Abstract
The time course and cellular basis for inflammation-induced hypertrophy of adipose tissue were investigated over 20 weeks in mature male rats. Mild inflammation was induced by subcutaneous injection of 20 µg lipopolysaccharide into one hind-leg three times/week for 4 or 8 weeks, followed by up to 12 weeks ‘rest’ without intervention. Mean volume and frequency of apoptosis (TUNEL assay) were measured in adipocytes isolated from sites defined by their anatomical relations to lymph nodes, plus numbers of CCL21-stimulated lymph node-derived and adipose tissue-derived dendritic cells. Experimental inflammation increased dendritic cells and adipocyte apoptosis in the locally stimulated popliteal depot and the lymphoid tissue-associated regions of the contralateral popliteal and mesentery and omentum. Responses declined slowly after inflammation ended, but all measurements from the locally stimulated popliteal depot, and the omentum, were still significantly different from controls after 12 weeks rest. The locally stimulated popliteal adipose tissue enlarged by 5% within 4 weeks and remained larger than the control. We conclude that prolonged inflammation induces permanent enlargement, greater adipocyte turnover and increased dendritic cell surveillance in the adjacent adipose tissue and the omentum. The experiment suggests a mechanism for selective hypertrophy of lymphoid tissue-associated adipose tissue in chronic stress and inflammatory disorders, including impaired lymph drainage, Crohn's disease and HIV-associated lipodystrophy, and a link between evolutionary fitness, sexual selection and aesthetically pleasing body symmetry. It would be useful for further study of molecular mechanisms in inflammation-induced local hypertrophy of adipose tissue and development of specific therapies that avoid interference with whole-body lipid metabolism.
Keywords: apoptosis, dendritic cells, mesentery, omentum, perinodal adipose tissue, popliteal, rats, site-specific properties
Introduction
The involvement of adipose tissue in inflammatory processes is now widely recognized (Lyon et al. 2003; Trayhurn & Wood, 2004; Trayhurn, 2005). However, most current research is directed towards investigating the infiltration of macrophages and certain kinds of lymphocytes (Weisberg et al. 2003; Curat et al. 2004; Robker et al. 2004) and adipocyte secretions (Rajala & Scherer, 2003) that may contribute to whole-body disorders such as diabetes and obesity (Wellen & Hotamisligil, 2005) and cardiovascular disease (Khovidhunkit et al. 2004). Little is known about how chronic inflammatory conditions alter the structure of the adipose tissue itself.
Functional specialization of adipocytes and the possibility that minor depots may have site-specific properties are becoming more generally accepted in vascular biology (Lohn et al. 2002; Verlohren et al. 2004), cardiology (Montani et al. 2004) and immunology (Taub & Longo, 2005). Adipocytes in depots that enclose lymph nodes or other dense masses of lymphoid tissue have many site-specific physiological properties (Pond, 2003), some of which can be positively identified in humans (Westcott et al. 2005) and persist in tissue culture (Tchkonia et al. 2005).
Adipose depots that incorporate lymph nodes or omental milky spots are much more richly perfused with dendritic cells (DCs) than the more widely studied nodeless depots (Mattacks et al. 2004a), and perinodal adipocytes respond strongly and specifically to tissue-derived and to node-derived DCs (Mattacks et al. 2005). Previous experiments have demonstrated the formation of more adipocytes near the site of inflammation after as little as 6 weeks (Mattacks et al. 2003), but the cellular mediators of this response, and how, if at all, the process is reversed after inflammation ends was not investigated. During low-grade inflammation, more DCs are extracted from node-containing depots (Mattacks et al. 2004a), and they elicit higher rates of lipolysis in perinodal adipocytes (Mattacks et al. 2005). Higher extracellular concentrations of fatty acids promote the proliferation and maturation of adipocytes, and hence adipose tissue hypertrophy (Hausman et al. 2001; MacDougald & Mandrup, 2002). Similar mechanisms operating locally in response to prolonged inflammation would lead to proliferation of adipocytes that are associated anatomically and metabolically with activated lymphoid tissues, and thus to permanent hypertrophy of those adipose depots (Pond, 2003).
This study investigates cellular bases for long-term changes in the cellular structure of node-containing adipose tissue during and after chronic local inflammation of a peripheral lymph node. The small adipose depots enclosing the popliteal lymph nodes are chosen for study because they are very consistent in size and structure, thus facilitating the identification of exactly homologous samples from the left and right hind legs, and are composed of adipocytes that interact strongly with lymphoid cells (Pond & Mattacks, 1995). The popliteal lymph nodes are quiescent in healthy animals but can be stimulated locally by minimally invasive procedures (Smith & Morris, 1970).
Materials and methods
Animals
This experiment used 129 adult male Sprague–Dawley rats that were kept from weaning onwards in permanent groups of three of similar age, usually littermates. The rats were fed ad libitum on RM3 chow (Special Diet Services, Witham, UK) and were housed with environmental enrichment (cardboard tunnels, materials to chew, etc.) that promotes welfare and minimizes boredom and stress. To keep body mass and total fatness as constant as possible, the rats were about 5 months old and at least 400 g at the start of the experiment. Chronic, local, low-level immune disturbance was generated by injecting 20 µg lipopolysaccharide (LPS) into the lateral skin of the left lower leg (the ‘catchment’ area of the left popliteal lymph node) three times a week for 4 or 8 weeks. Of those injected for 8 weeks, seven groups (i.e. 3 × 7 = 21 rats) were killed at once, and the others were ‘rested’ for 4, 8 or 12 weeks under the same conditions as the unaltered controls. All cage-mates were subject to the same procedures at the same time.
Animals were killed via carbon dioxide anaesthesia, after which each rat was weighed and the epididymal, perirenal/retroperitoneal, mesenteric, omental, inguinal, interscapular and popliteal adipose depots were dissected out, washed in Hank's balanced salt solution (HBSS) and weighed. These depots together comprise almost all visible adipose tissue and can be used to calculate fatness (fresh weight of adipose tissue as percentage total body mass). The stimulated and unstimulated popliteal lymph nodes, and some conspicuous mesenteric lymph nodes, were also dissected out and weighed.
