Abstract
NG2 is a high-molecular-weight chondroitin sulphate proteoglycan found on the surfaces of oligodendrocyte precursor cells (OPCs). Here we review the history and biology of OPCs with an emphasis on their functions after experimentally induced CNS injury. Injury to brain or spinal cord results in the rapid accumulation of NG2-expressing OPCs in the glial scar that forms at the injury site. The glial scar is considered a biochemical and physical barrier to successful axon regeneration and the functional properties of NG2 suggest that it, along with other macromolecules, participates in the creation of this growth-inhibitory environment. NG2 is an important target for therapies designed to promote successful axon regrowth.
Keywords: chondroitin sulphate proteoglycan, oligodendrocyte precursor cell, regeneration, spinal cord injury
Introduction
The glial composition of the adult mammalian central nervous system (CNS) is complex. In addition to astrocytes, oligodendrocytes and microglia, there is a fourth glial type that has been referred to as a β-astrocyte (Reyners et al. 1982), polydendrocyte (Nishiyama et al. 2002), synaptocyte (Butt et al. 2002) and most commonly, oligodendrocyte precursor cell (OPC) (Levine et al. 2001). This unusual and enigmatic cell type can be identified in brain slices by virtue of the expression of several marker antigens, in particular, the NG2 chondroitin sulphate proteoglycan (CSPG) and the α-receptor for platelet-derived growth factor (PDGF). Here we briefly review our studies of NG2-expressing cells in the adult CNS with an emphasis on their possible functions after brain injury and during attempts by the CNS to undergo repair.
Evidence for the existence of a specific precursor or progenitor cell for the oligodendrocyte lineage was first described by Martin Raff and colleagues in a series of tissue culture studies beginning in the mid-1980s (for review, see Raff, 1989). These pioneering studies showed that this precursor cell, which they referred to as an O2A progenitor cell, was phenotypically plastic and could respond to environmental factors by developing into either an oligodendrocyte or a glial fibrillary acidic protein (GFAP)-positive astrocyte. Additional studies showed that cells with the properties of O2A progenitor cells could be isolated from adult tissues, and, like their neonatal and embryonic counterparts, they too displayed a phenotypic plasticity and had the capacity to develop into either oligodendrocytes or GFAP-expressing cells (Levine et al. 1993; Shi et al. 1998). These cells also slowly self-renewed when placed in dissociated cell culture (Wren et al. 1992).
In these early studies, O2A progenitor cells or, as we prefer, OPCs, were identified in tissue culture due to their expression of the A2B5 ganglioside antigens. As they differentiated into oligodendrocytes, they began to express additional glycolipid antigens including 04 and galactocerebroside and lost A2B5 reactivity. Unfortunately, these marker antigens are difficult to localize in intact brain slices, leaving unanswered the important questions of whether OPCs existed in vivo, where they could be found in both developing and adult CNS, and whether they displayed the same phenotypic plasticity in vivo as they did in a tissue culture environment.
NG2 is a marker for adult OPCs
Our laboratory has been using anti-NG2 antibodies to identify and study the functions of adult OPCs. NG2 is a high-molecular-weight CSPG that was first identified as a cell surface antigen of neural tumour-derived cell lines that had properties intermediate between those of a neuron and a glial cell (hence NG for nerve-glia) (Wilson et al. 1981). The primary sequence of NG2 indicates that it is a type I transmembrane protein with a single membrane spanning domain, a large extracellular domain and a short cytoplasmic tail. NG2 contains several potential sites for the attachment of glycosaminoglycan (GAG) chains, but it appears that only one of these sites, serine999, is used (Nishiyama et al. 1991; Stallcup & Dahlin-Huppe, 2001). In OPCs, it is likely that this attached GAG chain is chondroitin 4-sulphate but its presence or absence is highly variable, making NG2 a ‘part-time’ proteoglycan.
The large extracellular domain (ECD) can be divided into three separate functional and structural domains that will be considered below. This large ECD is shed or secreted from the surfaces of cells and can be found in soluble extracts prepared from brain and in medium conditioned by cells in culture (Nishiyama et al. 1985; Morgenstern et al. 2002). The sequence of NG2 is quite distinct, indicating that NG2 is not a member of a large family of related molecules. This is unusual because many proteoglycans fall into such large family groups. However, NG2 is highly conserved and is identical to the human melanoma proteoglycan antigen and mouse AN2 (Staub et al. 2002). Furthermore, NG2-like molecules have been identified across the animal kingdom in organisms as diverse as sea-urchin embryos and primates. This high level of conservation suggests that NG2 mediates important cellular functions.
