Abstract
The phytoestrogenic compound trans-resveratrol (trans-3,5,4′-trihydroxystilbene) is found in appreciable quantities in grape skins and wine. It has been shown that both products rich in trans-resveratrol and pure trans-resveratrol inhibit platelet aggregation both in vivo and in vitro. However the mechanism of this action still remains unknown.
An essential component of the aggregation process in platelets is an increase in intracellular free Ca2+ ([Ca2+]i). Ca2+ must enter the cell from the external media through specific and tightly regulated Ca2+ channels in the plasma membrane. The objective of this study was to characterize what effect trans-resveratrol had on the Ca2+ channels in thrombin stimulated platelets.
In this study we showed that trans-resveratrol immediately inhibited Ca2+ influx in thrombin-stimulated platelets with an IC50 of 0.5 μM. trans-Resveratrol at 0.1, 1.0 and 10.0 μM produced 20±6, 37±6 and 57±4% inhibition respectively of the effect of thrombin (0.01 u ml−1) to increase [Ca2+]i.
trans-Resveratrol also inhibited spontaneous Ba2+ entry into Fura-2 loaded platelets, with 0.1, 1.0 and 10.0 μM trans-resveratrol producing 10±5, 30±5 and 50±7% inhibition respectively. This indicated that trans-resveratrol directly inhibited Ca2+ channel activity in the platelets in the absence of agonist stimulation.
trans-Resveratrol also inhibited thapsigargin-mediated Ca2+ influx into platelets. This suggests that the store-operated Ca2+ channels are one of the possible targets of trans-resveratrol. These channels rely on the emptying of the internal Ca2+ stores to initiate influx of Ca2+ into the cell.
The phytoestrogens genistein, daidzein, apigenin and genistein-glucoside (genistin) produced inhibitory effects against thrombin similar to those seen with trans-resveratrol.
We conclude that trans-resveratrol is an inhibitor of store-operated Ca2+ channels in human platelets. This accounts for the ability of trans-resveratrol to inhibit platelet aggregation induced by thrombin.
Keywords: trans-Resveratrol, platelets, aggregation, calcium, barium, channels, thapsigargin, phytoestrogen, thrombosis, thrombin
Introduction
Platelets, the major hemostatic cell in blood, are formed in the bone marrow from the precursor cell megacaryocytes (Heyns, 1994). Structurally, platelets contain no nucleus and have a large number of granules (dense granules and α-granules) for the storage of biologically active compounds such as serotonin, ADP and platelet derived growth factor (White, 1994). The contents of the granules are released upon platelet activation. The intracellular dense tubular system (analogous to the endoplasmic reticulum) serves as a calcium storage site (Crawford & Scrutton, 1994).
Platelets are activated by multiple factors (agonists) produced at the site of vascular injury (Brass et al., 1993; Hourani & Cusack, 1991). These factors include ADP, collagen, thromboxane A2 and thrombin, the most potent platelet activator. Upon activation, platelets undergo shape change, express fibrinogen receptors, aggregate and release their granule contents. As a result a hemostatic plug is formed on a fibrin meshwork thus sealing the damaged blood vessel. Excessive platelet aggregation however plays a role in the development of thrombosis and therefore it is important that this process is properly regulated for the efficient functioning of the cardiovascular system.
The major intracellular stimulus involved in platelet aggregation is an increase in free cytosolic calcium concentration ([Ca2+]i) (Sage & Rink, 1990). As in most non-excitable cells the increase in platelet [Ca2+]i induced by various agonists involves both influx of extracellular calcium through plasma membrane calcium channels and mobilization of intracellular calcium from the dense tubular system (Berridge, 1997; Heemskerk & Sage, 1994; Sage, 1997).
The pathway(s) of the calcium entry in platelets share common properties with the calcium entry pathways found in other non-excitable cells. The mechanism of calcium release from the myo-inositol 1,4,5-P3 (IP3)-sensitive internal stores is well characterized. On the other hand, the mechanism of the calcium influx pathway in platelets is still not completely resolved (Sage, 1997). Calcium from the external medium enters the cell through specific ion channels of several different types. Platelets, according to Sage (1997), lack voltage-gated calcium channels, so the change in the membrane potential does not generate calcium influx, and platelets are reported not to bind verapamil and nifedipine. Instead, receptor-mediated calcium entry (RMCE) through the plasma membrane channel following binding of a ligand to its membrane receptor seems to be responsible for calcium influx into platelets. RMCE channels may be one of the following types: ROC (receptor-operated channel), SMOC (second messenger operated channel) and SOCC (store-operating calcium channel) (Sage, 1997). SOCC channels are activated to promote calcium influx upon the emptying of the internal calcium stores (dense tubular system). The mechanism which links the depletion of the IP3-sensitive internal calcium stores to the SOCC have not yet been elucidated (Parekh & Penner, 1997; Clementi & Meldolesi, 1996). There are currently two models proposed for activating SOCC: (1) the two kinds of channels (plasma membrane calcium channel and the intracellular endoplasmic reticulum IP3 sensitive calcium channel) are coupled to each other by physical proximity or (2) the emptying of the internal stores generates a calcium influx factor (CIF), which then acts on the plasma membrane calcium channels. The specific nature of CIF is poorly defined, however it may involve arachidonate metabolites and S-nitrosylation (e.g. Rzigalinski et al., 1996; 1999; Favre et al., 1998).
A very important tool used to study SOCC is a plant derived tumor promoter known as thapsigargin (Thastrup et al., 1990). Thapsigargin effectively inhibits the calcium ATPase pump of the endoplasmic reticulum without increasing the level of IP3. When the pump is inhibited, calcium is prevented from entering the intracellular stores. Stored calcium leaks into the cytoplasm, some of which is extruded from the cell by the plasma membrane Ca2+-ATPase pump. Consequently, store emptying evokes calcium influx through SOCC in the plasma membrane (Parekh & Penner, 1997).
It has been reported numerous times that consumption of certain foods, grapes and red wine, serve as a protective factor against coronary heart disease (CHD). The ‘French paradox' correlates the lower prevalence of CHD with the regular moderate consumption of red wine (Soleas et al., 1997). The beneficial effects of this product have been attributed to their high antioxidant/phytoestrogenic content (e.g. Miksicek, 1995; Williams & Rutledge, 1998).
Phytoestrogens are naturally occurring antioxidant compounds, usually polyphenols, which are present in the variety of foods and beverages. Phytoestrogens derived their name for their ability to mimic the actions of oestrogens (female sex hormones). They can bind to the human oestrogen receptor and activate genomic oestrogen-response elements, although at a much higher concentration than the natural oestrogens (Miksicek, 1995; Gehm et al., 1997). trans-Resveratrol (trans-3,5,4′-trihydroxystilbene) (Figure 1) is a phytoestrogenic compound found in grapes (Miksicek, 1995), it possesses antitumor, antioxidative and antifungal activities (Jang et al., 1997; Tham et al 1998).
Figure 1.
