Abstract
The NG2 glycoprotein is a type I membrane protein expressed in the developing and adult central nervous system (CNS) by subpopulations of glia including oligodendroglial precursor cells (OPCs), and in the developing CNS additionally by pericytes. In the mouse CNS, expression of NG2 protein is already observed at embryonic day 13 and peaks between postnatal days 8 and 12. NG2+ cells persist in grey and white matter in adult mouse brain: cells in the developing and adult brain show clear differences in migration, cell-cycle length and lineage restriction. Several groups have provided evidence that subpopulations of NG2+ cells can generate neurons in vivo. Neuronal stimulation in the developing and adult hippocampus leads to Ca2+ signals in apposing NG2+ glia, suggesting that these cells may modulate synaptic activity, and NG2+ cells often ensheath synapses. The structure of the protein with two N-terminal LamininG/Neurexin/Sex-hormone-binding globulin domains suggests a role in adhesion. The C-terminal PSD-95/DiscsLarge/Zona Occludens-1 (PDZ)-binding motif has been found to associate with several PDZ proteins including the Glutamate Receptor Interacting Protein GRIP: NG2 may thus act to position AMPA receptors on glia towards sites of neuronal glutamate release. Furthermore, the NG2 proteoglycan plays a role in cell migration and spreading and associates with actin-containing cytoskeletal structures.
Keywords: astrocyte, glutamate, GRIP, myelin, synapse
Initial characterization of the NG2 proteoglycan as a marker for immature cells
NG2 was first characterized as a high-molecular-weight type 1 membrane proteoglycan in rat (Stallcup, 1981; Nishiyama et al. 1991; Levine & Nishiyama, 1996). It was subsequently found to be present in humans as Melanoma Chondroitin Sulphate Proteoglycan (MCSP; Pluschke et al. 1996). It was independently rediscovered as the AN2 molecule in mouse (Niehaus et al. 1999; Schneider et al. 2001; see Fig. 1). Homologues exist in Drosophila as CG10275 and Caenorhabditis elegans as C48E7.6.p.
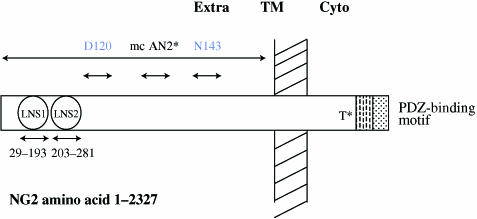
Fig. 1.
Schematic structure of NG2 with approximate locations of antibody-binding sites. Extra, TM and Cyto refer to the extracellular, transmembrane and cytoplasmic portions of the molecule. Modified from Fang et al. (1999). Dashed and dotted areas denote the proline-rich region and PDZ-binding region, respectively. T indicates threonine 2256, which has been shown to be phosphorylated, thereby leading to enhanced motility (Makagiansar et al. 2004). The mc AN2 (Niehaus et al. 1999) binds to a region within the amino acids 1237–1531. The other two mentioned antibodies discussed in the text were shown to compartmentalize NG2 with distinct cytoskeletal structures (Fang et al. 1999).
NG2 is a marker for immature oligodendrocytes in vitro (Levine & Nishiyama, 1996), overlapping partially with O4 and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) but absent in cells that express later stage markers such as myelin-associated glycoprotein (MAG) and myelin oligodendrocyte glycoprotein (MOG). Western blots of whole mouse brain homogenates using the AN2 monoclonal antibody recognizing mouse NG2 show expression starting from embryonic day (E)13/E14, peaking within the period of postnatal day (P)8–P12 and gradually falling thereafter (Niehaus et al. 1999). NG2 expression is, however, not just limited to the oligodendroglial precursor cells (OPCs) and pericytes of the developing central nervous system (CNS) (see below). A subpopulation of NG2+ cells is present in adult brain (see Fig. 2). It is also expressed by immature Schwann cells (Schneider et al. 2001), and fibroblast-like cells in the peripheral nervous system (PNS) (Morgenstern et al. 2003). Outside the nervous system many immature cell types including developing cartilage, immature smooth muscle cells, skeletal myoblasts, epidermal stem cells and human melanoma cells express NG2 (Stallcup, 2002; Nishiyama et al. 2005).
Fig. 2.
Morphology of NG2-expressing cells in grey and white matter in the adult mouse brain. (A) Low-magnification view of NG2+-labelled cells. (B) High-magnification view of an NG2+-labelled cell in corpus callosum (CC). The labelled cell appears to have an elongated morphology. (C) Low-magnification view of NG2+-labelled cells in the grey matter. (D) High-magnification view of an NG2+-labelled cell in the cortex (Cor). The cell appears to have a stellate morphology. Dashed lines signify separation between grey and white matter. Scale bar = 10 µm.
