Abstract
The mammalian adult central nervous system (CNS) is known to respond rapidly to demyelinating insults by regenerating oligodendrocytes for remyelination from a dividing precursor population. A widespread population of cells exists within the adult CNS that is thought to belong to the oligodendrocyte lineage, but which do not express proteins characteristic of mature myelinating oligodendrocytes, such as myelin basic protein (MBP) and 2,3-cyclic nucleotide 3-phosphodiesterase (CNP). Instead, these cells have phenotypic characteristics of a more immature stage of the oligodendrocyte lineage. They express the NG2 chondroitin sulphate proteoglycan, in addition to O4 and the platelet-derived growth factor α-receptor, all widely accepted as markers for oligodendrocyte progenitor cells (OPCs) throughout development. However, NG2+ cells residing in the adult CNS do not resemble embryonic or neonatal NG2+ cells in terms of their morphology or proliferation characteristics, but instead represent a unique type of glial cell that has the ability to react rapidly to CNS damage. In this review, we present the evidence that adult NG2+ cells are part of the oligodendrocyte lineage and are capable of giving rise to new oligodendrocytes under both normal and demyelinating conditions. We also review the literature that these cells may have multiple functional roles within the adult CNS, notwithstanding their primary role as OPCs.
Keywords: cell proliferation, glia, myelination, remyelination, stem cells
Introduction
The majority of mammalian tissues contain cells that are able to respond to local or global insult and become involved in repair processes. This regenerative process invariably involves cell proliferation and migration, two characteristics that are normally associated with immature or transformed cells. These repair processes are thought to be generally very efficient unless the tissue insult interferes directly with the regenerative mechanisms or repeated insults exhaust this capacity. Although the mammalian central nervous system (CNS) was for many years thought to be incapable of endogenous repair, we are now more aware of the many regenerative processes that can occur following a wide variety of insults to the brain and spinal cord. Neuronal replacement following degeneration in the adult CNS is, however, relatively limited and occurs predominantly in those areas that have an inherent plasticity vital to their normal physiological function, for example in the hippocampus and olfactory system. Nevertheless, rapid advances in stem cell biology are demonstrating the multipotential nature of small populations of cells in the mature CNS, usually only in vitro, and how these cells might be manipulated both to enhance endogenous repair and for cell transplantation therapies. Despite this relatively limited endogenous capacity for neuronal replacement, there is one regenerative process in the CNS that is both rapid and efficient: the capacity for remyelination. Remyelination is a rapid consequence of a wide variety of insults that result in demyelination (Levine et al. 2001; Franklin, 2002). The main reason for this remarkable endogenous repair capacity is undoubtedly the presence of a large population of cells, found throughout the adult CNS, that has the capability of regenerating oligodendrocytes. Even in the demyelinating disease multiple sclerosis (MS), in which remyelination appears eventually to fail in some lesions, there is an underlying robust endogenous repair that may continue for many years until rendered inoperative by the ongoing disease process.
In this review we look at the nature and functional characteristics of the NG2-expressing cells that are thought to regenerate oligodendrocytes in the mature mammalian CNS following demyelination and discuss additional roles that they might play in normal physiology.
Oligodendrocytes arise from NG2+ cells during development
It is generally accepted that NG2+ cells that arise during embryonic development of the CNS give rise to the majority of oligodendrocytes, the evidence for which has been gained through a combination of in vitro and in vivo experimental work. Neural precursors that give rise to cells of the oligodendrocyte lineage are first identified at embryonic day 12–14 (E12–14) in the rodent by their expression of mRNA for the platelet-derived growth factor alpha receptor (PDGFαR; Pringle et al. 1992; Pringle & Richardson, 1993) and DM-20, an isoform of the major myelin proteolipid protein (PLP; Timsit et al. 1995). Initially these cells are found in the ventral half of the ventricular zones of the spinal cord, diencephalon and telencephalon (Pringle & Richardson, 1993; Woodruff et al. 2001), but migrate quickly to populate other regions of the CNS so that by E17 PDGFαR+ cells are found throughout the spinal cord, hindbrain and basal forebrain (Nishiyama et al. 1996). At this stage, all PDGFαR+ cells are also immunopositive for NG2 (Nishiyama et al. 1996). Upon birth NG2+ PDGFαR+ cells are more or less evenly distributed throughout the CNS, in both putative white and putative grey matter regions. Before the onset of oligodendrocyte differentiation and myelination, the NG2+ cells in any one region of the CNS appear to display a homogeneous morphology and phenotype suggestive of homologous function.