The distribution of NG2 on neural cells in dissociated culture has been studied extensively. These studies show that the A2B5-positive populations of OPCs (O2A cells) express NG2 but lose it as they differentiate into oligodendrocytes (Levine & Stallcup, 1987; Beasley & Stallcup, 1987). As these cells also express the α-receptor for PDGF, one conclusion from these early studies was that NG2 serves as a valuable marker for cells at the early stages of the oligodendrocyte lineage.
Staining of tissue sections prepared from adult brain reveals that NG2 is found on the surfaces of a population of process-bearing cells that are abundant in both white and grey matter (Fig. 1). Numerous studies from several different laboratories have shown that these NG2+ cells do not contain GFAP-positive intermediate filaments and do not express antigens associated with myelinating oligodendrocytes or resting microglia (for review, see Dawson et al. 2000). Under normal conditions, they slowly divide (Dawson et al. 2003). Although these adult NG2-expressing cells are likely to be the precursors or progenitors that replenish the slowly turning over pool of oligodendrocytes in the adult brain, their abundance, their highly differentiated appearance, and the presence of cellular processes at synapses (Ong & Levine, 1999) and at nodes of Ranvier (Butt et al. 1999) has led to the speculation that they carry out additional, as yet unknown, functions.
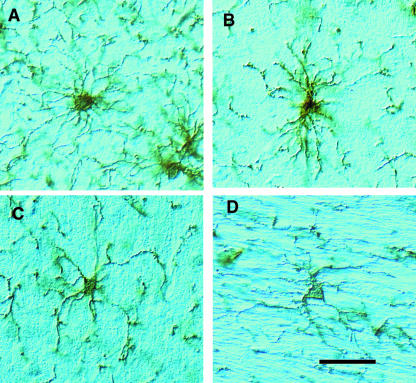
Fig. 1.
The appearance of NG2-labelled OPCs in the adult rat brain. Free-floating 30-µm-thick sections of adult rat brain were immunocytochemically stained with rabbit anti-NG2 antibodies. The individual cells shown are from (A) cortex, (B) suprapyramidal blade of the hippocampus, (C) external granule layer of cerebellum and (D) corpus callosum. The cells have irregularly shaped cell bodies from which numerous highly branched processes extend. The pattern of these processes is highly variable, being roughly spherical in the cortex but orientated parallel to axon bundles in the corpus callosum. The major processes give rise to many small filopodia. Scale bar = 50 µm.
NG2 cells form the glial scar
Given that the NG2-expressing cells are a dividing population of glial precursor or progenitor cells, we asked whether these cells participate in the glial reaction to injury. When the brain or spinal cord is damaged, glial cells at the site of injury begin to divide. The astrocytes and other cell types generated form a dense cellular plaque known as the glial scar. As shown in Fig. 2, after small stab wounds to the cerebellar cortex, there is an increase in the number of NG2-positive cells at the lesion site. Within 48 h of the initial injury, irregularly shaped cells with dense deposits of anti-NG2 immunoreactivity on their surfaces appear along the margins of the wound. Many of these cells incorporate 3H-thymidine and over the next 3–5 days there is a substantial increase in the number of NG2-positive cells (Levine, 1994). Not all these cells are OPCs because meningeal cells and some invading macrophages also express NG2 (Bu et al. 2001). Nevertheless, by 7–10 days post-injury, the glial scar contains high levels of NG2. Glial scars that form after hemisections of the spinal cord (Jones et al. 2002) and knife cuts to the cortex (Hampton et al. 2004) also contain high numbers of NG2-expressing cells.
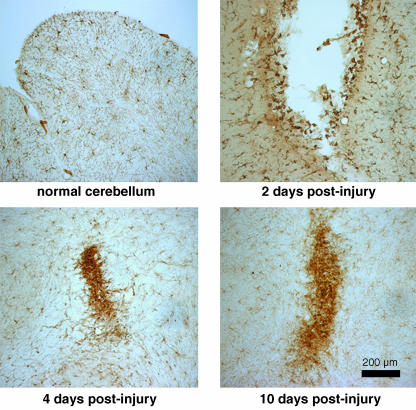
Fig. 2.
