Abstract
The present study was undertaken to investigate the effect of Δ9-tetrahydrocannabinol (Δ9-THC) and possible serotoninergic involvement on the extracellular level of dopamine (DA) in the striatum using microdialysis in conscious, freely-moving rats.
A dose-dependent increase in striatal DA release occurred after i.v. administration of 0.5–5 mg kg−1 Δ9-THC when compared with vehicle (n=5–8, P<0.05). Maximum increases, ranging from 42.1±5.4% to 97.4±5.9% (means±s.e.mean) of basal levels occurred 20 min after Δ9-THC. This effect was abolished by pretreatment with the cannabinoid CB1 receptor antagonist, SR 141716 (2.5 mg kg−1 i.p.).
Pretreatment with fluoxetine (10 mg kg−1 i.p.) abolished the Δ9-THC-induced DA release. Fluoxetine 10 mg kg−1 i.p. administered 40 min after Δ9-THC had no significant effect on Δ9-THC-induced DA release. However, fluoxetine perfused locally into the striatum by adding it to the microdialysis perfusion fluid (10 μM) 40 min after Δ9-THC significantly potentiated the Δ9-THC-induced DA release (n=6–8, P<0.05).
These results suggest that DA release induced by Δ9-THC is modulated by serotoninergic changes induced by fluoxetine, the effect of which depends on the time of its administration relative to that of Δ9-THC. Fluoxetine induces an acute increase in extracellular 5-HT through reuptake inhibition, which can activate autoreceptors which may decrease serotoninergic neuronal activity. This may be the reason fluoxetine pretreatment abolished the Δ9-THC-induced DA release. The potentiation of Δ9-THC-induced DA release by fluoxetine perfusion added 40 min after Δ9-THC may be due to an acute increase in 5-HT produced by reuptake inhibition.
Keywords: Δ9-THC, 5-HT, dopamine, microdialysis, fluoxetine
Introduction
Δ9-Tetrahydrocannabinol (Δ9-THC) is the major psychoactive constituent of the plant Cannabis sativa, one of the most commonly used drugs of abuse in the world (Grinspoon & Bakalar, 1993). Δ9-THC produces stereotypical behaviours and has a pronounced depressive effect on locomotor activity. It has been suggested that the nigrostriatal dopaminergic system plays an important role in the manifestation of these behaviours (Navarro et al., 1994). In support of the role of dopamine (DA) in these actions, the results from a number of studies using in vivo microdialysis have reported an increase in extracellular levels of DA following Δ9-THC administration in various regions of the nigrostriatal and mesolimbic systems (Chen et al., 1990a,1990b; Ng Cheong Ton et al., 1998; Tanda et al., 1997). In addition, in vitro experiments using rat striatal slices have shown that Δ9-THC can facilitate the release of DA (Sakurai Yamashita et al., 1989).
There is also considerable evidence to suggest that various effects of Δ9-THC are mediated by serotoninergic mechanisms. A number of studies using rat forebrain (Johnson et al., 1976) or whole brain homogenates (Sofia et al., 1971) have reported increased levels of extracellular 5-hydroxytryptamine (5-HT) following Δ9-THC administration. However, another study using rat striatal slices showed no significant effect (Navarro et al., 1994). Nevertheless, further evidence of Δ9-THC affecting serotoninergic mechanisms is apparent through the alteration of the behavioural effects of Δ9-THC, using drugs affecting 5-HT systems (Davies & Graham, 1980; Taylor & Fennessy, 1978; Verberne et al., 1980). More recently, we published a study that showed that the CB1 receptor mediated hypothermia can be augmented or antagonized by the addition of the selective serotonin reuptake inhibitor (SSRI), fluoxetine (Malone & Taylor, 1998b). The direction of the observed modulation was dependent on the time of administration of fluoxetine relative to that of Δ9-THC. From these results, we hypothesized that the amount of extracellular 5-HT influenced the postjunctional effects observed by altering the activity of the 5-HT neurones by acting prejunctionally. Furthermore, we speculated that CB1 receptor activation by Δ9-THC results in an increase in extracellular 5-HT in order to produce hypothermia.
