Abstract
Connective tissue plays a key role in the scaffolding and development of skeletal muscle. Pilot studies carried out in our laboratory have shown that the smallest porcine littermate has a higher content of connective tissue within skeletal muscle compared with its largest littermate. The present study investigated the prenatal development of intralitter variation in terms of collagen content within connective tissue and intramuscular fat of the M. semitendinosus. Twenty-three pairs of porcine fetuses from a Large White–Landrace origin were used aged from 36 to 86 days of gestation. The largest and smallest littermates were chosen by weight and the M. semitendinosus was removed from each. Complete transverse muscle sections were stained with Oil Red O (detection of lipids) and immunocytochemistry was performed using an antibody to collagen I. Slides were analysed and paired t-Tests revealed the smallest littermate contained a significantly higher proportion of fat deposits and collagen I content compared with the largest littermate. Recent postnatal studies showing elevated levels of intramuscular lipids and low scores for meat tenderness in the smallest littermate corroborate our investigations. It can be concluded that the differences seen in connective tissue elements have a fetal origin that may continue postnatally.
Keywords: connective tissue, fetal origins, M. semitendinosus, myofibres, porcine
Introduction
The early fetal environment plays a key role in development of skeletal muscle. Several studies have shown that intralitter variation, in utero, affects postnatal growth of skeletal muscle in the pig (Dwyer et al. 1994). It is well known that muscle growth is influenced by the number, size and type of the muscle fibres. The total number of skeletal muscle fibres (hyperplasia) is fixed before birth in most mammalian species. Postnatally, skeletal muscle fibres only grow by hypertrophy (increase in size). Marked differences in fibre number have been demonstrated in the smallest and largest porcine littermates prenatally, with the smallest littermate having significantly fewer fibres than the largest littermate (Wigmore & Stickland, 1983).
Pilot studies carried out in our laboratory have indicated that the smallest littermate may also have a higher proportion of connective tissue in its muscle (Clelland, 2001). It has also been demonstrated in other studies that ovine fetuses possess more adipose tissue after being exposed to undernutrition in utero (Bispham et al. 2003, 2005; Symonds et al. 2003). Fat and connective tissue components have also been suggested as a default pathway when muscle is unable to form (Kablar et al. 2003). Muscle connective tissue provides a structure to the muscle and is composed of ground substance, fibres and connective tissue cells. A proportion of these elements of the connective tissue comprise collagen I and fat cells. However, at fetal stages in the pig significant lipid deposition occurs within myofibres (Hauser et al. 1997). Collagen and intramuscular fat are important parameters to the meat industry as an increased amount of these components may impact on meat toughness and meat quality.
The primary objective of this study was to investigate the prenatal development of intralitter variation in collagen and fat content of muscles. In the pig, it can be argued that differing levels of nutrition received, in utero, are a major cause of the observed intralitter variation. In the present prenatal study the smallest and largest littermates were chosen and the content of collagen I and fat were analysed in the M. semitendinosus of both. It is hypothesized that the smallest littermate muscles will contain more non-muscle, specifically more fat deposition and collagen I fibres, compared with its largest sibling. Recent studies have shown that the heaviest and lightest pigs taken at birth and grown to slaughter weight exhibit a difference in intramuscular lipid content and tenderness (Gondret et al. 2005). This present study investigates whether the difference has a fetal origin.
Materials and methods
Fetal selection and preparation
A total of 23 pairs of porcine fetuses from a Large White–Landrace origin were used in this study. A range of fetuses aged from 36 to 86 days of gestation were chosen. This age range includes the periods of primary fibre formation (which occurs at about 30–50 days of gestation) and secondary fibre formation (which occurs at about 50–80 days of gestation) (Wigmore & Stickland, 1983), and comprises a broad representation of the important events in muscle development. At slaughter all fetuses were weighed and the largest and smallest littermates (excluding the runt if any in the litter) from each uterus were chosen by weight (g) for further analysis. The largest littermate was approximately 1.5 times heavier than the smallest littermate in each uterus. Runts were excluded from the study as outliers (Hegarty & Allen, 1978; Powell & Aberle, 1980). The M. semitendinosus muscles were dissected from the left hind limb and snap frozen in liquid nitrogen and stored at −80 °C until sectioning. Complete transverse sections (10 µm) were taken from the mid-belly region of the M. semitendinosus using a cryostat (Bright, UK) at −25 °C. The sections were mounted on 3′aminopropyltriethoxysilane (APTES) (Sigma Chemical Co., Poole, Dorset, UK)-coated slides for enhanced adhesion. Histochemistry and immunocytochemistry techniques were employed on the frozen transverse sections.
