Abstract
Cellullar deficits are replenished within the central nervous system (CNS) by progenitors to maintain integrity and recover function after injury. NG2 proteoglycan-expressing progenitors replenish oligodendrocyte populations, but the nature of NG2 proteoglycan may not indicate a restricted population of progenitors. After injury, restorative spatiotemporal cues have the potential ability to regulate divergent fate-choices for NG2 progenitors, and NG2 progenitors are known to produce multiple cell types in vitro. Recent data suggest that NG2 expression is attenuated while protein levels remain high within injurious tissue; thus, NG2 expression is not static but transiently controlled in response to a dynamic interplay of environmental cues. Therefore, NG2 proteoglycan expression could label newly generated cells or be inherited by resident cell populations that produce oligodendrocytes for remyelination, astrocytes that provide trophic support and other cells that contribute to CNS function.
Keywords: CNS progenitors, NG2 proteoglycan expression, oligodendrocyte populations
Introduction
Unlike their multipotent predecessor, progenitors are classified as a population of fate-restricted cells that proliferate (self-renew) and differentiate into a mature cell phenotype when instructed by their environmental niche. In this capacity, progenitors maintain the integrity and function of the central nervous system (CNS) by replenishing a cellular deficit. In the wake of traumatic injury, for example, progenitor cells are stimulated to proliferate. A cascade of post-injury inflammatory molecules, cytokines and growth factors then direct progenitor migration and differentiation to restore functions to damaged CNS regions. As such, spatiotemporal cues orchestrate restorative cascades in damaged tissue. However, the regulation of progenitor cell fate remains to be substantiated. Experiments are needed to examine whether temporal signals regulate spatially isolated populations of fate-restricted progenitors, or whether progenitors become restricted to a specific lineage according to temporally expressed spatial cues.
Data from prior studies have suggested that with age progenitors become limited in their capacity to generate multiple cell types. Proliferating cells that express the protein chondroitin sulphate proteoglycans (NG2-expressing progenitors), for example, are classified as oligodendrocyte progenitor cells (OPCs) during development and in the adult. However, recent transplantation studies have demonstrated that NG2 precursor cells retain neurogenic potential, which quixotically suggests that NG2 is not a static label for a single cell type. Several studies have published data demonstrating many distinct cells, of different classes, that express NG2 proteoglycan in the adult. Therefore, it is counter to the tenet that NG2 progenitors are restricted to produce a single cell type if it is assumed that these disparate progeny inherit NG2 expression from a single progenitor. However, one can rationalize the existence of heterogeneous populations if environmental cues are assumed to direct NG2 expression on multiple progenitors (and potentially other cells types). The effects of temporal and spatial signals on NG2 expression have yet to be investigated, but these studies are necessary in order to rationalize the perplexing lineage and to justify the questionable classification of progenitors that express NG2 proteoglycan as restricted (for example).
Although the signal that stimulates the proliferation of progenitors remains unknown, many studies over the past decade have examined the molecules and mechanisms that control progenitor differentiation following injury. These suggest that new-born glia originate from a fate-restricted progenitor. As a class, NG2-expressing progenitors are known to proliferate after CNS insult and the progeny differentiate and mature into myelin-producing oligodendrocytes. Immunofluorescent studies have demonstrated that NG2 proteoglycan co-localizes with markers for immature oligodendroglia and transient microglia populations. Accordingly, Jones et al. (2002) have demonstrated that glial progenitor cells, which co-express PDGFαR following spinal cord injury, primarily express NG2. After traumatic insult, NG2 cells have been shown to incorporate bromodeoxyuridine (BrdU) into their DNA, thus demonstrating that NG2 cells proliferate in response to injury. Additionally, NG2 proteoglycan has been shown to co-localize in a fraction of all dividing cells, in the intact CNS (Levine & Reynolds, 1999; McTigue et al. 2001). Together, these experiments demonstrate that the NG2 protein labels a dividing progenitor that differentiates into oligodendrocytes, and these progenitors have been shown to migrate into the lesion environment following forebrain injury to aid repair (Kruger & Morrison, 2002). Despite evidence for the participation of astrocytes in a gliogenic repair programme, the majority of these studies do not report NG2 expression in astrocytes, which is surprising given that NG2 is a predominant protein within the glial scar. Thus, NG2 progenitors are considered to be restricted progenitors that function as OPCs in both the intact and the injured CNS. Regardless of their fates, the regulation of OPC cells following injury, their derivation and the role they play in the repair process are poorly understood.