Several lines of evidence suggest that the serotoninergic fibres projecting from the dorsal raphe nucleus (DRN) can alter dopaminergic activity in the mesolimbic and nigrostriatal systems (Benloucif & Galloway, 1991; Blandina et al., 1989; Jacocks & Cox, 1992; Nedergaard et al., 1988; Schmidt et al., 1992; Sinton & Fallon, 1988; Yadid et al., 1994). Through neuroanatomical studies, the projection of serotoninergic neurones from the rostral DRN to DA cell bodies in the ventral tegmental area (VTA) and the substantia nigra (SN) as well as their respective terminal projections in the NAcc and the striatum have been identified (Benloucif & Galloway, 1991). Furthermore, administration of 5-HT or the SSRI, alaproclate, perfused into the striatum has been reported to dose-dependently facilitate striatal DA release (Benloucif & Galloway, 1991; Yadid et al., 1994). In light of this, and the neuroanatomical evidence for serotoninergic modulation of DA release in the striatum discussed previously, the present study was undertaken to examine the effect of fluoxetine on the Δ9-THC-induced DA release. This study was undertaken using microdialysis in the striatum of conscious, freely-moving rats. A preliminary account of these findings has been presented at the Fourth IUPHAR Satellite Meeting on Serotonin (Malone & Taylor, 1998a).
Methods
Animals and housing
Male Glaxo-Wistar rats weighing between 260 and 380 g were used. Prior to experiments, they were housed in group cages and kept at 22°C with a 12 h light–dark cycle. Food and water were available ad libitum.
Surgery
Two days prior to experimentation, rats were anaesthetized with a sodium methohexitone (18 mg kg−1 i.p.)/sodium amylobarbitone (30 mg kg−1 i.p.) mixture. A polyethylene (PE 50) catheter was surgically inserted into the external jugular vein for i.v. administration. The skull was exposed and a hole (2 mm i.d.) was drilled for the implantation of a 21 gauge microdialysis guide cannula above the left striatum (co-ordinates: L +20 mm, R +2.3 mm, V −5.0 mm). The co-ordinates were chosen according to the stereotaxic atlas of Paxinos & Watson (1986). The guide cannula was held in place using dental cement anchored to two stainless steel screws implanted into the skull. A stainless steel 25-gauge needle, cut to size, was inserted into the cannula as an obturator. Body temperature was maintained at 37°C with a heating pad used throughout the course of the operation. Following the operation, rats were injected with ticarcillin 15 mg kg−1 i.v. to prevent infection.
Microdialysis
Prior to implantation, each rat was lightly anaesthetized with halothane and a 25-gauge microdialysis probe (4.5 mm longer than the guide cannula) with a 3 mm dialysis membrane at the tip was inserted. The probe was then connected via Teflon tubing to a 1.0 ml Exmire micro syringe (Ito Corporation, Fuji, Japan), filled with an artificial cerebrospinal fluid (composition in mM): NaCl, 125; KCl, 2.5; CaCl2.2H20, 1.5; NaHCO3, 27; NaH2PO4.H20, 0.5; Na2HPO4, 1.2; Na2SO4, 0.5; MgCl2.6H20, 1.0; pH 6.2. The probe was continuously perfused at a rate of 2.0 μl min−1 by means of a CMA/100 microinjection pump (Carnegie Medecin, Stockholm, Sweden). The perfusate fractions (40 μl) were collected every 20 min in Eppendorf microcentrifuge tubes containing 10 μl 1 M perchloric acid to prevent the oxidation of the monoamines. The mean of the two perfusate collections immediately prior to Δ9-THC or vehicle injection was used to calculate the basal level of DA (100%).
At the end of the experiment, the rat was killed with an overdose of pentobarbitone. The brain was quickly removed, frozen and coronal sections were cut to verify the correct position of the probe. Only results obtained from rats with correctly positioned guide cannulae are presented.
Drugs
Δ9-THC (National Institute on Drug Abuse, U.S.A.) or vehicle was injected i.v. (t=0). Δ9-THC was incorporated into the triglyceride/phospholipid emulsion vehicle Intralipid® (Baxter) as described previously (Malone & Taylor, 1998b). A concentration of 4 mg ml−1 Δ9-THC in Intralipid® was utilized for the 2 and 5 mg kg−1 doses and 0.5 mg ml−1 Δ9-THC in Intralipid® was used for 0.5 mg kg−1 dose. SR 141716 (N-piperidino-5-(4-chlorphenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide; Sanofi Recherche) was incorporated in a similar manner into Intralipid® and, when used, was injected 40 min before Δ9-THC at a dose of 2.5 mg kg−1 i.p..