The perinodal adipose tissue is defined as that within 2 mm of the popliteal lymph nodes, ‘middle’ as that about 5 mm from the nodes and ‘remote’ as that more than 10 mm from the lymph nodes (Mattacks et al. 2003). Samples were chosen entirely on the basis of their anatomical relations to the nodes; apart from small differences in adipocyte volume, the microscopic appearance of fresh perinodal adipose tissue is similar to that of the rest of the depot. Perinodal, middle and remote samples were dissected from both popliteal depots together with samples of the nodeless perirenal depots. The average yield of perinodal adipose tissue from each popliteal depot was only 27 mg wet weight (i.e. about 80 mg from each group of three rats), so all methods were adapted for use on such small samples.
Quantification of DCs
DCs were isolated from adipose tissue, identified and counted by established methods (Mattacks et al. 2004a,b, 2005). Samples (50 mg) of adipose tissue were cut into 1–2-mm3 fragments and washed for 5 min in HBSS containing 0.1% bovine serum albumin (BSA) and 1 ng/mL of the chemokine CCL21 (also called C6kine, R&D Systems, Abingdon, UK), which stimulates migration of DCs (Randolph, 2001). After rinsing with HBSS, each adipose sample and the lymph nodes, cut open to release their contents, were re-suspended in 5 mL HBSS with 0.1% BSA in 15-mL Falcon tubes. The tubes shaken at 100 cycles min−1 in a waterbath at 37 °C for 4 h, allowing the dendritic cells to emerge from the adipose tissue and sink. After incubation, the adipose tissue and 4.5 mL of media were removed from each sample, leaving the dendritic cells in 0.5 mL. They were washed three times by filtering with a 10 µm centrifuge filter and resuspended in 0.5 mL HBSS + 0.1% BSA. DCs were identified by their size, distinctive crinkled outline and selective binding of specific antibodies: 5 min with 0.01% peroxidase indicator reagent (Sigma) and a 1 : 20 solution of FITC anti-rat MHC-II (I-E) marker [BD Biosciences (Pharmingen) Oxford, UK]. The cells were counted with a haemocytometer using bright-field and fluorescence microscopy, to a precision of ± 10 cells, from six separate 10 µL samples from each mesenteric and omental adipose tissue preparation and the lymph nodes, and three from each popliteal adipose site (pooling material from homologous sites of cage-mate rats). A sample of perirenal adipose tissue was also examined. The results were calculated as DCs per 0.1 mL (i.e. those that migrated from 50 mg of adipose tissue).
The left and right large popliteal lymph nodes and two large mesenteric lymph nodes were cut open, and the leukocytes released into HBSS. Each sample of leukocytes was centrifuged and layered in Histopaque (Sigma), and the DCs were separated from the other classes of leukocytes and counted using the method described above.
Adipocyte isolation
Collagenase isolation of adipocytes and cell sizing were performed within 4 h of dissection. The adipose tissue samples were cut up with scissors into approximately 2-mm pieces, washed in HBSS and incubated in 10 mL Krebs–Henseleit buffer with 2 mg collagenase at 37 °C for 30 min in a shaking waterbath (approximately 100 cycles min−1). The released adipocytes floated to the surface and were removed using a siliconized pasteur pipette, washed in fresh HBSS twice and then resuspended in 0.1 mL HBSS. The remaining stroma was spun at 1500 r.p.m. in an MSE Coolspin centrifuge, washed twice with HBSS and resuspended into 0.1 mL HBSS.
Adipocyte volume
The diameters of about 50 adipocytes were measured from three 10 µL aliquots from each tissue sample using a haemocytometer on a light microscope with a low-power objective and an eye-piece graticule. The mean adipocyte volume of each such sample was calculated from the mean and standard deviation of measurements of adipocyte diameter using Goldrick's equations (Goldrick, 1967).
Apoptotic adipocytes
Apoptotic nuclei were identified with terminal deoxynucleotidyl transferase (TdT) stain with red/fluorecein label using Guava TUNEL kits. Assays were carried out at about 1, 6 and 24 h post mortem, on cells freshly prepared from tissue that had been incubated as 1–2-mm3 explants at 37 °C in a 5% CO2/air incubator suspended in HBSS. The TdT stain was mixed with samples of about 5000 collagenase-isolated adipocytes and allowed to stand for 10 min. The cells were washed twice through centrifuge-filters and resuspended in 0.1 mL HBSS for counting with a haemocytometer.
Data analysis and display
Data were analysed with the PC version of Statistical Package for Social Sciences (SPSS) and Microsoft Excel. Differences between means were assessed using Student's t-test. Where possible, measurements from each of the 129 rats were recorded and analysed, but for the reasons explained above, it was usually necessary to pool homologous samples from cage-mates. SEs that are missing from graphs are too small to be displayed.
Results
Gross anatomy
Table 1 lists data on the body composition of the rats at the time of death. Small differences in mean age of the six groups arose from the need to keep littermates together throughout life. As often happens in long-term experiments, one rat usually became larger (though not necessarily fatter) than his cage-mates, increasing the variance of body mass and fatness. The average fatnesses of the groups were not significantly different except for the rats that were rested for 12 weeks, which were slightly fatter (P < 0.05). The data indicate that the experimental regimes did not impair appetite or induce significant stress, fever or emaciation.
Table 1.