As PDGFαR+/NG2+ cells mature into oligodendrocytes they gradually lose expression of these antigens and enter an intermediate pro-oligodendrocyte stage recognized by O4 immunoreactivity (Sommer & Schachner, 1981; Bansal & Pfeiffer, 1992; Pfeiffer et al. 1993), before finally expressing markers of mature, fully differentiated oligodendrocytes, such as 2′,3′-cyclic nucleotide phosphohydrolase (CNP), myelin bask protein (MBP), PLP and myelin oligodendrocyte glycoprotein (MOG). This is clearly demonstrated in the developing rat cerebral cortex where there is an even distribution of NG2+ cells before a wave of differentiation begins at approximately 4 days postnatal, outwards from the corpus callosum to the pial surface (Reynolds & Hardy, 1997). A gradual progression from NG2+/O4− to NG2+/O4+ cells to O4+/GC+ cells then occurs between postnatal days 4 and 20. Small numbers of NG2+CNP+ and NG2+GalC+ cells have been reported in the forebrain and cerebellum, indicating an intermediate stage of differentiation of NG2+ cells into oligodendrocytes (Levine et al. 1993; Dawson et al. 2003).
The in vitro studies are in agreement with in vivo studies, showing that PDGFαR+ cells isolated from E17 spinal cord by immunoselection are both NG2+ and A2B5+ and are capable of giving rise to both astrocytes and oligodendrocytes depending on environmental conditions (Hall et al. 1996). Cells isolated by PDGFαR immunopanning were both O4 and galactocerebroside (GC) negative, yet when cultured for 48 h in low-serum medium without growth factors, greater than 95% of these cells differentiated into GC+ cells with the multiprocessed ramified morphology characteristic of oligodendrocytes. Moreover, in cultures depleted of PDGFRα+/A2B5+ cells by antibody-mediated complement lysis, very few oligodendrocytes arose, indicating that the PDGFαR+NG2+ cells are indeed oligodendrocyte progenitors (Hall et al. 1996).
However, several groups have recently questioned the homogeneity of NG2+ cells during development, suggesting that the NG2+ cell population may be divergent at early stages of CNS maturation. Utilizing a transgenic mouse model in which expression of enhanced fluorescent green protein (EGFP) is driven by the promoter for PLP, Mallon et al. (2002) have proposed the existence of two distinct NG2+ cell populations during development, distinguished by expression of PLP/DM-20 gene promoter activity. In embryonic spinal cord (E14.5) EGFP expression was localized to a ventral position consistent with the spatio-temporal origins of oligodendrocyte progenitor cells (Noll & Miller, 1993; Hall et al. 1996). Co-localization of NG2 with EGFP was examined in the frontal cortex from postnatal day 1 (P1) to P10, the onset of myelination. Throughout the developing neocortex the presence of EGFP+/NG2+ cells and EGFP−/NG2+ cells was observed, implying the presence of two separate NG2+ cell populations. It is suggested that only those cells with PLP/DM-20 gene promoter activity may become myelinating oligodendrocytes whilst those negative for the promoter persist in the adult CNS as NG2-expressing cells, although this was not demonstrated. Both populations appeared to have similar morphologies, and migration and proliferation characteristics, indicating they are closely related. Whether they represent two distinct lineages or rather two different stages of differentiation of a single lineage within the oligodendrocyte continuum remains to be demonstrated.
A further transgenic model in which EGFP expression was driven by the promoter for glial fibrillary acidic protein (GFAP) identified two AN2+ (mouse homologue of NG2) cell phenotypes during development, one EGFP+/S100β+/AN2+, the other EGFP−/GFAP−/AN2+ (Matthias et al. 2003), suggesting a shared astrocyte/oligodendrocyte lineage. Previous in vitro studies of postnatal optic nerve identified a progenitor cell capable of giving rise to both oligodendrocytes and astrocytes depending upon culture conditions (Raff et al. 1983). Bipotential progenitors isolated from postnatal cerebellum and optic nerve also expressed NG2 on their cell surface (Levine & Stallcup, 1987; Stallcup & Beasley, 1987). Although other studies have shown no overlap between NG2 and the astrocyte lineage markers S100β and GFAP during normal CNS development (Nishiyama et al. 1996; Reynolds & Hardy, 1997), it will be of considerable interest to determine whether the PLP-EGFP−/NG2+ population described by Mallon and colleagues contains the EGFP+/S100β+/AN2+ population identified by Matthias et al., thereby providing in vivo evidence for the bipotential progenitors described in vitro. A shared oligodendrocyte/neuronal lineage has also been suggested for NG2+ cells during development of the hippocampus. A proportion of NG2+ cells in the hippocampus of transgenic mice in which GFP expression is driven by the CNP gene promoter are able to generate GABAergic neurons in vitro and in vivo (Belachew et al. 2003).
