Abstract
Classic studies have recognized neurons and three glial elements in the central nervous system (CNS) – astrocytes, oligodendrocytes and microglia. The identification of novel glia that specifically express the NG2 chondroitin sulphate proteoglycan (CSPG) raises the possibility of a fifth element. Until recently, all NG2-expressing glia were considered to be oligodendrocyte precursor cells (OPCs) that persist in the adult CNS to generate oligodendrocytes throughout life. However, this narrow view of the function of ‘NG2-glia’ is being challenged. The majority of NG2-expressing glia in the adult CNS are a distinct class of cells that we have called ‘synantocytes’ (from the Greek synanto for contact). Synantocytes are stellate cells, with large process arborizations, and are exquisitely related to neurons. Individual cells traverse white and grey matter and form multiple contacts with neurons, astrocytes, oligodendrocytes and myelin. Synantocytes are an integral component of the ‘tetrapartite’ synapse, and provide a potential integrative neuron-glial communications pathway. Neuronal activity, glutamate and adenosine triphosphate (ATP) act on synantocyte receptors and evoke raised intracellular calcium. It remains to be seen whether this serves a physiological function, but synantocytes may be specialized to monitor signals from neurons and glia, and to respond to changes in the integrity of the CNS via their specific contacts and ion channel and receptor profiles. The general consequences of synantocyte activation are proliferation and phenotypic changes, resulting in glial scar formation, or regeneration of oligodendrocytes, and possibly neurons.
Keywords: astrocyte, ATP, glia, glutamate, neuron glial signalling, NG2, oligodendrocyte, optic nerve, synantocyte
Introduction
Historically, NG2 was one of a panel of molecules derived by Stallcup and colleagues from mixed cultures of neurons (N) and glia (G) that was subsequently shown to be a novel chondroitin sulphate proteoglycan (CSPG) (reviewed by Stallcup, 2003). Antibodies raised against NG2 labelled cells in vitro with the antigenic phenotype of bipotential oligodendrocyte-type-2-astrocyte (O-2A) progenitor cells (Levine & Stallcup, 1987; Stallcup & Beasley, 1987). O-2A cells were first shown by Raff and colleagues to develop into oligodendrocytes or astrocytes depending on the culture medium (Raff et al. 1983). In the absence of evidence for type-2 astrocytes in vivo, O-2A cells have been referred to as oligodendrocyte precursor or progenitor cells (OPCs). Notably, when tested in sections of adult brain, NG2 antibodies labelled a substantial population of cells that had the morphological features associated with protoplasmic astrocytes (Levine & Card, 1987). However, these cells did not express glial fibrillary acidic protein (GFAP), or other recognized markers of mature glia (Levine et al. 1993; Reynolds & Hardy, 1997; Butt et al. 1999). NG2-expressing cells were shown to be immunopositive for platelet-derived growth factor α receptors (PDGFαR) and O4, considered diagnostic for OPCs (Nishiyama et al. 1996; Reynolds & Hardy, 1997). In addition, NG2-expressing cells have been shown to differentiate into oligodendrocytes in vitro and in vivo, and accordingly all NG2-expressing cells have been considered to be OPCs (Dawson et al. 2000). There is also evidence that resident adult NG2-expressing cells can regenerate oligodendrocytes following demyelinating insults, and that they do the same in multiple sclerosis (Reynolds et al. 2002). However, NG2-expressing cells exhibit properties apart from their assumed function as OPCs and we have proposed that they comprise a separate class of glia, termed synantocytes (Butt et al. 2003). This fourth type of glial cell is distinct from fibrous and protoplasmic astrocytes, and has features of glial cells that were previously described as beta astrocytes (Berry et al. 2002; Peters & Sethares, 2004; Peters, 2004). The issue is compounded by recent evidence that not all NG2-expressing cells in the developing central nervous system (CNS) are of the oligodendrocyte lineage (Mallon et al. 2002), and may be a subclass of astrocyte (Matthias et al. 2003), or neural stem cells (Belachew et al. 2003; Aguirre & Gallo, 2004; Aguirre et al. 2004). A number of reviews have discussed the lineage of NG2-expressing glia and their relations to OPCs, astrocytes and neural stem cells (Berry et al. 2002; Nishiyama et al. 2002; Belachew & Gallo, 2004; Kimelberg, 2004; Peters, 2004), and these questions are also addressed elsewhere in this issue (Nishiyama et al. 2005; Polito & Reynolds, 2005). The aim of the present review is to examine the distinguishing features of what we call ‘synantocytes’, which we argue make up the bulk of resident NG2-expressing glia in the adult CNS and are phenotypically and functionally distinct from other glia and neurons.