Histochemistry
The extent of fat deposition in the smallest and largest littermates was determined using Oil Red O stain. Stock Oil Red O solution was prepared by adding 0.5 g Oil Red O powder to 100 mL 98% isopropanol. Prior to staining, 60 mL of this solution was diluted in 40 mL of water and left to stand for 24 h. The working solution was then filtered the following day using grade number 42 Whatman filter paper (Whatman, Maidstone, UK) to remove any Oil Red O crystals. Slides were air-dried for 30 min and stained in Oil Red O solution for 10 min. Slides were then de-differentiated in 60% isopropanol (Sigma Chemical Co.) and washed in distilled water. Slides were mounted in glycerol jelly and analysed using Kontron Image Analysis software (Carl Zeiss, Oberkochen, Germany). Analysis of fat deposition was made by measuring the area of stained fat within a known area of muscle section using a × 20 objective. A representative portion (at least 2%) (Clelland, 2001) across the muscle from the deep part to the superficial part was used to perform the analysis. Figures are expressed as area of fat per mm2 of muscle section.
Immunocytochemistry
An antibody to collagen I (Sigma Chemical Co.) was used to identify collagen fibres within the connective tissue matrix. Slides were air-dried for 30 min. Sections were incubated in ice-cold acetone for 10 min and then air-dried overnight at −20 °C. This treatment was carried out in order to make the connective tissue more permeable to the collagen I antibody. The following day sections were washed in phosphate-buffered saline (PBS; 100 mm NaCl, 20 mm phosphate buffer, pH 7.4) and blocked in 10% goat serum (Invitrogen, Paisley, UK) to prevent non-specific binding. A monoclonal anti-collagen I antibody raised in mouse was diluted according to recommendations from the manufacturer (1 : 100) (Sigma Chemical Co.) in 10% goat serum and incubated on slides for 30 min. Sections were washed in PBS containing 0.1% Tween (Sigma Chemical Co.). A biotinylated rabbit anti-mouse IgG secondary antibody diluted according to recommendations from the manufacturer (1 : 200) (Vector Laboratories Inc., Burlingame, CA, USA) in 10% goat serum was incubated on sections for 30 min and again washed with PBS containing 0.1% Tween. Additionally, positive and negative controls were included. Vectorstain ABC (avidin/biotin) kit (Vector Laboratories) was applied onto sections for 30 min and washed in PBS. Colour was developed using mixed VIP kit reagents (Vector Laboratories) on the sections for 2 min. The colour reaction was stopped in PBS and dehydrated in a progression of alcohols ranging from 30% to 100% ethanol. Slides were mounted in DPX (BDH, UK) and analysed using Kontron Image Analysis software. Staining analysis was performed by comparing collagen I content within a known area of muscle section using a × 10 objective. Again, a representative portion (at least 2%) (Clelland, 2001) across the muscle from the deep part to the superficial part was used to perform the analysis. Figures are expressed as area of collagen per mm2 of muscle section.
Statistical analysis
Paired t-Tests were performed on all results collected to determine significant differences, if any, between the smallest and largest littermates analysed. Fetuses were grouped from day 36 to 60 (including the initiation of primary and secondary muscle fibre proliferation) and from day 61 to 86 (including the period of differentiation of fibres and the ending of myogenesis). A P-value of ≤ 0.05 was deemed to be of significance.