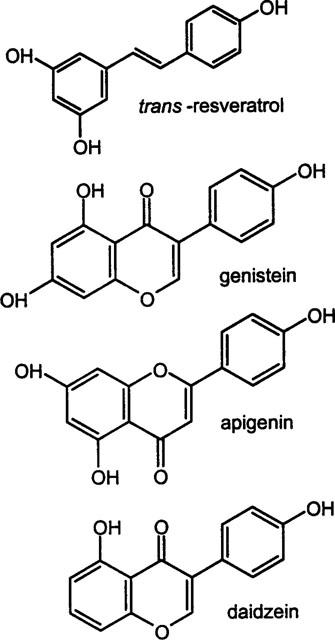
Structures of the phytoestrogens trans-resveratrol, genistein, apigenin and daidzein.
trans-Resveratrol inhibits human platelet aggregation both in vitro and in vivo (Pace-Asciak et al., 1995; 1996; Bertelli et al., 1995). This has important implications in ascribing how the intake of phytoestrogens in food can help prevent thrombosis, essentially serving as a blood thinner, having potentially beneficial effects on the cardiovascular system. However, the mechanism by which trans-resveratrol inhibits platelet aggregation has not been elucidated. Since aggregation can not occur without a sufficient increase in the [Ca2+]i, the goal of the present study was to establish if dietary phytoestrogens, such as trans-resveratrol, could have an effect on the calcium channels in stimulated human platelets.
Phytoestrogenic isoflavonoids genistein and daidzein and the flavonoid apigenin (Figure 1) are also encountered in foods such as soy beans and were also examined briefly in this study. Genistein is also known as a potent tyrosine kinase inhibitor (Jayatilake et al., 1993), and is also known to inhibit certain tumor cell growth (e.g. Fioravanti et al., 1998). Previous studies have shown that 100 μM genistein but not daidzein was able to inhibit ADP and thrombin induced calcium influx in human platelets (Sargeant et al., 1993a,1993b), it was concluded that tyrosine phosphorylation controlled calcium entry. Our findings are more consistent with genistein and daidzein having a more direct effect on calcium channels.
Methods
Reagents and material sources
The following were from Sigma Chemical Co: trans-resveratrol, apigenin (4′,5,7-trihydroxyflavone), genistein (4′,5,7-trihydroxyisoflavone), genistein-7-O-D-glucoside (genistin), daidzein (4′,7-dihydroxyisoflavone), thrombin, Me2SO (dimethylsulphoxide) and EGTA [ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′-tetraacetic acid]. The following were from Calbiochem: thapsigargin and ionomycin. Fura-2 A/M was from Molecular Probes. Other chemicals were from Fisher Scientific and Sigma Chemical Co.
Blood donors and platelet preparation
All donors were healthy volunteers (aged 20–40 years) who had not consumed any medication known to affect platelet function (e.g. calcium channel blockers and aspirin) for at least 10 days prior to the study. Venous blood was collected into 1/10 volume of ACD (74.8 mM sodium citrate, 38.1 mM citric acid and 123 mM dextrose pH 6.4) (Baxter Healthcare Corp.). The blood was centrifuged at 250×g for 10 min at room temperature to obtain platelet rich plasma (PRP). The PRP was centrifuged at 550×g for 12 min to sediment the platelets. The platelets were then re-suspended in a modified Tyrodes physiological salt solution (in mM): NaCl 145, KCl 4, MgSO4 1, Na2HPO4 0.5, Na/HEPES 10, glucose 6, pH 7.4, containing 1.0 mM EGTA which acted to prevent spontaneous aggregation during the various experimental manipulations by binding extracellular Ca2+ (Nolan & Lapetina, 1990). The platelets were washed once (500×g for 15 min) and finally re-suspended at a count of approximately 3×108 platelets ml−1.
Phytoestrogen solution preparations
Stock solutions of the phytoestrogens (trans-resveratrol, genistein, daidzein, apigenin) in Me2SO (3 mg ml−1) were prepared and stored at −20°C. Just before each experiment aliquots were thawed and diluted with Me2SO up to a desirable concentration (see specific figure legends). Stock solutions of phytoestrogens were diluted 200 fold into the spectrofluorometric cuvette to give the desired final concentration.
Platelet loading with fura-2 and measurement of [Ca2+]i
Intracellular calcium measurements [Ca2+]i employed the fluorescent dye fura-2, which involved incubating the platelets with the cell permeant acetoxymethyl ester (fura-2/AM) (e.g. Sargeant et al., 1992). A suspension of human platelets (isolated as described above) was incubated with 2 μM fura-2/AM for 1 h at room temperature (on a rocking platform). Excess fura-2/AM was removed by centrifugation (500×g for 10 min) and the platelets suspended in fresh buffer, without added EGTA. Aliquots of platelet suspension (0.5 ml) were added to 1.0 ml cuvettes containing a Teflon coated stirrer bar (Chrono-log, Havertown, PA, U.S.A.). Just before [Ca2+]i measurements were performed, Ca2+ was added back to the buffer to a final concentration of 2 mM, then phytoestrogens (various concentrations in 2.5 μl) and thrombin 0.01 u ml−1 were added (see individual Figure legends for specific details). The effects of trans-resveratrol and other phytoestrogens to inhibit the actions of thrombin (see Results) were immediate. In preliminary experiments we added trans-resveratrol together with thrombin and the inhibitory effect was the same as if we preincubated the trans-resveratrol for 5–10 s or 120 min before thrombin. trans-Resveratrol therefore produced an immediate blockade of calcium influx channel. Also when trans-resveratrol was added 60 s after thrombin, the [Ca2+]i declined immediately to a value approaching the inhibitory effect observed when trans-resveratrol was added just prior to the addition of thrombin. The measurements of [Ca2+]i were performed at room temperature in a SPEX ARCM spectrofluorometer using excitation wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. Calibration was performed as previously described for human sperm (Blackmore et al., 1990). [Ca2+]i was calculated by using the SPEX dM3000 software package.
Measurement of [Ba2+]i
To assess basal Ca2+ channel activity without agonist stimulation, Ba2+ was added to platelets in Ca2+-free medium to act as a calcium surrogate. Ba2+ enters the cell through Ca2+ channels, but unlike Ca2+, it cannot be extruded from the cell by plasma membrane Ca2+-ATPase pump therefore it accumulates in the cell (Ozaki et al., 1992; Zschauer et al., 1988; Blache & Clavatti, 1987; Blache et al., 1987). The removal of Ba2+ from the cytoplasm by the endoplasmic reticulum may be limited when compared to Ca2+ and Sr2+ (Ozaki et al., 1992). When Ca2+ enters the cell through plasma membrane calcium channels it can be either extruded from the cell by the Ca2+-ATPase pump or sequestered within organelles such as the endoplasmic reticulum and mitochondria Therefore when Ca2+ is added to platelets suspended in low Ca2+ containing medium the [Ca2+]i changes very little. Once inside the cell, Ba2+ forms a fluorescent complex with the dye fura-2, in a similar manner to Ca2+, but with a different affinity (KD=1360 nM for Ba2+ and KD=227 nM for Ca2+, Ozaki et al., 1992). The intensity of the fluorescence is directly proportional to the [Ba2+]i. BaCl2 (5 mM) was added to the fura-2 loaded platelets in the absence of Ca2+ and in the absence of agonist. The fura-2/Ba2+ fluorescent complex was determined by measuring the 340/380 nm fluorescence ratio. The addition of thrombin with Ba2+ causes a large increase in 340/380 nm ratio, consistent with thrombin stimulating Ba2+ influx through SOCC. However it was not possible to determine how much of the fluorescence increase was due to Ba2+ and how much was due to Ca2+ being mobilized from intracellular stores, although mobilization of Ca2+ from intracellular Ca2+ stores only produces a small increase in [Ca2+]i (compare Figures 4 and 5).
Figure 4.