Distribution and morphology of synantocytes
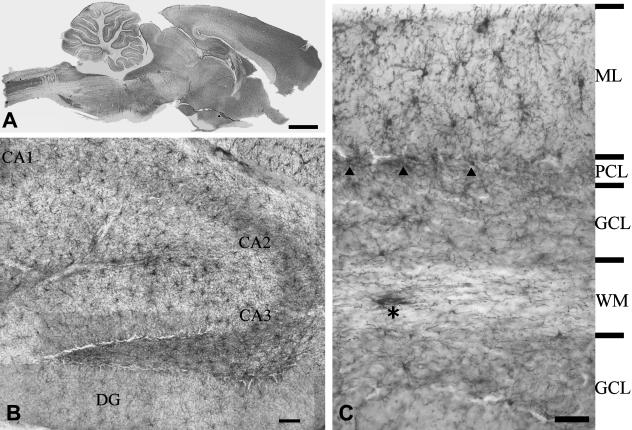
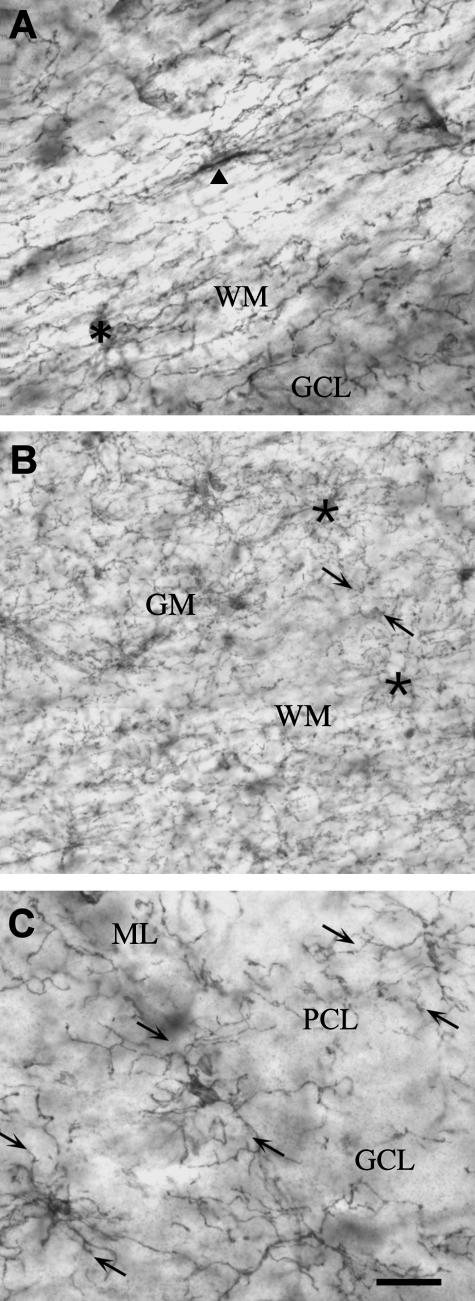
Immunolabelling for NG2 clearly defines the major structures of the brain (Fig. 1A), and at higher magnification distinguishes a mosaic of synantocytes (Fig. 1B). Synantocytes have a characteristic stellate morphology, with a centrally placed cell body from which extend numerous primary processes that pass radially and bifurcate three or more times to form a process field of approximately 100 µm diameter (Fig. 1B,C). The process arborizations of grey matter synantocytes tend to be symmetrical (Fig. 1C, ML), whereas those in white matter are often polarized, due to preferential extension of processes along the axonal axis (Fig. 1C, asterisk). The process fields of adjacent synantocytes overlap slightly (Fig. 1C), but cells are not dye-coupled via gap junctions (Bergles et al. 2000; Lin & Bergles, 2002, 2004; Chittajallu et al. 2004; Lin et al. 2005), unlike astrocytes and oligodendrocytes, which exhibit extensive dye-coupling (Butt & Ransom, 1993). NG2 is a transmembrane CSPG (Stallcup, 2002) and the bulk of NG2 immunolabelling in the CNS is cellular (Fig. 1C). However, diffuse and apparently extracellular NG2 immunolabelling was also observed in some grey matter areas (e.g. the CA2 and CA3 areas of the hippocampus), consistent with the possibility that synantocytes may also secrete NG2 (Stallcup, 2003). Notably, synantocytes are numerous in both grey and white matter (Fig. 1C), and it has been shown that there is no correlation between their population density and that of oligodendrocytes or myelin (Dawson et al. 2003). In the cerebellum, for example, synantocytes appear less abundant in the myelinated white matter than in the molecular layer, where there is little myelination and oligodendrocytes are rare (Fig. 1C).
Fig. 1.
Distribution of NG2-expressing glia – synantocytes – in the adult rat brain. (A) Mid-saggital section of whole brain ABC immunolabelled for NG2 defines functionally significant areas of the brain. (B) Synantocytes form a mosaic of stellate cells that clearly delineate the structure of the hippocampal formation, with dense labelling in the CA1, CA2 and CA3 areas, some of which appears extracellular. (C) Synantocytes are directly related to the neuronal layers of the cerebellum. Synantocytes are interspersed amongst the Purkinje cells (PCL, arrowheads) and extend processes radially to traverse the molecular layer (ML) and granular cell layer (GCL). Synantocytes in the white matter (WM) are more polarized and processes extend along the trajectory of axons (asterisk). Individual synantocytes extend processes through white and grey matter. Scale bars = 1 mm in A, 100 µm in B and 50 µm in C.
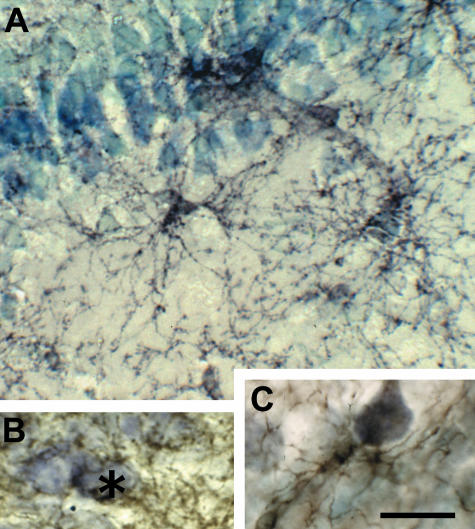
Grey matter synantocytes and their processes intertwine amongst and form multiple connections with neurons (Fig. 2). Synantocytes are often interspersed with and closely apposed to neuronal cell bodies, as illustrated in Purkinje cells in the cerebellum (Fig. 1C, arrowheads), pyramidal cells of the CA1 area of the hippocampus (Fig. 2A,B), and layer V of the cortical grey matter (Fig. 2C). Confocal imaging of double immunofluorescence labelled tissue shows that neurons are contacted by more than one synantocyte, and that individual synantocytes form multiple associations with individual neurons on their somata, dendritic trees and axons (Fig. 3A–C). Moreover, individual cells extend interlaminar processes that traverse neuronal layers, for example, in the cerebellum synantocytes traverse the Purkinje cell (PCL), molecular (ML) and granular cell (GCL) layers (Fig. 1C), and in the hippocampus contact neurons in the pyramidal, polymorphic and molecular layers (Fig. 2A). Electron microscopy (EM) immunocytochemistry has shown that these synantocyte processes contact and even form synapses (Bergles et al. 2000; Lin et al. 2005). Thus, a single synantocyte will contact multiple synapses with numerous neurons. Furthermore, electrophysiological studies have shown that synantocytes respond to neurotransmitters released from climbing fibres in the molecular layer of the cerebellum (Lin et al. 2005) and from hippocampal neurons (Bergles et al. 2000; Lin & Bergles, 2004), indicating functional neuron–syantocyte signalling (see below). In addition, the processes of synantocytes often encapsulate neuronal cell bodies (Figs 2B and 3A), suggesting that synantocytes are a component of the perineuronal nets that surround pyramidal cells and interneurons (Butt et al. 2002).