Age, body composition and properties of the lymph nodes of the rats. Means and standard deviations (SD) of age and masses are calculated from measurements from each rat (n = 24 for controls, n = 21 for other groups). Dendritic cell counts are means of values from tissues pooled from the three cage-mates (n = 8 × 3 for controls, N = 7 × 3 for other groups)
| LPS (20 µg) injected three times/week for 8 weeks | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uninjected controls | LPS (20 µg) injected for 4 weeks | no rest | +4 weeks rest | +8 weeks rest | +12 weeks rest | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age ( days) | 220 | 20 | 171 | 17 | 197 | 22 | 220 | 14 | 238 | 12 | 258 | 11 |
| Body mass (g) | 511 | 46 | 512 | 68 | 522 | 58 | 559 | 42 | 586 | 94 | 559 | 77 |
| Fatness (all adipose tissue %BM) | 1.92 | 0.19 | 1.79 | 0.17 | 1.81 | 0.10 | 1.96 | 0.38 | 2.08 | 0.03 | 2.27 | 0.21 |
| Fresh mass of single major lymph node (mg) | ||||||||||||
| Stimulated POP | 12.5 | 0.48 | 20.0 | 2.3 | 18.8 | 1.7 | 17.8 | 0.38 | 17.4 | 0.48 | 16.7 | 0.79 |
| Unstimulated POP | 12.6 | 0.45 | 15.8 | 2.0 | 14.7 | 1.4 | 14.0 | 0.09 | 13.7 | 0.30 | 13.2 | 0.35 |
| Mesenteric | 20.0 | 0.33 | 20.5 | 0.23 | 20.4 | 0.40 | 20.4 | 0.36 | 20.2 | 0.31 | 20.1 | 0.26 |
| CCL21-stimulated DCs from lymph node (×103) | ||||||||||||
| Stimulated POP | 5.84 | 0.15 | 9.10 | 0.55 | 9.64 | 0.32 | 7.77 | 0.34 | 7.36 | 0.11 | 6.96 | 0.10 |
| Unstimulated POP | 5.86 | 0.16 | 6.41 | 0.16 | 7.00 | 0.11 | 6.46 | 0.82 | 6.06 | 0.07 | 6.25 | 0.03 |
| Mesenteric | 6.72 | 0.15 | 6.63 | 0.10 | 7.02 | 0.07 | 7.06 | 0.39 | 6.56 | 0.05 | 6.82 | 0.44 |
LPS, lipopolysaccaride.
As intended, the injection of LPS increased the mass of the ipsilateral popliteal lymph node by up to 59%, but the response was rather variable, as indicated by the larger SDs of mean values for the treated rats compared with the controls (Table 1). The response seems to be complete within 4 weeks, as there was no difference between the sizes of homologous nodes after 4 or 8 weeks on LPS (P = 0.07 NS). As expected, the nodes were smaller in the ‘rested’ rats that in those culled while still on the pro-inflammatory regime, but the effects of immune stimulation were not fully reversed during the experimental period: even after 12 weeks rest, the locally stimulated popliteal lymph node was 33% larger than that of the controls, and 26% larger than its homologue in the other leg (P < 0.001). Enlargement of the contralateral popliteal lymph nodes followed an almost identical time course, although the increases were smaller (maximum 25%), and were not completely reversed after prolonged rest (5% larger than the unaltered controls, P < 0.001). However, the mean mass of the mesenteric lymph nodes was similar in all groups of rats.
Dendritic cells
The numbers of DCs extracted from the lymph nodes changed in a qualitatively similar way to their gross mass (Table 1). The injection of LPS increased the numbers of DCs extracted from the ipsilateral popliteal lymph node by 56% after 4 weeks (65% after 8 weeks), and numbers were still 19% higher than in the controls after 12 weeks of recovery after the stimulation regime (P < 0.001). Changes in the contralateral popliteal lymph nodes were delayed and much attenuated, but remarkably similar. By the end of the experiment, 7% more DCs emanated from this node. A small (5%) but significant (P < 0.001) increase in DCs emanating from the mesenteric lymph nodes was also recorded but the effect disappeared within 8 weeks after experimental inflammation ended.
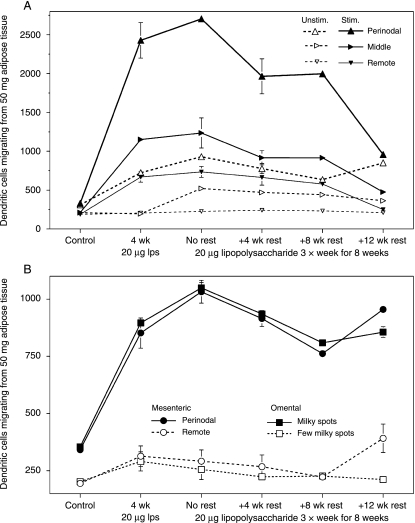
Figure 1 shows the numbers of DCs migrating from adipose tissue associated with lymphoid structures. Our procedures yielded no DCs from samples of the perirenal adipose tissue of these rats. In the resting state, the numbers of DCs were low and almost equal throughout the popliteal adipose tissue (Fig. 1A). Injecting LPS increased DCs throughout the ipsilateral popliteal depot, by up to 275% in the perinodal region, and in the perinodal sample of the contralateral depot. Prolonging the treatment from 4 to 8 weeks made no difference to DC numbers in the adipose tissue near to the site of stimulation, but it did increase DC permeation of the contralateral depot except in the remote-from-node samples. The increase in adipose tissue DCs reversed only slowly: 8 weeks after immune stimulation stopped, the perinodal adipose tissue from the stimulated leg still contained six times as many DCs as the controls. By the end of the experiment, DC numbers were similar in all three homologous samples from the ipsi- and contralateral popliteal depots, but there were almost three times as many DCs in the perinodal adipose tissue of both legs as in the homologous samples from the controls (t = 57.8, P < 0.001).
Fig. 1.
Time course of numbers of dendritic cells extracted from the adipose samples. Mean ± SE of numbers of CCL21-stimulated dendritic cells collected over 4 h from 50 mg adipose tissue. (A) Perinodal (large upright triangles), middle (right-pointing, middle-sized triangles) and remote (small inverted triangles) adipocytes from the popliteal adipose depots of the stimulated (solid symbols and lines) and unstimulated (open symbols and broken lines) legs. (B) Mesentery (circles) and omentum (squares), perinodal or milky spot-rich (closed symbols) and remote-from-lymphoid structure (open symbols). n = 8 × 3 cage-mate large male rats for controls, and 7 × 3 for other groups.
The experimental inflammation also raised the numbers of DCs emanating from the mesenteric perinodal and omental milky spot-rich adipose tissue by up to three-fold (Fig. 1B). Extending the LPS treatment from 4 to 8 weeks further increased DC numbers by 17–21%. In number of DCs, these intra-abdominal depots remote from the site of inflammation resembled the contralateral popliteal depot but contrasted with the locally stimulated popliteal depot (Fig. 1A). Changes in the remote-from-lymphoid tissue samples were much smaller, although significant (mesenteric: t = 2.84; omental: t = 2.24, P < 0.05), and reached their maximum after 4 weeks of treatment (Fig. 1B). In the perinodal and omental milky spot-rich tissue, DC numbers declined surprisingly little during the long rest period; all measurements recorded more than twice as many DCs as in the controls.