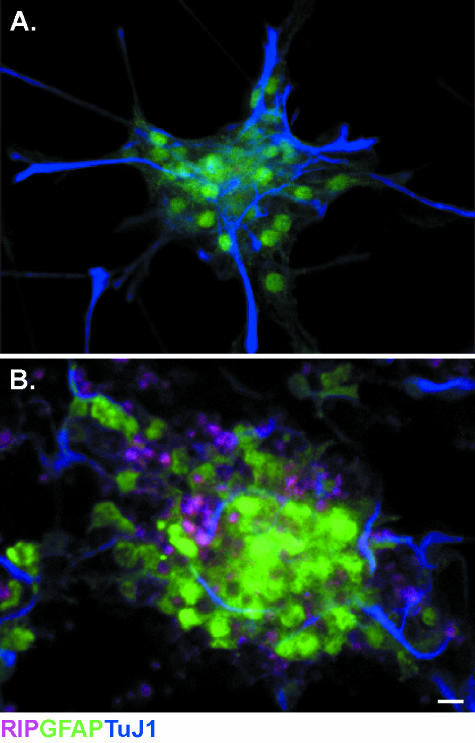
Recent studies demonstrating that the cellular niche instructs the fate-choice of progenitors contradict the conjecture that a cellular lineage is derived solely from a fate-restricted progenitor in the adult (recently reviewed by Palmer et al. 2000; Doetsch, 2003; Horner & Palmer, 2003). For example, NG2-expressing progenitors can be purified, via flow cytometry, from a population of spinal cord progenitors that have been maintained in vitro. Following treatments to induce neuronal differentiation, NG2 progenitors are able to express phenotypic markers for neurons and astrocytes (Fig. 1) despite being designated an OPC.
Fig. 1.
NG2-expressing spinal cord progenitors express astrocyte and neural markers. Spinal cord progenitors isolated from adult mice are maintained in vitro and fractionated according to NG2 expression via flow cytometry. The growth mediium is then supplemented to induce differentiation. (A) NG2-expressing cells differentiate into GFAP (green)-expressing astrocytes when grown in media supplemented with 1% FBS. (B) Under neural differentiation conditions (0.5 µmtrans-retinoic acid, 10 µg mL−1 BDNF), NG2 progenitors differentiate and express neural proteins (TuJ1, blue). Scale bar = 10 µm.
Similarly, isolated O2A progenitors express astrocyte or oligodendrocyte molecular markers when grown in different culture media (Raff et al. 1983). Together, these studies have led investigators to conclude that in vitro culture conditions result in phenotype expression artefacts (perhaps unjustly). Progenitors from the spinal cord can also be expanded and differentiated along neuronal, astroglial and oligodendroglial lineages in vitro, whereas these progenitors only produce glia when transplanted into the spinal cord. Yet the hippocampus is able to instruct the gliogenic progenitor to produce neurons (Shihabuddin et al. 2000). Regardless, among the data to demonstrate that progenitors are multipotent (in vitro) and retain neurogenic abilities, when transplanted into the hippocampus, adult progenitor cells are reported to be fate-restricted. When considered together, these experiments implicate the cellular niche in the alleged fate-restriction and mandate the need for future work to define the temporal nature of the instructive cues as well as the phenotype and capacity of endogenous progenitors to respond to these cues.
The recent debate over restrictive niches vs. homogenic progenitors as a cause of restricted lineage differentiation has prompted us to re-examine the systematic classification of fate-restricted progenitors. Experiments are needed to determine whether phenotypic molecular markers are dynamically expressed in response to instructions from the environment. We discuss many lines of evidence that provide compelling support for the view that NG2 expression by progenitors is derived from dynamic environmental cues and that, following differentiation, NG2 expression is heritable not intrinsic. We suggest that NG2 progenitors generate oligodendrocytes to fulfil tissue demands in addition to other cell types and, thus, these progenitors retain the ability to differentiate along multiple neural lineages.