CNS lineage
In the early CNS development, distinct lineage-restricted cells are generated from pluripotent precursors in an orderly manner to form intricate networks. Lineage specification of the neural precursors is associated with proliferation, migration and differentiation. Some of the pluripotent precursors persist throughout development into adulthood. It was generally thought that during development neuronal genesis takes place in the ventricular zone (VZ), an early embryonic layer, while the genesis of glia precursors takes place in a proliferating layer that is formed in late embryonic development persisting into adulthood, the subventricular zone (SVZ; Hirano & Goldman, 1988; Levison et al. 1993; Romanko et al. 2004). Both neurons and oligodendrocytes are postmitotic at the end of their development, whereas astrocytes retain the ability to proliferate, for example in lesion areas. After the majority of the cells have been generated during development, neural genesis still takes place at a very low level in the adult brain. Neurogenesis persists in the areas of the SVZ and the subgranular layer in the dentate gyrus throughout adulthood (Alvarez-Buylla et al. 2001; Seri et al. 2001). There has been ongoing discussion as to whether the different classes of neural cells share a common precursor cell. The identification of the neural stem cell(s) that generates these cells in vivo is still a matter of heated debate. What are these cells and what is their differentiation potential?
NG2-expressing cells
In the last few years NG2 has drawn a lot attention, owing to the fact that a large majority of cells expressing NG2 retain the ability to divide throughout development. This interesting property suggests that NG2-expressing cells have a precursor nature (Levine & Nishiyama, 1996; Levison et al. 1999). NG2+ cells play a role in myelination. Our own observations show that the depletion of AN2/NG2+ cells in myelinating cultures by lysis with AN2 monoclonal antibody plus complement prevents the development of MAG- and MOG-expressing cells (Niehaus et al. 2000). Interestingly, repeated lysis was required, suggesting that the NG2+ cells can be regenerated from an NG2 precursor cell. It has been demonstrated that in vitro NG2 cells behave like oligodendrocyte-type 2 astrocyte (O-2A) precursors. Antibodies to NG2 label O-2A cells (Raff et al. 1983), which give rise to oligodendrocytes and astrocytes. When NG2-expressing cells isolated from early postnatal mouse brain are grown in the presence of fetal calf serum, the cells differentiate into astrocytes, and when grown in the absence of serum they have the ability to differentiate into oligodendrocytes (Diers-Fenger et al. 2001). From these results NG2 cells could be considered glial precursor cells that generate oligodendrocytes and astrocytes in the developing and adult brain (Levison & Goldman, 1993; Levison et al. 1993, 1999). The fact that no neurons were seen in these studies does not rule out that these cells could generate neurons. It is possible that the culture conditions were not conducive to neuronal generation.
In vivo NG2+ cells express platelet-derived growth factor-α (PDGF-α) receptor, often considered a marker for immature oligodendrocytes but not proteins expressed by more mature oligodendrocytes (Levine & Nishiyama, 1996; Nishiyama et al. 1996). With the generation of a transgenic animal expressing enhanced green fluorescent protein (EGFP) under the proteolipid protein (PLP) promotor, Mallon et al. demonstrated two populations of NG2-expressing cells. One population of NG2+ cells were EGFP-positive, indicating that these cells in which the PLP promotor is active could be a source of myelinating oligodendrocytes in the developing brain. These cells migrate into the cortex from the SVZ and stay in an undifferentiated state for up to 3 weeks until myelination starts (Mallon et al. 2002).
Recent results have shown that radial glia give rise to neurons in the CNS and are indeed stem cells that have the ability to develop into distinct subpopulations of neurons depending on the brain region. In the developing mouse brain, different types of radial glia exist that differ in their expression of transcription factors and the type of neurons they give rise to (Gotz & Steindler, 2003; Kriegstein & Gotz, 2003). The patterns of transcription factor expression thus yield cues patterning the development of the neural tube. Could radial glia spawn NG2-expressing cells (see Fig. 3)? It has been shown that basic helix–loop–helix transcription factors Olig1 and Olig2 are important in both motorneuron and oligodendrocyte development (Lu et al. 2001, 2002; Zhou & Anderson, 2002). Knockout mice lacking expression of both of these transcription factors die during embryogenesis and are lacking both motorneuron and oligodendrocytes, further indicating that they share a common precursor. Recently it has been shown that cells expressing the NG2 glycoprotein also express the transcription factor Olig2 in vivo (Liu & Rao, 2004). It has been claimed that NG2 cells give rise to neurons expressing neuronal markers such as Neu-n and βIII Tubulin in the SVZ and also to (γ-amino-butyric acid) (GABA)ergic interneurons in vitro and in vivo in the hippocampus (Aguirre et al. 2004). These experiments rely heavily on the use of the CNP-EGFP transgenic mouse and the specificity of the polyclonal NG2 antibodies used. It has been recently reported that a small number of NG2+ cells that reside in the cortex of adult rats give rise to GABAergic neurons. This evidence further supports the idea that the NG2 cell is a precursor cell that can give rise to different cell lineages (Dayer et al. 2005).