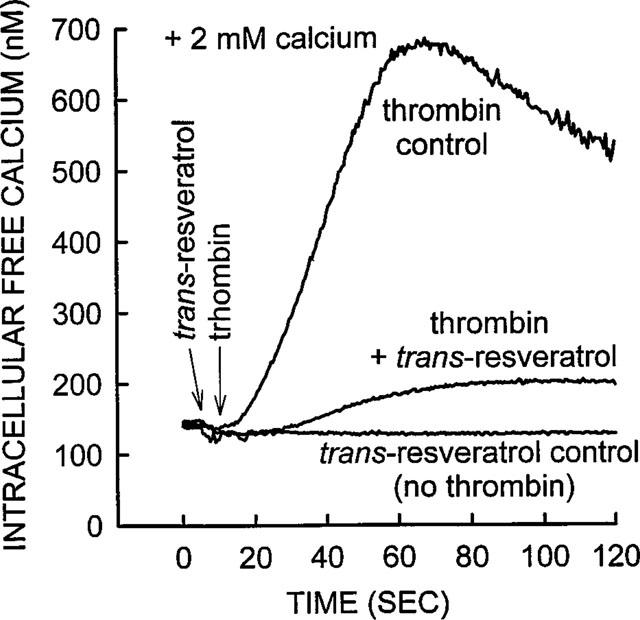
trans-Resveratrol does not affect [Ca2+]i level in platelets in the absence of thrombin stimulation. All experiments were performed in the presence of 2.0 mM external calcium. Thrombin control experiment represents [Ca2+]i elevation in platelets, stimulated with thrombin alone (0.01 u ml−1). trans-Resveratrol 10 μM significantly inhibited thrombin response, similar to data shown in Figure 2. trans-Resveratrol 10 μM, by itself did not influence [Ca2+]i. The trace shown is from a representative experiment; similar results were obtained from six other platelet preparations.
Figure 5.
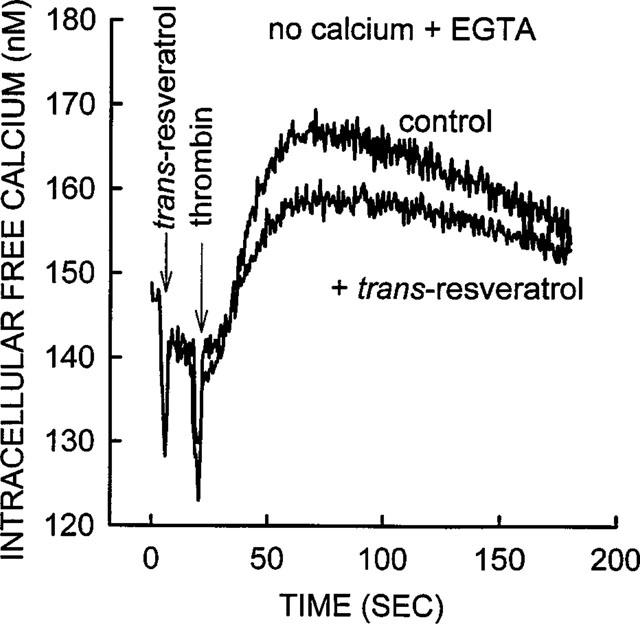
Effect of trans-resveratrol on the release of the internally stored calcium in thrombin stimulated platelets. External calcium was not added to the platelet suspension, 5 mM EGTA was also added 20 s prior to data collection (zero time). Thrombin (0.01 u ml−1) caused a very small increase in [Ca2+]i when calcium was removed from the external medium, compare to data in Figure 4. trans-Resveratrol 10 μM produced a small inhibition of thrombin to mobilize internal calcium, although the inhibitory effect was not statistically significant (see text for actual values). The data in Figure 5 (no calcium plus EGTA) is directly comparable to the data shown in Figure 4 (with 2.0 mM calcium) since the same platelet preparation was used in both experiments. The trace shown is from a representative experiment; similar results were obtained from three other platelet preparations.
Thapsigargin-induced [Ca2+]i entry
After Ca2+ was added back to the platelet suspensions, thapsigargin (dissolved in Me2SO) was added to the platelet suspension to a final concentration of 10−7 M and the fluorescence was monitored as described previously.
Statistical analysis
Data are reported as mean±s.e.mean for the number of individual experiments specified in the Figure legends. Comparisons were made using Student's t-test, with a P value <0.05 considered significant. Different platelet donors were used for each experiment.
Results
Effects of trans-resveratrol on thrombin-induced [Ca2+]i increase in human platelets
Treatment of the platelet suspension with trans-resveratrol markedly reduced the elevation of the [Ca2+]i in response to the thrombin. The data in Figure 2 shows a representative experiment in which three different concentrations of trans-resveratrol were added to platelets. The effect of trans-resveratrol was observed after a short 5–10 s incubation with trans-resveratrol before adding thrombin. It takes 5–10 s for complete mixing of the 2.5 μl aliquot of the trans-resveratrol solution in the 500 μl aliquot of platelet suspension in the spectrofluorometric cuvette using a magnetic stirrer bar. trans-Resveratrol reduced both the rate of [Ca2+]i rise together with the peak level of [Ca2+]i induced by thrombin. The data in Figure 3 is an average of six experiments, similar to that shown in Figure 1, and shows that trans-resveratrol produced a dose-dependent inhibition of the [Ca2+]i increase with an IC50 of 0.5 μM, see Figure 3 legend for details. The data shows that 0.1, 1.0 and 10.0 μM trans-resveratrol produced statistically significant inhibitory effects of 20±6% (P<0.01), 37±6% (P<0.001) and 57±4% (P<0.001). The maximum degree of inhibition (57±4%) was observed with 10 μM trans-resveratrol, higher concentrations of trans-resveratrol were not used because they caused large changes in fluorescence that interfered with the fura-2 fluorescence measurements (data not shown). Cholesterol (negative control steroid) at a concentration of 10 μM, and the Me2SO solvent (0.5% v v−1) used to dissolve trans-resveratrol did not influence [Ca2+]i levels (data are not shown). trans-Resveratrol did not interfere with the calcium ionophore ionomycin (10.0 μM) or digitonin (0.01%) induced [Ca2+]i elevations in human platelets, thereby demonstrating that trans-resveratrol does not influence fura-2 fluorescence and therefore rules out any non-specific effects of the phytoestrogen on the [Ca2+]i measurements. Both these agents increase [Ca2+]i by mechanisms independent of plasma membrane calcium channels.
Figure 2.