The evidence thus indicates that the majority of NG2-expressing cells generated during CNS development are indeed a part of the oligodendrocyte lineage and do give rise to oligodendrocytes. However, there is increasing evidence that a significant proportion of NG2+ cells do not differentiate into myelin-forming oligodendrocytes but remain in the mature CNS with an immature phenotype. It is the identity and function of these cells that the rest of this review addresses.
NG2+ cells constitute an abundant population in the adult CNS
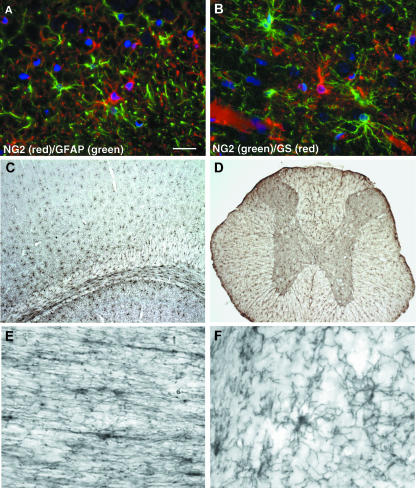
Our studies of the rat cerebral cortex indicated that an even distribution of O4+ oligodendrocyte progenitors that did not express proteins or lipids characteristic of mature oligodendrocytes existed in the adult (Reynolds & Hardy, 1997). We went on to demonstrate that the O4+ cells in the cortical grey matter that were not mature oligodendrocytes all expressed NG2. Expression of another protein characteristic of oligodendrocyte progenitors, PDGFαR, also delineates the same population of cells in the adult CNS that express NG2 (Nishiyama et al. 1997; Dawson et al. 2003). Recent evidence suggests that these cells also express the Nkx2.2 transcription factor and Olig1 (Fancy et al. 2004), both characteristic of oligodendrocyte progenitor cells. Numerous studies have demonstrated that NG2+ cells in the resting adult CNS do not express proteins characteristic of mature astrocytes or microglia (Nishiyama et al. 1997; Reynolds & Hardy, 1997; Dawson et al. 2000, 2003; Horner et al. 2000). Thus, they do not express GFAP (Fig. 1A) or OX42. They do express glutamine synthetase (Fig. 1B), as expected for cells that are able to transport glutamate actively (see later), but at much lower levels than protoplasmic astrocytes in the grey matter.
Fig. 1.
Immunofluorescence labelling of NG2+ cells (red) in the hippocampus (A) clearly shows that they do not express GFAP (green). In the cerebral cortex (B) a clear distinction is seen between the NG2+ cells (green) and the strongly glutamine synthetase+ protoplasmic astrocytes (red). The widespread and uniform distribution of NG2+ cells is illustrated in the forebrain (C) and spinal cord (D). NG2+ cells have an elongated morphology in white matter tracts (E; optic nerve) and a more radial arrangement of processes in the grey matter (F; cerebellar granule cell layer). Scale bars = 20 µm (A,B), 250 µm (C,D) and 25 µm (E,F).
The presence of a population of cells with a glial progenitor phenotype comprising 3–9% of the total cell number in the adult CNS (Dawson et al. 2003) is an exciting prospect, both for myelin repair and for the possibility of manipulating these cells to give rise to other cell types (Kondo & Raff, 2000). They are now considered a major glial cell type (Levine et al. 2001) unique from oligodendrocytes, astrocytes and microglia. Their distribution is relatively independent of the density of myelinated fibres and they are equally numerous in white and grey matter (Fig. 1C,D), a finding that has led to the suggestion that they are unlikely to perform the role of oligodendrocyte progenitors (Butt et al. 2002). However, NG2+PDGFαR+ glial progenitors migrate throughout the developing CNS at a stage before axons have fully matured and before myelination begins (Nishiyama et al. 1996), in response to cues that may be unrelated to myelination. Therefore, it would be expected that they would be localized in both developing white and developing grey matter. In fact they have a very even distribution in the early neonatal brain (Nishiyama et al. 1996; Reynolds & Hardy, 1997). Further division of NG2+ cells would then occur under the influence of neuronal- and astroglial-derived factors (Dawson et al. 2000). This would lead to a greater density in presumptive white matter tracts in order to provide the requisite number of oligodendrocytes. Those oligodendrocyte progenitor cells (OPCs) that did not receive signals to differentiate into premyelinating oligodendrocytes would either remain as OPCs or undergo apoptosis. Although it has been demonstrated that OPCs and immature oligodendrocytes undergo apoptosis in the optic nerve white matter (Barres et al. 1992) nothing is known about the relative frequency of this event in the different microenvironments of the grey and white matter.