Fig. 2.
Synantocytes are closely associated with neurons. NG2-immunolabelled sections of adult rat brain counterstained with toluidine blue. (A) Synantocytes in the CA1 area of the hippocampus, some of which are directly apposed to pyramidal cell bodies, and extending processes through multiple layers. (B) Synantocyte processes forming a perineuronal network and enwrapping hippocampal neurons (asterisk). (C) Synantocyte apposed to and forming multiple contacts with a cortical pyramidal neuron. Scale bar = 25 µm.
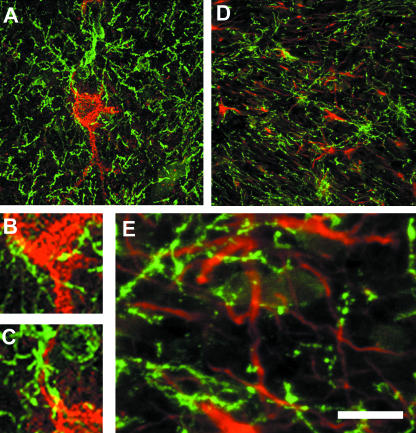
Fig. 3.
Synantocytes form multiple contacts with neurons and astrocytes. Confocal micrographs of cortex (A–C) and optic nerve (D,E) double immunofluorescence labelled for NG2 (green) and calbindin for neurons (red, A–C) or GFAP for astrocytes (red, D,E). Individual synantocytes form multiple contacts with neuronal somata (A), axons (B) and dendrites (C), and neurons are contacted by multiple synantocytes. In white matter, synantocytes are interspersed with astrocytes, which they contact (D), and their processes are interwined (E). Scale bar = 50 µm in A and D, and 12.5 µm in B, C and E.
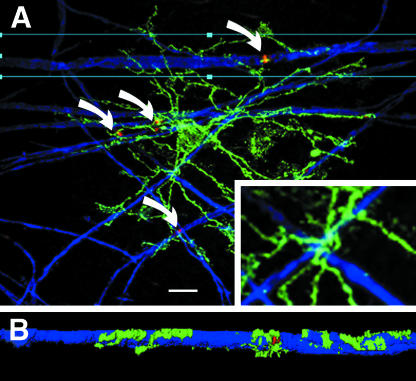
In white matter, synantocytes are interspersed with astrocytes within, or slightly offset from, rows of oligodendrocytes (Butt et al. 1999). Synantocyte processes intermingle with those of astrocytes and oligodendrocytes and form multiple contacts with the cell bodies of oligodendrocytes, astrocytes and their processes (Fig. 3D, E). Synantocyte processes extend along myelinated axons (Fig. 4), and EM immunocytochemistry indicates they pass to nodes of Ranvier (Butt et al. 1999). The relationships between synantocytes and axons is best illustrated in whole mounted anterior medullary velum (AMV), in which axons and glia are widely dispersed (Fig. 4). Triple immunofluorescence labelling for NG2, myelin basic protein (MBP) and ankyrin-3G, an axoskeletal protein that anchors sodium channels (NaCh) at nodes of Ranvier (Poliak & Peles, 2003), shows synantocytes extend processes along myelin sheaths to contact the paranodes and nodes of Ranvier (Fig. 4A,B). Notably, synantocyte processes are seen to track along myelinated axons as they cross in the AMV (Fig. 4A, inset), and to extend branches along the myelin sheaths which then terminate at nodes of Ranvier (Fig. 4B). Observations in cerebellar white matter, optic nerve and AMV indicate the close relationships between synantocytes and myelinated axons may be a feature common to most white matter. Morevover, individual synantocytes traverse grey and white matter throughout the brain (Fig. 5), suggesting a single cell may contact synapses, nodes, astrocytes, oligodendrocytes and myelin.
Fig. 4.
Synantocytes contact nodes of Ranvier. Confocal micrographs of whole-mounted anterior medullary velum triple immunofluoresecnce labelling for NG2 (green), myelin basic protein (blue) and ankyrin-3G (red). (A) Individual synantocytes contact multiple nodes of Ranvier (curved arrows) and their processes closely follow the path of myelinated axons (inset). All synantocytes observed in the velum formed similar associations with nodes of Ranvier. (B) Deconvolution shows the synantocyte process extending along the myelin sheath to form exquisite contacts with the paranodes and node of Ranvier. Scale bar = 10 µm in A, 30 µm in inset and 50 µm in B.
Fig. 5.