Results
Histochemistry
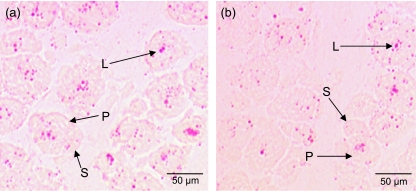
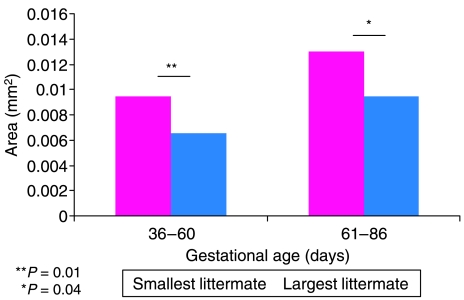
Figure 1 shows transverse cryosections from the mid-belly portion of the M. semitendinosus stained in Oil Red O solution for detection of lipid (fat) deposition. The majority of lipid droplets appeared to be located within the myofibres. Qualitatively, more fat deposition is seen in the smallest fetus (Fig. 1a) than in the largest fetus (Fig. 1b). Fat content per mm2 was determined for a representative portion of the section. The analysed values are shown in Fig. 2 A paired t-Test indicated that the smallest littermate had significantly more fat per mm2 present than the largest littermate (P = 0.01) in the group of fetuses aged 36–60 days of gestation. In the group aged 61–86 days of gestation, the smallest littermate also exhibited more fat per mm2 than the largest littermate (P = 0.04).
Fig. 1.
Oil Red O-stained M. semitendinosus sections from fetuses aged 57 days of gestation. Primary (P) and secondary (S) muscle fibres are apparent at this stage of development. The smallest littermate (a) illustrates increased lipid deposition (L) compared with the largest littermate (b).
Fig. 2.
Fat area density per mm2 of muscle for grouped fetal samples aged 36–60 days of gestation and 61–86 days of gestation. The smallest littermate has an increased amount of fat present compared with the largest littermate in the group aged 36–60 days of gestation (P = 0.01) and in the group aged 61–86 days of gestation (P = 0.04).
Immunocytochemistry
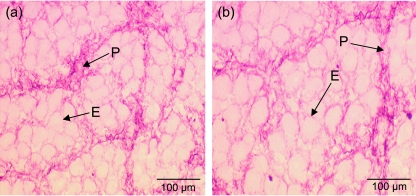
An antibody against collagen I was used (Fig. 3). Qualitatively it can be seen that in Fig. 3(a) the area of collagen I in the smallest fetus is more densely packed than in the largest fetus (Fig. 3b). The endomysium and perimysium of the connective tissue was observed (Fig. 3a,b). The endomysium is apparent around groups of fibres rather than individual fibres at this stage (60 days of gestation).
Fig. 3.
M. semitendinosus sections stained using collagen I antibody in fetuses aged 60 days of gestation. The smallest littermate (a) has more collagen I deposition than the largest littermate (b). Note the structure of the endomysium (E) and perimysium (P).
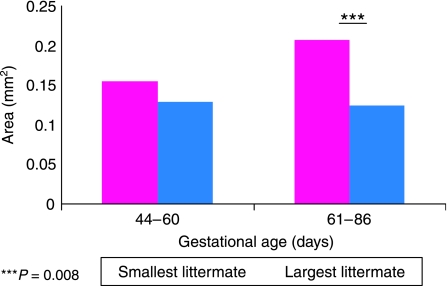
As a result of the acetone stage in the protocol, only fetuses of 44–86 days of gestation could be analysed because this treatment proved to be too harsh for the younger tissue sections. Values obtained through analysis are illustrated in Fig. 4. A paired t-Test revealed that there was no significant difference in collagen content between the smallest and largest littermate during the late embryonic stage to the fetal stage of muscle development (44–60 days of gestation). However, a paired t-test showed the smallest littermate had significantly more expression of collagen I area per mm2 than the largest littermate in the later stages of fetal life (61–86 days of gestation) (P = 0.008).
Fig. 4.
Collagen I area density per mm2 of muscle for grouped fetal samples aged 44–60 days of gestation and 61–86 days of gestation. The smallest littermate has a significantly increased amount of collagen I present compared with the largest littermate (P = 0.008) in the group aged 61–86 days of gestation.