Thus, the distribution of NG2+ cells in the adult CNS does not really inform the debate concerning the physiological role of these cells. Likewise, astrocytes perform many different functions in the CNS depending on the stage of development and the neuronal environment of a particular region. Indeed, the morphology of astrocytes is very different between white and grey matter. The same is likely to be the case for NG2+ glia.
In order to determine whether adult NG2+ cells function as oligodendrocyte progenitors, similar to their neonatal NG2+ counterparts, we need to compare their morphological and physiological characteristics. During embryogenesis the morphology of NG2+ glia changes rapidly from a simple oval or polygonal cell-shaped body with few unbranched processes to a more differentiated and highly branched cell with a smaller cell soma (Levine et al. 1993; Nishiyama et al. 1996). By the time of birth, NG2+ cells in the cerebral cortex extend multiple long processes in numerous directions whilst those in the presumptive white matter tracts have more elongated and oval-shaped cell bodies with long parallel processes associated with axon bundles. As the CNS matures these cells become more complex with greater arborizations so that each cell within the grey matter occupies a distinct domain, whilst those in the white matter lie between myelinated axons (Fig. 1E,F). Although such morphologically complex cells do not fit our concept of progenitor cells, we know that they are capable of cell division in the resting adult CNS (Dawson et al. 2003) and can revert to a simpler morphology following injury (Levine et al. 2001; Reynolds et al. 2002). Thus, such a complex morphology does not negate their role as oligodendrocyte progenitors but instead assumes further additional functional properties. Any variations in morphology are likely simply to reflect differing roles determined by the tissue environment. Whole-cell patch-clamp studies on isolated cortical slices from postnatal transgenic mice expressing EGFP driven by the CNP promoter (Yuan et al. 2002) demonstrated that cortical and white matter NG2+ cells have differing resting membrane potentials and ion channel expression profiles (Chittajallu et al. 2004). Further experiments are required to determine whether this remains the case in the fully mature CNS.
What physiological role do NG2-expressing glia play in the resting adult CNS?
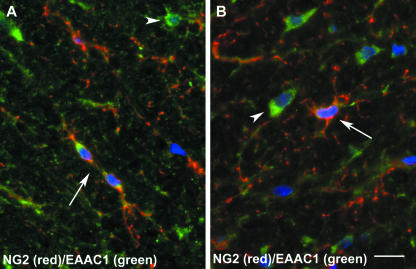
Defining the role of NG2-expressing cells in vivo has remained elusive despite their phenotypic characterization as part of the oligodendrocyte lineage and in vitro evidence that isolated adult OPCs give rise to oligodendrocytes (Levine et al. 1993; Tang et al. 2000). Although these cells have a modest rate of cell division, they represent the major dividing population of the adult CNS, accounting for 70% and 74% of bromodeoxyuridine (BrdU)-incorporating cells in the spinal cord and cerebral cortex, respectively (Horner et al. 2000; Dawson et al. 2003). As a mitotically active population, generation of new oligodendrocytes is an expected function, but it is likely that they also participate in homeostasis in the adult CNS. Evidence exists that they are involved in glutamate signalling, as NG2+ cell processes can interdigitate between pre- and post-synaptic terminals (Bergles et al. 2000; Ong & Levine, 1999). Furthermore, excitatory synapses mediated by AMPA receptors have been identified on NG2+ cells and such cells have been shown to respond to neuronally released glutamate (Bergles et al. 2000). In addition to the expression of glutamate receptors, cells of the oligodendrocyte lineage have been shown to transport glutamate actively (Reynolds & Herschkowitz, 1987; Domercq et al. 1999) and express the GLAST and EAAC1 glutamate transporter proteins (Kugler & Schmitt, 1999; Domercq et al. 1999). Preliminary experiments in this laboratory have shown that NG2+ cells in the adult rat cerebral cortex also express the EAAC1 glutamate transporter (Fig. 2; unpublished observations). In keeping with a role in glutamate neurotransmission, oligodendrocytes and NG2+ cells have both been shown to express glutamine synthetase (Domercq et al. 1999; Dawson et al. 2003). Thus, there is good evidence for a role for NG2+ cells in glutamate neurotransmission and the glutamate–glutamine cycle.