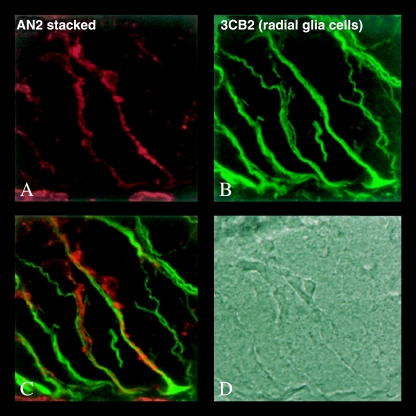
Fig. 3.
Association of NG2+ cells and radial glia in the spinal cord of E15 mouse. (A) NG2+-expressing cell lablled with AN2 antibody. (B) 3CB2+-labelled radial glia. (C) Overlay of A & B shows close association between NG2-expressing cell and radial glial cell. (D) Phase image of the section.
A subpopulation of NG2+ cells express S100-β, mRNA for excitatory amino acid carrier 1 (EAAC1), glutamate/aspartate transporter (GLAST) and glutamate transporter 1 (GLT-1). S100-β is a calcium-binding protein, which is unique to glia cells; EAAC1 is a neuronal glutamate transporter, GLAST is a glia-associated glutamate transporter and GLT-1 is glia associated. These NG2+ cells express α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors containing all the subunits GluR 1–4. These GluR cells could be precursor cells that give rise to neurons and glial cells (Matthias et al. 2003).
NG2 cells in the adult brain
A significant number of NG2-expressing cells exist in the adult brain after gliogenesis is complete (Fig. 2). These cells have two distinct morphologies: the first morphology resembles a protoplasmic astrocyte containing an oblong nucleus and limited cytoplasm with bipolar or unipolar processes. The second morphology resembles a microglia cell or a premyelinating oligodendrocyte with multipolar stellate processes (Horner et al. 2002). Their role in the function of the adult brain and the significance of their activation in injury or repair states is still not understood. There is evidence that a subpopulation of NG2-expressing cells play a progenitor role in the adult CNS by continuing to divide, and that they retain PDGF-α receptor expression. These adult cells exhibit clear differences to the embryonic cells in migration, cell-cycle length and lineage restriction. The cells have the antigenic phenotype of OPCs, but the morphological phenotype and distribution of astrocytes. They are antigenically distinct, however, from astrocytes, microglia, myelin-producing oligodendrocytes and neurons. The current view is that some of these NG2+ cells are oligodendrocyte progenitors, which lose their ability to label for the NG2 antibodies as they differentiate into mature oligodendrocytes.
NG2+ cells are often found contacting neurons. This includes perisynaptic wrappings of NG2+ cell processes around and between neuronal synaptic contacts in the cortex and hippocampus (Ong & Levine, 1999; Butt et al. 2002). It has been recently shown that NG2+ cells in the developing and adult hippocampus expressing AMPA receptors form glutamatergic and GABAergic synapses with neurons (Bergles et al. 2000; Lin & Bergles, 2004a,b). Stimulation of the neurons resulted in glutamate and GABA release and activation of the AMPA and GABA-A receptors, respectively, in the NG2+ cells. Synaptic structures such as synaptic density and synaptic vesicles could be seen in the neurons contacting the NG2+ cells. NG2 could thus be involved in the alignment and formation of glia–neuron synapses (Stegmuller et al. 2003) and play a role in the modulation of synaptic activity. NG2-expressing cells, in addition to GFAP+ astrocytes, also contact nodes of Ranvier in the white matter (Butt et al. 2002). When studying the morphology of these cells in sections, they seem to resemble astrocytes rather than oligodendrocytes. They comprise a highly complex and distinct adult glia population within the CNS. These cells could be considered the fourth type of neuroglia cell in the adult CNS. Under the electron microscope these cells are clearly distinct from any other type of cell in the adult CNS (Butt et al. 2002; see also this issue). The nucleus of these cells seems to be not as dense as astrocytes, but they seem always to be connected to astocytes, which are then closely associated with blood vessels. These cells can be seen to have dense areas that look like synapses, when making contact with axons (Peters, 2004).
In demyelination models, classical experiments demonstrated that the remyelinating cells were proliferative (Gensert & Goldman, 1997). Later studies showed an increase in the number of NG2+ cells as a primary response to demyelination around lesions, following a decrease in these cells weeks later when oligodendrocytes appear and remyelinate the lesions (Keirstead et al. 1998; Watanabe et al. 2002). In remyelinating lesions, studies from the group of Akiko Nishiyama showed that NG2+ cells divide and can be labelled with bromodeoxyuridine (BrdU). Many of the oligodendrocytes generated at later time points in these remyelinating lesions are BrdU-labelled, strongly suggesting that they derive from NG2+ cells (Watanabe et al. 2002).