Discussion
The results of the present study demonstrate that an increased proportion of fat and collagen I was present in the muscles of the smallest littermate compared with the largest littermate, at least in the later stages of fetal development. This confirms our initial hypothesis. A significant difference was seen using both histochemistry (Oil Red O stain) and immunocytochemistry (anti-collagen I antibody stain).
The results presented here extend previous pilot studies carried out in our laboratory (Clelland, 2001). The increased non-muscle found in these pilot studies can now be confirmed as including increased collagen I within the connective tissue composition of the smallest littermate. Additionally, there was more lipid deposition in the muscle of the smallest sibling compared with the largest.
Muscle connective tissue provides a structural framework for the muscle. Movements from muscle fibres are transmitted to the tendons via connective tissue (Purslow, 2002). Connective tissue found in skeletal muscle includes the following components: (1) ground substance, consisting mainly of glycoprotein and proteoglycans; (2) connective tissue fibres, consisting of collagen, elastin and reticulin; and (3) connective tissue cells, including fibroblasts (which secrete the fibres and the ground substance), and other cells in various numbers, including adipose cells, plasma cells, lymphocytes, macrophages and mast cells.
In the present study it was demonstrated that significantly higher levels of fat per mm2 were present in muscles of the smallest littermate compared with the largest littermate. The difference was seen at both the late embryonic to mid-fetal stages (36–60 days of gestation) and the late fetal stages (61–86 days of gestation). Increased adiposity has also been reported in undernourished sheep fetuses (Bispham et al. 2003, 2005; Symonds et al. 2003). In apparent contrast, Rehfeldt (2005) found that lower birthweight neonate pigs had less body fat than larger littermates. Nevertheless, the smallest littermates appear to put on more fat (Powell & Aberle, 1981), including within muscle (Gondret et al. 2005), by slaughter weight. This latter finding may be due to greater prenatal development of adipocytes in the smallest littermate rather than of muscle fibres. A reduction in muscle fibres in smaller fetuses has previously been reported in the pig (Wigmore & Stickland, 1983). Adipocytes and myocytes are derived from a common mesenchymal precursor (Sordella et al. 2003). Gene targeting studies have shown that when essential genes for myogenic determination, Myf5 and MyoD, are knocked out, the resulting progeny contain excessive amounts of adipose tissue (loose connective tissue) in place of muscle (Kablar et al. 2003). Although adipocytes could not be identified in the present study due to lack of differentiation at fetal stages, it has been shown that intramuscular fat is greater in the smallest littermate prenatally. Most of this fat appears to be within the myofibres, which is in agreement with Hauser et al. (1997). Large fat deposits within fibres have been associated with metabolic disorders (Hegarty et al. 2003). Interestingly, intrafibre fat increases with endurance training, which is an adaptation of the body to acquire more readily available fuel (Hoppeler, 1999). It is possible that in low nutrient situations the pig fetuses store fat within fibres so that energy is more readily available, i.e. an adaptation to low nutrition. As nutrition becomes more available postnatally, the small unfilled adipocytes start to fill. In a recent study by Gondret et al. (2005) the lightest and heaviest birthweight pigs were analysed at slaughter weight (mean weight of 111.8 kg) and the lightest littermates were found to contain higher levels of intramuscular lipid in the M. semitendinosus compared with the heaviest littermate. Although it has not been determined postnatally whether most of the muscle fat is found within or between fibres, we can conclude that high levels of intramuscular fat in the smallest birthweight pigs at slaughter (Gondret et al. 2005) can also be identified prenatally.