For intraperitoneal (i.p.) injection, fluoxetine HCl (Prozac®, Eli Lilly) was dissolved in water for injection to give a concentration of 5 mg ml−1. Rats were administered fluoxetine HCl (10 mg kg−1 i.p.) either 40 min before or 40 min after Δ9-THC administration. When fluoxetine was administered via the microdialysis probe, a 10 μM concentration of fluoxetine was added to the microdialysis perfusion fluid 40 min after Δ9-THC administration and remained in the perfusion fluid for the duration of the experiment, i.e. 200 min after Δ9-THC administration. Other drugs used were amylobarbitone sodium (Amytal®, Ely Lilly) and methohexitone sodium (Brietal3, Eli Lilly). All other reagents used were of analytical grade.
Chromatography
The striatal perfusates were assayed for DA using reverse-phase ion-pair high performance liquid chromatography (HPLC) coupled with electrochemical (EC) detection (Hurd & Ungerstedt, 1989). Perfusate samples (40 μl) were collected over 20 min fractions into microcentrifuge tubes containing 10 μl 1 M perchloric acid, and the resultant 50 μl samples were injected directly onto the HPLC column. The HPLC system consisted of a BAS PM-60 pump operating at a pressure of about 2200 p.s.i., a Rheodyne BH 7125 injector, a reverse-phase Spherisorb 5 ODS column (250 mm×4.6 mm i.d. Phase Sep. Ltd., Deeside, U.K.) which was encapsulated in a temperature-controlled oven set at 28°C (Waters, Division of Millipore, MA, U.S.A.) and a LC-4B dual electrode EC detector equipped with a TL-5A glassy carbon working electrode (Bioanalytical Systems Inc., IN, U.S.A.). The analysis was carried out at a flow rate of 1.3 ml min−1 and a detector potential of +750 mV (vs Ag/AgCl/3M NaCl) with a sensitivity of either 10.0 or 20.0 nA FS. Quantification was performed using a BBC Goerz Metrawatt SE 120 dual channel pen recorder.
The mobile phase consisted of methanol: 0.15 M sodium phosphate buffer containing 0.1 mM ethylenediaminetetraacetic acid (EDTA) and 0.5 mM sodium 1-octanesulphonic acid (14:86 v v−1). The 0.15 M sodium phosphate buffer was prepared by dilution of a stock solution of 1.0 M disodium hydrogen orthophosphate/phosphoric acid buffer pH 2.9. The final solution was adjusted to pH 3.7 with 10.0 M NaOH and was filtered through a 0.45 μm vacuum filter apparatus (Waters, Division of Millipore, MA, U.S.A.). An external standard (0.05 μM DA) was used for the identification of sample peaks.
Statistics
When comparing pretreatments to vehicle only, a Student's t-test was used. When comparing pretreatment or post treatment values plus Δ9-THC or vehicle, a one-way analysis of variance was used. When values were found to be significant, the Student-Newman-Keuls multi-comparison test was used to determine which treatment groups were different and the level of significance (P<0.05 or P<0.01).
Results
Intralipid® administration had no effect on the extracellular levels of DA. Δ9-THC caused a dose-dependent increase in basal DA levels following i.v. administration. Maximum increases were to 142.1±5.4, 178.7±9.4 and 197.4±5.9% of basal levels following the 0.5, 2 and 5 mg kg−1 doses of Δ9-THC respectively and occurred 20 min after Δ9-THC administration (Figure 1). The increase in basal DA levels was significant at all doses 20 min after Δ9-THC administration (n=5–7, P<0.05). Forty minutes after Δ9-THC administration DA levels remained significantly different from vehicle following the 5 mg kg−1 dose of Δ9-THC.
Figure 1.
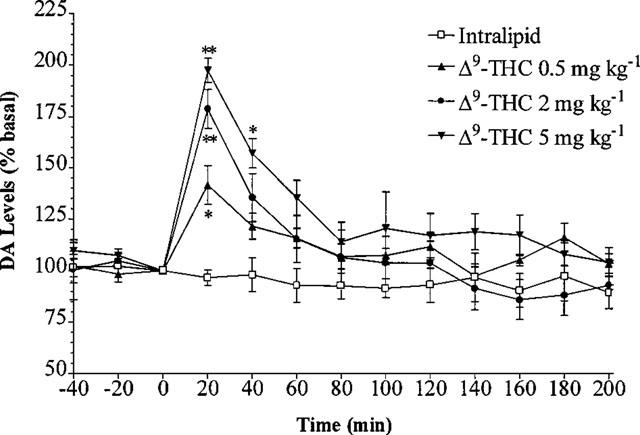
Effect of 0.5, 2 and 5 mg kg−1 Δ9-THC i.v. or vehicle on the level of extracellular DA in the rat striatum. Results are expressed as mean±s.e.mean of 5–7 experiments. (*P<0.05, **P<0.01 when compared with vehicle alone.)