Does NG2 expression validate a systematic classification: do feathers identify a duck?
When debating the characterization of a progenitor phenotype, it is logical to discuss the consistency of the phenotypic molecular markers and the fate-potential (as a function of the progeny derived from the dividing progenitor). One would like to define whether a designated protein is a static marker of a cell phenotype. Conversely, the expression of the molecular marker (such as NG2 proteoglycan) could fluctuate and be expressed dynamically in accord with tissue demands. In addition, we need to consider the tissue demands that would bias progeny differentiation (Gensert & Goldman, 1996). According to the stipulations of a restricted cell fate, NG2-expressing progenitors should produce one cell type. Logistically, however, the stipulations above are difficult to delineate owing to the diverse morphologies and molecular phenotypes adopted by presumed progeny of NG2 progenitors; current techniques cannot resolve whether the presence of NG2 is dynamically heritable or static (e.g. the temporal association of histochemical substrates that reflect proliferation and cell type).
The intact CNS is known to contain a small population of cells that incorporate bromodeoxyuridine (BrdU, a mitotic marker) and express NG2 on the cell surface (Genesert & Goldman, 1996, 2001). Progenitors expressing the NG2 proteoglycan comprise the largest population of dividing cells in the CNS (5–6%), and NG2-expressing cells constitute approximately 20% of white matter cells (Horner et al. 2002). Lineage analysis experiments have documented that BrdU+/NG2+ progenitors mature and express oligodendrocyte markers when analysed 14 days after their birth (Horner & Gage, 2000). In addition to their predominance, NG2 progenitors expand in response to traumatic injury (Levine & Reynolds, 1999; McTigue et al. 2001; Hampton et al. 2004), and NG2 progenitor expansion is required to recover function and to remyelinate axons in demyelination lesions (Keirstead et al. 1998; Levine & Reynolds, 1999; Chang et al. 2000; Gensert & Goldman, 2001). Therefore, a substantial number of studies have shown that a portion of progenitor cells express the NG2 proteoglycan, and the progeny derived from these progenitors participate in restoration of function after CNS insult. Despite the accounts of BrdU+/NG2+ progenitors expressing phenotypic markers for immature oligodendrocytes, NG2-expressing cells are not restricted to an oligodendrocyte lineage. Numerous phenotypes are known and have been described for cells that express NG2, which prompts one to question whether NG2 expression is instructional.
Since the original characterization of a progenitor that expressed NG2 but no mature glial markers (Beasley & Stallcup, 1987; Levine & Stallcup, 1987; Levison et al. 1999; Gensert & Goldman, 2001), NG2 has been shown to label protoplasmic astrocytes (Levine & Card, 1987), and to co-localize with GD3 (Levine et al. 1993) on O2A progenitors (Nishiyama et al. 1996) and RC2+ cells (Diers-Fenger et al. 2001). Electrophysiological studies have characterized distinct populations of NG2+/glial fibrillary acidic protein− (GFAP−) cells that have electrical properties similar to outward rectifying astrocytes (Schools et al. 2003). In corroboration, Matthias defined a class of s100β+ astrocytes in the mouse [transgenic GFAP/enhanced green fluorescent protein (EGFP)] hippocampus that express glutamate receptors and NG2 (Matthias et al. 2003). Experiments published by Vittorio Gallo suggest that NG2 progenitors produce neurons. NG2 proteoglycan-positive cells (co-expressing cyclic nucleotide phosphodiesterase, CNP) produced excitable neurons, in vitro (Belachew et al. 2003). Additionally, Aguirre and colleagues demonstrated that CNP+/NG2+ progenitors derived from the subventricular zone of CNP/EGFP mice [SVZ, type C-like multipotent cells (Doetsch et al. 1999; Aguirre & Gallo, 2004)] produced hippocampal GABAergic interneurons when transplanted into wild-type mice. To confuse matters further, Bergles and colleagues characterized a class of NG2 cells that elicited inward currents mediated by AMPA receptors in response to excitatory axon stimulation (Bergles et al. 2000). These oligodendrocyte precursors made synaptic junctions with vesicle-filled axon terminals similar to perisynaptic terminals of astrocytes and Bergmann glial cells (Ventura & Harris, 1999). The phylogenetic classification of the multiple cell types is confounded by the inability to discriminate and resolve whether NG2 can be dynamically expressed by cells or whether NG2 is only expressed statically by a particular cell: does nurture or nature dictate expression?