NG2-positive OPCs react to injury. Small stab wounds were made into the cerebellum of adult rats and the tissue was stained with anti-NG2 antibodies at the indicated survival times. By 2 days post-injury, many small intensely positive cells appear along the walls of the lesion cavity. At 4 and 10 days post-injury, the NG2-expressiong cells have formed small plaques at the injury site. These plaques correspond to the glial scar.
The formation of a glial scar is an attempt by the damaged nervous system to regenerate the glia limitans, which serves as a barrier between nervous and non-nervous tissue (Reier et al. 1983). The segregation of injured tissue from the non-injured parts of the CNS may help to re-establish metabolic and ionic homeostasis. Glial scars also act as physical and biochemical barriers to axon regeneration. After injury, the cut ends of axons reseal and the axons begin to form new growth cones. Although these growth cones can enter the glial scar, they fail to grow for any significant distance and subsequently become dystrophic. One major focus of spinal cord injury research is to identify the molecules responsible for this barrier function and to understand the mechanisms by which they retard endogenous axonal repair.
Three families of macromolecules are thought to participate in the formation of this barrier to axon regeneration: myelin-associated growth-inhibitory molecules, including myelin-associated glycoprotein (MAG), nogo and oligodendrocyte-myelin glycoprotein (OMgp); repulsive axon guidance molecules, such as the semaphorins and the ephrins; and CSPGs. Several lines of evidence implicate the CSPGs in the barrier properties of the glial scar. First, CSPGs are abundant in those areas through which regenerating axons do not grow (Jones et al. 2002). This correlation was demonstrated by Davies et al. (1999), who atraumatically transplanted adult dorsal root ganglion (DRG) neurons into the CNS. These cells extended long axons through the undamaged white matter. When the white matter was experimentally damaged and a CSPG-rich glial scar allowed to form, the axons stopped growing when they encountered this scar. Second, glial scar tissue is a poor substrate for axon growth in vitro and this non-permissiveness correlates with the presence of CSPGs (McKeon et al. 1991). Third, treatment of damaged CNS tissue with chondroitinase ABC, a bacterial enzyme that removes the chondroitin sulphate GAG chains from proteoglycan core proteins and promotes axon regrowth and functional recovery (Moon et al. 2001; Bradbury et al. 2002). As glial scars contain cells that express high levels of NG2, we became interested in how growing neurons react to NG2. We did this in two ways: first, we studied the effects of purified NG2 and recombinant fragments of NG2 on living neurons and second, we studied the effects of membranes from NG2-expressing OPCs and transfected cell lines on growing neurites.
NG2 inhibits neurite growth in vitro
We purified NG2 in a native state from B49 cells, the neural tumour cell line on which NG2 was first identified (Dou & Levine, 1994). Neurons will not attach to NG2-coated surfaces unless provided with adhesive molecules such poly-l-lysine, laminin or the L1 cell adhesion molecule. These cell adhesion molecules all promote the growth of neuritic processes; however, when NG2 is a component of the substrate, the extent of neurite outgrowth is reduced by 40–45%. Removal of the chondroitin sulphate GAG chains from the NG2 core protein by digestion with chondroitinase ABC did not alter the ability of substrate-bound NG2 to inhibit neurite extension. On the other hand, treatment of the substrate with polyclonal antibodies against NG2 did reduce the extent of growth inhibition. When neurons were grown on growth-promoting substrates with either stripes or spots of NG2, the axons avoided the NG2-coated regions but grew extensively on the permissive surfaces (Chen et al. 2003). We also purified the large ECD of NG2 and, when added to cultures of newborn rat dorsal root ganglion neurons, this fragment rapidly induced the collapse of growth cones (Uhgrin et al. 2003). These experiments demonstrated that NG2 has all the properties associated with repulsive axon guidance cues and suggest that the dense accumulation of NG2 at glial scars could contribute to the creation of a barrier against successful axon regrowth.