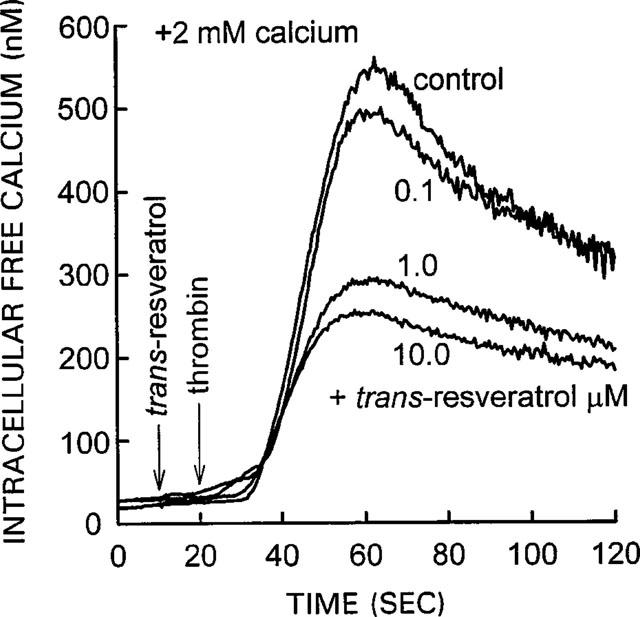
Effect of trans-resveratrol on [Ca2+]i in thrombin-stimulated platelets. Calcium 2 mM was added to the platelet suspension 20 s before data collection started (zero time). Ten seconds later, trans-resveratrol solution was added to yield a final concentration in the platelet suspension of either 0.1, 1.0 or 10 μM. Thrombin (0.01 u ml−1) was added 10 s later. Thrombin caused a large increase in [Ca2+]i in the control condition, platelets started to aggregate at 60–80 s. All three concentrations of trans-resveratrol inhibited the thrombin-induced rise in [Ca2+]i in a dose-dependent manner. The trace shown is from a representative experiment; similar results were obtained from six other platelet preparations and are presented in Figure 3.
Figure 3.
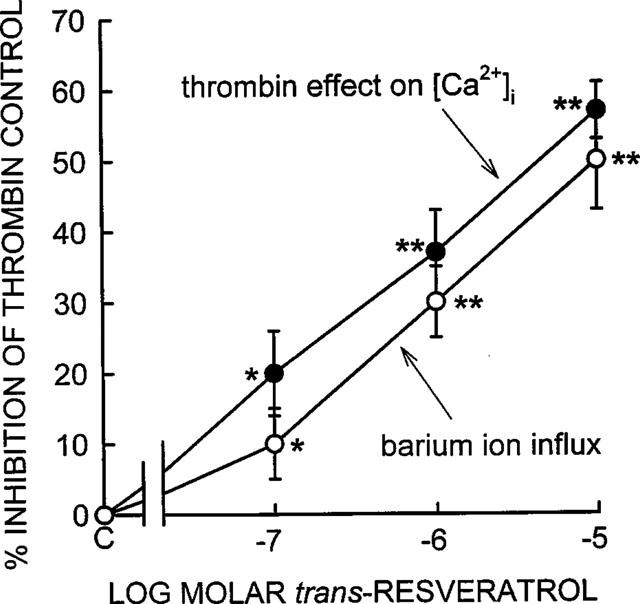
Dose-response of trans-resveratrol to inhibit thrombin-induced elevation of [Ca2+]i and Ba2+ ion influx. The % inhibitory effect of trans-resveratrol was calculated by subtracting the basal or pre-stimulated value from the peak effect with trans-resveratrol present and comparing it to the effect observed in the control with thrombin and without trans-resveratrol. The IC50 was 0.5 μM for both parameters, and was calculated by estimating the concentration of trans-resveratrol that produced 50% inhibition of the maximum inhibitory effect which was 57% inhibition of the [Ca2+]i response, and 50% inhibition of the barium ion influx response. These values were 28.5% for [Ca2+]i (i.e. 1/2 of 57%) and 25% for barium ion influx (i.e. 1/2 of 50%). The data represent mean±s.e.mean from six separate experiments with different platelet donors, typical experiments are shown in Figures 2 and 7. Statistical significance of trans-resveratrol effect when compared to thrombin control: *P<0.05, **P<0.01.
The data in Figure 4 depicts a representative experiment that indicates trans-resveratrol alone did not have an effect on [Ca2+]i level in the absence of thrombin stimulation. In the same experiment trans-resveratrol blocked the thrombin effect similar to that shown in Figure 2, although in this particular experiment the inhibitory effect was larger. The elevation of [Ca2+]i in thrombin stimulated platelets, without the addition of Ca2+ to the medium together with 5.0 mM EGTA added to the buffer, was substantially smaller (25 nM increase, Figure 5), compared with [Ca2+]i elevation in the presence of the external Ca2+ (500 nM increase, Figure 4). The elevation of [Ca2+]i induced by thrombin in EGTA containing media was 7±2% of that seen in the presence of 2.0 mM calcium (average from three separate experiments). This suggested that the majority of the [Ca2+]i increase, due to thrombin (0.01 u ml−1) stimulation, was primarily due to Ca2+ influx, and not the result of the emptying of the internal Ca2+ stores. The data in Figure 5 showed that 10 μM trans-resveratrol produced a very small inhibition of the thrombin-induced internal mobilization of calcium. The average of four separate experiments showed that 10.0, 1.0 and 0.1 μM trans-resveratrol produced an 8±10, 2±11 and 12±11% inhibition respectively of thrombin, these small inhibitory effects were not statistically significant (P>0.05).
Extracellular calcium in the concentration range of 50–4000 μM did not overcome the trans-resveratrol inhibition of Ca2+ influx into the thrombin-stimulated platelets (Figure 6). The effect of trans-resveratrol to inhibit thrombin-induced increases in [Ca2+]i was very small (not statistically significant, P>0.05) when the extracellular calcium was in the 50–250 μM range. This is consistent with the data shown in Figure 5 in which trans-resveratrol produced a small but not statistically significant inhibition (see above) of thrombin in the presence of extracellular EGTA and no added calcium. When the extracellular calcium was increased to 500–4000 μM, thrombin was then able to promote a much larger increase in [Ca2+]i due to a greater calcium influx and the effect of trans-resveratrol was much more pronounced (Figures 4 and 6). The degree of inhibition induced by trans-resveratrol remained constant as the Ca2+ concentration increased in the 1000–4000 μM range (Figure 6).
Figure 6.
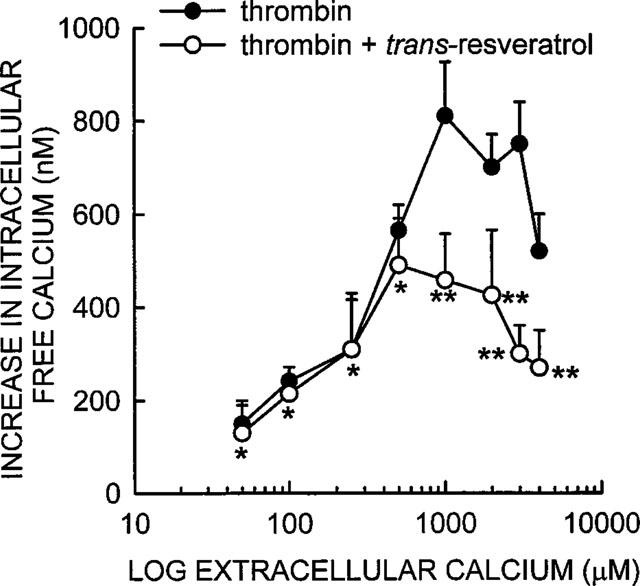
Dose response of extracellular calcium on the ability of thrombin to increase [Ca2+]i in the presence and absence of trans-resveratrol. Various concentrations of calcium were added to the medium 20 s before data collection was started (zero time). Thrombin 0.01 u ml−1 was then added to the platelets in the presence and absence of 10 μM trans-resveratrol as described in the legend to Figure 2. The data shown is the mean±s.e.mean from three separate experiments.*P>0.05, **P<0.05.