SR 141716 (2.5 mg kg−1) given 40 min before vehicle injection decreased DA levels when compared with vehicle. This decrease reached significance at 120 and 140 min after SR 141716 administration (n=5, P<0.05) (Figure 2). Following pretreatment with i.p. SR 141716, there was no significant change in DA levels produced by 0.5, 2 or 5 mg kg−1 Δ9-THC (n=5–7, P>0.05) (Figure 3).
Figure 2.
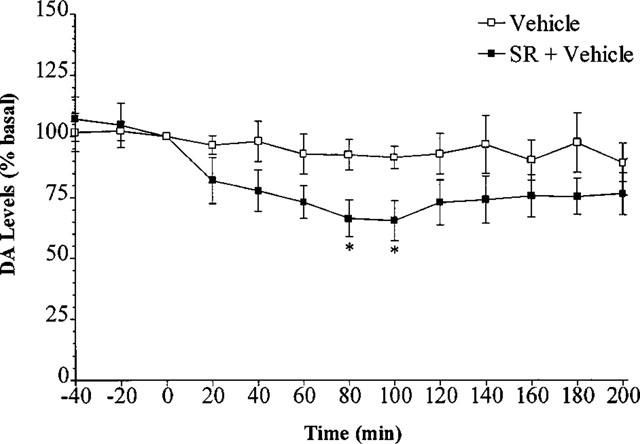
Effect of SR 141716 or vehicle on the level of extracellular DA in the rat striatum. Results are expressed as mean±s.e.mean of five experiments. (*P<0.05 when compared with vehicle alone.)
Figure 3.
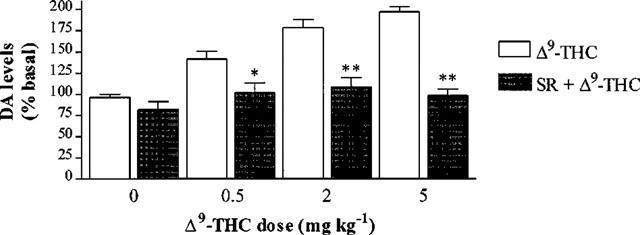
Effect of SR 141716 (SR) 2.5 mg kg−1 i.p. injected at time =−40 min on the extracellular DA levels in the rat striatum 20 min after Δ9-THC or vehicle injection. Results are expressed as mean±s.e.mean of 5–7 experiments. (*P<0.05, **P<0.01 when compared with Δ9-THC or vehicle alone.)
When fluoxetine was added to the microdialysis perfusion fluid at a concentration of 10 μM, a highly significant increase in basal DA levels occurred (n=5–6, P<0.01) compared with vehicle alone 20 min after the fluoxetine perfusion commenced (Figure 4). Also, a significant increase in basal DA levels occurred 40 min after the perfusion commenced (n=5–6, P<0.05).
Figure 4.
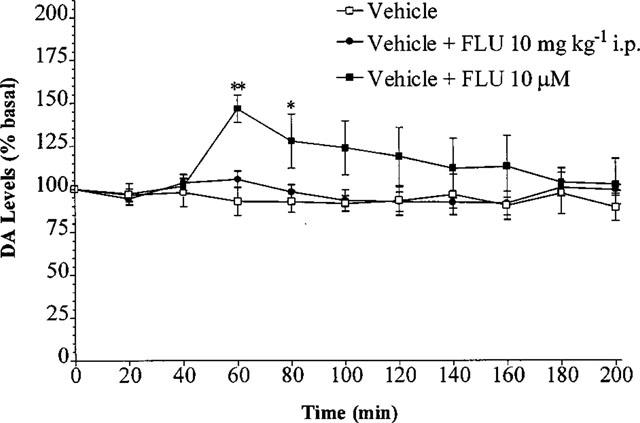
Effect of fluoxetine perfusion 10 μM and fluoxetine 10 mg kg−1 i.p., commencing at t=40 min, on the level of extracellular DA in the rat striatum. Results are expressed as mean±s.e.mean of 5–6 experiments. (*P<0.05, **P<0.01 when compared with vehicle alone.)