The cloak and dagger of NG2 progenitors: the mask of protein expression
The conundrum regarding the identity of NG2 cells and their predecessor could necessitate a paradigmatic shift in thinking. Taking into consideration the data detailed above, it is difficult to rationalize a lineage map based on the phylogenetic expression of the chondroitin-sulphate proteoglycan NG2. However, if NG2 expression is considered to result from an environmental cue, as opposed to a cell-type-specific phenomenon, a progenitor's genealogy becomes less of a logistical challenge. Factors throughout development, ageing and injury have the ability to influence spatial and temporal protein expression patterns. Unfortunately, because of the method of observation, the exact time and location/source of protein expression cannot be known simultaneously with the impending phenotypic fate of a particular cell. However, by taking a cause-and-effect approach to examine protein expression, NG2 proteoglycan can be evaluated in the intact CNS and compared with injurious conditions to evaluate how environmental cues alter NG2 expression.
The morphology of NG2 cells within the intact CNS varies from region to region, despite homogeneous populations in isolated grey and white matter regions (as discussed previously), and NG2 proteoglycan protein expression levels remain relatively low (Dawson et al. 2003). Irrespective of the method used to induce an injury (i.e. chemical, immunological or traumatic lesion), all injuries to the CNS produce a hallmark increase in proteoglycan expression that participates in scar formation. Spinal cord injury (SCI) produces an appreciable increase in NG2 expression, which has been shown to peak at 7 days post-injury (PI) via Western blot analysis (Jones et al. 2002). In an ethidium bromide-induced demyelination lesion, Levine & Reynolds (1999) showed a 175-fold increase (compared with controls) in brain-stem progenitors that expressed NG2 at 2 days PI. Other experiments demonstrating NG2+/BrDU+ co-localization support this finding and show that NG2 protein labels progenitors following lysolecithin-induced demyelination (Gensert & Goldman, 1996; Keirstead et al. 1998), in immunologically stimulated demyelination lesions (Nishiyama et al. 1997) and in transgenic animals that lack myelination (Chang et al. 2000; Wu et al. 2000). Together, these experiments demonstrate that NG2 expression is dramatically increased and co-labels immature oligodendrocytes and dividing progenitors (with occasional expression by microglia). As a result, the assertion that oligodendroglia are derived from NG2 progenitors is more consistent when analysing the progeny generated in the context of injury. Considering the lesion, the oligodendrocyte cell fate would be appropriate to promote recovery. However, a close examination of recent stab wound experiments show a quantifiable and persistent increase in NG2+ cells from 15 cells (2 days PI) to 22 cells per 0.01 mm2 at 14 days PI while the number of BrdU+-labelled NG2 progenitors remained steady (5 cells per 0.01 mm2), and the number of NG2+ cells decayed with distance from the lesion epicentre (Hampton et al. 2004). Studies in our laboratory have also produced an appreciable increase in NG2 expression following SCI that cannot be assigned to a particular progenitor or group of immature oligodendrocytes (unpublished observations).