Fig. 2.
Expression of the EAAC1 glutamate transporter by NG2+ cells (A,B). NG2+/EAAC1+ cells (arrows) are shown in the spinal cord white matter together with EAAC1-expressing astrocytes (arrowheads). Scale bar = 10 µm.
In addition to the presence of glutamate receptors, NG2+ cells in several CNS regions have been shown to express functional GABA-A receptors (Lin & Bergles, 2004). Rather than acting to generate action potentials, these neurotransmitter receptors are more likely to act as a rapid means of altering ion concentrations within NG2+ cells, thereby altering their physiological properties. Earlier in vitro experiments showed that activation of these receptors on developing oligodendrocyte progenitor cells induced calcium influx, inhibiting their proliferation and progression along the oligodendrocyte lineage (Kirchhoff & Kettenmann, 1992; Gallo et al. 1996). This allows for the possibility that in the adult CNS neurons may be able to signal and direct physiological changes in NG2+ cells, perhaps controlling cell division and preventing their differentiation.
It has been suggested that adult NG2-expressing cells develop postnatally following myelination and as such have different functional roles to perinatal OPCs. One group has termed these cells ‘synantocytes’ (Butt et al. 2002), suggesting they function to monitor neuronal function and to provide periaxonal and perinodal processes. Given that NG2+ cell processes are known to make contact with nodes of Ranvier (Butt et al. 1999), these suggested functions would not be inconsistent with the idea that these cells may have a possible neuromodulatory role, as described above for glutamate neurotransmission. However, it should also be pointed out that such glial–neuronal communications are not incompatible with their role as oligodendrocyte progenitors, which would require them to be sampling the electrical environment for alterations in activity and to react and respond as necessary.
The physiological characteristics described for NG2+ cells thus suggest that they have a real and significant role in cellular homeostasis. Astrocytes and microglia are also thought to contribute to neuronal homeostasis by controlling the extracellular microenvironment and reacting to any changes. Therefore, it is not unreasonable to expect NG2+ glia to be playing a similar role.
Are NG2+ cells a heterogeneous population?
There has recently been much debate concerning the possible heterogeneous nature of the NG2+ cell population with respect to developmental origin and functional role. The possibility of multiple and varied functions might suggest such a heterogeneous population, although this is also undoubtedly true for astrocytes. One also has to consider that the NG2 protein may be expressed by multiple cell lineages in the adult CNS, although, as described earlier in this review, the vast majority of NG2+ cells display an oligodendrocyte lineage phenotype. A number of studies have suggested that there may be two distinct populations of NG2-expressing cells in the adult CNS: one is able to generate oligodendrocytes whereas the other has undefined roles (Keirstead et al. 1998; Mallon et al. 2002; Belachew et al. 2003). Keirstead et al. (1998) examined the response of NG2+ cells following demyelination in the rat spinal cord and identified the presence of two NG2+ cell populations, one responding to the insult by dividing and the other non-responsive, suggesting that the responsive population act as oligodendrocyte progenitors. Studies of NG2 expression in the developing CNS of mice in which the PLP/DM-20 gene promoter drives EGFP expression (Mallon et al. 2002) have suggested the presence of one population of NG2+ cells that gives rise to oligodendrocytes and a second population that is closely related yet which subserves a function other than myelination. However, these studies have yet to show the presence of two distinct NG2+ populations in the adult CNS.