Fluoxetine perfusion significantly potentiated the increase in DA levels produced by the 2 mg kg−1 (n=5–6, P<0.01) and 5 mg kg−1 (n=5–6, P<0.05) doses of Δ9-THC (Figure 5). Fluoxetine perfusion had no significant effect on DA levels following the 0.5 mg kg−1 dose of THC (n=5–6, P>0.05) although a slight increase was observed.
Figure 5.
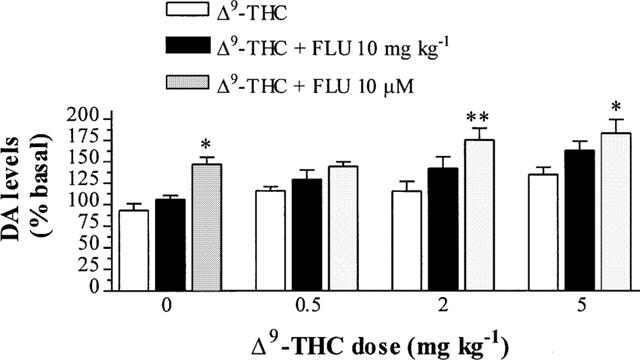
Effect of fluoxetine perfusion 10 μM and fluoxetine 10 mg kg−1 i.p., commencing at t=40 min on Δ9-THC-induced DA levels in the rat striatum 60 min after Δ9-THC or vehicle injection. Results are expressed as mean±s.e.mean of 5–10 experiments (*P<0.05, **P<0.01 when compared with Δ9-THC or vehicle alone.)
Fluoxetine (10 mg kg−1 i.p.) given 40 min after Δ9-THC administration caused a slight but non-significant increase in DA levels produced by 0.5, 2 or 5 mg kg−1 Δ9-THC (n=5–10, P>0.05) (Figure 5).
Fluoxetine (10 mg kg−1 i.p.) given 40 min before vehicle injection had no significant effect on DA levels (Figure 6). Fluoxetine (10 mg kg−1 i.p.) given 40 min before Δ9-THC administration abolished the change in basal DA levels produced by 0.5, 2 or 5 mg kg−1 Δ9-THC (n=5–7, P>0.05) (Figure 6).
Figure 6.
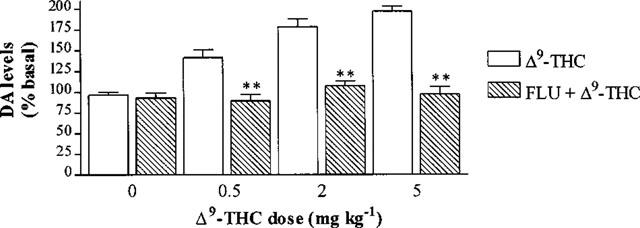
Effect of 10 mg kg−1 fluoxetine (FLU) i.p. injected 40 min before Δ9-THC or vehicle on the extracellular DA levels in the rat striatum 20 min after Δ9-THC or vehicle injection. Results are expressed as mean±s.e.mean of 5–8 experiments. (*P<0.05, **P<0.01 when compared with Δ9-THC or vehicle alone.)
Discussion
The release of DA observed following Δ9-THC administration is comparable with previous studies using microdialysis. Chen et al. (1990a) observed an increase of approximately 125 and 160% from basal levels of DA in the NAcc following 0.5 and 1 mg kg−1 Δ9-THC respectively. An increase to 160% of basal levels was reported in the medial prefrontal cortex following 2 mg kg−1 Δ9-THC i.p. (Chen et al., 1990b). In the present study, increases to approximately 142, 179, and 197% following 0.5, 2 and 5 mg kg−1 doses of Δ9-THC occurred, respectively (Figure 1). Although the magnitude of increase in DA levels was comparable, one difference in the results of previous studies and the present study was the time taken for the increase in DA levels to reach a maximum. In the present study, the maximum effect was determined in the first sample collected, ie within 20 min after Δ9-THC administration whereas 40–60 min elapsed before a maximum effect was observed in previous studies. The studies described above used the i.p. route of administration but in the present experiment, the i.v. route was chosen, which provides a faster absorption to Δ9-THC's sites of action in the brain (Agurell et al, 1986). In other experiments mentioned, DA release peaked 40 min after Δ9-THC i.p.. This may account for the more rapid onset of action in the present study. The i.v. route of administration was chosen as this route more accurately mimics the inhaled route of administration, which is the method that most recreational users choose when using cannabis (Agurell et al., 1986), presumably because of the fast absorption rate produced.