Structure of NG2
The mouse homologue of NG2, first termed AN2, comprises 2327 amino acids (aa; Fig. 1). The extreme C-terminal consists of a Postsynaptic density protein-95, Discs-large, Zona occludens-1 (PDZ) motif, which specifies binding to a class II PDZ domain (Sheng & Sala, 2001) followed by a 25-aa transmembrane helix and a large extracellular domain. Two Laminin G, Neurexin, Sex-hormone-binding globulin (LNS) domains are present at the amino-terminal end (aa 47–179 and 224–364). The presence of two high-affinity LNS domains suggests that NG2 probably has ligands interacting with its extracellular domain in trans; so far these have not been identified unambiguously. NG2 carries a single chondriotin sulfate gylcosaminoglycan chain attached at serine 999, although there are several more potential sites (Stallcup & Dahlin-Huppe, 2001). The attachment of glycosaminoglycan chains is developmental time and cell-type specific (Schneider et al. 2001).
Given that NG2 is present on multiple cell types at differing stages of development, it is likely to possess more than one role and interacting partner.
Functions of NG2: migration, spreading and and process outgrowth
Multiple studies have confirmed that NG2 is involved in cell attachment and migration. The Oli-neu cell line having glial precursor properties developed in our laboratory (Jung et al. 1991) is remarkably motile. Human melanomas over-expressing the protein MCSP show invasive properties (Eisenmann et al. 1999) and endothelial cell motility is promoted by the NG2 proteoglycan (Fukushi et al. 2004). Addition of the IgG fraction of the polyclonal antibodies generated against the whole NG2 molecule shows retardation in the rate of migration of the cells (Niehaus et al. 1999). The monoclonal AN2 antibody, which binds to NG2 in the region between aa 1237 and 1531, does not affect the rate of migration. Polyclonal antibodies against the F3 glycoprotein, which is also expressed by these cells, do not affect the migration. The rate of migration also differs with the substrate engaging the NG2 extracellular domain, as shown by work from the Stallcup group.
Integrins are likely to cooperate with NG2 in migration and spreading: α2β1, αvβ1, α4β1 and α3β1 have all been implicated. The integrin profile varies according to cell type: the wide variety of integrins thought to be engaging NG2 might be explained by this diversity. It has been shown that oligodendrocyte precursors express αvß1 whose down-regulation correlates with loss of migratory potential (Milner et al. 1996). Similarly, incubation with anti-integrin antibodies leads to an inhibition in migration of melanoma cells (Iida et al. 2001). The chondroitin sulfate glycosaminoglycan chains have been shown to be required for αvβ1 binding to NG2 (Iida et al. 1998). Integrin clustering triggers a cascade of intracellular signalling pathways and phosphorylation of intracellular and cytoskeletal molecules. While β1 integrin seems to be involved in many migration pathways, β1 integrin-independent extension of ruffling lamellipodia and rearrangement of the actin cytoskeleton has also been demonstrated (Tillet et al. 2002). Multiple publications have shown the involvement of integrins in the signalling cascade through which NG2 may mediate cytoskeletal reorganization and motility.
During spreading, MCSP clustering activates Cdc42 to its GTP-bound form. Cdc42 is a Rho-family GTPase. Ack-1 (activated Cdc-42-associated kinase) recognizes activated Cdc42 and phosphorylates a tyrosine residue on p130cas with further recruitment of α4β1 (Eisenmann et al. 1999). It is has been proposed that a supra-molecular complex is formed by recruiting target proteins to be phosphorylated, thus propagating the signal. A similar complex of NG2, GTP-bound rac and p130cas have been shown to be involved in cell spreading and morphology changes in other NG2+ cells (Majumdar et al. 2003).
Engaging NG2 and α4β1 by plating NG2+ cells on a ‘chimeric substrate’ consisting of a fibronectin synthetic peptide and anti-NG2 mAb 9.2.27 leads to cell spreading and formation of focal contacts (Iida et al. 1995). The same publication showed that two selective tyrosine kinase inhibitors, genistein and herbimycin A, totally inhibited cell spreading, indicating that tyrosine kinase(s) is important for cell spreading and focal contact formation. Makagiansar et al. (2004) have shown that protein kinase C-alpha (PKC-α) phosphorylates the cytoplasmic domain of NG2. Phosphorylation of threonine 2256 leads to redistribution of NG2 on the surface of astrocytomas, polarization of the cell and a significant increase in cell motility. PMA (phorbol myristate-acetate, a PKC stimulator) treatment leads to a co-localization of NG2 with the ezrin-radixin-moesin (ERM) protein ezrin and α3β1 integrin in lamellipodia. Selective inhibition of PKC showed significantly reduced phosphorylation of threonine 2256 with a reduction in cell motility.
Movements of pericytes in newly formed blood vessels involve a cell surface complex of NG2, galectin-3 and α3β1. Pericytes form the outer layer of blood vessels. It has been suggested that NG2 mediates communication between pericytes and vascular endothelial cells (ECs) probably in its soluble, proteolytically cleaved form. Migration was reduced when antibodies against α3 integrin and galectin-3 were used (Fukushi et al. 2004).