Collagen produced from fibroblasts is a major component of connective tissue. The fibrous material forms a continuous mesh within the extracellular matrix and provides support to the muscle bundle. Collagen is found abundantly within connective tissue and is divided into seven different subtypes. Within fetal skeletal muscle collagen I and collagen III have been previously identified (Listrat et al. 1999). Collagen I is the most abundant subtype of collagen found in mature muscles and could be suggested to be the most important for meat quality. Although collagen III is abundantly found within young tissue, there is a general shift with chronological age to increased amounts of collagen I (Kovanen, 1989). In the present study elevated levels of collagen I were found in the smallest littermate compared with the largest in later fetal life (61–86 days of gestation). Although somewhat debatable, collagen content has been linked with meat toughness, using a wide range of postnatal animals (Fang et al. 1999). In other studies, e.g. DeVol et al. (1988) and Avery et al. (1996), a link was not observed between collagen content and meat toughness. However, the connective tissue differences seen in the present study do appear to reflect a difference in meat tenderness between low and high birthweight littermates at slaughter. In a study by Gondret et al. (2005) low birthweight pigs at slaughter exhibited a low score for loin meat tenderness compared with the heaviest littermate. These results may indicate increased connective tissue in the less tender muscles. Our results indicate that differences in collagen content between littermates may therefore have a prenatal origin.
Arguably, intralitter variation provides us with a good naturally occurring model of differing levels of nutrients reaching the offspring. A variation in blood flow and hence nutrients, as a consequence of good and restricted access from the placenta, results in a wide range of littermate sizes within the entire litter (Warwick, 1928; McLaren & Michie, 1960; Perry & Rowell, 1969). Previous studies have investigated the influence of prenatal nutrition, either directly or by studying intralitter variation on muscle development and consequences for postnatal growth (Dwyer et al. 1994). These studies have shown that low nutrition (by either method) impedes fibre number development and correlates with future postnatal growth (Wigmore & Stickland, 1983; Ward & Stickland, 1991; Dwyer & Stickland, 1992; Yamaguchi et al. 1993; Dwyer et al. 1994). In this study we have shown a prenatal nutritional influence also on the connective tissue and fat components of muscle and, from other work, this appears to continue postnatally. In this way prenatal programming is influencing the longer term postnatal phenotype of muscle.
Acknowledgments
This work was funded by the BBSRC. Thanks also to Dr Stephanie Bayol and the Muscle Unit team at the Royal Veterinary College for support.
References
- Avery NC, Sims TJ, Warkup C, Bailey AJ. Collagen cross-linking in porcine m. longissimus lumborum: Absence of a relationship with variation in texture at pork weight. Meat Sci. 1996;42:355–369. doi: 10.1016/0309-1740(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- Bispham J, Gardner DS, Gnanlingham MG, Stephenson T, Symonds ME, Budge H. Maternal nutritional programming of fetal adipose tissue development: differential effects on mRNA abundance for uncoupling proteins, peroxisome proliferators activated and prolactin receptors. Endocrinology. 2005 doi: 10.1210/en.2005-0246. in press. [DOI] [PubMed] [Google Scholar]
- Clelland A. Intra-litter variation in early porcine muscle development. The Royal Veterinary College, University of London; 2001. PhD thesis. [Google Scholar]
- DeVol DL, McKeith FK, Bechtel PJ, Novakofski J, Shanks RD, Carr TR. Variation in composition and palatability traits and relationships between muscle characteristics and palatability in a random sample of pork carcasses. J Anim Sci. 1988;66:385–395. [Google Scholar]
- Dwyer CM, Stickland NC. Does the anatomical location of a muscle affect the influence of undernutrition on muscle fibre number? J Anat. 1992;181:373–376. [PMC free article] [PubMed] [Google Scholar]