The antagonism of the Δ9-THC-induced DA release by SR 141716 indicates that the Δ9-THC-induced DA release is CB1 receptor-mediated (Figure 3). This is in agreement with Tanda et al. (1997) who showed the increase in DA in the NAcc was prevented by SR 141716A, the hydrochloride salt of SR 141716. The dose of SR 141716 used in this study was shown to antagonize the catalepsy produced by the CB1 agonist, CP 55940 (Anderson et al., 1996).
Interestingly, SR 141716 administration 40 min before vehicle decreased basal DA to 66% of basal levels (Figure 2). This effect reached a maximum decrease at 120 to 140 min after SR 141716. The reason for this decrease is unclear, but may be due to either SR 141716 antagonising an endogenous cannabinoid system in the brain, or that it is an inverse agonist. In vitro, SR 141716A has been shown to display properties opposite to a CB1 receptor agonist (Pertwee & Fernando, 1996) whilst in vivo, SR 141716A causes hyperalgesia in a rodent model of thermal pain (Richardson et al., 1997). In cell lines expressing human recombinant CB1 receptors, SR 141716A reduced basal incorporation of [35S]-GTPγS (Landsman et al., 1997; MacLennan et al., 1998) whereas cannabinol had no effect, ruling out the possibility of SR 141716A blocking the effect of an endogenous agonist (MacLennan et al., 1998). This raises the possibility that SR 141716A (and indeed the base form SR 141716) is an inverse agonist. However, the physiological relevance of these results remains to be determined.
Previous studies show that 10 μM fluoxetine added to microdialysis perfusion fluid causes substantial 5-HT reuptake inhibition (Matsumoto et al., 1995; Pullar & Findlay, 1992). Based on these studies, a concentration of 10 μM fluoxetine was added to the perfusion fluid 40 min after Δ9-THC or vehicle injection, and remained in the perfusion fluid until the end of the experiment. Fluoxetine 10 μM perfused into the striatum via the microdialysis probe facilitated DA release (Figure 4). This effect of increasing basal DA levels has been reported previously with alaproclate perfusion, another SSRI (Yadid et al., 1994). An increase in extracellular 5-HT in the striatum resulting in an increase in extracellular DA is in agreement with a study that showed that direct perfusion of 5-HT into the striatum dose-dependently facilitates DA release (Benloucif & Galloway, 1991). Also, it has been reported that an increase in local serotoninergic activity results in an increase in DA release (Jacocks & Cox, 1992; Yadid et al., 1994) whereas a decrease in serotoninergic activity can reduce striatal DA release (Blandina et al., 1989; Carboni et al., 1989). As well as fluoxetine increasing extracellular 5-HT acutely by blocking 5-HT reuptake (Perry & Fuller, 1992), there is also evidence to suggest that selective 5-HT reuptake inhibitors may decrease 5-HT release (Adell & Artigas, 1991; Gartside et al., 1995; Invernizzi et al., 1997; Rutter & Auerbach, 1993). This is thought to occur following the increased extracellular 5-HT (produced by reuptake inhibition) stimulating autoreceptors on cell bodies and dendrites in the DRN to inhibit firing of 5-HT neurones, and stimulating terminal autoreceptors to inhibit neuronal release of 5-HT (Adell & Artigas, 1991; Invernizzi et al., 1997; Rutter & Auerbach, 1993). The DA release reported by Yadid et al. (1994) with alaproclate was only apparent in the first hour after the perfusion commenced. Similarly, in the present study, the DA release induced by fluoxetine was only significant in the first hour. It is therefore possible that the increased DA levels produced by these 5-HT reuptake inhibitors were not sustained because of the elevated 5-HT activating inhibitory autoreceptors on the 5-HT nerve terminal and hence decreasing 5-HT release.