Synantocytes are interlaminar. NG2 immunolabelled sections of cerebellum (A, C) and cortex (B). (A) Individual cerebellar synantocytes with cell bodies in the white matter (WM, arrowhead) or at the interface between white and grey matter (GM, asterisk) extend processes into both. (B) Synantocytes in the cortical grey matter and subcortical white matter (asterisks) extend processes that traverse both layers and intermingle at the interface. (C) Interlaminar synantocytes extend processes into all layers of the cerebellum (arrows). Synantocytes are not specialized for either white or grey mater, and the same cells subserve both. Scale bar = 30 µm in A, C and 50 µm in B.
The morphology and distribution of synantocytes are not consistent with the argument that all NG2-expressing cells are OPCs (Dawson et al. 2000). The counter-argument is supported by a study in mice in which expression of enhanced green fluorescent protein (EGFP) is driven by the promoter for proteolipid protein (PLP), a myelin-related gene, which found that some (EGFP)PLP+/NG2+ cells were committed to the oligodendrocyte lineage, whereas a second population of synantocytes was (EFGP)PLP− (Mallon et al. 2002). Further confusion on the oligodendroglial lineage of synantocytes has arisen from studies in which EGFP is driven by the promoters for GFAP or 2′−3′-cyclic nucleotide 3′-phosphodiesterase (CNP), which, respectively, identified a subclass of NG2+ astrocyte (Matthias et al. 2003) and NG2+ neural stem cells (Belachew et al. 2003). These findings have raised the possibility that NG2 is expressed by heterogeneous cell populations, at least in the developing brain. However, it may be premature to make this conclusion until the behaviour of the inserted constructs is better understood (Kimelberg, 2004; Nishiyama et al. 2005). On the basis of morphology, NG2-expressing cells in the adult brain neuropil have the appearance of a single class of mature glial cell that forms multifarious contacts with neurons and glia, and which we call synantocytes.
Synantocytes express voltage-gated ion channels
The functions of synantocytes in relation to their neuronal and glial contacts will be determined in part by the ion channels and receptors they express. There are a growing number of reports on the physiological properties of NG2-expressing cells in situ (Bergles et al. 2000; Matthias et al. 2003; Schools et al. 2003; Chittajallu et al. 2004; Grass et al. 2004; Lin & Bergles, 2004; Lin et al. 2005). In addition, there were numerous earlier studies on OPCs or O-2A cells in vitro and in situ, which are likely to correspond to synantocytes (reviewed by Verkhratsky & Steinhauser, 2000; Belachew & Gallo, 2004). Studies on NG2-expressing glia and OPCs concur that two major features is that they express significant voltage-gated K+ currents (Kv) and ionotropic receptors, in particular AMPA-type glutamate receptors.
Electrophysiological studies on NG2-expressing cells in situ generally show that they have a high membrane resistance and negative membrane potentials of −70 to −90 mV (Bergles et al. 2000; Lin & Bergles, 2003, 2004; Chittajallu et al. 2004; Lin et al. 2005). In situ, NG2+ cells in the postnatal hippocampus with the morphological features of synantocytes, as defined above, express dominant outward rectifying (KDR) and transient (KA) K+ currents, but not appreciable inward rectifying K+ currents (KIR) (Schools et al. 2003). However, another study found they exhibit inward K+ currents to a variable degree, most likely resulting from the activity of both ATP-sensitive K+ channels (KATP) and KIR (Lin & Bergles, 2003). These differences could reflect developmental changes in K+ channels (KCh) expression or functional heterogeneity in NG2-expressing cells. A developmental down-regulation of outward K+ currents and an increase in inward K+ currents has been demonstrated in OPCs (Sontheimer et al. 1989; Kettenmann et al. 1991; Berger et al. 1995). A study on postnatal day (P)10–26 hippocampal NG2+ cells has provided further evidence of a developmental decrease in KDR, but that KA remained dominant and KIR was negligible at all ages (Schools et al. 2003). Heterogeneity between postnatal NG2+ cells has been demonstrated in situ, whereby those in subcortical white matter possess significant KDR and KA, with no appreciable KIR, whereas NG2+ cells in cortical grey matter also expressed KIR to variable degrees (Chittajallu et al. 2004). There is also heterogeneity in the expression of TTX-sensitive sodium currents (INa) in synantocytes. In the hippocampus and subcortical white matter, synantocytes exhibited a small depolarization-induced INa and did not fire action potentials (Bergles et al. 2000; Chittajallu et al. 2004). By contrast, cortical synantocytes expressed larger INa and a proportion were capable of generating action potentials (Chittajallu et al. 2004).
The functional significance of the apparent heterogeneity and possible developmental changes in K+ and Na+ currents in synantocytes is unclear. KIR are important in setting the membrane potential of glia and are important in extracellular K+ regulation by glia (Kofugi & Newman, 2004). The differential expression of KIR in white and grey matter NG2-expressing cells is consistent with those in subcortical white matter being significantly depolarized at −70 mV, compared with at −90 mV in cortical grey matter, and suggests the latter may play a role in K+ uptake during neuronal activity (Chittajallu et al. 2004). In addition, there is a clear correlation between K+ currents and the proliferative and maturation state of glia, whereby proliferative cells express KDR and KA, which are then down-regulated, and KIR up-regulated in postmitotic cells (Sontheimer et al. 1989; Kettenmann et al. 1991; Knutson et al. 1997). Differences in K+ channel expression in synantocytes may therefore reflect differences in their proliferative states. The role of INa in synantocytes is more obscure, but they may provide a path for sodium entry and maintaining activity of Na+–K+ pumps (Sontheimer et al. 1994). This could be important in K+ uptake during neuronal activity, although it has been questioned whether there is significant Na+ entry by this route (Verkhratsky & Steinhauser, 2000). Perhaps more importantly, raised [Na+]i and depolarization inhibits the proliferative activity of OPCs (Knutson et al. 1997), and so activation of NaCh in response to neuronal activity may help maintain syantocytes in a quiescent state (see below). In cultured OPCs, INa are at sufficient density to fire action potentials (Barres et al. 1990), but these are lost as they mature, concomitantly with a switch from outward to inward K+ currents (Sontheimer et al. 1989). Thus, the expression of significant inward K+ currents and Na+ currents in some NG2-expressing cells in situ raises the possibility that they may represent more mature synantocytes (Chittajallu et al. 2004). However, in the absence of electrophysiological studies on adult synantocytes, the balance of evidence is that in the quiescent state they generally have predominantly outward K+ currents and negligible inward K+ currents, with small Na+ currents.