The experiments reviewed above demonstrated that NG2 can inhibit axon growth and can convert a growth-permissive surface into one that is non-permissive for axon extension. Because NG2 is one of many different molecules expressed on the surface of OPCs, we wondered whether growth inhibition was a property of the OPC surface and whether or not NG2 was involved. We initially tried to co-culture neonatal OPCs and neurons, but the high intrinsic mobility of the OPCs made these experiments difficult to analyse. We therefore turned to membrane-based assays for axon growth (Chen et al. 2002). OPCs were grown in bulk culture and membranes were prepared from the cells. The membrane fragments were then used as substrates for the growth of explants of postnatal rat cerebellum. When explants were grown on membranes prepared from OPCs, neurite growth was reduced by 35% compared with growth on membranes from HEK293 cells. As was the case with purified NG2, treatment of the OPC membranes with anti-NG2 antibodies significantly reversed this growth-inhibitory effect. The reversal by anti-NG2 antibodies was not complete, most likely due to the expression of other growth-inhibitory molecules, such as semaphorin 5A by the OPCs (Goldberg et al. 2004). Thus, the surface of OPCs is not permissive to axon elongation and NG2 participates in the creation of this non-permissive environment. Using the same membrane-based assay, we also demonstrated that the expression of NG2 is sufficient to convert a relatively growth-permissive surface (the membranes of HEK293 cells) into a surface that is non-permissive for neurite growth (Chen et al. 2002).
NG2 inhibits the growth of adult sensory neurons
The in vitro experiments described above suggest that the accumulation of NG2 at sites of CNS injury could inhibit axon regrowth. However, because these experiments utilized either newborn or embryonic neurons, it remained unknown whether adult neurons would also be responsive to the inhibitory influences of NG2. We therefore tested the effects of NG2 on axon growth from adult DRG neurons. Because adult neurons grow only short, highly branched processes in short-term culture, we first took adult rat Schwann cells, which had been purified and propagated in culture, and transfected the cells to express NG2. As shown in Fig. 3, adult Schwann cells, which do not express NG2, support the growth of an extensive network of neurites from the adult DRG cells. By contrast, Schwann cells transfected to express NG2 on their surfaces are not very supportive of neurite growth and the cells extend many short neurites with stubby branches. This suggests that, after injury, the centrally projecting axons of damaged sensory neurons would be unable to grow through the deposits of NG2-immunoreactivity. To confirm this conjecture, we transected bilaterally the dorsal spinal cords of adult rats at level T8 and examined the distribution of retrogradely labelled mechanosensory axons after injecting the sciatic nerve with cholera toxin B subunit. As shown in Fig. 4, cut axons retract from the lesion centre. These transected nerve fibres have enlarged end-bulbs characteristic of non-growing neurons, and these end-bulbs are embedded in the NG2-rich region that surrounds the lesion centre. This observation is similar to those of Jones et al. (2002) and Zhang et al. (2001), who found that lesioned axons end in areas rich in the NG2 proteoglycan. Thus, there is a strong correlation between those areas into which axons do not sprout or extend processes and the presence of NG2.
Fig. 3.
Adult DRGs grow poorly on beds of NG2-expressing Schwann cells. Left panel: normal adult rat sciatic nerve Schwann cells were maintained in culture and transfected with a full-length NG2 cDNA under control of the CMV promoter (pcDNA3.1). Both populations of Schwann cells express p75, the low-affinity neurotrophin receptor, but only the transfected cells express immunodetectable NG2. Right panel: adult rat DRG neurons were plated on the living Schwann cells and allowed to grow for 40 h and then stained with anti-βIII tubulin antibodies. Normal Schwann cells support extensive neurite growth: growth on the NG2-expressing Schwann cells was poor.
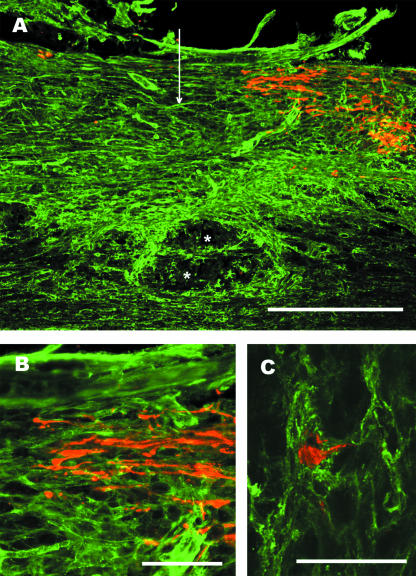
Fig. 4.