Effect of trans-resveratrol on the basal Ca2+ channel activity measured by monitoring Ba2+ uptake
Non-stimulated (control) platelets exhibit a biphasic (an early and a late component) rise in Ba2+ possibly due to at least two different populations of Ca2+ channels, which are accessible for Ba2+ entry (Figure 7). In most of the experiments the influx of Ba2+ began to induce platelet aggregation after 2 min. The rational for examining basal Ba2+ influx in the absence of thrombin was that the influence of various second messenger pathways (e.g. tyrosine phosphorylation, Ca2+ store depletion, cyclic nucleotides) would not be involved and hence the effect of the various phytoestrogens would only be on the Ca2+ influx channels and not on any agonist stimulated signal transduction pathway(s). trans-Resveratrol produced a large inhibition of Ba2+ influx into the platelets, affecting mostly the later component of entry. Three different concentrations (0.1, 1.0 and 1.0 μM) of trans-resveratrol are shown in this representative experiment (Figure 7). The dose-response of trans-resveratrol to inhibit Ba2+ influx from six different experiments is shown in Figure 3, with 0.1, 1.0 and 10.0 μM trans-resveratrol producing 10±5 (P<0.05), 30±5 (P<0.01) and 50±7% (P<0.01) inhibition respectively. Thapsigargin stimulated Ba2+ influx, both the rate of influx and the magnitude of the influx (Figure 7). This result shows that at least some of the Ba2+ that enters the platelet is through SOCC (see also Figure 8). When thapsigargin was added to platelets in the absence of both calcium and Ba2+ there was a small increase in the 340/380 nm ratio (Figure 7). This result demonstrates that thapsigargin was able to mobilize intracellular calcium stores and that these stores were relatively small in platelets. This result is consistent with the data in Figures 4 and 5 in which chelation of extracellular calcium with EGTA reduced the ability of thrombin to increase [Ca2+]i by 93%. Even though thapsigargin only produced a small mobilization of intracellular calcium, it was sufficient to promote a large Ba2+ ion influx through SOCC (Figure 7).
Figure 7.
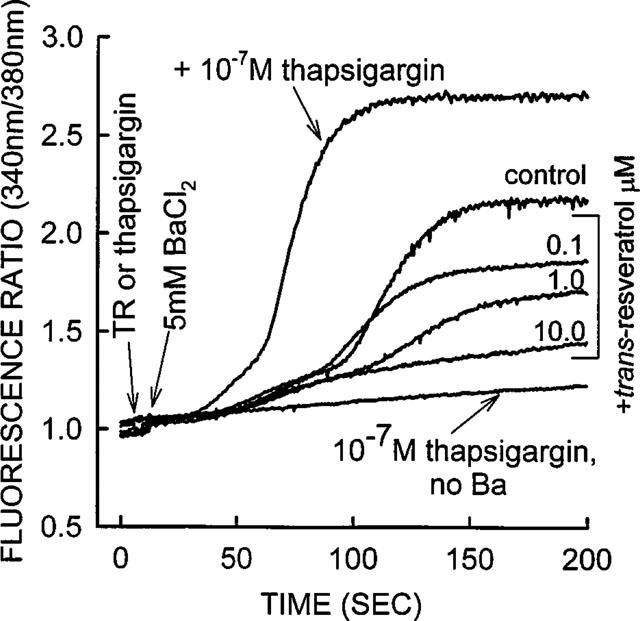
Effect of thapsigargin and trans-resveratrol on Ba2+ ion influx into platelets in calcium-free medium. Control represents the experiment where 5.0 mM Ba2+ was added alone to the platelet suspension. trans-Resveratrol concentrations 10, 1.0 and 0.1 μM inhibited basal Ba2+ influx into platelets. Results are expressed as 340/380 fluorescence ratios. Thapsigargin 10−7 M stimulated Ba2+ ion influx over control levels. When no calcium or Ba2+ was added, thapsigargin 10−7 M produced a small elevation in [Ca2+]i as indicated by a small increase in the 340/380 nm ratio. The trace shown is from a representative experiment, similar results were obtained from six other platelet preparations. The dose response of trans-resveratrol to inhibit Ba2+ influx into platelets is shown in Figure 3.
Figure 8.

Effect of trans-resveratrol on thapsigargin-induced [Ca2+]i in platelets. Experiments were conducted in the presence of 2.0 mM external calcium. The control trace represents the [Ca2+]i increase in platelets stimulated with thapsigargin 10−7 M alone. trans-Resveratrol concentrations of 0.1, 1.0, and 10 μM dramatically decreased the rate of [Ca2+]i increase in thapsigargin-exposed platelets. The trace shown is from a representative experiment, similar results were obtained in seven other platelet preparations. trans-Resveratrol, 0.1, 1.0 and 10.0 μM, increased the time to reach the EC50 for maximum [Ca2+]i elevation by 20±6, 30±7 and 94±10% respectively.
Effect of trans-resveratrol on thapsigargin-induced Ca2+ entry
trans-Resveratrol markedly decreased, in a dose-dependent manner, thapsigargin-induced Ca2+ influx, which suggested that SOCC are one of the possible targets for trans-resveratrol in the platelets (Figure 8). This result is consistent with the data on Ba2+ influx shown in Figure 7. Thapsigargin produced a delayed response to increase [Ca2+]i, compared to that seen with thrombin (also see Figure 2), with [Ca2+]i values reaching 1 μM, approximately 100 s after stimulation. In most of the experiments, even though the trans-resveratrol inhibited the rate of increase in [Ca2+]i and delayed the onset of the increase in [Ca2+]i, the increase in [Ca2+]i eventually approached that obtained in the control. We therefore expressed the inhibition data as the time to reach the half maximum [Ca2+]i value (EC50) for the thapsigargin control. The basal [Ca2+]i value was subtracted from the maximum [Ca2+]i value obtained in each run, this number was divided by 2, then this number was added to the basal value. The time it takes to reach this 50% value, after thapsigargin addition with various concentrations of trans-resveratrol, was the time to reach the EC50. To normalize each experiment we expressed the data as % increase in time over the control. Therefore when 0.1, 1.0 and 10.0 μM trans-resveratrol was added the time for thapsigargin to reach the EC50 was 20±6 (P<0.01), 30±7 (P<0.01) and 94±10% (P<0.001), data from seven separate experiments. This data shows that the greater the inhibition by trans-resveratrol the greater the % increase in time to reach the EC50.
Effect of other phytoestrogens on the thrombin-induced [Ca2+]i increase in human platelets
The phytoestrogens apigenin, daidzein, genistein, (Figure 1) as well as genistein-glycoside did not cause a change in the [Ca2+]i when added to non-stimulated platelet suspension, similar to that observed with trans-resveratrol (Figure 4). All the above compounds caused a marked decrease in [Ca2+]i, in thrombin-stimulated platelets (Figure 9). Daidzein (10 μM) produced a 42±4% inhibition of thrombin, average from four experiments, genistein (10 μM) produced a 50±5% inhibition of thrombin, average from seven experiments and in three separate experiments 10 μM apigenin produced a 50±8% inhibition of thrombin. The degree of the inhibition produced by each phytoestrogen was similar to the inhibition caused by 10 μM trans-resveratrol, which was 57±4% (see Figures 2 and 3). The IC50 was 1 μM for each phytoestrogen tested.
Figure 9.