Neuron–synantocyte and astrocyte–synantocyte signalling
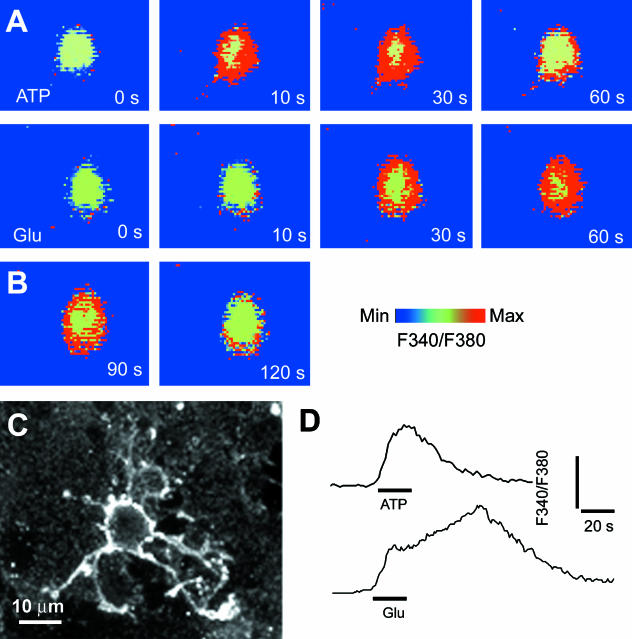
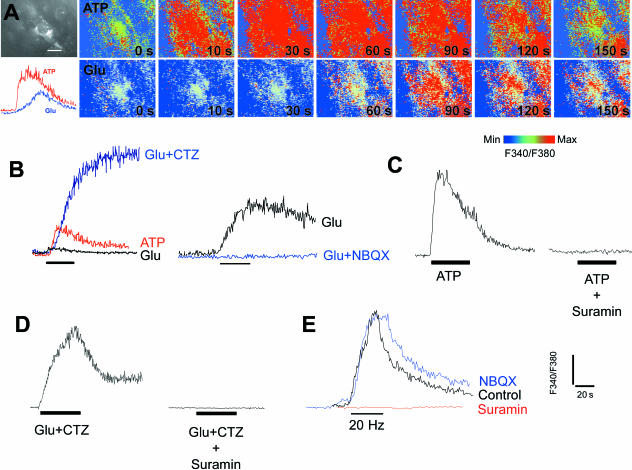
A major physiological feature of OPCs in vitro and NG2-expressing cells in situ is their expression of AMPA-type GluR (Barres et al. 1990; Fulton et al. 1992; Steinhauser & Gallo, 1996; Verkhratsky & Steinhauser, 2000). There is evidence that activation of AMPA receptors in OPCs and in NG2-expressing cells in situ are linked to Ca2+ entry (Holzwarth et al. 1994; Steinhauser et al. 1994; Borges et al. 1995; Holtzclaw et al. 1995; Bergles et al. 2000; Lin & Bergles, 2003). In addition, OPCs have been shown to express the Ca2+-permeable AMPA receptor subunit GluR4 (Gallo et al. 1994; Ong et al. 1996), and synantocytes in situ had AMPA receptors with properties of those lacking the GluR2 subunit, which allow Ca2+ influx (Bergles et al. 2000). In astrocytes, calcium waves are propagated by the release of the ‘gliotransmitter’ ATP, which acts on P2Y purinoceptors to evoke raised [Ca2+]i in neighbouring cells (Newman, 2004; Zhang & Haydon, 2005). It was not known whether synantocytes express functional purinoceptors (James & Butt, 2002), and so we have examined the actions of glutamate and ATP on [Ca2+]i in optic nerve synantocytes, both in vitro (Fig. 6) and in situ (Fig. 7). Application of glutamate or ATP for 30 s evoked a rapid and transient rise in [Ca2+]i in immunohistochemically identified synantocytes in explants of optic nerve glia (n = 3; Fig. 6) and in situ (n = 6; Fig. 7A). Synantocytes responded to ATP with a rapid and large increase in [Ca2+]i, which was transient and decayed during the application, whereas glutamate had a markedly smaller effect, evoking a delayed increase in [Ca2+]i that was sustained for minutes after wash-out of the agonist (Fig. 7A). All cells analysed responded similarly, and so we have examined these responses further in unidentified optic nerve glia. The glutamate-mediated increase in glial [Ca2+]i was dramatically increased by cyclothiazide (CTZ), which acts on AMPA-type GluR to maintain them in an open state (Fig. 7B), and was blocked by the AMP receptor antagonist NBQX (Fig. 7B). Suramin, a general antagonist for P2X and P2Y purinoceptors, decreased responses to both ATP (Fig. 7C) and glutamate (Fig. 7D). There is circumstantial evidence that axonal electrical activity in the optic nerve also triggers glutamate- and ATP-mediated glial Ca2+ signalling (Kreigler & Chiu, 1993; Butt et al. 2004). To examine this, we stimulated optic nerves using suction electrodes and showed that stimulation at 20 Hz for 20 s resulted in a rapid rise in glial [Ca2+]i, of the same amplitude as that observed for 1 mm ATP (Fig. 7E). Notably, the activity-evoked increase in glial [Ca2+]i was decreased by suramin, but was unaffected by NBQX (Fig. 7E). We have not yet confirmed that identified synantocytes respond to axonal activity, but this seems likely, because the vast majority of cells analysed in the optic nerve responded similarly. The results provide evidence that the effects of glutamate and axonal activity on glial [Ca2+]i are mediated by ATP, presumably released from astrocytes and acting as a ‘gliotransmitter’ (Zhang & Haydon, 2005), to propagate calcium signals in synantocytes, as well as astrocytes and oligodendrocytes.