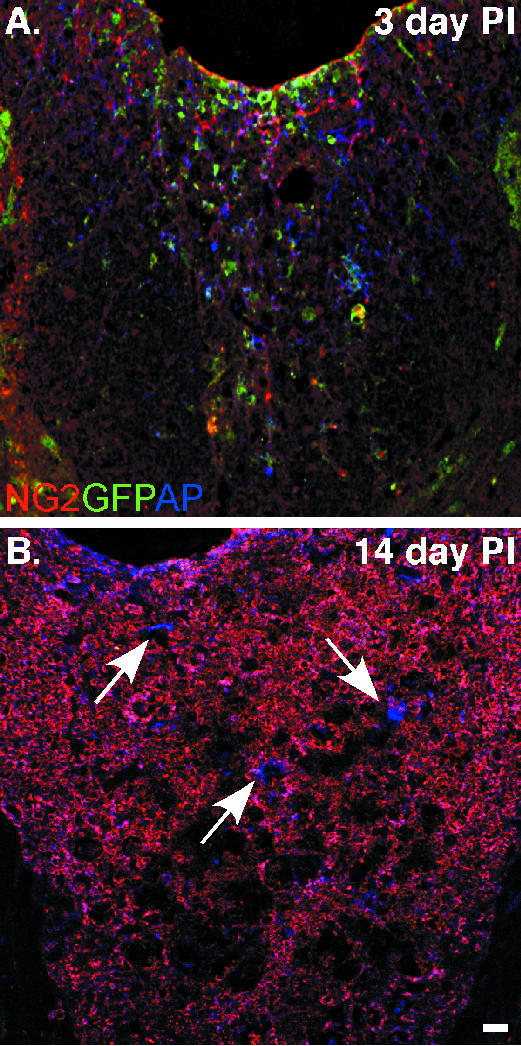
Notably, immunological lineage-tracing studies do not refute conclusions made about the predecessor of a cell that adopts a specific fate. Therefore, our laboratory has constructed a promoter-specific retrovirus in order to analyse the lineage derived from NG2-expressing progenitors. The NG2 promoter region is utilized to regulate the expression of green fluorescent protein (GFP) from the inserted viral genome. To expand the breadth of the analysis, alkaline phosphatase (AP) is also expressed, and internal viral promoters regulate AP expression. Therefore, AP labels all infected cells and GFP is expressed in NG2 progenitors. Through the expression of the two reporter genes, our analysis suggests that NG2 expression becomes attenuated in progenitors after a spinal cord hemisection. Early after injury, GFP and AP expression is seen throughout the dorsal columns. However, GFP cannot be detected in cells labelled 24 h PI and analysed at 14 days PI, whereas AP expression is still robust (Fig. 2). This demonstrates that the integrated viral genome remains active. In addition, we know that NG2 progenitors continue to proliferate in the cord because NG2-expressing progenitors can be infected 7 days PI for analysis at 14 days PI (data not shown). NG2 protein is stable and persists in all tissues analysed; yet the reporter expression (GFP) suggests that progenitors born 24 h PI attenuate NG2 expression. These observations bring into question how environmental cues might regulate NG2 expression and the half-life of the proteins. Consequently, future studies are needed to resolve whether NG2 is dynamically expressed in accordance with environmental instruction (i.e. post-injury).
Fig. 2.
NG2 proteoglycan expression is attenuated in nascent progenitors 14 days after spinal cord injury. Spinal cord progenitors are labelled in vivo by a retrovirus engineered to regulate GFP expression by the NG2 promoter. (A) Spinal cord sections analysed 3 days post-injury (PI) have numerous cells expressing GFP, which co-localizes with NG2 protein. The expression of alkaline phosphatase (AP, blue), from an SV40 promoter, labels all cells infected by the retrovirus. (B) GFP expression is attenuated when the tissue is analysed 14 days PI. NG2 protein (red) persists in the lesion, but the lack of GFP suggests that NG2 transcriptional activation has been down-regulate while AP (blue) expression persists (white arrows). Continued AP expression suggests that the incorporated viral genome remains active. Scale bar = 10 µm.
Lamark of a niche: the nature by which inherited characteristics affect proteomic expression
The central tenet of the questions posed is whether a tissue's cellular niche is responsible for the dynamic regulation of NG2 proteoglycan expression or progenitor fate. Given the diverse cell types labelled by NG2, we cannot assert the existence of a single NG2 cell type without investigating whether environmental cues affect NG2 expression. Therefore, if a cell's niche does direct NG2 expression, progenitors would express NG2 as a consequence of environmental location and instruction and not because of their genomic restriction. As a result, NG2 would not label a select progenitor and, thus, would not be a valid marker to identify and tag a cell as lineage-restricted. It would be useful to examine if transplanted progenitors inherit or attenuate NG2 protein expression as a result of their new niche, which would indicate that current opinions of fate restriction with NG2 expression would be an artefact of the paradigmatic models used to analyse lineage progression after injury. These caveats, coupled with recent finds to demonstrate that OPCs can produce multiple cell fates, necessitate the need for experiments to examine how niches regulate a single cell's or progenitors’ proteomic expression.