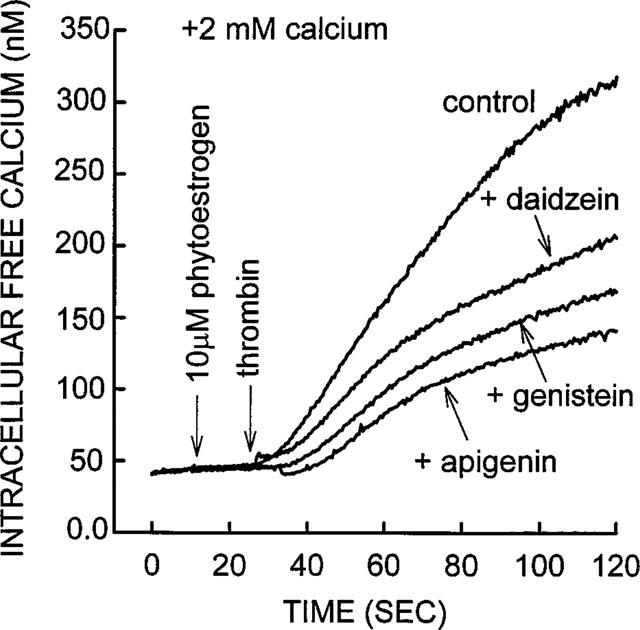
Effect of phytoestrogens genistein, daidzein and apigenin on [Ca2+]i level in thrombin-stimulated platelets. Calcium was added to the platelet suspension to a concentration of 2.0 mM 20 s before data collection was started (zero time). Approximately 10 s later, either genistein, daidzein or apigenin was added to yield final concentrations in the platelet suspension of 10 μM. Thrombin (0.01 u ml−1) was added approximately 10 s later. All three phytoestrogenic compounds inhibited the thrombin-induced rise in [Ca2+]i in a similar manner to that seen with trans-resveratrol (Figures 2, 3 and 4). The trace shown is from a representative experiment; similar results were obtained from four other platelet preparations.
The very rapid inhibitory action of apigenin, daidzein, and genistein (immediately upon contact with platelets) suggested that these compounds produced their effects on the platelet surface. To confirm this suggestion, the addition of hydrophilic conjugates of these compounds should produce a similar inhibitory effect, since they are unlikely to be able to traverse the hydrophobic plasma membrane and hence will be confined to the extracellular space. One such analogue was commercially available, namely genistein-7-O-D-glucoside (4′,5,7-trihydroxyisoflavone-7-glucoside). The data in Figure 10 shows that genistein-7-O-D-glucoside produced an inhibitory effect similar to that of genistein, which implied that the effect was mediated by genistein-7-O-D-glucoside acting on the platelet surface and not on some intracellular target. Genistein-glucoside (10 μM) produced a 37±6% inhibition of thrombin in three separate experiments, while genistein (10 μM) produced a 50±5% inhibition of thrombin in seven separate experiments. The inhibitory effects of both agents were not significantly different from each other.
Figure 10.
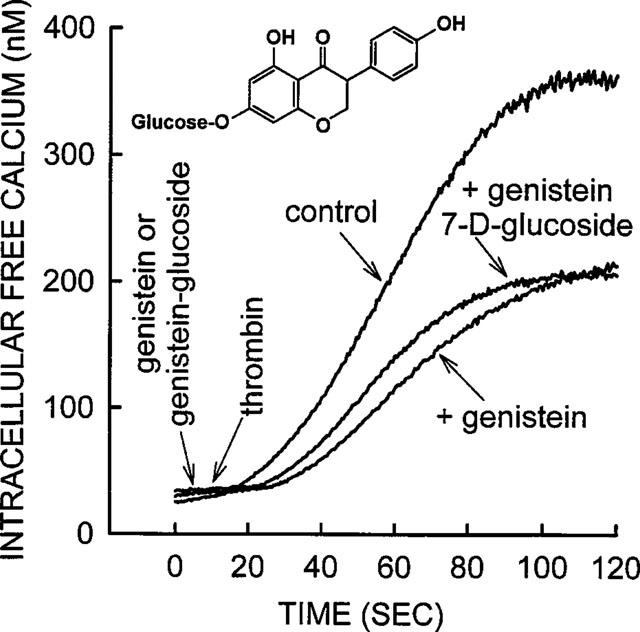
Genistein-7-D-glucoside inhibited calcium influx into thrombin-stimulated platelets in a manner similar to that of genistein. Calcium was added to the platelet suspension to a concentration of 2.0 mM 20 s before data collection starts (zero time). Approximately 5 s later, either genistein or genistein-7-D-glucoside was added to yield final concentration in the platelet suspension of 10 μM. Thrombin (0.01 u ml−1) was added at approximately 10 s after data collection started. No phytoestrogens were added to the platelets in the thrombin control experiment. Both compounds inhibited the thrombin-induced rise in [Ca2+]i in a similar manner. The trace shown is from a representative experiment; similar results were obtained from three other platelet preparations.
Discussion
The inhibitory effect of trans-resveratrol on platelet aggregation has been described (Pace-Asciak et al., 1995; 1996; Bertelli et al., 1995). However, little is known about the actual mechanism of this phenomena. The present study demonstrates for the first time that trans-resveratrol significantly inhibited Ca2+ influx into thrombin-stimulated platelets. Since a rise in the [Ca2+]i is a necessary step for aggregation, we suggest that the Ca2+ blocking action of trans-resveratrol was responsible for the observed inhibition of thrombin induced platelet aggregation in platelet rich plasma (Pace-Asciak et al., 1995; 1996; Bertelli et al., 1995), which we have also confirmed using washed human platelets. The mode of inhibition was very rapid (several seconds preincubation), essentially at the moment of contact with the platelets. Incubating the platelets for longer periods (up to 120 min) with trans-resveratrol did not produce any further inhibitory effect (data not shown). Studies performed in polymorphonuclear leukocytes (PMN) demonstrated that trans-resveratrol was able to inhibit formyl methionyl leucyl phenylalanine (fMLP) induced elevations of [Ca2+]i (Rotondo et al., 1998). The PMN's do not appear to be as responsive to trans-resveratrol as the platelets are in our present study. The rise in [Ca2+]i in the PMN's was inhibited slightly by 22 μM trans-resveratrol, with near maximum inhibition seen with 110 μM trans-resveratrol (Rotondo et al., 1998). The elevation of [Ca2+]i in platelets induced by thrombin was seen with as little as 0.1 μM trans-resveratrol, with a maximum 57±4% inhibition seen with 10 μM trans-resveratrol (Figure 3). We did not use amounts of trans-resveratrol higher than 10 μM due to its insolubility at higher concentrations, and the quenching of the fura-2 fluorescence.
The question remains whether trans-resveratrol acts immediately on the platelet Ca2+ channels or whether inhibition of Ca2+ influx is a secondary phenomenon to some other unknown primary effect of trans-resveratrol. It is possible that trans-resveratrol and other phytoestrogens generate an inhibitory second messenger to block calcium influx, such as cyclic AMP or cyclic GMP (Sage, 1997). However, the finding that trans-resveratrol inhibited the basal activity of Ca2+ channels, measured by measuring Ba2+ ion influx in non-stimulated platelets, suggested that trans-resveratrol may have a binding site on the cell surface, thus directly affecting the channel itself. The first component of Ba2+ entry seems to remain unaffected by trans-resveratrol, however the second component was inhibited dramatically. An IC50 of 0.5 μM was estimated for trans-resveratrol to block Ba2+ influx (Figure 3). The phenomena, where trans-resveratrol immediately inhibited Ba2+ influx into platelets, supports the hypothesis that ionic channels are involved. Since basal Ba2+ influx is independent from the generation of second messengers (no agonist used), trans-resveratrol probably acts on the channel directly.