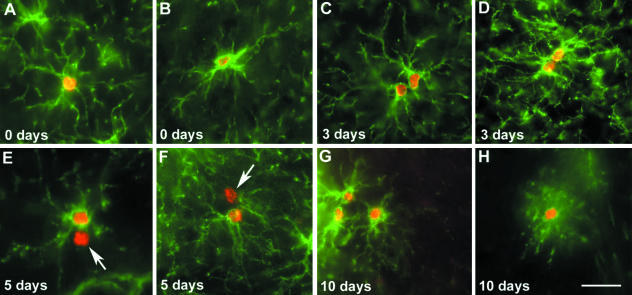
Following the fate of cycling NG2+ cells in vivo would provide invaluable evidence in determining the lineage of these cells. However, there are little data available concerning the fate of cycling NG2+ cells. In view of this, we have undertaken an investigation of NG2+ cell cycling and followed their fate over 10 days in adult rats. In accordance with previous studies (Horner et al. 2000; Dawson et al. 2003), we find that NG2+ cells account for approximately 70% of BrdU+ cells after a short pulse (Fig. 3A,B). However, we do observe the presence of two NG2+ cell populations that differ in their cycling properties (Fig. 3C–F; our unpublished observations). In one population, division gives rise to symmetrical pairs of BrdU+/NG2+ cells that remain close to one another over the 10-day period (Fig. 3C,D). In the other population, cell division gives rise to asymmetrical pairs of one BrdU+/NG2+ cell and one BrdU+/NG2− cell (Fig. 3E,F). Whether this occurs as a result of asymmetrical division has yet to be determined. Although this study and previously published data (Wu et al. 2001; Dawson et al. 2003) indicate that there is an increase in BrdU+/CNP+ oligodendrocytes over the 10-day period (Fig. 3H), the identity and fate of the BrdU+/NG2− cells in asymmetric pairs have yet to be determined.
Fig. 3.
BrdU incorporation by NG2+ cells following a 6-h pulse. Immediately after the pulse, single NG2+BrdU+ cells were seen in both the grey (A) and the white (B) matter. Between 3 and 5 days increasing numbers of paired cells were seen, both symmetrical pairs of NG2+/BrdU+ cells (C,D) and asymmetrical pairs of one NG2+/BrdU+ cell and one NG2−/BrdU+ cell (arrows) (E,F). Triplets of NG2+/BrdU+ cells were first observed between 7 and 10 days post-pulse (G). Small numbers of CNP+/BrdU+ cells began to be seen between 5 and 10 days (H). Scale bar = 30 µm.
Are NG2-expressing cells multipotential stem cells?
If oligodendrocyte replacement is an important capacity for the mature CNS to maintain then one would expect this to be reflected in the cell cycle characteristics of NG2+ cells in the adult. This would require the cells to maintain a slow turnover in the resting state but to revert to a rapidly dividing motile population following injury. Culture experiments have shown that OPCs isolated from the adult CNS divide and migrate more slowly than their neonatal counterparts (Wolswijk & Noble, 1989; Shi et al. 1998). They are also capable of reverting to a rapidly dividing motile population upon addition of the right combination of growth factors in vitro (Shi et al. 1998). In vivo they display a slow resting turnover (Horner et al. 2000; Dawson et al. 2003) but following demyelination display a simpler reactive morphology and rapidly divide to repopulate the lesion site (Levine & Reynolds, 1999; Cenci di Bello et al. 1999; Reynolds et al. 2002; Watanabe et al. 2002). There is also evidence that the NG2+ cells are able to replenish their number following remyelination (Cenci di Bello et al. 1999; Levine & Reynolds, 1999), which implies the ability to divide asymmetrically. For this reason it has been suggested that NG2+ cells in the adult CNS may possess stem cell-like characteristics, including multipotentiality.
The lineage potential of a cell becomes progressively restricted as development proceeds, and hence in the adult most cell types are unipotent. However, adult neural stem cells (NSCs) found within restricted regions of the CNS [subventricular zone (SVZ) and hippocampus] have been described (Alvarez-Buylla & Temple, 1998), although it is unknown whether these stem cells are the same as the neuroepithelial-derived stem cells seen during development. What is the relationship of adult NSCs to the adult NG2-expressing cells? It has recently been suggested that adult NG2+ cells may in fact be multipotent NSCs (Belachew et al. 2003). Belachew et al. (2003) investigated the possible multipotentiality of NG2+ cells in the postnatal CNS both in vitro and in vivo, in a transgenic mouse model in which GFP expression is under the control of the CNP gene promoter. NG2+/CNP-GFP+ cells isolated from the postnatal day 2 (P2) brain were able to generate neurons, oligodendrocytes and astrocytes in culture. In vivo, NG2+/CNP-GFP+ cells in the hippocampus generated predominantly oligodendrocytes but never astrocytes. In addition, 10% of the NG2+/CNP-GFP+ cells in the P30 hippocampus also expressed a marker of immature neurons, TOAD-64, indicating that a proportion of newly differentiated neurons were derived from NG2+ precursors. NG2+ cells isolated from P2 brain could also generate hippocampal neurons when transplanted into the lateral ventricles of mice (Aguirre et al. 2004). These studies clearly show that a proportion of NG2+ cells seen during early postnatal development are intrinsically multipotent in vitro and can generate small numbers of neurons in the hippocampus in vivo. It was suggested that NG2+ cells might represent a sizeable pool of progenitor cells that could generate new GABAergic neurons in the adult hippocampus. However, the study provides no evidence that the NG2+ cell population that remains in the fully mature CNS is actually multipotent in vivo. Whether a similar multipotentiality might exist in CNS regions in which neurogenesis does not occur in the adult also remains to be investigated.