Recent studies suggest cellular niches in the CNS have instructive powers (Palmer et al. 2000; Horner & Palmer, 2003), and transplantation experiments have demonstrated that glial-restricted progenitors retain neurogenic potential when implanted into the hippocampus. Prior experiments, in vitro, have fuelled debates about whether adult or postnatal progenitors are multipotent or restricted (Raff et al. 1983; Levine & Stallcup, 1987; Palmer et al. 1999), as a result of the homogenic fates that these progenitors adopted when transplanted into injury models and the intact spinal cord (Jeffery et al. 1999; Shihabuddin et al. 2000; Nunes et al. 2003; Zerlin et al. 2004). The nature of these studies suggests that these cells are glial-restricted despite the cells’ ability to express a neuronal marker when treated in vitro. Other experiments have refuted the assertion that certain progenitors are fate-restricted. When transplanted into neurogenic regions, these cells integrate into the CNS and produce functional neurons in the hippocampus (Shihabuddin et al. 2000; Seri et al. 2001; van Praag et al. 2002; Belachew et al. 2003) and GABAergic interneurons (Aguirre & Gallo, 2004). Therefore, instead of supporting the theory of a fate-restricted progenitor, these studies demonstrate the susceptibility of these cells to instructive cues, and implicate the powers of the niche in directing a multipotential progenitor to adopt a select phenotype.
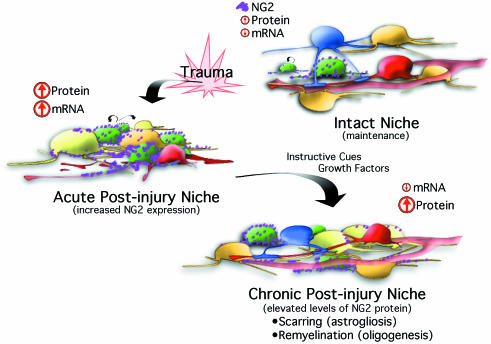
Relatively few experiments have investigated the environmental changes that occur with injury (or transplantation) or how these alterations could affect progenitor fates or how a cell's proteome is expressed. Studies have been conducted to investigate how ectopic expression of developmental cues directs adult progenitor fate and have shown how bone morphogen proteins (BMPs) or sonic hedgehog (Shh) affect cell fate (Lim et al. 2000; Gomes et al. 2003; Agius et al. 2004). However, physiological changes to the endogenous niche have gone largely unexplored. For example, one would like to know if instructive cues produced by injury induce a physiological up-regulation of NG2 proteoglycan expression on established cells or dividing progenitors, and whether this increased expression persists to mark immediate and subsequent progeny. Given the questions that surround how a niche affects proteomic expression, we have proposed a model to direct studies to investigate the hypothesis that NG2 proteoglycan expression is dynamic and is directed by cues within a niche (Fig. 3). A stimulus, such as traumatic injury, produces a transient increase in NG2 mRNA, which subsequently increases NG2 protein. Thus, the physiological response results in an increased number of progenitors, and thus of NG2 progenitors. As these cells mature to become an oligodedrocyte (for example), protein expression is inherited passively, and NG2 proteoglycan expressed by the progeny and the progenitor result from the emergent requirements of the damaged tissue. The cellular deficits incurred by the injury models above could bias fate selection and result in a predominant injurious phenotype. Therefore, progenitors in the intact CNS would be more likely to have a diverse lineage compared with the homogeneous phenotypes seen in the context of injury.
Fig. 3.