The increase in extracellular DA observed following local perfusion of fluoxetine was not observed following systemic fluoxetine (Figure 4). A possible explanation for this difference is that, when injected peripherally, fluoxetine would be expected to inhibit 5-HT reuptake in the striatum, as with local fluoxetine perfusion, but also at the 5-HT cell bodies in the DRN. Peripheral administration of 5-HT reuptake inhibitors has been shown to inhibit the firing of 5-HT neurones through activation of somatodendritic autoreceptors in the DRN in less than 5 min (Gartside et al., 1995). This effect could be long-lasting since the increase in extracellular 5-HT levels in the DRN following 5-HT reuptake inhibition has been reported to persist for hours (Adell & Artigas, 1991; Gartside et al., 1995). In contrast, when fluoxetine is administered 40 min before Δ9-THC, the Δ9-THC-induced DA release was abolished (Figure 4). As SSRIs can inhibit the firing of 5-HT neurones in minutes (Gartside et al., 1995), when Δ9-THC is administered 40 min later, the firing rate of raphe-striatal 5-HT neurones would be minimal. Hence, Δ9-THC-induced DA release may be dependent on a normal firing rate of serotoninergic neurones.
Although there is considerable evidence of the involvement of 5-HT and DA in the actions of Δ9-THC, the exact nature of how Δ9-THC produces its pharmacological effects remains to be elucidated. In the present study, it appears that increasing the amount of extracellular 5-HT in the striatum (as seen when fluoxetine was perfused into the striatum after Δ9-THC) potentiates Δ9-THC-induced DA release. Conversely, Δ9-THC-induced DA release is attenuated when the activity of raphe-striatal serotoninergic neurones is decreased (as seen with fluoxetine pretreatment). Previously, we have hypothesized that Δ9-THC increases extracellular 5-HT in the hypothalamus in order to produce hypothermia (Malone & Taylor, 1998b). It is possible that Δ9-THC also produces an increase in 5-HT, in the striatum, in order to increase extracellular DA.
In autoradiographic studies using [3H]-CP 55,940 as a radiolabel, the distribution of CB1 receptors in the brain has been shown to correlate well with some of the pharmacological effects of Δ9-THC (Herkenham et al., 1990). For example, the high density of CB1 receptors in the forebrain and the cerebellum correlate with the altered cognition and movement produced by cannabinoids (Dewey, 1986). Fewer receptors were found in the areas controlling cardiovascular and respiratory functions in the lower brainstem, which may explain why high doses of Δ9-THC, unlike other drugs of abuse such as morphine are not lethal (Dewey, 1986).
The existence of CB1 receptors in the striatum suggests an association with the same DA neurones activated by other drugs of abuse. However, when these neurones were lesioned with 6-OHDA, DA but not CB1 receptors were lost (Herkenham et al., 1991). This suggests that the CB1 receptors found in the striatum are not localized on DA neurones. The existence of cannabinoid receptors on presynaptic nerve endings has been suggested on noradrenergic neurones, as CB1 receptor mRNA was found to be present in sympathetic ganglion (Ishac et al., 1996). Hence, the possibility exists that CB1 receptors are located presynaptically on other types for neurones. Several reports have suggested that striatal CB1 receptors are located on opioid (Gardner & Lowinson, 1991) or GABA (Herkenham et al., 1991) neurones that are thought to modulate striatal DA release. In the present study, it was found that altering striatal 5-HT output could modulate the Δ9-THC-induced DA release. In light of this discovery, it may be hypothesized that CB1 receptors are located on striatal serotoninergic neurones and the stimulation of these receptors by Δ9-THC may modulate striatal 5-HT output in order to evoke DA release. In addition, CB1 receptors are quite dense in the SN and the DRN (Herkenham et al., 1990) and local application of 5-HT agonists and antagonists to the SN modulates the firing of DA neurones (Benloucif & Galloway, 1991; Sinton & Fallon, 1988). Hence, the SN and the DRN are other potential sites for Δ9-THC to act in order to increase striatal DA levels. Further studies are required to test the hypothesis that Δ9-THC increases extracellular 5-HT in order to increase striatal DA.
Conclusion
It is suggested from the results of the present study that Δ9-THC administration results in a dose-dependent increase in extracellular levels of DA which occurs via activation of CB1 receptors. Since an apparent increase in serotoninergic activity in the striatum resulted in an increase in DA release induced by Δ9-THC, whereas an apparent decrease in serotoninergic activity attenuated the Δ9-THC-induced DA release, it is hypothesized that Δ9-THC increases extracellular 5-HT in the striatum in order to increase DA release.
Acknowledgments
We thank the National Institute on Drug Abuse, U.S.A., Sanofi Recherche and Eli Lilly for the generous gifts of Δ9-THC, SR 141716 and fluoxetine respectively.