Lesioned mechanosensory axons end in an NG2-rich region of the glial scar. Adult rats were subjected to dorsal-over hemisections of the thoracic spinal cord and ascending mechanosensory axons retrogradely labelled by the injection of cholera toxin B subunit into the sciatic nerve 5 days post-lesion. Two days later the animals were killed and the tissue prepared for anti-NG2 (green) and anti-cholera toxin (red) immunofluorescence labelling. (A) NG2 immunoreactivity is increased surrounding and within the lesion centre. The vertical arrow marks the lesion centre and the asterisks indicate a cyst forming near the central canal. Scale bar = 500 µm. (B) Higher power view showing that the retrogradely labelled axons end with dystrophic end bulbs in a area with dense anti-NG2 stain. Scale bar = 100 µm. (C) A dystrophic end bulb is encased in NG2-positive processes. This is a z-stack made from 18 1-µm-thick scans taken with a Ziess 510 confocal microscope. Scale bar = 50 µm.
Multiple regions of NG2 inhibit neurite growth
As mentioned above, the ECD of the NG2 core protein can be divided into three separate structural domains. In a series of studies, Stallcup analysed the functional properties of these different domains of NG2 and showed that while some domains have their own specific functions, other functions are redundant and can be mediated by regions of the NG2 core protein that are structurally dissimilar to each other. For example, the central domain 2 binds to several extracellular matrix molecules but both domains 2 and 3 each independently bind cationic growth factors such as bFGF and PDFG (Goretzki et al. 1999). Because of this domain-specific functional specialization, we were interested in determining which regions of the NG2 core protein are responsible for the inhibition of neurite growth.
We prepared a series of Fc and myc/his fusion proteins encoding individual domains of the NG2 core protein and combinations of adjacent domains (Uhgrin et al. 2003). Each fusion protein was tested for its ability to induce growth cone collapse of newborn DRG neurons and to inhibit the growth of axons from neonatal cerebellar granule neurons. As shown in Table 1, fusion proteins containing either domains 1 or 3 are inhibitory in both of these assays. Domain 2, which contains the sites of GAG attachment, is modestly inhibitory only when the GAG chains were present. Removal of the chondroitin sulphate GAG chains with chondroitinase ABC renders domain 2 inactive. Curiously, chondroitinase digestion has no effect on the activity of a domain 1 and 2 Fc-fusion protein that also contains chondroitin sulphate GAG chains. Clearly, the GAG chains are not required for the inhibitory activity of large fragments of NG2 containing either domains 1 or 3 in these in vitro assays. The presence of inhibitory chondroitin sulphate GAG chains at glial scars in association with CSPGs other than NG2 may by one reason why chondroitinase ABC is effective in stimulating axon regeneration.
Table 1.
Quantitative comparison of different fragments of the NG2 proteoglycan. The data shown are taken from Uhgrin et al. (2003) and compare the effects of different regions of NG2 on the inhibition of neurite extension from postnatal cerebellar neurons and on the collapse of the growth cones of newborn rat DRG neurons. Cerebellar neurons were grown on substrates containing either the L1 cell adhesion molecule or a mixture of L1 and NG2 and neurite lengths were measured after 24 h of growth. For growth cone collapse, DRG neurons were plated onto laminin-coated surfaces and soluble proteins added 6 h later. After 20 min, the number of collapsed growth cones was determined. C’ase treated indicates that the protein was treated with chondroitinase ABC prior to testing
| Fusion protein | Amino acid residues | EC50 (nm) for growth inhibition | EC50 (nm) for growth cone collapse |
|---|---|---|---|
| ECD | 30–6738 | 3.5 | 1.8 |
| domain 1-Fc | 30–640 | 4.5 | 3.1 |
| domain 2-Fc | 636–1591 | 9 | 3.5 |
| domain 2-Fc | 22 | >20 | |
| C’ase treated | |||
| domain 1,2-Fc | 30–1591 | 3 | 2.5 |
| domain 1,2-Fc | 3.5 | 2.4 | |
| C’ase treated | |||
| domain 3-Fc | 1592–2222 | 6 | 2.4 |
Although NG2 is considered an integral membrane proteoglycan, a considerable portion (between one-third and one-half) of the total NG2 in brain is easily extractable in low-salt, aqueous buffers (Morgenstern et al. 2002). Nishiyama et al. (1985) showed that some cell lines shed or secrete a truncated form of NG2 into the medium. This truncated form arises from cleavage of the ECD at sites close to the external face of the plasma membrane and a similar mechanism may be responsible for the accumulation of extracellular NG2. The observation that two separate domains of NG2 (domains 1 and 3) mediate most of the inhibition of neurite growth and that NG2 can be secreted suggested to us a model of NG2 conformation as illustrated in Fig. 5. When NG2 is present as an integral membrane protein, domain 1, which extends away from the plasma membrane, may be available to interact with the growth cones of damaged and sprouting neurons. Domain 3 lies close to the plasma membrane and may be inaccessible (Uhgrin et al. 2003). When cleaved from the membrane and incorporated into the ECM, domains 1 and 3 would both be accessible. Thus, secretion or shedding of NG2 at glial scars could amplify the inhibitory signals transmitted by NG2.