- Dwyer CM, Stickland NC, Fletcher JM. The influence of maternal nutrition on muscle fiber number development in the porcine fetus and on subsequent postnatal growth. J Anim Sci. 1994;72:911–917. doi: 10.2527/1994.724911x. [DOI] [PubMed] [Google Scholar]
- Fang SH, Nishimura T, Takahashi K. Relationship between development of intramuscular connective tissue and toughness of pork during growth of pigs. J Anim Sci. 1999;77:120–130. doi: 10.2527/1999.771120x. [DOI] [PubMed] [Google Scholar]
- Gondret F, Lefaucheur L, Louveau I, Lebret B. The long-term influences of birth weight on muscle characteristics and eating meat quality in pigs individually reared and fed during fattening. Arch Tierz Dummerstorf. 2005;48:68–73. [Google Scholar]
- Hauser N, Mourot J, De Clercq L, Genart C, Remacle C. The cellularity of developing adipose tissues in Pietrain and Meishan pigs. Reprod Nutr Dev. 2005;37:617–625. doi: 10.1051/rnd:19970601. [DOI] [PubMed] [Google Scholar]
- Hegarty PVJ, Allen C. Effect of pre-natal runting on the postnatal development of skeletal muscles in swine and rats. J Anim Sci. 1978;46:1634–1640. doi: 10.2527/jas1978.4661634x. [DOI] [PubMed] [Google Scholar]
- Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178:373–383. doi: 10.1046/j.1365-201X.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- Hoppeler H. Skeletal muscle substrate metabolism. Int J Obes Relat Metab Disord. 1999;23:S7–S10. doi: 10.1038/sj.ijo.0800878. [DOI] [PubMed] [Google Scholar]
- Kablar B, Krastel K, Tajbakhsh S, Rudnicki MA. Myf5 and MyoD activation define independent myogenic compartments during embryonic development. Dev Biol. 2003;258:307–318. doi: 10.1016/s0012-1606(03)00139-8. [DOI] [PubMed] [Google Scholar]
- Kovanen V. Effects of ageing and physical training on rat skeletal muscle. An experimental study on the properties of collagen, laminin, and fibre types in muscles serving different function. Acta Physiol Scand Suppl. 1989;577:1–56. [PubMed] [Google Scholar]
- Listrat A, Picard B, Geay Y. Age-related changes and location of type I, III, IV, V and VI collagens during development of four foetal skeletal muscles of double-muscles and normal bovine muscles. Tissue Cell. 1999;31:17–27. doi: 10.1054/tice.1998.0015. [DOI] [PubMed] [Google Scholar]
- McLaren A, Michie D. Control of pre-natal growth in mammals. Nature. 1960;4735:363–365. [Google Scholar]
- Perry JS, Rowell JO. Variations in foetal weight and vascular supply along the uterine horn of the pig. J Reprod Fertil. 1969;19:527–534. doi: 10.1530/jrf.0.0190527. [DOI] [PubMed] [Google Scholar]
- Powell SE, Aberle ED. Effects of birthweight on growth and carcass composition of swine. J Anim Sci. 1980;50:860–868. doi: 10.2527/jas1980.505860x. [DOI] [PubMed] [Google Scholar]
- Powell SE, Aberle ED. Skeletal muscle and adipose tissue cellularity in runt and normal birth weight swine. J Anim Sci. 1981;52:748–756. doi: 10.2527/jas1981.524748x. [DOI] [PubMed] [Google Scholar]
- Purslow P. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol Part A. 2002;133:347–966. doi: 10.1016/s1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- Rehfeldt C. Prenatal events that determine the number of muscle fibres are important for lean growth and meat quality in pigs. Arch Tierz Dummerstorf. 2005;48:11–22. [Google Scholar]
- Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signalling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Gopalakrishnan G, Bispham J, et al. Maternal nutrient restriction during placental growth, programming of fetal adiposity and juvenile blood pressure control. Arch Physiol Biochem. 2003;111:45–52. doi: 10.1076/apab.111.1.45.15141. [DOI] [PubMed] [Google Scholar]
- Ward SS, Stickland NC. Why are slow and fast muscles differentially affected during prenatal undernutrition? Muscle Nerve. 1991;14:259–267. doi: 10.1002/mus.880140310. [DOI] [PubMed] [Google Scholar]
- Warwick BL. Prenatal growth of the swine. J Morph Physiol. 1928;46:59–84. [Google Scholar]
- Wigmore PM, Stickland NC. Muscle development in large and small pig fetuses. J Anat. 1983;137:235–245. [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Horio Y, Sakuma K, Katsuta S. The effect of nutrition on the size and proportion of muscle fibre types during growth. J Anat. 1993;182:29–36. [PMC free article] [PubMed] [Google Scholar]