Fig. 6.
Synantocytes in vitro respond to glutamate and ATP with raised intracellular calcium. Explants of optic nerve glia were loaded with the calcium-sensitive dye fura-2 and imaged during bath application of ATP (A) or glutamate (B), and cells were identified at the end of the experiment by immunolabelling for NG2 (C). ATP and glutamate evoked a rapid increase in cytosolic [Ca2+]i in immunohistochemically identified synantocytes. (D) The response to ATP was transient and began to decay during exposure to the agonist, whereas the response to glutamate was slower to peak and was sustained after washout of the agonist.
Fig. 7.
Calcium signalling in synantocytes in situ. Optic nerves were isolated intact and loaded with fura-2 for calcium imaging, and at the end of the experiment cells were identified by immunolabelling for NG2 (A). Both ATP and glutamate (Glu) evoked a rise in cytosolic [Ca2+]i in immunohistochemically identified synantocytes, although the response to ATP was rapid and transient, whereas that to glutamate was slow and sustained (A). These responses were analysed in greater detail in unidentified glia, but all cells in the optic nerve responded similarly (n > 100), and it reasonable to conclude that the findings reflect synantocytes as well as astrocytes and oligodendrocytes. (B) The response to glutamate was markedly increased by incubation with cyclothiazide (CTZ), which acts on AMPA glutamate receptors to maintain them in an open state, and was blocked by the AMPA receptor antagonists NBQX. (C) The ATP response was blocked by suramin, a general antagonist for P2X and P2Y purinoceptors. (D) Suramin also inhibited the response to Glu plus CTZ, indicating the increase in [Ca2+]i was partly mediated by ATP released in response to activation of AMPA receptors. (E) Electrical stimulation of the optic nerve at 20 Hz for 20 s induced an increase in glial [Ca2+]i that was inhibited by suramin but not by NBQX. The results indicate that glial calcium signals are evoked by ATP, presumably released from astrocytes in response to axonal electrical activity and activation of AMPA glutamate receptors, and support a primary role for ATP as a ‘gliotransmitter’ in the optic nerve.
Functions of neuron– and glial–synantocyte interactions
Synantocytes traverse grey and white matter throughout the brain and form a potential communications pathway between neurons, astrocytes and oligodendrocytes. It is not clear how this relates to the physiological distinctions between cortical grey and white matter synantocytes described above (Chittajallu et al. 2004), but these interlaminar synantocytes may exhibit intermediate properties. Moreover, synantocytes cross several neuronal layers and will therefore receive input from numerous neurons and express multiple neurotransmitter receptors. Synantocytes contact glutamatergic and GABAergic synapses in the hippocampus, and activation of their AMPA and GABAA receptors occurs under physiological conditions in response to activity of excitatory CA3 pyramidal neurons and inhibitory interneurons in the stratum radiatum of area CA1, respectively (Bergles et al. 2000; Lin & Bergles, 2004). As well as responding to glutamate, GABA and ATP, it is likely that synantocytes will express other receptors, like OPCs in culture which have been shown to express receptors for glycine, acetyl choline, monoamines and substance P (Verkhratsky & Steinhauser, 2000; Belachew & Gallo, 2004). Expression will be related to the neurons that synantocytes contact, and so it is likely that there will be functional heterogeneity in synantocytes from different brain regions. The physiological function of synantocytes at synapses is unresolved, but it is clear that they respond dynamically to neuronal activity and they may also release neurotransmitters (Bergles et al. 2000). Signalling between synantocytes, neurons and astrocytes could therefore be important in integrative neuronal function. The phrase ‘the tripartite synapse’ was coined in acknowledgement that astrocytes are an important component of the synapse (Araque et al. 1999). Evidently, synantocytes are also an integral element of the ‘tetrapartite’ synapse.
Neuron– and astrocyte–synantocyte signalling also provide mechanisms for regulating synantocyte functions. Activation of both AMPA and GABAA receptors depolarizes synantocytes, resulting from sodium influx in the former and chloride efflux in the latter (Bergles et al. 2000; Lin & Bergles, 2004). There is a direct link between activation of AMPA and GABAA receptors and the inhibition of OPC proliferation and lineage progression (Yuan et al. 1998), and so glutamate or GABA released from neurons could function to maintain synantocytes in a quiescent state, and this may involve activation of NaCh during neuronal activity (Knutson et al. 1997). Calcium also plays a central role in glial cell physiology, proliferation, growth, differentiation and death (Finkbeiner, 1993; Verkhratsky et al. 1998), and ATP released from astrocytes may therefore stimulate synantocytes by evoking raised [Ca2+]i. We have provided evidence that synantocytes exhibit a similar injury response to axon transection and to blocking axonal electrical activity (Butt et al. 2002, 2004), supporting the possibility that axonal action potential propagation maintains synantocytes in a quiescent state. By contrast, synantocytes may be activated by sustained increases in [Ca2+]i evoked by enhanced activation of AMPA receptors or purinoceptors in response to high levels of glutamate or ATP, such as occur following injury and ischaemia.
