2005). Focal grafting of labelled donor neural crest from individual migratory streams into unlabelled host embryos confirmed and extended initial observations regarding derivation of the frontoparietal bone obtained with GFP (see above): neural crest contributes to the frontoparietal bone along its entire length; all three cranial neural crest streams contribute to the same bone; and contributions from the three streams are regionally distinct and arrayed in a rostrocaudal sequence that reflects the order of these streams in the neural fold (Fig. 6). Preliminary evidence of neural crest contribution to three additional skull bones (nasal, parasphenoid and squamosal) was also obtained.
Fig. 6.
Neural crest derivation of the frontoparietal bone in Xenopus laevis, assessed by using fluorescein dextran. Fluorescent (A, C, E and G, I, K) and bright-field (B, D, F and H, J, L) images of cryosections through the frontoparietal bone in chimeric froglets, which earlier received labelled unilateral grafts of cranial neural crest. Frontal sections; anterior at top. Successive panels correspond to squares that are superimposed on the skull outlined in M (dorsal view; frontoparietal bone grey). Grafting labelled mandibular neural crest yields brightly labelled bone matrix in the rostral frontoparietal (G, green punctate clusters); labelling of bone matrix is confirmed by TriChrome staining in the following section (H, red). Similarly, intermediate (I, J) and caudal (K, L) portions of the bone are labelled by cells derived from hyoid and branchial crest stream grafts, respectively. Fluorescent marker is absent on the control (left) side of the skull (A–F), which did not receive labelled grafts. Horizontal lines in M demarcate three equal-sized zones in the frontoparietal bone, which were defined for the purposes of analysis. Scale bars: A–L, 25 mm; M, 1 mm. Reproduced with permission from Gross & Hanken (2005).
The above analyses are being extended with the use of a newly derived strain of transgenic Xenopus as the source of labelled donor cells for chimeric grafting experiments. The transgenic construct contains a fully constitutive promoter gene, which drives expression of GFP in all cells at all developmental stages. Preliminary results are very encouraging, and we are confident that this labelling method will both enable us to map the full extent of neural crest contribution to the anuran osteocranium and facilitate additional detailed studies of cranial cartilage and other derivatives (Gross & Hanken, unpublished data).
All these studies are designed to assess directly the contribution of neural crest to individual cranial bones in frogs. The possible contribution of cranial mesoderm to the same elements remains to be assessed in similar fashion. Until these additional data are in hand, neural crest cannot be interpreted properly as the sole embryonic source of any bone.
Discussion
Recent data from amphibians, as summarized above, combined with results of contemporary studies of fishes, reptiles and other non-standard amniote models are gradually enabling more reliable and rigorous assessments of the comparative biology of the neural crest, especially as it applies to cranial evolution in vertebrates (e.g. Hou & Takeuchi, 1994; Peterson et al. 1996; Hirata et al. 1997; Horigome et al. 1999; Falck et al. 2000; Kuratani & Horigome, 2000; Kimmel et al. 2001; Vaglia & Smith, 2003). In general, basic features of neural crest biology appear to be highly conserved, even stereotypic; broad patterns of neural crest derivation of cranial tissues (e.g. the list of crest- vs. noncrest-derived components) reveal relatively few obvious differences among species. Thus, the initial (and laudable) choice to confine to ‘higher tetrapods’ the neural crest's direct contribution to cranial muscle development when it was first discovered in amniotes (Noden, 1983a) is no longer required, as this important property has now been extended to both frogs and salamanders among living amphibians (see above). The most parsimonious interpretation of existing data suggests that a direct and extensive contribution of neural crest to skeletal and muscular connective tissues (the former including both cartilage and bone) as well as a prominent role of neural crest in musculoskeletal patterning are fundamental properties of cranial development that evolved early in vertebrate history and are retained in living forms.
One important exception to the overall broad similarity among species regarding patterns of neural crest contribution concerns the derivation of the bony skull roof, or cranial vault. In most vertebrates, and in all living tetrapods, the skull roof comprises principally the frontal and parietal bones (either separate or fused), yet recent analyses have yielded contrasting accounts of the embryonic derivation of these bones in different tetrapod species. Experiments using Wnt1-Cre/R26R transgenic mouse embryos document neural crest derivation of the frontal but not of the ossified parietal bones (although the unossified sutural membrane between paired parietals is neural-crest-derived; Morriss-Kay, 2001; Jiang et al. 2002). In chicken, two comprehensive fate maps for the osteocranium derived ostensibly by using the same chick–quail chimera labelling system claim different origins of the same two bones, and neither is identical to the pattern seen in mouse. According to one map, virtually the entire skull roof is neural-crest-derived, including both frontal and parietal bones (Couly et al. 1993; Le Douarin & Kalcheim, 1999), whereas in the other map neural-crest-derived territory is restricted to a relatively small rostral portion of the frontal bone; the rest of the frontal, and all of the parietal, is derived from mesoderm (Le Lièvre, 1978; Noden, 1978). Derivation of the anuran frontoparietal bone, which receives contributions from neural crest along its entire length (Gross & Hanken, 2005), most closely resembles one of the two avian patterns (Couly et al. 1993), although the anuran map is incomplete pending direct assessment of potential contributions from cranial mesoderm.