Several studies have shown that NG2 reorganizes the actin cytoskeleton (Lin et al. 1996a,b; Fang et al. 1999; Stallcup & Dahlin-Huppe, 2001). However, direct interaction with actin has not been shown. Such an interaction is probably mediated by adaptor protein(s). The cytoplasmic domain of MCSP is highly conserved between species. Experiments with cells expressing a chimeric protein where the cytoplasmic portion had been replaced with CD8 showed decreased keratinocyte cohesiveness without affecting adhesiveness to the extracellular matrix or motility. The adherens junctions in such cells were disorganized and it is suggested that a disruption of E-cadherin-based adherens junction occurs (Legg et al. 2003). Stallcup's group found that U251MG human astrocytomas transfected with wild-type NG2 spread better than those expressing chimeric proteins where the cytoplasmic/transmembrane portion of NG2 had been replaced. They concluded that the proximal-membrane segment of NG2 is necessary for actin cytoskeleton reorganization and cell motility. Furthermore, the cytoplasmic region and the chondriotin sulfate chain appear to direct the localization of NG2 to distinct subcellular microdomains, thus playing a role in cell polarity (Fang et al. 1999).
Lin et al. (1996b) suggest that NG2 can associate with two distinct types of actin-containing structures, depending on the stimulus. This may define cell polarity during migration and morphogenesis. NG2 monoclonal antibodies engaging different epitopes of the proteoglycan trigger distinct forms of actin reorganization (Fang et al. 1999). Engaging the amino-terminal region of NG2 (by the D120 monoclonal) induces close apposition to radial actin spikes characteristic of filopodia, while addition of an antibody (N143) against the extracellular portion near the transmembrane leads to the appearance of cortical actin bundles usually seen in lamellipodia. These NG2+ filopodial extensions lack both myosin and focal adhesion plaques. Myosin is absent in the radial processes of motile cells while in static cells NG2 co-localizes with myosin-containing stress fibres (Lin et al. 1996a). Because NG2–actin interaction has not been directly shown, it is probable that adaptor proteins help to direct the cytoskeletal reorganization on reception of appropriate cues.
NG2 and neurite growth
Whether NG2 has an inhibitory or attractive role for outgrowing neurites is still unclear. Although work from the Stallcup laboratory with antibodies against discrete sections and chimeric proteins shows that NG2 serves as a transducer of signalling between the substratum and helps in migration of whole cells, results from Joel Levine's group demonstrate inhibition of neurite outgrowth by immunopurified NG2 from the B49 cell line (Dou & Levine, 1994) as well as by NG2 fusion proteins covering the whole molecule (Ughrin et al. 2003). Our work supports the idea that NG2 might have dual roles: immunoaffinity purified NG2 from early postnatal mouse brain showed neither inhibition nor enhancement of neurite outgrowth (Schneider et al. 2001). Cerebellar granule cells extend neurites on NG2-coated as well as NG2-free areas of the coverslip (Niehaus et al. 1999). Furthermore, the expression of NG2 by immature Schwann cells (Schneider et al. 2001) and the up-regulation in regenerating PNS (Rezajooi et al. 2004) as well as a recent paper comparing neurite outgrowth in vivo in the NG2 knockout vs. wild-type mice (de Castro et al. 2005) argue against the concept of a purely inhibitory role of NG2 on neurite outgrowth. In view of the ability of molecules such as MAG to act as inhibitors or promotors of neurite outgrowth depending on age and type of neuron and priming of intracellular cyclic nucleotide levels (Henley et al. 2004), perhaps NG2 can be either inhibitory or repulsive depending on the individual situation. The close proximity of NG2 cells to neurons during myelination, at the nodes of Ranvier and also around synaptic structures suggests the existence of a neuronal receptor.
Adaptor proteins
ELISA and surface plasma resonance have shown that some extracellular matrix components, chief among which is Type VI and V collagen (Stallcup et al. 1990; Burg et al. 1996; Tillet et al. 1997), growth factors such as PDGF AA and bFGF, and kringle domain proteins such as plasminogen (Goretzki et al. 1999) associate with NG2. The presence of a PDZ-binding motif raised the idea of PDZ-domain proteins as likely scaffolding candidates. MUPP1, a protein with 13 PDZ modules, was shown to interact with NG2 (Barritt et al. 2000) as well as with GRIP (glutamate receptor interaction protein), which has seven PDZ domains (Stegmuller et al. 2003). Recently, we have identified another protein, Syntenin-1, containing a tandem repeat of PDZ domains, as another interacting partner of NG2 (unpublished data). The functional importance of MUPP1-NG2 is yet to be determined. However, the multiple PDZ domains provide a wealth of potential sites for clustering NG2 with other structural and/or signalling molecules.