The apparent target for trans-resveratrol action seems to be the SOCC, since thapsigargin mediated increases in [Ca2+]i are also inhibited by trans-resveratrol (Figure 8). This is consistent with the fact that thrombin stimulated Ca2+ influx is primarily through the SOCC. Experiments also suggested that trans-resveratrol was unable to inhibit thrombin induced release of intracellular stored Ca2+ (Figure 5), although a very small, not statistically significant, inhibition was observed. Though it appears that trans-resveratrol acts on the platelet surface, there is still a possibility that a very small inhibition of internal Ca2+ release may translate into an inhibition of the plasma membrane Ca2+ channels, since the Ca2+ influx process through SOCC depends on the degree of filling of the internal Ca2+ stores (Parekh & Penner, 1997). The data in Figure 6 suggests that trans-resveratrol and Ca2+ are not competing for the same site on the calcium channel, since the trans-resveratrol inhibition of thrombin induced elevation of [Ca2+]i could not be overcome by increasing the extracellular Ca2+.
Other types of phytoestrogens (apigenin, daidzein, genistein, genistein-glucoside) showed a similar mode of action to trans-resveratrol on thrombin-stimulated platelets, by inhibiting Ca2+ influx. Genistein-glucoside, being a very hydrophilic compound, is a tool to explore indirectly if the phytoestrogens have their primary targets on the platelet surface rather than on an intracellular target. Genistein and genistein-glucoside have a very similar time course of action, inhibiting Ca2+ influx apparently at the moment of their contact with the platelet (Figure 10). This evidence also indirectly supports the hypothesis that phytoestrogens act upon the platelet surface, directly or indirectly changing Ca2+ channel activity.
The key point to resolve is whether trans-resveratrol and other phytoestrogens act directly on the cell surface or must penetrate the inside of the platelet. This could be clarified by a direct radiolabelled ligand binding and affinity labelling experiment to platelet membranes. It would be of interest to explore what intracellular signal transduction pathways are involved in the trans-resveratrol action. Examining whether trans-resveratrol and other phytoestrogens change the phosphorylation state of the intracellular targets would be important since several phytoestrogens have been shown to act as tyrosine kinase inhibitors, although high concentrations are required to inhibit a variety of kinases. For genistein the IC50 for the epidermal growth factor receptor tyrosine kinase is 2.6 μM, for p60v-src the IC50=25 μM and for protein kinase C and protein kinase A the IC50>100 μM (Akiyama et al., 1987). In our experiments we observed inhibition of thrombin effects by genistein and the other phytoestrogens daidzein, apigenin and trans-resveratrol in the 0.1–10 μM concentration range (Figure 9 and data not shown). Since daidzein is used as an inactive analogue of genistein in tyrosine kinase experiments (Akiyama et al., 1987), and since both daidzein and genistein produced the same degree of inhibition of thrombin (Figure 9) this would argue against these compounds producing the inhibition of Ca2+ influx in platelets by inhibiting a tyrosine kinase.
The levels of trans-resveratrol in red wine are typically in the 10–100 μM (0.4–4 mg l−1) range (Soleas et al., 1997), with wine from Cabernet Savignon having levels as high as 7 mg l−1. Also the presence of trans-resveratrol-glucoside (piceid) in wine may also contribute to the levels of trans-resveratrol in the blood. Even though much experimental data supports the biological activity of trans-resveratrol in vivo, following regular wine consumption in man (e.g. Tham et al., 1998), no serum levels have been reported. Also there is no data on clearance from the blood and first-pass effects in the portal circulation of the liver (e.g. Gehm et al., 1997). The data presented by Pace-Asciak et al. (1996), in which humans had consumed 2 mg of trans-resveratrol per day, showing decreased platelet activity was very convincing. This amount of trans-resveratrol is equivalent to consuming approximately 1 L of a typical red wine per day (Soleas et al., 1997). We observed that genistein and genistein-glucoside (Figures 9 and 10) were able to inhibit thrombin induced elevations of [Ca2+]i similar to that seen with trans-resveratrol (Figures 2 and 3). After consuming a soybean flour-based meal on two occasions, 6 days apart, the levels of genistein and daidzein reached peak plasma levels of 4 and 3 μM respectively, with elimination half-lives of 5.7 and 4.7 h, respectively (King & Bursill, 1998). These levels of genistein and daidzein were able to inhibit the ability of thrombin to elevate [Ca2+]i in platelets in vitro (Figure 9). In another study involving 14 Japanese men, average age 55 years on an uncontrolled diet, the plasma phytoestrogen levels were approximately 0.4 μM (Adlercreutz et al., 1993).
In conclusion, since phytoestrogenic compounds are present in a variety of foods (Mikscicek, 1995), their Ca2+-channels blocking action may be potentially beneficial in the prevention and management of cardiovascular disease and other cardiovascular dysfunctions in which thrombosis plays a role.
Acknowledgments
Thanks to Ms Patricia G. Loose for her expert technical assistance. The laboratory of P.F. Blackmore was supported by a grant from the American Heart Association, VA affiliate. A part of this project was supported by the Virginia Academy of Science.
Abbreviations
- ADP
adenosine diphosphate
- [Ca2+]i
intracellular free calcium
- EGTA
ethylene glycol bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid
- IP3
myo-inositol 1,4,5-trisphosphate
- Me2SO
dimethylsulphoxide
- RMCE
receptor-mediated calcium entry
- ROC
receptor-operated channel
- SMOC
second messenger operated channel
- SOCC
store operated calcium channel
References
- ADLERCREUTZ H., MARKKANEN H., WATANABE S. Plasma concentrations of phytoestrogens in Japanese men. Lancet. 1993;342:1209. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- BERRIDGE M.J. Elementary and global aspects of calcium signalling. J. Physiol. 1997;499.2:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTELLI A.A., GIOVANNINI L., GIANNESSI D., MIGLIORI M., BERNINI W., FREGONI M., BERTELLI A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tiss. React. 1995;17:1–3. [PubMed] [Google Scholar]
- BLACHE D., CIAVATTI M. Rat platelet arachidonate metabolism in the presence of Ca2+, Sr2+ and Ba2+: studies using intact platelets and semi-purified phospholipase A2. Biochim. Biophys. Acta. 1987;921:541–551. doi: 10.1016/0005-2760(87)90082-8. [DOI] [PubMed] [Google Scholar]