The evolutionary significance of apparent interspecific differences in derivation of the skull roof is difficult to establish at this time. Until there is consensus regarding the correct pattern of derivation in chicken, comparison with other species is problematic regardless of whether the other species are birds or more distantly related vertebrates. Published data for Xenopus (see above) most closely resemble the avian results in which frontal and parietal bones are derived exclusively from neural crest (Couly et al. 1993). Moreover, both these accounts differ from that in the mouse, in which the parietal bone is derived exclusively from mesoderm. This pattern of similarity and difference among taxa might indicate that amphibians and birds share the primitive tetrapod condition of derivation of the skull roof, which was subsequently altered in the clade leading to mammals. Alternatively, avian results that report a dual neural crest/mesodermal origin of the frontal bone (Noden, 1978) are distinct from results for both Xenopus and mouse. If these avian results are correct, and in the absence of comparable data from additional species of birds, amphibians and mammals, as well as from outgroup taxa (e.g. fishes), then none of these three patterns of derivation can be favoured at this time over the remaining two as the likely primitive tetrapod condition.
The above interpretation assumes that the neural crest–mesoderm boundary has shifted among bones of the skull roof during vertebrate evolution: for example, from the caudal edge of the parietal bone in birds to the rostral edge of the parietal in mammals. An alternative interpretation is that the neural crest–mesoderm boundary has remained fixed in place and that frontal and parietal bones are identified differently (i.e. non-homologously) in the species involved. Jiang et al. (2002) suggest that the avian ‘frontal’ may in fact represent the fused frontal and parietal bones of reptilian ancestors. This interpretation is attractive in that it would readily reconcile the chicken fate map of Noden (1978), which posits a dual origin of the frontal, with Jiang et al.'s fate map for mouse, which posits contrasting embryonic origins for the mammalian frontal and parietal. In each case, the anterior region (or entire bone) is derived from neural crest, whereas the posterior region is derived from mesoderm. However, this interpretation also implies that other bones as currently identified in the avian skull may not be homologous with bones of the same name in other vertebrates; for example, the avian ‘parietal’ might actually be homologous to the postparietal of other vertebrates. A similar proposal would reconcile the seemingly contrasting patterns of embryonic derivation of the skull roof between mouse and Xenopus, i.e. the anuran ‘frontoparietal’ is homologous with the frontal of other tetrapods, including mammals. Interestingly, the same proposal has been offered previously, based on comparative patterns of ossification at metamorphosis among extant frogs (Eaton, 1942; Sedra, 1948), although it is rejected on the same grounds by most authors (de Beer, 1937; Griffiths, 1954; Trueb, 1973). Questions regarding the homology of the cranial vault have spurred some of the most lively and contentious debates in the history of vertebrate anatomy and palaeontology (e.g. Jollie, 1981; Bjerring, 1995). For the most part, these questions are regarded as having been answered long ago, but the debates may need to be revived in light of results from modern studies of embryonic cell lineage and cranial fate mapping, which are forcing reassessment of many widely held assumptions and conventions.
Interspecific comparisons in evolutionary morphology require robust understanding of homology, and developmental data have tremendous potential to inform such assessments among similar structures. Yet when potential variation in developmental parameters is the central subject of analysis, one must look to other kinds of data for help in deriving critical statements of similarity and difference. A more complete delineation of the degree of evolutionary conservatism vs. lability of the pattern of neural crest contribution to cranial ontogeny in vertebrates awaits additional comparative data of many kinds from a wide array of taxa with diverse morphologies and developmental modes.
Acknowledgments
Research support was provided by the US National Science Foundation (grant no. EF-0334846, AmphibiaTree), the Milton Fund of Harvard University and Sigma Xi. Tim Carl and Lennart Olsson graciously provided permission to reproduce images from their published work. Revisions suggested by two anonymous reviewers greatly improved the manuscript.