Adipocyte volume and adipose mass
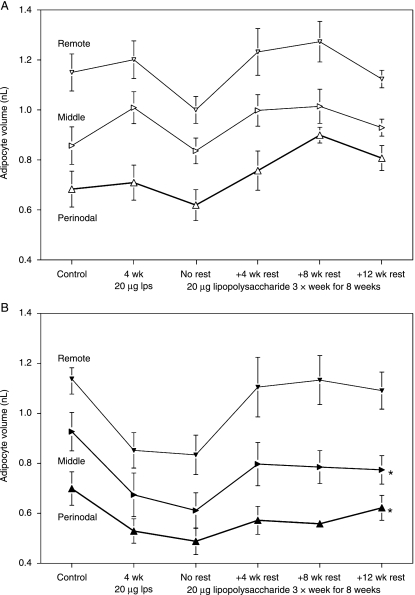
Figure 2 shows the mean volume of about 50 adipocytes from the three sample sites of both popliteal depots. The fairly large standard errors (SEs) of the means reflect the substantial variation between individual rats in the cellular composition of all adipose depots studied (i.e. the adipose tissue of some rats consists of many small adipocytes, whereas other specimens of similar overall body composition have fewer, larger adipocytes). However, the very high concordance between the measurements from homologous samples from the left and right legs of the controls demonstrates the reproducibility of the technique. As expected from previous measurements from wild and laboratory animals (Pond, 1992; Mattacks et al. 2003), there were clear and consistent gradients in adipocyte size within the popliteal depots, with the smallest adipocytes closest to the lymph nodes.
Fig. 2.
Site-specific differences in adipocyte volume and time course of its changes. Mean ± SE of volume (nl) of perinodal (upright large triangles), middle (right-pointing, middle-sized triangles) and remote (small inverted triangles) adipocytes from the popliteal adipose depots of the unstimulated (A) and stimulated leg (B). n = 8 × 3 cage-mate rats for controls, and 7 × 3 for other groups. *Differences between homologous samples from stimulated and unstimulated legs significant at P < 0.05.
Mean adipocyte volume declined by up to 30% following experimental inflammation throughout the popliteal depot of the locally stimulated leg, with the largest changes recorded from the remote-from-node samples (Fig. 2B). A similar pattern of changes in the contralateral popliteal depot did not occur until 8 weeks of LPS treatment (Fig. 2A). In both popliteal depots, mean adipocyte volume at all sites sampled recovered after inflammation ended. However, recovery was most efficient in samples furthest from the inflamed lymph node. By the end of the experiment, the mean volumes of the perinodal and middle adipocytes from the stimulated popliteal depot were still significantly different from those of the corresponding samples from the unstimulated leg (P < 0.05). Measurements (data not shown) from the omentum and mesentery produce a similar picture, with remote inflammation leading to a small decline in mean adipocyte volume that is completely abolished by rest.
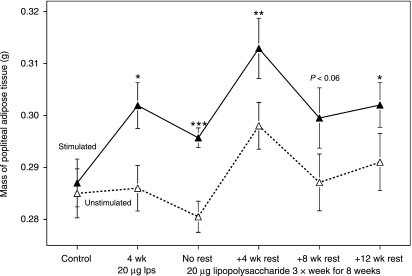
Although mean adipocyte volume declined, especially in the popliteal depot adjacent to the site of immune stimulation, the total mass of this depot increased (Fig. 3). As expected, the two popliteal depots were of almost identical size in the controls, and their absolute masses followed the minor changes in body size and total fatness (see Table 1). However, comparison of the two popliteal depots (Fig. 3) showed that local stimulation induced small but significant selective enlargement of the adjacent adipose tissue in a dose-dependent way and the effect was not completely reversed even after 12 weeks of rest. Particularly striking is the 5% increase in mass of the stimulated popliteal adipose depot after 4 weeks of local LPS administration relative to the controls and to the homologous unstimulated depot, even though the rats’ total fatness decreased by 7% (Table 1). Adipocyte volume changes (Fig. 2) and increased DC infiltration (Fig. 1A) were not fully developed in the contralateral unstimulated depot until 8 weeks of treatment, but the differences in mass of the two popliteal depots were similar at 4 and 8 weeks (Fig. 3).
Fig. 3.
Time course of changes in mass of the popliteal depots. Means ± SE of the gross mass (g) of the popliteal adipose depots from the stimulated (solid symbols and lines) and unstimulated (open symbols and broken lines) legs. n = 24 for controls, and 21 for other groups. Differences between popliteal depots from stimulated and unstimulated legs significant at: *P < 0.05; **P < 0.01; ***P < 0.001.
The total mass of the popliteal depot adjacent to the site of immune stimulation increased (Fig. 3) in spite of transient decreases in the mean volume of adipocytes (Fig. 2), so more adipocytes must have formed. Infiltrating DCs and other immune cells may contribute to the increased mass, but their presence is unlikely to explain all the changes in Fig. 3. The mass of the popliteal lymph nodes was only 4–7% of that of the surrounding adipose tissue (Table 1 and Fig. 3), so the total mass of lymphoid cells emanating from them would be much less than the 15-mg increase in average mass of the adipose tissue. The abundance of DCs (and probably other infiltrating lymphoid cells) declined considerably during the rest period (Fig. 1A), much more than the mass of the adipose tissue (Fig. 3).
The total mass of the omentum also increased by about 10% after 4 weeks of LPS treatment and remained enlarged for at least 4 weeks after experimental inflammation ended, possibly due in part to expansion of the lymphoid tissues. The masses of all the large adipose depots (inguinal, mesenteric, perirenal, etc.) are so variable between individuals that significant permanent changes during this brief experiment could not be demonstrated.