GRIP probably plays such a clustering role. GRIP1 binds to the GluR B and C subunits of AMPA receptors. Recent work from our group (Stegmuller et al. 2003) showed that NG2, GRIP and the AMPA receptor subunit GluRB, all of which are co-expressed in glial progenitor cells, form a complex. A triple complex of these proteins was co-precipitated from lysates of whole brain and from co-transfected cells (Stegmuller et al. 2003). GRIP may help to cluster and orientate AMPA receptors towards neurons releasing glutamate whereby NG2 acts as an adhesion molecule. In view of the hypothesis that AMPA receptors in immature oligodendrocytes regulate cell differentiation and proliferation (Gallo et al. 1996; Yuan et al. 1998) and electron microscopic and electrophysiological evidence of synapses between NG2+ cells and CA3 neurons (Bergles et al. 2000), this triple protein complex is likely to play a role in glial–neuronal signalling.
NG2 and disease
NG2 and tumours
The human homologue of NG2, MCSP, is expressed by human melanomas and many human gliomas (Ferrone & Kageshita, 1988; Pluschke et al. 1996; Shoshan et al. 1999; Chekenya & Pilkington, 2002). NG2 has been shown to interact with extracellular matrix proteins such as collagen VI and tenascin C (Stallcup, 2002: see above). Cell–cell and cell–extracellular matrix interactions are particularly important for invasion and metastasis. Antibodies against the MCSP core protein inhibit invasion by melanoma cells in vitro (Harper and Reisfeld, 1983). Invasion involves cell adhesion and degradation of ECM proteins. MCSP is linked to MT3-MMP, a matrix-metalloproteinase that degrades Type I collagen. Antisense MCSP oligonucleotides and antisense MT3-MMP constructs significantly suppress invasion in a three-dimensional gel as do the inhibitors rTIMP2 and BB94 (Iida et al. 2001). The presence of the chondriotin sulfate chains appears necessary for this invasive potential, because CS-affinity columns alone could bind tagged MT3-MMP, and enzymatic digestion or treatment of melanoma cells with chondroitinase ABC to remove glycosaminoglycan chains or inhibition of their synthesis with β-d-xylopyransoside effectively inhibited the process. MT3-MMP was also isolated from a CS-affinity column to which biotinylated melanoma cell surface proteins were applied. Another metalloprotease, MMP 9, has also been shown to degrade NG2 (Larsen et al. 2003).
Several studies have examined the potential role of MCSP as a tumour target antigen in melanoma (see discussion in Wagner et al. 2005): although this may well be feasible for melanoma it is difficult to imagine how it could function for glioma as many NG2+ cells exist in the normal mature brain.
NG2 and multiple sclerosis
In multiple sclerosis (MS), the most common human demyelinating disease, oligodendrocytes and myelin are lost from the CNS combined with an increasing tissue sclerosis (Raine, 1997). The issue of neuronal loss has recently been re-addressed and it is apparent that long-term demyelination results in axonal transection and loss of neurones (Ferguson et al. 1997; Trapp et al. 1998; reviewed in Bjartmar & Trapp 2001). In recent years the essential role of the myelinating glia in communicating to and maintaining the health of the axons they myelinate has become increasingly clear (de Waegh et al. 1992). In many mouse models where myelin is reduced in quantity or shows even subtle abnormalities, axonal pathology as evidenced by swelling and organelle accumulation at the nodes of Ranvier has been demonstrated (e.g. see Griffiths et al. 1998; Edgar et al. 2004). The molecular signals mediating this cross-talk between axons and glia remain largely undetermined.
In MS patients, there is a progressive inability to remyelinate demyelinated axons with disease progression, in contrast to animal models where remyelination of demyelinated lesions occurs efficiently (Franklin, 2002). NG2+ cells exist in MS lesions (Chang et al. 2000) but whether they are indeed OPCs is open to debate. Apart from the age difference between human patients and animal models, which is known to influence the capacity to remyelinate, this poses the question as to whether a part of the immune attack in MS patients is targeted at the progenitor cells that mediate remyelination. With this concept in mind we analysed a small group of MS patients for the presence of cerebral spinal fluid (CSF) antibodies against mouse NG2. Strikingly some patients showed CSF reactivity against NG2: these patients all had active relapsing remitting MS. Serum levels of antibody did not appear to correlate with disease status. NG2 antibodies could cause pathology by interfering with OPC generation, migration and differentiation, or by interacting with NG2+ cell processes at nodes of Ranvier or synapses, thus possibly interfering with glial–neuronal signalling. The role of such antibodies in disease progression can only be elucidated by understanding the functional role of the NG2 glycoprotein, as well as examining the effect of NG2 autoantibodies on pathology in controllable models of CNS inflammation and de- and remyelination such as experimental allergic encephalitis.
Acknowledgments
Our work described in this review was supported by grants from the Deutsche Forschungsgemeinschaft and the Gemeinnützige Hertie-Stiftung.