References
- de Beer GR. The Development of the Vertebrate Skull. Oxford: Clarendon Press; 1937. [Google Scholar]
- Bjerring HC. The parietal problem: how to cut this Gordian knot. Acta Zool (Stockholm) 1995;76:193–203. [Google Scholar]
- Carl TF, Vourgourakis Y, Klymkowsky MK, Hanken J. Green fluorescent protein used to assess cranial neural crest derivatives in the frog, Xenopus laevis. In: Jacobson CO, Olosson L, editors. Regulatory Processes in Development: the Legacy of Sven Hörstadius (1898–1996) Vol. 76. London: Portland Press; 2000. pp. 167–172. [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail–chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of the skull in higher vertebrates: a study in quail–chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Eaton TH., Jr Are ‘frontoparietal’ bones in frogs actually frontals? J Wash Acad Sci. 1942;32:151–153. [Google Scholar]
- Edgeworth FH. The Cranial Muscles of Vertebrates. Cambridge: Cambridge University Press; 1935. [Google Scholar]
- Ericsson R, Cerny R, Falck P, Olsson L. Role of cranial neural crest cells in visceral arch muscle positioning and morphogenesis in the Mexican axolotl, Ambystoma mexicanum. Dev Dynam. 2004;231:237–247. doi: 10.1002/dvdy.20127. [DOI] [PubMed] [Google Scholar]
- Falck P, Joss J, Olsson L. Cranial neural crest cell migration in the Australian lungfish, Neoceratodus forsteri. Evol Dev. 2000;2:179–185. doi: 10.1046/j.1525-142x.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–274. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Graham A, Köntges G, Lumsden A. Neural crest apoptosis and the establishment of craniofacial pattern: an honorable death. Mol Cell Neurosci. 1996;8:76–83. doi: 10.1006/mcne.1996.0046. [DOI] [PubMed] [Google Scholar]
- Griffiths I. On the nature of the fronto-parietal in Amphibia, Salientia. Proc Zool Soc Lond. 1954;123:781–792. [Google Scholar]
- Gross JB, Hanken J. Use of fluorescent dextran conjugates as a long-term marker of the osteogenic neural crest in frogs. Dev Dynam. 2004;230:100–106. doi: 10.1002/dvdy.20036. [DOI] [PubMed] [Google Scholar]
- Gross JB, Hanken J. Cranial neural crest contributes to the bony skull vault in adult Xenopus laevis: insights from cell labeling studies. J Exp Zool (Mol Dev Evol) 2005;304B:1–8. doi: 10.1002/jez.b.21028. [DOI] [PubMed] [Google Scholar]
- Hall BK, Hörstadius S. The Neural Crest. Oxford: Oxford University Press; 1988. [Google Scholar]
- Hall BK. The Neural Crest in Development and Evolution. New York: Springer; 1999. [Google Scholar]
- Hanken J, Hall BK. Skull development during anuran metamorphosis. I. Early development of the first three bones to form – the exoccipital, the parasphenoid, and the frontoparietal. J Morphol. 1988;195:247–256. doi: 10.1002/jmor.1051950303. [DOI] [PubMed] [Google Scholar]
- Hanken J, Hall BK, editors. The Skull, Vol. 3: Functional and Evolutionary Mechanisms. Chicago: University of Chicago Press; 1993. [Google Scholar]
- Hanken J. Larvae in amphibian development and evolution. In: Hall BK, Wake MH, editors. The Origin and Evolution of Larval Forms. San Diego: Academic Press; 1999. pp. 61–108. [Google Scholar]
- Hirata M, Ito K, Tsuneki K. Migration and colonization patterns of HNK-1 immunoreactive neural crest cells in lamprey and swordtail embryos. Zool Sci. 1997;14:305–312. [Google Scholar]
- Horigome N, Myojin M, Ueki T, Hirano S, Aizawa S, Kuratani S. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Dev Biol. 1999;207:287–308. doi: 10.1006/dbio.1998.9175. [DOI] [PubMed] [Google Scholar]
- Hörstadius S. The Neural Crest: its Properties and Derivatives in the Light of Experimental Research. London: Oxford University Press; 1950. [Google Scholar]
- Hou L, Takeuchi T. Neural crest development in reptilian embryos, studied with monoclonal antibody HNK-1. Zool Sci. 1994;11:423–431. [Google Scholar]
- Ishii M, Merrill AE, Chan YS, et al. Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development. 2003;130:6131–6142. doi: 10.1242/dev.00793. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Jollie MT. Segment theory and the homologizing of cranial bones. Am Nat. 1981;118:785–802. [Google Scholar]
- Kimmel CV, Miller CT, Moens CB. Specification and morphogenesis of the zebrafish larval head skeleton. Dev Biol. 2001;233:239–257. doi: 10.1006/dbio.2001.0201. [DOI] [PubMed] [Google Scholar]
- Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Horigome N. Developmental morphology of branchiomeric nerves in a cat shark, Scyliorhinus torazame, with special reference to rhombomeres, cephalic mesoderm, and distribution patterns of cephalic crest cells. Zool Sci. 2000;17:893–909. [Google Scholar]
- Langille RM, Hall BK. Role of the neural crest in the development of the trabeculae and branchial arches in embryonic sea lamprey, Petromyzon marinus (L.) Development. 1988;102:301–310. [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Le Lièvre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Le Lièvre CS. Participation of neural crest-derived cells in the genesis of the skull in birds. J Embryol Exp Morphol. 1978;47:17–37. [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee GJ, Ferguson MWJ. The effects of mesencephalic neural crest cell extirpation on the development of chick embryos. J Anat. 1984;139:491–512. [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay GM. Derivation of the mammalian skull vault. J Anat. 2001;199:143–151. doi: 10.1046/j.1469-7580.2001.19910143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. The control of avian cephalic neural crest cytodifferentiation. I. skeletal and connective tissues. Dev Biol. 1978;67:296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983a;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983b;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Olsson L, Hanken J. Cranial neural crest migration and chondrogenic fate in the Oriental fire-bellied toad, Bombina orientalis: defining the ancestral pattern of head development in anuran amphibians. J Morphol. 1996;229:105–120. doi: 10.1002/(SICI)1097-4687(199607)229:1<105::AID-JMOR7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Olsson L, Ericsson R, Falck P. Neural crest contributions to cranial muscle fate and patterning in the Mexican axolotl (Ambystoma mexicanum) In: Jacobson CO, Olloson L, editors. Regulatory Processes in Development: the Legacy of Sven Hörstadius (1898–1996) Vol. 76. London: Portland Press; 2000. pp. 159–166. [Google Scholar]
- Olsson L, Falck P, Lopez K, Cobb J, Hanken J. Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Dev Biol. 2001;237:354–367. doi: 10.1006/dbio.2001.0377. [DOI] [PubMed] [Google Scholar]
- Peterson PE, Blankenship TN, Wilson DB, Hendrickx AG. Analysis of hindbrain neural crest migration in the long-tailed monkey (Macaca fascicularis) Anat Embryol. 1996;194:235–246. doi: 10.1007/BF00187134. [DOI] [PubMed] [Google Scholar]
- Sadaghiani B, Thiébaud CH. Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Dev Biol. 1987;124:91–110. doi: 10.1016/0012-1606(87)90463-5. [DOI] [PubMed] [Google Scholar]
- Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806–818. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- Scherson T, Serbedziya G, Fraser S, Bronner-Fraser M. Regulative capacity of the cranial neural tube to form neural crest. Development. 1993;118:1049–1062. doi: 10.1242/dev.118.4.1049. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120:483–494. doi: 10.1242/dev.120.3.483. [DOI] [PubMed] [Google Scholar]
- Schilling TF. Genetic analysis of craniofacial development in the vertebrate embryo. Bioessays. 1997;19:459–468. doi: 10.1002/bies.950190605. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:55–58. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Schwenk K, editor. Feeding: Form, Function, and Evolution in Tetrapod Vertebrates. San Diego: Academic Press; 2000. [Google Scholar]
- Sedra SN. On the homology of certain elements in the skull of Bufo regularis Reuss (Salientia) Proc Zool Soc Lond. 1948;119:633–641. [Google Scholar]
- Stone LS. Experiments showing the role of migrating neural crest (mesectoderm) in the formation of head skeleton and loose connective tissue in Rana palustris. Roux's Arch Entw Mech Org. 1929;118:40–77. doi: 10.1007/BF02108871. [DOI] [PubMed] [Google Scholar]
- Thorogood PV. Embryos, Genes and Birth Defects. London: John Wiley and Sons Ltd; 1997. [Google Scholar]
- Trueb L. Bones, frogs, and evolution. In: Vial JL, editor. Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. Columbia: University of Missouri Press; 1973. pp. 65–132. [Google Scholar]
- Vaglia JL, Hall BK. Regulation of neural crest cell populations: occurrence, distribution and underlying mechanisms. Int J Dev Biol. 1999;43:95–110. [PubMed] [Google Scholar]
- Vaglia JL, Smith KK. Early differentiation and migration of cranial neural crest in the opossum, Monodelphis domestica. Evol Dev. 2003;5:121–135. doi: 10.1046/j.1525-142x.2003.03019.x. [DOI] [PubMed] [Google Scholar]