NG2+ cells respond to demyelination by rapid division
Understanding and elucidating the mechanisms underlying remyelination is essential if we are ever to understand the reasons for the development of the chronic demyelinated plaques seen in MS. In experimental models of demyelination, remyelination of denuded axons is usually an extremely rapid and efficient repair process, regardless of the method of induction of demyelination (Levine et al. 2001; Franklin, 2002). It has been demonstrated that cell proliferation is required in order to replenish lost oligodendrocytes (Blakemore & Keirstead, 1999). Oligodendrocytes that survive the demyelinating insult are not thought to be able to contribute to remyelination as evidence demonstrates they are not induced to proliferate (Keirstead & Blakemore, 1997; Redwine & Armstrong, 1998). Instead, endogenous oligodendrocyte progenitor cells local to the lesion are believed to be the source of new myelinating cells. Retroviral labelling studies have shown that a mitotically active population of immature cells were able to give rise to myelinating oligodendrocytes following lysolecithin-induced demyelination (Gensert & Goldman, 1997). Previous sections of this review have illustrated that NG2+ cells are the major cycling cell within the resting adult CNS (Horner et al. 2000; Dawson et al. 2003) and express many characteristics of oligodendroglial lineage cells, not least their ability to differentiate into oligodendrocytes when isolated into culture. Their role in demyelination and remyelination has been extensively studied and much suggests that they are the cells responsible for myelin repair.
In response to a variety of demyelinating insults, NG2+ cells undergo well-characterized reactive changes. Morphologically their cell bodies become enlarged, processes become shorter and thicker, and there is up-regulation of NG2 immunoreactivity (Keirstead et al. 1998; Cenci di Bello et al. 1999; Levine & Reynolds, 1999; Reynolds et al. 2002; Watanabe et al. 2002). In addition, NG2+ cells within the lesion and at the lesion edge rapidly proliferate as assessed by BrdU incorporation (Keirstead et al. 1998; Levine & Reynolds, 1999; Watanabe et al. 2002) or the presence of mitotic figures (Cenci di Bello et al. 1999; Reynolds et al. 2002). This results in a 3–10-fold increase in NG2+ cell number in the lesion after several days. In comparison with other glial cell types, NG2+ cells are the first to react to the damage (Levine & Reynolds, 1999) and show the highest proliferation rate in response to demyelination (Watanabe et al. 2002). Similar reactive and proliferative changes have been demonstrated in PDGFαR-expressing cells in mouse hepatitis virus- (Redwine & Armstrong, 1998) and ethidium bromide- (Sim et al. 2002) induced demyelination.
Although there has yet to be a direct demonstration that NG2+ cells give rise to remyelinating oligodendrocytes in these demyelinating models, the presence of newly generated oligodendrocytes within remyelinated lesions, some of which co-express NG2 and CNP (Reynolds et al. 2002), implicates them in such a role. More importantly, in various experimental demyelinating models, NG2+ cell number decreases upon onset of remyelination, suggesting that at least some of the NG2+ cells have developed into oligodendrocytes (Levine & Reynolds, 1999; Reynolds et al. 2002; Watanabe et al. 2002), although the degree of apoptosis in this cell population has yet to be investigated. More convincing evidence that NG2+ cells are the source of remyelinating oligodendrocytes comes from Watanabe et al. (2002), who demonstrated the presence of BrdU+/APC+ cells prior to remyelination concomitant with a decrease in BrdU+/NG2+ cells indicating their differentiation into oligodendrocytes.
This rapid proliferative response of NG2+ cells is only observed when the insult to the CNS causes demyelination or generalized tissue destruction. It is not seen for example when inflammation is not accompanied by demyelination (Cenci di Bello et al. 1999) or following viral infection (Levine et al. 1998). In these circumstances NG2+ cells demonstrate reactive changes but do not increase in number, suggesting that proliferation is initiated by signalling from the exposed axon or tissue debris, in combination with synergistic signalling from astrocytes and microglia/macrophages (Franklin, 2002).