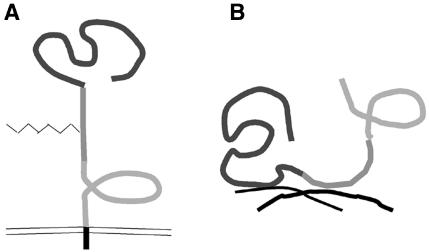
Fig. 5.
NG2 can adapt in different conformations. These schematics illustrate a model for the different conformations of NG2. The three individual domains of the ECD are indicated by the different shades of grey. (A) As an integral membrane proteoglycan, NG2 may extend away from the membrane surface. In this conformation, domain 1 is available but domain 3 may be shielded and inaccessible to neural growth cones. (B) When shed or secreted from the membrane, NG2 can become incorporated into the extracellular matrix via the collagen binding domain 2. Both domains 1 and 3 are now accessible. Because both domains 1 and 3 can independently inhibit neurite growth, extracellular NG2 may amplify the growth-non-permissive properties of the glial scar.
Conclusion and perspectives
Although the function of adult OPCs in the normal CNS is not well understood, the abundance of these unique progenitor cells throughout the brain and spinal cord suggests their function extends beyond acting simply as a pool of undifferentiated precursor cells that replace the slowly turning over oligodendrocyte population. As adult OPCs often have processes associated with synapses and nodes of Ranvier, both sites of ion exchange across neural membranes, they may be poised to sense changes in normal physiological activity and respond quickly to those changes. Whether these responses modulate or maintain synaptic activity is unknown.
Despite uncertainties concerning their functions in the normal brain, it is clear that adult OPCs are one of several cell types to react rapidly to CNS injury. In addition to the stab wounds and spinal cord hemisections described here, OPCs proliferate in response to ischaemia, excitoxicity and experimentally induced demyelination (Levine et al. 2001). A major feature of this response is the rapid proliferation of OPCs. The signals that initiate this proliferation of OPCs are obscure, but because the reaction is localized only to damaged areas, whatever mechanisms regulate the rapid re-entry of OPCs into the cell cycle must operate over short distances. One possibility is that signals from neighbouring neurons, oligodendrocytes, or both, maintain the slow normal proliferation of OPCs, perhaps through a notch-based signalling mechanism (Zhang & Miller, 1996; Wang et al. 1998). Physical damage or demyelination may remove these inhibitory signals and allow OPCs to proliferate rapidly. This conjecture does not rule out a role for signalling factors that can enter into the injury site through the disrupted blood–brain barrier.
The ability of these cells to re-enter the cell cycle may aid in wound healing by contributing to the re-creation of the glial limitans or by increasing the size of the pool of available precursor cells required for the remyelination of damaged axons. However, the rapid accumulation of OPCs following traumatic CNS injury leads to elevated levels of the NG2 proteoglycan. Given the large body of evidence reviewed here that NG2 is a potent inhibitor of axon growth in vitro, the dense deposits of NG2 at glial scars may perturb growth cone function and prevent newly sprouting axons from regenerating into and past the injury site. It is therefore important to determine whether removing or blocking the functions of NG2 can promote CNS axon repair after injury. Our laboratory has developed monoclonal anti-NG2 antibodies capable of neutralizing the growth-inhibitory properties of the core protein (Uhgrin et al. 2003) and we are currently using these antibodies to test this hypothesis (Tan et al. 2003).