Apoptosis of adipocytes
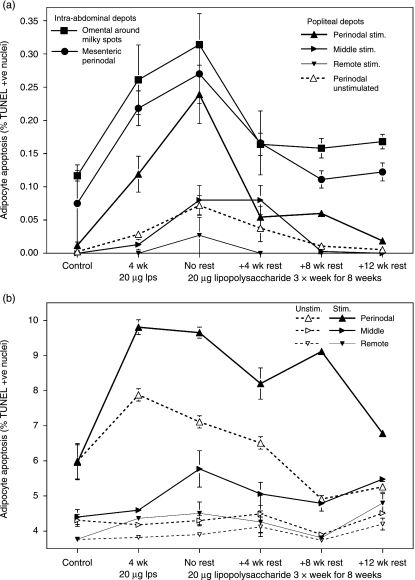
TUNEL kits were used to identify apoptotic nuclei among collagenase-isolated adipocytes, i.e. with lymphoid and other stromal cells in all samples at 1, 6 and 24 h post mortem. No apoptotic adipocytes were detected in any site at 1 h post mortem, and none was ever found in any of the samples from the perirenal (nodeless) adipose depot. At 6 h post mortem, an average of 14 ± 3 such adipocytes were recorded in the popliteal perinodal samples after 8 weeks of local stimulation with LPS (16 ± 3 in the mesenteric perinodal). At 24 h post mortem, an average of 557 ± 13 apoptotic nuclei were recorded in the popliteal perinodal adipocytes after 8 weeks of local LPS stimulation (average 450 ± 22 in the mesenteric perinodal, maximum 910 apoptotic adipocytes per sample).
No apoptotic adipocytes were detected in the resting popliteal depots at 6 h post mortem (Fig. 4A). Although a few apoptotic adipocytes could be seen in the omentum and mesentery, the numbers were still very low compared with those of many other tissues. Local inflammation increased the frequency of apoptosis among perinodal adipocytes near the site of stimulation, reaching a peak value similar to those measured from the intra-abdominal depots. Delayed, attenuated changes were found in the adipose samples from elsewhere in the ipsilateral popliteal depot, and in the contralateral perinodal tissues (all rates in the other contralateral samples were too low to be measured). Numbers of apoptotic adipocytes in the popliteal and mesenteric depots increased with more prolonged inflammation but declined quickly after the regime ended and disappeared by the end of the experiment. However, after 12 weeks without intervention, the rate of apoptosis in adipocytes from the milky spot-rich region of the omentum was still significantly higher than that of the controls (t = 3.82, P < 0.01).
Fig. 4.
Site-specific differences in adipocyte apoptosis and time course of its changes. Mean ± SE of the relative abundance (%) of TUNEL-positive apoptotic adipocytes isolated from perinodal (large upright triangles), middle (middle-sized right-pointing triangles) and remote (small inverted triangles) samples from the popliteal depots of the stimulated (solid symbols and lines) and unstimulated (open symbols and broken lines) legs, and from the perinodal mesenteric (circles) and omental milky spot-rich tissue (squares). (A) Within 6 h post mortem; (B) 24 h post mortem (data from popliteal depots only shown). n = 8 × 3 cage-mate rats for controls, and 7 × 3 for other groups.
Apoptotic adipocytes were much more abundant after 24 h of incubation ex vivo (Fig. 4B). At least 3% of adipocyte nuclei that stained positive for TdT could be found in all popliteal samples (and in all the intra-abdominal samples studied, including perirenal; data not shown). The similarities of the site-specific differences and time courses of changes between the data from freshly excised (Fig. 4A) and incubated material (Fig. 4B) indicate that the latter data reflect natural processes. Basal rates of apoptosis were much higher in perinodal samples than in those remote from nodes and they increased fastest and most extensively following LPS stimulation. As for other parameters (Figs 1a,2A and 4A), all changes in the contralateral popliteal adipose depot were delayed and attenuated relative to the ipsilateral. By the end of the experiment, rates of apoptosis in all samples from the locally stimulated popliteal depot were still significantly (P < 0.001) higher than those of their homologues in the other depot, and (except the perinodal) than in the controls. This effect was not observed in the contralateral popliteal depot or in any of the intra-abdominal depots studied.
Discussion
We tried to keep the rats’ body mass and fatness as constant as possible during this 5-month experiment. Each dose of 20 µg LPS was less than half that required to generate measurable hyperthermia, and 1–10% of that used to generate fever in adult rats (Rosenthal et al. 1996). The immune stimulation regimes were sufficient for signals to pass from the locally stimulated lymph node to the adipose tissue surrounding other nodes, as has previously been found (Pond & Mattacks, 2002; Mattacks et al. 2003, 2004b), but did not produce a systemic immune response involving fever.
Lymphoid cells comprise only a very small fraction of the total mass of mature adipose tissue (Weisberg et al. 2003; Robker et al. 2004), so the numbers of DCs that could be extracted from the anatomically defined tissue samples were orders of magnitude too low for further separation and characterization of surface proteins. The constant but comparatively low abundance of DCs in the popliteal lymph nodes (Table 1) and surrounding adipose tissue (Fig. 1A) of unaltered rats is consistent with the fact that these nodes are immunologically quiescent in healthy animals (Smith & Morris, 1970). The experimental regime increased DC numbers in the perinodal adipose tissue of remote (Fig. 1A, B) as well as contiguous (Fig. 1A) depots much more than in the lymph nodes themselves (Table 1). The data in Fig. 1 confirm reports that even very mild inflammation increases the numbers of DCs in associated adipose tissue (Mattacks et al. 2004a), and demonstrate that DC surveillance remains high in perinodal adipose tissue in depots remote from, as well as adjacent to, the inflammatory focus long after the stimulus ends.
At around 10 µm3 in volume, DCs are minute compared with mature adipocytes (see Fig. 2) and the numbers recorded (Fig. 1) are low compared with those reported for other tissues. So the mass of the DCs would make only a tiny contribution to the mass of adipose tissue as a whole, not enough to explain more than a very small fraction of the growth of the stimulated popliteal depot (Fig. 3).
Macrophages and certain kinds of lymphocytes become more numerous in epididymal, parametrial and other large nodeless adipose depots of genetically obese mice (Weisberg et al. 2003; Robker et al. 2004). These findings have generated much interest in the possible role of chronic inflammation in the pathology of long-term obesity (Fantuzzi, 2005; Wellen & Hotamisligil, 2005). However, more recent investigations showed that diet-induced obesity in genetically unaltered rats failed to induce similar changes in markers of inflammation (Bedoui et al. 2005). Our rats were not obese and the changes in DC numbers were limited to anatomically defined adipose sites that together comprised only a small fraction of the total adipose mass. The relationship between macrophage accumulation and other indicators of inflammation in ‘general-purpose’ adipose tissue in long-term obesity and inflammation-induced increase in DC surveillance remains to be explored.