The functions of synantocytes are likely to involve interactions with extracellular matrix (ECM) and cellular molecules expressed at synapses, nodes of Ranvier and on myelin. Synantocytes produce a number of ECM molecules that comprise the perineuronal nets (Sandvig et al. 2004), such as hyaluronan, versican, phosphacan, neurocan and tenascins (Celio et al. 1998; Brückner et al. 2000). These function to stabilize synapses and form a link with an intracellular net formed by the neuronal intracellular cytoskeleton, composed of spectrin and ankyrin, which anchor ion channels and neurotransmitter receptors at synapses. In addition, synantocytes may have a repulsion-guidance effect on axon growth (P. Hubbard, B. I. Berry and A. M. Butt, unpublished observations), and their presence at synapses would influence synaptogenesis and synaptic refinement. ECM and intracellular cytoskeletal molecules are also localized to nodes of Ranvier and are important in nodal specialization and the clustering of ion channels (Poliak & Peles, 2003). For example, syantocytes produce and interact with tenascins and phosphacan, which bind to the β2 subunits of NaCh and are important in their clustering at nodes. Oligodendrocytes also produce ECM and transmembrane proteins that interact with NG2 and other molecules expressed by synantocytes, such as tenascin, which would provide a mechanism by which synantocytes monitor myelin integrity and mediate their response to demyelination and degeneration. The response of synantocytes to CNS injury is significant because NG2, together with other CNS CSPGs, is deposited at the glial scar and is considered a potent axon growth inhibitor (Sandvig et al. 2004). In vivo evidence is largely indirect, in that NG2 has been shown to be present at the right place at the right time to inhibit regeneration (Jones et al. 2002; Tang et al. 2002). Direct evidence comes from in vitro studies using stripe assays and NG2-expressing cells which have shown that NG2 reduces neurite outgrowth and causes growth cone collapse (Chen et al. 2002; Levine et al. 2005). However, we have found that regenerating axons grow directionally along the processes of NG2-glia following optic nerve crush and disinhibition of retinal ganglion cells (P. Hubbard, B. I. Berry and A. M. Butt, unpublished observations). Following optic nerve crush, axons do not normally grow through the glial scar. By contrast, following disinhibition by grafting peripheral nerve tissue into the vitreous of the eye, regenerating axons grow through the glial scar and down the nerve in a directional manner. Remarkably, the paths of regenerating axons extend along the processes of NG2-glia, which are aligned longitudinally along the nerve axis. The growing extremities of regenerating axons do not avoid NG2-glia, which might be expected if they have an exclusively inhibitory role, but instead NG2-glia form exquisite associations with the growth cones of regenerating axons, giving the appearance that those axons are drawn along NG2-glia. These results indicate that synantocytes may act in a repulsion-guidance manner and provide guidance cues for growing axons. The mechanisms by which synantocytes regulate axon growth are unresolved, but may involve the same molecular interactions that are important at synapses and nodes.
Conclusions
It is difficult to reconcile the morphology, distribution, physiology and pathological responses of synantocytes with their being OPCs with the sole function of regenerating oligodendrocytes. The exquisite relationships between synantocytes and neurons indicates a significant degree of specialization. Glia and neurons are functionally interdependent and their physiology and pathology are integrated by extracellular signals, including glutamate and ATP. Synantocytes are an integral component of the ‘tetrapartite’ synapse, and appear to be specialized to monitor and respond rapidly to changes in CNS integrity, via their multiple contacts with neurons, astrocytes, oligodendrocytes and myelin sheaths. There are several consequences to synantocyte activation. Synantocytes respond to the loss of neuronal activity and to physical injury with a rapid gliosis, helping to form the protective glial scar and possibly providing guidance cues for regenerating axons. Conceivably, activated synantocytes may regenerate functional neurons. Synantocytes can also regenerate oligodendrocytes, and this may be triggered by synanocytes sensing changes in axon conduction or the loss of myelin at nodes and paranodes, via receptors, ion channels and interactions with ECM molecules. Notably, electroconvulsive seizures have been shown to induce proliferation of synantocytes and the generation of neurons and oligodendrocytes in the adult rat hippocampus and amygdala (Hellsten et al. 2002; Wennström et al. 2005). It is clear from these studies that synantocytes are not simple passive OPCs, but interact with neurons and other glia in a dynamic fashion, which may have particular importance in pathology.
Acknowledgments
This work wupported in part by grants from the BBSRC, International Spinal Research Trust, and the Anatomical Society of the UK and Ireland.