References
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Barritt DS, Pearn MT, Zisch AH, et al. The multi-PDZ domain protein MUPP1 is a cytoplasmic ligand for the membrane-spanning proteoglycan NG2. J Cell Biochem. 2000;79:213–224. doi: 10.1002/1097-4644(20001101)79:2<213::aid-jcb50>3.0.co;2-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Trapp BD. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr Opin Neurol. 2001;14:271–278. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Burg MA, Tillet E, Timpl R, Stallcup WB. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996;271:26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- de Castro R, Jr, Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol. 2005;192:299–309. doi: 10.1016/j.expneurol.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekenya M, Pilkington GJ. NG2 precursor cells in neoplasia: functional, histogenesis and therapeutic implications for malignant brain tumours. J Neurocytol. 2002;31:507–521. doi: 10.1023/a:1025795715377. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia. 2001;34:213–228. doi: 10.1002/glia.1055. [DOI] [PubMed] [Google Scholar]
- Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Yool D, et al. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J Cell Biol. 2004;166:121–131. doi: 10.1083/jcb.200312012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann KM, McCarthy JB, Simpson MA, et al. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat Cell Biol. 1999;1:507–513. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- Fang X, Burg MA, Barritt D, Dahlin-Huppe K, Nishiyama A, Stallcup WB. Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol Biol Cell. 1999;10:3373–3387. doi: 10.1091/mbc.10.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Ferrone S, Kageshita T. Human high molecular weight-melanoma associated antigen as a target for active specific immunotherapy – a phase I clinical trial with murine antiidiotypic monoclonal antibodies. J Dermatol. 1988;15:457–465. doi: 10.1111/j.1346-8138.1988.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem. 1999;274:16831–16837. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- Gotz M, Steindler D. To be glial or not– how glial are the precursors of neurons in development and adulthood? Glia. 2003;43:1–3. doi: 10.1002/glia.10251. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Harper JR, Reisfeld RA. Inhibition of anchorage – independent growth of human melanoma cells by a monoclonal antibody to a chondroitin sulfate proteoglycan. J Natl Cancer Inst. 1983;71(2):259–263. [PubMed] [Google Scholar]
- Henley JR, Huang KH, Wang D, Poo MM. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron. 2004;44:909–916. doi: 10.1016/j.neuron.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Goldman JE. Gliogenesis in rat spinal cord: evidence for origin of astrocytes and oligodendrocytes from radial precursors. J Neurosci Res. 1988;21:155–167. doi: 10.1002/jnr.490210208. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–480. doi: 10.1023/a:1025739630398. [DOI] [PubMed] [Google Scholar]
- Iida J, Meijne AM, Spiro RC, Roos E, Furcht LT, McCarthy JB. Spreading and focal contact formation of human melanoma cells in response to the stimulation of both melanoma-associated proteoglycan (NG2) and alpha 4 beta 1 integrin. Cancer Res. 1995;55:2177–2185. [PubMed] [Google Scholar]
- Iida J, Meijne AM, Oegema TR, Jr, et al. A role of chondroitin sulfate glycosaminoglycan binding site in alpha4beta1 integrin-mediated melanoma cell adhesion. J Biol Chem. 1998;273:5955–5962. doi: 10.1074/jbc.273.10.5955. [DOI] [PubMed] [Google Scholar]
- Iida J, Pei D, Kang T, et al. Melanoma chondroitin sulfate proteoglycan regulates matrix metalloproteinase-dependent human melanoma invasion into type I collagen. J Biol Chem. 2001;276:18786–18794. doi: 10.1074/jbc.M010053200. [DOI] [PubMed] [Google Scholar]
- Jung M, Krämer E, Grzenkowski M, et al. Lines of murine oligodendrocyte precursor cells immortalised via an activated neu tyrosine kinase show distinct degrees of interaction with axons in vitro and in vivo. Eur J Neurosci. 1991;7:1245–1265. doi: 10.1111/j.1460-9568.1995.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Kriegstein AR, Gotz M. Radial glia diversity: a matter of cell fate. Glia. 2003;43:37–43. doi: 10.1002/glia.10250. [DOI] [PubMed] [Google Scholar]
- Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci. 2003;23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg J, Jensen UB, Broad S, Leigh I, Watt FM. Role of melanoma chondroitin sulphate proteoglycan in patterning stem cells in human interfollicular epidermis. Development. 2003;130:6049–6063. doi: 10.1242/dev.00837. [DOI] [PubMed] [Google Scholar]
- Levine JM, Nishiyama A. The NG2 chondroitin sulfate proteoglycan: a multifunctional proteoglycan associated with immature cells. Perspect Dev Neurobiol. 1996;3:245–259. [PubMed] [Google Scholar]
- Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119:611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004a;47:290–298. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004b;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lin XH, Dahlin-Huppe K, Stallcup WB. Interaction of the NG2 proteoglycan with the actin cytoskeleton. J Cell Biochem. 1996a;63:463–477. doi: 10.1002/(sici)1097-4644(19961215)63:4<463::aid-jcb8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Lin XH, Grako KA, Burg MA, Stallcup WB. NG2 proteoglycan and the actin-binding protein fascin define separate populations of actin-containing filopodia and lamellipodia during cell spreading and migration. Mol Biol Cell. 1996b;7:1977–1993. doi: 10.1091/mbc.7.12.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rao MS. Olig genes are expressed in a heterogeneous population of precursor cells in the developing spinal cord. Glia. 2004;45:67–74. doi: 10.1002/glia.10303. [DOI] [PubMed] [Google Scholar]