NG2 expression is transiently induced according to environmental instructions. Progenitor cells (green) divide in the intact CNS to maintain and replenish populations of cells in accord with tissue requirements. NG2 proteoglycan mRNA levels are low and NG2 protein (magenta) is expressed at basal levels on the plasma membrane of multiple cells and select progenitors. Trauma induces a transient increase in NG2 mRNA and a dramatic increase in proteoglycan protein expression. Therefore, injury results in both an increase in progenitor proliferation and coincident increases in NG2 protein expression as instructed by acute post-injury conditions, which are known to persist for 14–21 days PI. Progenitor proliferation decreases as the transition from acute to chronic post-injury is made. In conjunction, viral studies suggest that NG2 transcription levels decrease. Despite the reduced mRNA (to basal levels), NG2 protein levels persist and become inherited by cells derived from progenitors and from extracellular milieu of the injurious conditions. Tissue restoration continues after injury and begins with the formation of a gliotic scar. Other newly generated cells respond to the instructive cues and growth factors within the post-injury niche and replenish oligodendrocytes that remyelinate axons, and sprouting neurites and astrocytes that provide trophic support.
Experiments designed to investigate how niche physiology impacts cellular behaviour will facilitate a better understanding of a progenitor cell's potential. As technology progresses, issues about the exact time and location/source of protein expression can be resolved to correlate the expression with a phenotypic fate. In the absence of these studies, assumptions about cell fate restriction under conditional stresses are premature. A better understanding of the niche that supports and directs a progenitor's fate will help to elucidate the cues that direct a progenitor to proliferate or to differentiate. This will greatly facilitate the therapeutic potential bestowed upon adult progenitors.
References
- Agius E, Soukkarieh C, Danesin C, et al. Converse control of oligodendrocyte and astrocyte lineage development by Sonic hedgehog in the chick spinal cord. Dev Biol. 2004;270:308–321. doi: 10.1016/j.ydbio.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley L, Stallcup WB. The nerve growth factor-inducible large external (NILE) glycoprotein and neural cell adhesion molecule (N-CAM) have distinct patterns of expression in the developing rat central nervous system. J Neurosci. 1987;7:708–715. doi: 10.1523/JNEUROSCI.07-03-00708.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia. 2001;34:213–228. doi: 10.1002/glia.1055. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. In vivo characterization of endogenous proliferating cells in adult rat subcortical white matter. Glia. 1996;17:39–51. doi: 10.1002/(SICI)1098-1136(199605)17:1<39::AID-GLIA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48:75–86. [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Rhodes KE, Zhao C, Franklin RJ, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–480. doi: 10.1023/a:1025739630398. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Palmer TD. New roles for astrocytes: the nightlife of an ‘astrocyte’. La vida loca! Trends Neurosci. 2003;26:597–603. doi: 10.1016/j.tins.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Crang AJ, O'Leary T, Hodge M, SJ Blakemore WF. Behavioural consequences of oligodendrocyte progenitor cell transplantation into experimental demyelinating lesions in the rat spinal cord. Eur J Neurosci. 1999;11:1508–1514. doi: 10.1046/j.1460-9568.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Kruger GM, Morrison SJ. Brain repair by endogenous progenitors. Cell. 2002;110:399–402. doi: 10.1016/s0092-8674(02)00899-1. [DOI] [PubMed] [Google Scholar]
- Levine JM, Card JP. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci. 1987;7:2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT, et al. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Drazba M, Yu JA, Tuohy VK. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res. 1997;48:299–312. doi: 10.1002/(sici)1097-4547(19970515)48:4<299::aid-jnr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Electrophysiologically ‘complex’ glial cells freshly isolated from the hippocampus are immunopositive for the chondroitin sulfate proteoglycan NG2. J Neurosci Res. 2003;73:765–777. doi: 10.1002/jnr.10680. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Miller RH, Ransohoff RM, Robinson S, Bu J, Nishiyama A. Elevated levels of the chemokine GRO-1 correlate with elevated oligodendrocyte progenitor proliferation in the jimpy mutant. J Neurosci. 2000;20:2609–2617. doi: 10.1523/JNEUROSCI.20-07-02609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlin M, Milosevic A, Goldman JE. Glial progenitors of the neonatal subventricular zone differentiate asynchronously, leading to spatial dispersion of glial clones and to the persistence of immature glia in the adult mammalian CNS. Dev Biol. 2004;270:200–213. doi: 10.1016/j.ydbio.2004.02.024. [DOI] [PubMed] [Google Scholar]