The rates of apoptosis shown in Fig. 4 A are similar to those reported in mature adipose tissue from the epididymal and perirenal depots of adult rats (0.3–0.5%) (Duff et al. 2004) and human omental and superficial abdominal adipose tissue (less than 1%) (Li et al. 2004). The very low frequency of apoptotic adipocytes in the popliteal depots of the controls, and the slightly greater numbers in the omentum and mesentery, are consistent with the quiescent state of the popliteal lymph nodes (Smith & Morris, 1970), and the continuous, low-level activity of the omental and gut-associated lymphoid tissue (Stagg et al. 2003). The higher rates of apoptosis in the mesenteric and omental adipocytes are consistent with intrinsic site-specific differences in pre-adipocyte proliferation and maturation that have been described for human samples in tissue culture (Tchkonia et al. 2002): omental adipocytes accumulate less lipid and differentiate more slowly than mesenteric adipocytes, with the subcutaneous samples that are not associated with lymphoid tissue in vivo maturing most readily.
The clear increase in adipocyte apoptosis in all perinodal samples following mild inflammation could be mediated by secretions from the lymphoid cells themselves, or locally produced adipokines (Trayhurn, 2005). Rapid growth in adipose tissue has been postulated to lead to anoxia or insufficiency of other blood-borne nutrients (Trayhurn & Wood, 2004). Consistent with this interpretation is the observation (Fig. 4B) that after 24 h of incubation ex vivo, the maximum recorded abundance of apoptotic adipocytes was about 10%, 30 times higher than the values found in freshly excised material (Fig. 4A), suggesting that pro-apoptotic signals persist and are amplified within the intact tissue.
The data in Figs 2 and 4 demonstrate changes in the adipocytes themselves. Adipocytes are by far the largest component of adipose tissue in mature rats, so their responses to local inflammation must be the main contributors to the growth of the whole adipose depot (Fig. 3). Lipolysis in adipocytes in node-containing depots, especially perinodals, is stimulated by dendritic cells that permeate adipose tissue (Mattacks et al. 2005) and by cytokines, including tumour necrosis factor-α and various interleukins (Mattacks & Pond, 1999). Higher concentrations of non-esterified fatty acids outside adipocytes would promote the maturation and enlargement of pre-adipocytes (Hausman et al. 2001; MacDougald & Mandrup, 2002), thus increasing adipocyte complement. Maturation of pre-existing and/or newly formed pre-adipocytes changes the proportions of large, replete adipocytes and smaller maturing cells (Gullicksen et al. 2003b) and thus lowers the mean adipocyte volume (Fig. 2). Permanent increases in adipose tissue mass (Fig. 3) must entail proliferation and maturation of pre-adipocytes that is associated with increased apoptosis (Fig. 4).
Lymph node-derived lymphoid cells (Pond & Mattacks, 1995) and adipose tissue-derived DCs (Mattacks et al. 2005) stimulate lipolysis from perinodal adipocytes in vitro. In adipocyte cell lines and adipocytes isolated from nodeless depots, sirtuins both stimulate lipolysis and inhibit adipogenesis (Picard et al. 2004), leading to the suggestion (Unger, 2005) that fatty acid mobilization accompanies reduced apoptosis. The site-specific differences in apoptosis (Fig. 4) point to the opposite link: apoptosis is highest among adipocytes associated in vivo with the most DCs (Fig. 1) that best stimulate lipolysis (Mattacks et al. 2005). Lipotoxicity has been postulated as a promotor of apoptosis (Unger, 2003), but is unlikely to be a factor in the processes documented in Fig. 4 because the most strongly affected depot was growing (Figs 2 and 3) and the lipid content of perinodal adipocytes is less, not more, than that of adipocytes from nodeless depots of the same rats (Mattacks et al. 2003). Weak correlations between the changes in adipocyte volume (Fig. 2) and apoptosis (Fig. 3) in both time (i.e. responses to experimental treatments) and space (i.e. site-specific differences) are also not consistent with the hypothesis (Gullicksen et al. 2003a) that leptin stimulates adipocyte apoptosis.
These inconsistencies suggest that, as in other respects (Pond, 2003, 2005), adipocytes associated with lymphoid structures, especially perinodal adipocytes, do not share all the properties found in those from nodeless depots. Specializations for local interactions with lymphoid tissues should be considered when interpreting intrinsic site-specific properties of adipocytes (Tchkonia et al. 2005). The selective enlargement of the popliteal adipose tissue (Fig. 3) can be interpreted as an adaptive response to the adjacent inflammation and is consistent with the hypothesis (Pond, 2003; Pond, 2005) that the main function of anatomically minor, node-containing depots such as the popliteal node is to support immunological processes in adjacent lymphoid tissues.
Implications for human biology
These findings suggest explanations for the wide variation between conspecifics in the relationship between the size and abundance of adipocytes that is found in both humans (Sjöström & Björntorp, 1974) and wild and domesticated animals (Pond & Mattacks, 1985). Chronic inflammation, especially early in life while the adipose tissue is forming, could stimulate adipocyte proliferation more strongly or for longer than normal. ‘Hyperplastic’ obesity in humans, in which more mature adipocytes appear, has long been recognized as more difficult to treat than ‘hypertrophic’ obesity, in which adipocytes are enlarged (Strain et al. 1984; Hausman et al. 2001). Natural, seasonal obesity in wild animals appears to be almost always hypertrophic and, although we find much variation between individuals, there is no consistent relationship between adipose tissue cellularity and age (Pond, 1992). Local proliferation of adipocytes contiguous to chronically inflamed lymphoid tissue may also account for reports of an association between markers of viral infection and enlargement of certain adipose depots in domesticated birds, captive primates and humans (Dhurandhar et al. 2000). Such ‘obesity’ may not involve the usual metabolic correlates of obesity (Dhurandhar et al. 1997, 2000, 2002), and could be due to selective enlargement of adipose depots that enclose lymphoid tissue.