Acknowledgments
Work in our laboratory has been supported by grants from the National Institutes of Health, the Christopher Reeve Paralysis Foundation and the New York State Spinal Cord Injury Research Board. We thank all past and present members of the lab for their contributions to this work.
References
- Beasley L, Stallcup WB. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;9:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a sub-population of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34:296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synaptocyte: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Chen Z, Uhgrin Y, Levine JM. Axon growth inhibition by oligodendrocyte precursor cells. Mol Cell Neurosci. 2002;20:125–139. doi: 10.1006/mcne.2002.1102. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Negra M, Levine AK, Ughrin Y, Levine JM. Oligodendrocyte precursor cells: reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2003;31:481–495. doi: 10.1023/a:1025791614468. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ML, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dawson ML, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:787–802. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Dou CL, Levine JM. Inhibition of neurite outgrowth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem. 1999;274:16831–16837. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Seitz A, Chen P, Heber-Katz E, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studies with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon LDF, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their original target following treatment of adult rat brain with chondroitinase ABC. Nature Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Morgenstern DEA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Stallcup WB. Generation of truncated forms of the NG2 proteoglycan by cell surface proteolysis. Mol Biol Cell. 1985;6:1819–1832. doi: 10.1091/mbc.6.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary sequence of NG2, a novel membrane spanning proteoglycan. J Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Zhang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Ong WY, Levine JM. A light and electron microscopic analysis of NG2 positive oligodendrocyte precursor cells in the normal and kainate lesioned hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- Raff MC. Glial cell diversification in the rat optic nerve. Science. 1989;243:1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Stensaas LJ, Guth L. The astrocytic scar as an impediment to regeneration in the central nervous system. In: Kao CC, Bunge RP, Reier PJ, editors. Spinal Cord Reconstruction. New York: Raven Press; 1983. pp. 163–195. [Google Scholar]
- Reyners H, Gianfelici de Reyners E, Hooge R, Vankerkom J, Maisin JR. The beta astrocyte: a newly recognized radiosensitive glial cell type in the cerebral cortex. J Neurocytol. 1982;11:967–983. doi: 10.1007/BF01148311. [DOI] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres B. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promote retraction fiber formation and cell polarization. J Cell Sci. 2001;114:2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- Staub E, Hinzmann B, Rosenthal A. A novel repeat in the melanoma-associated chondroitin sulfate proteoglycan defines a new protein family. FEBS Lett. 2002;527:111. doi: 10.1016/s0014-5793(02)03195-2. [DOI] [PubMed] [Google Scholar]
- Tan AM, Levine AK, Chen Z, Levine JM. Neutralizing antibodies against the NG2 proteoglycan promote sensory axon regeneration in the injured adult rat spinal cord. Neurosci. Abstract. 2003:245.12. [Google Scholar]
- Uhgrin Y, Chen ZJ, Levine JM. Multiple domains of the NG2 proteoglycan mediate axon growth inhibition. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wilson SS, Baetge EE, Stallcup WB. Antisera specific for cell lines with mixed neuronal and glial properties. Dev Biol. 1981;83:146–153. doi: 10.1016/s0012-1606(81)80017-6. [DOI] [PubMed] [Google Scholar]
- Wren D, Wolswijk G, Noble M. In vitro analyis of the origin and miantenance of O2Aadult progenitor cells. J Cell Biol. 1992;116:167–1776. doi: 10.1083/jcb.116.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Miller RH. Density-dependent feedback inhibition of oligodendrocyte precursor expansion. J Neurosci. 1996;16:6886–6895. doi: 10.1523/JNEUROSCI.16-21-06886.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tohyama K, Winterbottom JK, et al. Correlation between putative inhibitory molecules at the dorsal root entry zone and failure of dorsal root axonal regeneration. Mol Cell Neurosci. 2001;17:444–459. doi: 10.1006/mcne.2000.0952. [DOI] [PubMed] [Google Scholar]