References
- Aguirre A, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells that contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Swartz KJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990;4:507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Gallo V. Synaptic and extrasynaptic neurotransmitter receptors in glial precursors’ quest for identity. Glia. 2004;48:185–196. doi: 10.1002/glia.20077. [DOI] [PubMed] [Google Scholar]
- Berger T, Muller T, Kettenmann H. Developmental regulation of ion channels and receptors on glial cells. Perspect Dev Neurobiol. 1995;2:347–356. [PubMed] [Google Scholar]
- Bergles DE, Roberts JDB, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Berry M, Hubbard P, Butt AM. Cytology and lineage of NG2-positive glia. J Neurocytol. 2002;31:457–467. doi: 10.1023/a:1025735513560. [DOI] [PubMed] [Google Scholar]
- Borges K, Wolswijk G, Ohlemeyer C, Kettenmann H. Adult rat optic nerve oligodendrocyte progenitor cells express a distinct repertoire of voltage- and ligand-gated ion channels. J Neurosci Res. 1995;40:591–605. doi: 10.1002/jnr.490400504. [DOI] [PubMed] [Google Scholar]
- Brückner G, Grosche J, Schmidt S, et al. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428:616–629. doi: 10.1002/1096-9861(20001225)428:4<616::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Butt AM, Ransom BR. Morphology of astrocytes and oligodendrocytes during development in the intact rat optic nerve. J Comp Neurol. 1993;338:141–158. doi: 10.1002/cne.903380110. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Hubbard P, Kiff J, Ibrahim M, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2003;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Butt AM, Pugh M, Hubbard P, James G. Functions of optic nerve glia: axoglial signalling in physiology and pathology. Eye. 2004;18:1110–1121. doi: 10.1038/sj.eye.6701595. [DOI] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Negra M, Levine A, Ughrin Y, Levine JM. Oligodendrocyte precursor cells: reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2002;31:481–495. doi: 10.1023/a:1025791614468. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Aguiree A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson RL, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendrocyte progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Finkbeiner SM. Glial calcium. Glia. 1993;9:83–104. doi: 10.1002/glia.440090202. [DOI] [PubMed] [Google Scholar]
- Fulton BP, Burne JF, Raff MC. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Patneau DK, Mayer ML, Vaccarino FM. Excitatory amino acid receptors in glial progenitor cells: molecular and functional properties. Glia. 1994;11:94–101. doi: 10.1002/glia.440110204. [DOI] [PubMed] [Google Scholar]
- Grass D, Pawlowski PG, Hirrlinger J, et al. Diversity of functional astroglial properties in the respiratory network. J Neurosci. 2004;24:1358–1365. doi: 10.1523/JNEUROSCI.4022-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten J, Wennstrom M, Mohapel P, Ekdahl CT, Bengzon J, Tingstrom A. Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosteron treatment. Eur J Neurosci. 2002;16:283–290. doi: 10.1046/j.1460-9568.2002.02093.x. [DOI] [PubMed] [Google Scholar]
- Holtzclaw LA, Gallo V, Russell JT. AMPA receptors shape Ca2+ responses in cortical oligodendrocyte progenitors and CG-4 cells. J Neurosci Res. 1995;42:124–130. doi: 10.1002/jnr.490420114. [DOI] [PubMed] [Google Scholar]
- Holzwarth JA, Gibbons SJ, Brorson JR, Philipson LH, Miller RJ. Glutamate receptor agonists stimulate diverse calcium responses in different types of cultured rat cortical glial cells. J Neurosci. 1994;14:1879–1891. doi: 10.1523/JNEUROSCI.14-04-01879.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Blankenfeld GV, Trotter J. Physiological properties of oligodendrocytes during development. Ann NY Acad Sci. 1991;633:64–77. doi: 10.1111/j.1749-6632.1991.tb15596.x. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochem Int. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Knutson P, Ghiani CA, Zhou JM, Gallo V, McBain CJ. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J Neurosci. 1997;17:2669–2682. doi: 10.1523/JNEUROSCI.17-08-02669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofugi P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129:1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreigler S, Chiu SY. Calcium signaling of glial cells along mammalian axons. J Neurosci. 1993;13:4229–4245. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Card JP. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci. 1987;7:2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- Levine J, Tan A, Zhang W. NG2: a component of the glial scar that inhibits axon growth. J Anat. 2005;in press doi: 10.1111/j.1469-7580.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-C, Bergles DE. Physiological characteristics of NG2-expressing glial cells. J Neurocytol. 2003;31:537–549. doi: 10.1023/a:1025799816285. [DOI] [PubMed] [Google Scholar]
- Lin S-C, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lin S-C, Huck JHJ, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial modulation of synaptic transmission in the retina. Glia. 2004;15:268–274. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J NeurosciRes. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2003;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yang Z, Butt AM. Astrocytes: what's in a name? J Anat. 2005;in press doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Leong SK, Garey LJ, Reynolds R. A light- and electron-microscopic study of GluR4-positive cells in cerebral cortex, subcortical white matter and corpus callosum of neonatal, immature and adult rats. Exp Brain Res. 1996;110:367–378. doi: 10.1007/BF00229137. [DOI] [PubMed] [Google Scholar]
- Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345–357. doi: 10.1023/B:NEUR.0000044195.64009.27. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cereb Cortex. 2004;14:995–1007. doi: 10.1093/cercor/bhh060. [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;in press doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, et al. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2003;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt AM, Logan A. Reactive glia and scar derived CNS axon growth inhibitors. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Electrophysiologically ‘complex’ glial cells freshly isolated from the hippocapampus are immunopositive for the chondroitin sulphate proteogllycan NG2. J Neurosci Res. 2003;73:765–777. doi: 10.1002/jnr.10680. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Trotter J, Schachner M, Kettenmann H. Channel expression correlates with differentiation stage during the development of oligodendrocytes from their precursor cells in culture. Neuron. 1989;2:1135–1145. doi: 10.1016/0896-6273(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Fernandez-Marques E, Ullrich N, Pappas CA, Waxman SG. Astrocyte Na+ channels are required for maintenance of Na+/K(+)-ATPase activity. J Neurosci. 1994;14:2464–2475. doi: 10.1523/JNEUROSCI.14-05-02464.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2003;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Jabs R, Kettenmann H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus. 1994;4:19–35. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Gallo V. News on glutamate receptors in glial cells. Trends Neurosci. 1996;19:339–345. doi: 10.1016/0166-2236(96)10043-6. [DOI] [PubMed] [Google Scholar]
- Tang X, Davies JE, Davies JA. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2002;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Wennström M, Hellsten J, Tingström A. Electroconvulsive seizures induce proliferation of NG2-expressing glial cells in adult rat amygdale. Biol Psychiatry. 2005;55:464–471. doi: 10.1016/j.biopsych.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for the glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Haydon PG. Roles for gliotransmission in the nervous system. J Neural Transm. 2005;112:121–125. doi: 10.1007/s00702-004-0119-x. [DOI] [PubMed] [Google Scholar]