The selective hypertrophy of adipose tissue near the site of chronic, low-grade inflammation (Fig. 3) could underlie fat wrapping in Crohn's disease (Sheehan et al. 1992; Schäffler & Herfarth, 2005; Westcott et al. 2005), the growth of adipose tissue associated with chronic disorders of the lymphatic system (Herpertz, 2001; Rockson, 2004) and other diseases characterized by anomalous local growth of adipose tissue, such as HIV-associated adipose redistribution syndrome (Pond, 2003). Local inflammation-induced changes in the cellular structure (Fig. 2) and/or in the gross mass (Fig. 3) would lead to asymmetries in the size, shape and/or mechanical texture of paired adipose depots. The visual and tactile symmetry of many such paired depots, including those in the limbs and breasts, buttocks and cheeks, is important to sexual selection in many higher animals, including humans (Grammer et al. 2003). The formation of additional adipocytes seems to contribute more to adipose tissue growth in primates (Pond & Mattacks, 1987; Pereira & Pond, 1995) than in other mammals (Pond, 1992). This property, together with primates’ social habits and longevity and the importance of visual cues in communication, may increase the evolutionary significance of adipose depot symmetry.
The rapid and extensive response of the mesentery and omentum to inflammation of peripheral tissues (Figs 1 and 4A) would eventually produce abdominal hypertrophy and high waist/hip ratios in humans (Björntorp, 1996). Thick waists are common among people of average body mass who smoke heavily, which continually exposes them to toxins and irritants (Seidell et al. 1991), or who are frequently exposed to a wide variety of parasites and other pathogens (Singh, 1993). Low waist/hip ratio in women is consistently associated with bodily attractiveness and with a variety of biological measures of health and fertility, a finding that Grammer et al. (2003) deem ‘surprising’. Our experiment suggests how repeated or chronic infections, long-term stress or other sources of persistent inflammation could lead to higher waist/hip ratios.
Conclusions
This experiment reveals changes in the cellular structure of adipose tissue that long outlast the low-grade inflammation that induced them. The data are consistent with the hypothesis that perinodal adipose tissue is specialized for local interactions with lymphoid tissues. The processes described may contribute to selective hypertrophy of certain adipose depots in chronic inflammatory disorders, including impaired lymph drainage and Crohn's disease, and some forms of obesity, especially the slow expansion of the intra-abdominal mesentery and omentum, adipose depots that incorporate much lymphoid tissue. This experimental protocol would be useful for further study of the molecular mechanisms in the hypertrophy of adipose tissue associated with chronic inflammation and for the development of therapies that prevent or correct local problems without interfering with whole-body energy balance or lipid metabolism.
Acknowledgments
Dr R. H. Colby contributed helpful comments on an earlier version of the manuscript; Steve Walters and Karen Evans are thanked for their skilled technical assistance.
References
- Bedoui S, Velkoska E, Bozinovski S, Jones JE, Anderson GP, Morris MJ. Unaltered TNF-α production by macrophages and monocytes in diet-induced obesity in the rat. J Inflamm. 2005;2 doi: 10.1186/1476-9255-2-2. 10.1186/1476-9255-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björntorp P. The regulation of adipose-tissue distribution in humans. Int J Obesity. 1996;20:291–302. [PubMed] [Google Scholar]
- Curat CA, Miranville A, Sengenès C, et al. From blood monocytes to adipose tissue-resident macrophages – Induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obes Res. 1997;5:464–469. doi: 10.1002/j.1550-8528.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. Increased adiposity in animals due to a human virus. Int J Obesity. 2000;24:989–996. doi: 10.1038/sj.ijo.0801319. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Whigham LD, Abbott DH, et al. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002;132:3155–3160. doi: 10.1093/jn/131.10.3155. [DOI] [PubMed] [Google Scholar]
- Duff E, Li CL, Hartzell DL, Choi Y-H, Della-Ferra MA, Baile CA. Ciliary neurotrophic factor injected icv induces adipose tissue apoptosis in rats. Apoptosis. 2004;9:629–634. doi: 10.1023/B:APPT.0000038042.31683.7b. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Goldrick RB. Morphological changes in the adipocyte during fat deposition and mobilization. Am J Physiol. 1967;212:777–782. doi: 10.1152/ajplegacy.1967.212.4.777. [DOI] [PubMed] [Google Scholar]
- Grammer K, Fink B, Møller AP, Thornhill R. Darwinian aesthetics: sexual selection and the biology of beauty. Biol Rev. 2003;78:385–407. doi: 10.1017/s1464793102006085. [DOI] [PubMed] [Google Scholar]
- Gullicksen PS, Della-Ferra MA, Baile CA. Leptin-induced adipose apoptosis: implications for body weight regulation. Apoptosis. 2003a;8:327–335. doi: 10.1023/a:1024112716024. [DOI] [PubMed] [Google Scholar]
- Gullicksen PS, Hausman DB, Dean RG, Hartzell DL, Baile CA. Adipose tissue cellularity and apoptosis after intracerebroventricular injections of leptin and 21 days of recovery in rats. Int J Obes. 2003b;27:302–312. doi: 10.1038/sj.ijo.0802205. [DOI] [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Herpertz U. The most common forms of leg oedema, differentiation between venous oedema, lymphoedema and lipoedema. Phlebologie. 2001;30:48–52. [Google Scholar]
- Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- Li X, Chen R, Lindquist S, Hernell O. Expression of cellular inhibitor of apoptosis protein-2 in human subcutaneous and omental adipose tissue. Int J Obes. 2004;28:352–356. doi: 10.1038/sj.ijo.0802566. [DOI] [PubMed] [Google Scholar]
- Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- Lyon CJ, Law RE, Hsueh WA. Adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- MacDougald OA, Mandrup S. Adipogenesis: forces that tip the scales. Trends Endocrinol Metab. 2002;13:5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Pond CM. Interactions of noradrenalin and tumour necrosis factor-α, interleukin-4 and interleukin-6 in the control of lipolysis from adipocytes around lymph nodes. Cytokine. 1999;11:334–346. doi: 10.1006/cyto.1998.0442. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Sadler D, Pond CM. The cellular structure and lipid/protein composition of adipose tissue surrounding chronically stimulated lymph nodes in rats. J Anat. 2003;202:551–561. doi: 10.1046/j.1469-7580.2003.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattacks CA, Sadler D, Pond CM. The effects of dietary lipids on dendritic cells in perinodal adipose tissue during chronic mild inflammation. Br J Nutr. 2004a;91:883–891. doi: 10.1079/BJN20041147. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Sadler D, Pond CM. Site-specific differences in the fatty acid compositions of dendritic cells and associated adipose tissue in popliteal depot, mesentery and omentum, and their modulation by chronic inflammation and dietary lipids. Lymph Res Biol. 2004b;2:107–129. doi: 10.1089/lrb.2004.2.107. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Sadler D, Pond CM. The control of lipolysis in perinodal and other adipocytes by lymph node and adipose tissue-derived dendritic cells in rats. Adipocytes. 2005;1:43–56. [Google Scholar]
- Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes. 2004;28:S58–S65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- Pereira ME, Pond CM. Organization of white adipose tissue in lemuridae. Am J Primatol. 1995;35:1–13. doi: 10.1002/ajp.1350350102. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung NJ, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. Body mass and natural diet as determinants of the number and volume of adipocytes in eutherian mammals. J Morph. 1985;185:183–193. doi: 10.1002/jmor.1051850204. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. The anatomy of adipose tissue in captive Macaca monkeys and its implications for human biology. Folia Primatol. 1987;48:164–185. doi: 10.1159/000156293. [DOI] [PubMed] [Google Scholar]
- Pond CM. An evolutionary and functional view of mammalian adipose tissue. Proc Nutr Soc. 1992;51:367–377. doi: 10.1079/pns19920050. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. Interactions between adipose tissue around lymph nodes and lymphoid cells in vitro. J Lipid Res. 1995;36:2219–2231. [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. The activation of adipose tissue associated with lymph nodes during the early stages of an immune response. Cytokine. 2002;17:131–139. doi: 10.1006/cyto.2001.0999. [DOI] [PubMed] [Google Scholar]
- Pond CM. Paracrine relationships between adipose and lymphoid tissues: implications for the mechanism of HIV-associated adipose redistribution syndrome. Trends Immunol. 2003;24:13–18. doi: 10.1016/s1471-4906(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Pond CM. Adipose tissue and the immune system. Prostagland Leukot Essent Fatty Acids. 2005;73:17–30. doi: 10.1016/j.plefa.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Rajala MW, Scherer PE. Minireview: The adipocyte – at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- Randolph GJ. Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Semin Immunol. 2001;13:267–274. doi: 10.1006/smim.2001.0322. [DOI] [PubMed] [Google Scholar]
- Robker RL, Collins RG, Beaudet AL, Mersmann HJ, Smith CW. Leukocyte migration in adipose tissue of mice null for ICAM-1 and Mac-1 adhesion receptors. Obes Res. 2004;12:936–940. doi: 10.1038/oby.2004.114. [DOI] [PubMed] [Google Scholar]
- Rockson SG. The elusive adipose connection. Lymph Res Biol. 2004;2:105–106. doi: 10.1089/lrb.2004.2.105. [DOI] [PubMed] [Google Scholar]
- Rosenthal M, Roth J, Störr B, Zeisberger E. Fever response in lean (Fa/-) and obese (fa/fa) Zucker rats and its lack to repeated injections of LPS. Physiol Behav. 1996;59:787–793. doi: 10.1016/0031-9384(95)02158-2. [DOI] [PubMed] [Google Scholar]
- Schäffler A, Herfarth H. Creeping fat in Crohn's disease: travelling in a creeper lane of research? Gut. 2005;54:742–744. doi: 10.1136/gut.2004.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidell JC, Cigolini M, Deslypere J-P, Charzewska J, Ellsinger B-M, Cruz A. Body-fat distribution in relation to physical activity and smoking habits in 38-year-old European men. The European fat distribution study. Am J Epidemiol. 1991;133:257–265. doi: 10.1093/oxfordjournals.aje.a115870. [DOI] [PubMed] [Google Scholar]
- Sheehan AL, Warren BF, Gear MWL, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–958. doi: 10.1002/bjs.1800790934. [DOI] [PubMed] [Google Scholar]
- Singh D. Adaptive significance of female physical attractiveness: the role of waist-to-hip ratio. J Personal Soc Psychol. 1993;654:293–307. doi: 10.1037//0022-3514.65.2.293. [DOI] [PubMed] [Google Scholar]
- Sjöström L, Björntorp P. Body composition and adipose tissue cellularity in human obesity. Acta Med Scand. 1974;195:201–211. doi: 10.1111/j.0954-6820.1974.tb08123.x. [DOI] [PubMed] [Google Scholar]
- Smith JB, Morris B. The response of the popliteal lymph node of the sheep to swine influenza virus. Aust J Exp Biol Med. 1970;48:47–55. doi: 10.1038/icb.1970.5. [DOI] [PubMed] [Google Scholar]
- Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut. 2003;52:1522–1529. doi: 10.1136/gut.52.10.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain GW, Strain JJ, Zumolf B, Knittle J. Do fat cell morphometrics predict weight loss maintenance? Int J Obesity. 1984;8:53–59. [PubMed] [Google Scholar]
- Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Abundance of two human preadipocyte subtypes with Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Tchoukalova YD, Giorgadze N, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–E277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. The biology of obesity. Proc Nutr Soc. 2005;64:31–38. doi: 10.1079/pns2004406. [DOI] [PubMed] [Google Scholar]
- Unger RH. The physiology of cellular liporegulation. Ann Rev Physiol. 2003;65:333–347. doi: 10.1146/annurev.physiol.65.092101.142622. [DOI] [PubMed] [Google Scholar]
- Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie. 2005;87:57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Dubrovska G, Tsang SY, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott EDA, Windsor ACJ, Mattacks CA, Pond CM, Knight SC. The fatty acid compositions of lipids in mesenteric adipose tissue and lymphoid cells in patients with and without Crohn's disease and their therapeutic implications. Inflamm Bowel Dis. 2005;11:820–827. doi: 10.1097/01.mib.0000179213.80778.9a. [DOI] [PubMed] [Google Scholar]