- BLACHE D., CIAVATTI M., OJEDA C. Platelet aggregation and endogenous 5-HT secretion in presence of Ca2+, Sr2+ and Ba2+. Effect of calcium antagonists. Thromb. Res. 1987;46:779–791. doi: 10.1016/0049-3848(87)90070-3. [DOI] [PubMed] [Google Scholar]
- BLACKMORE P.F., BEEBE S.J., DANFORTH D.R., ALEXANDER N. Progesterone and 17 α-hydroxyprogesterone: Novel stimulators of calcium influx in human sperm. J. Biol. Chem. 1990;265:1376–1380. [PubMed] [Google Scholar]
- BRASS L.F., HOXIE J.A., KIEBER-EMMONS T., MANNING D.R., PONCZ M., WOOLKALIS M. Mechanisms of Platelet Activation and Control 1993Plenum Press: New York; 17–36.(eds). Authi et al., pp [DOI] [PubMed] [Google Scholar]
- CLEMENTI E., MELDOLESI J. Pharmacological and functional properties of voltage-independent Ca2+ channels. Cell Calc. 1996;19:269–279. doi: 10.1016/s0143-4160(96)90068-8. [DOI] [PubMed] [Google Scholar]
- CRAWFORD N., SCRUTTON M.C. Haemostasis and Thrombosis 1994London: Churchill Livingstone; 89–114.(eds). Bloom, A.L., Forbes, C.D., Thomas, D.P. & Tuddenham, E.G.D. pp [Google Scholar]
- FAVRE C.J., UFRET-VINCENTY C.A., STONE M.R., MA H.T., GILL D.L. Ca2+ pool emptying stimulates Ca2+ entry activated by S-nitrosylation. J. Biol. Chem. 1998;273:30855–30858. doi: 10.1074/jbc.273.47.30855. [DOI] [PubMed] [Google Scholar]
- FIORAVANTI L., CAPPELLETTI V., MIODINI P., RONCHI E., BRIVIO M., DI FRONZO G. Genistein in the control of breast cancer cell growth: insights into the mechanism of action in vitro. Cancer Lett. 1998;130:143–152. doi: 10.1016/s0304-3835(98)00130-x. [DOI] [PubMed] [Google Scholar]
- GEHM B.D., MCANDREWS J.M., CHIEN P.Y., JAMESON J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEEMSKERK J.W.M., SAGE S.O. Calcium signalling in platelets and other cells. Platelets. 1994;5:295–316. doi: 10.3109/09537109409006439. [DOI] [PubMed] [Google Scholar]
- HEYNS A., DU P.Thrombopoiesis and platelet kinetics Haemostasis and Thrombosis 1994London: Churchill Livingstone; 31–47.(eds). Bloom, A.L., Forbes, C.D., Thomas, D.P. & Tuddenham, E.G.D. pp [Google Scholar]
- HOURANI S.M.O., CUSACK N.J. Pharmacological receptors on blood platelets. Pharmacol. Rev. 1991;43:243–298. [PubMed] [Google Scholar]
- JANG M., CAI L., UDEANI G.O., SLOWING K.V., THOMAS C.F., BEECHER C.W.W., FONG H.H.S., FARNSWORTH N.R., KINGHORN A.D., MEHTA R.G., MOON R.C., PEZZUTO J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- JAYATILAKE G.S., JAYASURIYA H., LEE E.-S., KOONCHANOK N.M., GEAHLEN R.L., ASHENDEL C.L., MCLAUGHLIN J.L., CHANG C.-J. Kinase inhibitors from Polygonum Cuspidatum. J. Nat. Prod. 1993;56:1805–1810. doi: 10.1021/np50100a021. [DOI] [PubMed] [Google Scholar]
- KING R.A., BURSILL D.B. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am. J. Clin. Nutr. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- MIKSICEK R.J. Estrogenic flavonoids: structural requirements for biological activity. Proc. Soc. Exp. Biol. Med. 1995;208:44–50. doi: 10.3181/00379727-208-43830. [DOI] [PubMed] [Google Scholar]
- NOLAN R.D., LAPETINA E.G. Thrombin stimulates the production of a novel polyphosphoinositide in human platelets. J. Biol. Chem. 1990;265:2441–2445. [PubMed] [Google Scholar]
- OZAKI Y., YATOMI Y., KUME S. Evaluation of platelet calcium ion mobilization by the use of various divalent ions. Cell Calc. 1992;13:19–27. doi: 10.1016/0143-4160(92)90026-o. [DOI] [PubMed] [Google Scholar]
- PACE-ASCIAK C.R., HAHN S., DIAMANDIS E.P., SOLEAS G., GOLDBERG D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin. Chimica. Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- PACE-ASCIAK C.R., ROUNOVA O., HAHN S.E., DIAMANDIS E.P., GOLDBERG D.M. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin. Chimica. Acta. 1996;246:163–182. doi: 10.1016/0009-8981(96)06236-5. [DOI] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- ROTONDO S., RAJTAR G., MANARINI S., CELARDO A., ROTILIO D., DE GAETANO G., EVANGELISTA V., CERLETTI C. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br. J. Pharmacol. 1998;123:1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RZIGALINSKI B.A., BLACKMORE P.F., ROSENTHAL M.D. Arachidonate mobilization is coupled to depletion of intracellular calcium stores and influx of extracellular calcium in differentiated U937 cells. Biochim. Biophys. Acta. 1996;1299:342–352. doi: 10.1016/0005-2760(95)00224-3. [DOI] [PubMed] [Google Scholar]
- RZIGALINSKI B.A., WILLOUGHBY K.A., HOFFMAN S.W., FALCK J.R., ELLIS E.F. Calcium influx factor, further evidence it is 5,6-epoxyeicosatrienoic acid. J. Biol. Chem. 1999;274:175–182. doi: 10.1074/jbc.274.1.175. [DOI] [PubMed] [Google Scholar]
- SAGE S.O. Calcium entry mechanisms in human platelets. Exp. Physiol. 1997;82:807–823. doi: 10.1113/expphysiol.1997.sp004066. [DOI] [PubMed] [Google Scholar]
- SAGE S.O., RINK T.J. Calcium signaling in human platelets. Annu. Rev. Physiol. 1990;52:431–449. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- SARGEANT P., CLARKSON W.D., SAGE S.O., HEEMSKERK J.W. Calcium influx evoked by Ca2+ store depletion in human platelets is more susceptible to cytochrome P-450 inhibitors than receptor-mediated calcium entry. Cell Calc. 1992;13:553–564. doi: 10.1016/0143-4160(92)90035-q. [DOI] [PubMed] [Google Scholar]
- SARGEANT P., FARNDALE R.W., SAGE S.O. The tyrosine kinase inhibitors methyl 2,5-dihydroxycinnamate and genistein reduce thrombin-evoked tyrosine phosphorylation and Ca2+ entry in human platelets. FEBS Lett. 1993a;315:242–246. doi: 10.1016/0014-5793(93)81172-v. [DOI] [PubMed] [Google Scholar]
- SARGEANT P., FARNDALE R.W., SAGE S.O. ADP- and thapsigargin-evoked Ca2+ entry and protein-tyrosine phosphorylation are inhibited by the tyrosine kinase inhibitors genistein and methyl-2,5-dihydroxycinnamate in Fura-2-loaded human platelets. J. Biol. Chem. 1993b;268:18151–18156. [PubMed] [Google Scholar]
- SOLEAS G.J., DIAMANDIS E.P., GOLDBERG D.M. Resveratrol: a molecule whose time has come? and gone. Clin. Biochem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- THAM D.M., GARDNER C.D., HASKELL W.L. Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. 1998;83:2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- THASTRUP O., CULLEN P.J., DROBAK B.K., HANLEY M.R., DAWSON A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE J.G.Platelet ultrastructure Haemostasis and Thrombosis 1994London: Churchill Livingstone; 49–87.(eds). Bloom, A.L., Forbes, C.D., Thomas, D.P. & Tuddenham, E.G.D. pp [Google Scholar]
- WILLIAMS R.L., RUTLEDGE T. Recent phytoestrogen research. Chem. Industry. 1998;1:14–16. [Google Scholar]
- ZSCHAUER A., VAN BREEMEN C., BUHLER F.R., NELSON M.T. Calcium channels in thrombin-activated human platelet membrane. Nature. 1988;334:703–705. doi: 10.1038/334703a0. [DOI] [PubMed] [Google Scholar]