- Lu QR, Cai L, Rowitch D, Cepko CL, Stiles CD. Ectopic expression of Olig1 promotes oligodendrocyte formation and reduces neuronal survival in developing mouse cortex. Nat Neurosci. 2001;4:973–974. doi: 10.1038/nn718. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Majumdar M, Vuori K, Stallcup WB. Engagement of the NG2 proteoglycan triggers cell spreading via rac and p130cas. Cell Signal. 2003;15:79–84. doi: 10.1016/s0898-6568(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Makagiansar IT, Williams S, Dahlin-Huppe K, Fukushi JI, Mustelin T, Stallcup WB. Phosphorylation of NG2 proteoglycan by PKC-alpha regulates polarized membrane distribution and cell motility. J Biol Chem. 2004;279:55262–55270. doi: 10.1074/jbc.M411045200. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Edwards G, Streuli C, ffrench-Constant C. A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors. J Neurosci. 1996;16:7240–7252. doi: 10.1523/JNEUROSCI.16-22-07240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Naidu M, Carlstedt T, Levine JM, Fawcett JW. Expression and glycanation of the NG2 proteoglycan in developing, adult, and damaged peripheral nerve. Mol Cell Neurosci. 2003;24:787–802. doi: 10.1016/s1044-7431(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Niehaus A, Stegmuller J, Diers-Fenger M, Trotter J. Cell-surface glycoprotein of oligodendrocyte progenitors involved in migration. J Neurosci. 1999;19:4948–4961. doi: 10.1523/JNEUROSCI.19-12-04948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus A, Shi J, Grzenkowski M, et al. Patients with active relapsing-remitting multiple sclerosis synthesize antibodies recognizing oligodendrocyte progenitor cell surface protein: implications for remyelination. Ann Neurol. 2000;48:362–371. [PubMed] [Google Scholar]
- Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yang Z, Butt A. Astrocytes and NG2-glia: what's in a name? JAnat. 2005;207:687–694. doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345–357. doi: 10.1023/B:NEUR.0000044195.64009.27. [DOI] [PubMed] [Google Scholar]
- Pluschke G, Vanek M, Evans A, et al. Molecular cloning of a human melanoma-associated chondroitin sulfate proteoglycan. Proc Natl Acad Sci USA. 1996;93:9710–9715. doi: 10.1073/pnas.93.18.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raine CS. The Norton Lecture: a review of the oligodendrocyte in the multiple sclerosis lesion. J Neuroimmunol. 1997;77:135–152. doi: 10.1016/s0165-5728(97)00073-8. [DOI] [PubMed] [Google Scholar]
- Rezajooi K, Pavlides M, Winterbottom J, et al. NG2 proteoglycan expression in the peripheral nervous system: upregulation following injury and comparison with CNS lesions. Mol Cell Neurosci. 2004;25:572–584. doi: 10.1016/j.mcn.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Romanko MJ, Rola R, Fike JR, et al. Roles of the mammalian subventricular zone in cell replacement after brain injury. Prog Neurobiol. 2004;74:77–99. doi: 10.1016/j.pneurobio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Schneider S, Bosse F, D'Urso D, et al. The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J Neurosci. 2001;21:920–933. doi: 10.1523/JNEUROSCI.21-03-00920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shoshan Y, Nishiyama A, Chang A, et al. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci USA. 1999;96:10361–10366. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 antigen, a putative lineage marker: immunofluorescent localization in primary cultures of rat brain. Dev Biol. 1981;83:154–165. doi: 10.1016/s0012-1606(81)80018-8. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin K, Healy P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J Cell Biol. 1990;111:3177–3188. doi: 10.1083/jcb.111.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J Cell Sci. 2001;114:2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- Stegmuller J, Werner H, Nave KA, Trotter J. The proteoglycan NG2 is complexed with alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells. Implications for glial-neuronal signaling. J Biol Chem. 2003;278:3590–3598. doi: 10.1074/jbc.M210010200. [DOI] [PubMed] [Google Scholar]
- Tillet E, Ruggiero F, Nishiyama A, Stallcup WB. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J Biol Chem. 1997;272:10769–10776. doi: 10.1074/jbc.272.16.10769. [DOI] [PubMed] [Google Scholar]
- Tillet E, Gential B, Garrone R, Stallcup WB. NG2 proteoglycan mediates beta1 integrin-independent cell adhesion and spreading on collagen VI. J Cell Biochem. 2002;86:726–736. doi: 10.1002/jcb.10268. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Ughrin YM, Chen ZJ, Levine JM. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- Wagner S, Hafner C, Allwardt D, et al. Vaccination with a human high molecular weight melanoma-associated antigen mimotope induces a humoral response inhibiting melanoma cell growth in vitro. J Immunol. 2005;174:976–982. doi: 10.4049/jimmunol.174.2.976. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]