Conclusions
NG2-expessing cells represent an abundant, slowly cycling population of cells that are distributed throughout the adult CNS. Their immunological phenotype suggests that the majority of these cells are related to the oligodendrocyte lineage, although the time point during development at which they diverged from cells destined to become myelinating oligodendrocytes is not yet clear. When isolated from the adult CNS into culture they can be differentiated into oligodendrocytes or astrocytes, but their potential for differentiation down multiple lineages in vivo has yet to be fully demonstrated in the adult. Their widespread distribution and physiological and molecular characteristics suggest a role in the maintenance of the extracellular microenvironment of neurons, both in the white and the grey matter, in addition to their role as OPCs. They are ideally located to monitor and respond to a wide variety of injurious insults but their response to these events other than to give rise to new oligodendrocytes for remyelination is as yet unknown.
Acknowledgments
Work described in this review carried out by the authors was funded by The Wellcome Trust, The Multiple Sclerosis Society of Great Britain and Northern Ireland and the Berkeley Group plc. We thank Dr Mary Dawson and Mr Arsalan Zamir for supplying images.
References
- Aguirre A, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Temple S. Stem cells in the developing and adult nervous system. J Neurobiol. 1998;36:105–110. [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE. Novel stage in the oligodendrocyte lineage defined by reactivity of progenitors with R-mAb prior to O1 anti-galactocerebroside. J Neurosci Res. 1992;32:309–316. doi: 10.1002/jnr.490320303. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Keirstead HS. The origin of remyelinating cells in the central nervous system. J Neuroimmunol. 1999;98:69–76. doi: 10.1016/s0165-5728(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Cenci di Bello I, Dawson MRL, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol. 1999;28:365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MRL, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dawson MRL, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Domercq M, Sanchez-Gomez MV, Areso P, Matute C. Expression of glutamate transporters in rat optic nerve oligodendrocytes. Eur J Neurosci. 1999;11:2226–2236. doi: 10.1046/j.1460-9568.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Zhao C, Franklin RJ. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27:247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Hall A, Giese NA, Richardson WD. Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development. 1996;122:4085–4094. doi: 10.1242/dev.122.12.4085. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Kirchhoff F, Kettenmann H. GABA triggers a [Ca2+]i increase in murine precursor cells of the oligodendrocyte lineage. Eur J Neurosci. 1992;4:1049–1058. doi: 10.1111/j.1460-9568.1992.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff MC. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kugler P, Schmitt A. Glutamate transporter EAAC1 is expressed in neurons and glial cells in the rat nervous system. Glia. 1999;27:129–142. doi: 10.1002/(sici)1098-1136(199908)27:2<129::aid-glia3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- Levine JM, Enquist LW, Card JP. Reactions of oligodendrocyte precursor cells to alpha herpesvirus infection of the central nervous system. Glia. 1998;23:316–328. [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004;47:290–298. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yu M, Drazba JA, Tuohy VK. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res. 1997;48:299–312. doi: 10.1002/(sici)1097-4547(19970515)48:4<299::aid-jnr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Noll E, Miller RH. Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development. 1993;118:563–573. doi: 10.1242/dev.118.2.563. [DOI] [PubMed] [Google Scholar]
- Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Herschkowitz N. Oligodendroglial and astroglial heterogeneity in mouse primary CNS culture as demonstrated be differences in GABA and d-aspartate transport and immunocytochemistry. Dev Brain Res. 1987;36:13–25. doi: 10.1016/0165-3806(87)90061-7. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Dawson MRL, Papadopoulos D, et al. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJM. The age-related decrease in CNS remyelination efÆciency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Raff MC. Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol. 2000;148:971–984. doi: 10.1083/jcb.148.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit S, Martinez S, Allinquant B, Peyron F, Puelles L, Zalc B. Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J Neurosci. 1995;15:1012–1024. doi: 10.1523/JNEUROSCI.15-02-01012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Tekki-Kessaris N, Stiles CD, Rowitch DH, Richardson WD. Oligodendrocyte development in the spinal cord and telencephalon: common themes and new perspectives. Int J Dev Neurosci. 2001;19:379–385. doi: 10.1016/s0736-5748(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Wu HY, Dawson MRL, Reynolds R, Hardy RJ. Expression of QKI proteins and MAP1B distinguishes actively myelinating oligodendrocytes in adult rat brain. Mol Cell Neurosci. 2001;17:292–302. doi: 10.1006/mcne.2000.0941. [DOI] [PubMed] [Google Scholar]
- Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. J Neurosci Res. 2002;70:529–545. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]