Abbreviations
- DA
dopamine
- FLU
fluoxetine
- 5-HT
5-hydroxytryptamine
- SR
SR 141716
- Δ9-THC
Δ9-tetrahydrocannabinol
References
- ADELL A., ARTIGAS F. Differential effects of clomipramine given locally or systemically on extracellular 5-hydroxytryptamine in raphe nuclei and frontal cortex. An in vivo brain microdialysis study. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:237–244. doi: 10.1007/BF00251121. [DOI] [PubMed] [Google Scholar]
- AGURELL S., HALLDIN M., LINDGREN J.E., OHLSSON A., WIDMAN M., GILLESPIE H., HOLLISTER L. Pharmacokinetics and metabolism of Δ1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol. Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- ANDERSON J.J., KASK A.M., CHASE T.N. Effects of cannabinoid receptor stimulation and blockade on catalepsy produced by dopamine receptor antagonists. Eur. J. Pharmacol. 1996;295:163–168. doi: 10.1016/0014-2999(95)00661-3. [DOI] [PubMed] [Google Scholar]
- BENLOUCIF S., GALLOWAY M.P. Facilitation of dopamine release in vivo by serotonin agonists: studies with microdialysis. Eur. J. Pharmacol. 1991;200:1–8. doi: 10.1016/0014-2999(91)90658-d. [DOI] [PubMed] [Google Scholar]
- BLANDINA P., GOLDFARB J., CRADDOCK ROYAL B., GREEN J.P. Release of endogenous dopamine by stimulation of 5-hydroxytryptamine3 receptors in rat striatum. J. Pharmacol. Exp. Ther. 1989;251:803–809. [PubMed] [Google Scholar]
- CARBONI E., ACQUAS E., FRAU R., DI CHIARA G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur. J. Pharmacol. 1989;164:515–519. doi: 10.1016/0014-2999(89)90259-8. [DOI] [PubMed] [Google Scholar]
- CHEN J.P., PAREDES W., LI J., SMITH D., LOWINSON J.H., GARDNER E.L. Δ9-tetrahydrocannabinol produces naloxone-blockade enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990a;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- CHEN J.P., PAREDES W., LOWINSON J.H., GARDNER E.L. Δ9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur. J. Pharmacol. 1990b;190:259–262. doi: 10.1016/0014-2999(90)94136-l. [DOI] [PubMed] [Google Scholar]
- DAVIES J., GRAHAM J. The mechanism of action of Δ9-tetrahydrocannabinol on body temperature in mice. Psychopharmacology. 1980;69:299–305. doi: 10.1007/BF00433100. [DOI] [PubMed] [Google Scholar]
- DEWEY W.L. Cannabinoid pharmacology. Pharmacol. Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- GARDNER E.L., LOWINSON J.H. Marijuana's interaction with brain reward systems: update 1991. Pharmacol. Biochem. Behav. 1991;40:571–580. doi: 10.1016/0091-3057(91)90365-9. [DOI] [PubMed] [Google Scholar]
- GARTSIDE S.E., UMBERS V., HAJOS M., SHARP T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br. J. Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRINSPOON L., BAKALAR J. Marihuana: The Forbidden Medicine. New Haven: Yale University Press; 1993. [Google Scholar]
- HERKENHAM M., LYNN A.B., DE COSTA B.R., RICHFIELD E.K. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- HERKENHAM M., LYNN A.B., LITTLE M.D., JOHNSON M.R., MELVIN L.S., DE COSTA B.R., RICE K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURD Y.L., UNGERSTEDT U. Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse. 1989;3:48–54. doi: 10.1002/syn.890030107. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., VELASCO C., BRAMANTE M., LONGO A., SAMANIN R. Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular serotonin in the frontal cortex, striatum and dorsal hippocampus. Neuropharmacology. 1997;36:467–473. doi: 10.1016/s0028-3908(97)00060-9. [DOI] [PubMed] [Google Scholar]
- ISHAC E.J., JIANG L., LAKE K.D., VARGA K., ABOOD M.E., KUNOS G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOCKS H.M.D., COX B.M. Serotonin-stimulated release of [3H]dopamine via reversal of the dopamine transporter in rat striatum and nucleus accumbens: a comparison with release elicited by potassium, N-methyl-D-aspartic acid, glutamic acid and D-amphetamine. J. Pharmacol. Exp. Ther. 1992;262:356–364. [PubMed] [Google Scholar]
- JOHNSON K., HO B., DEWEY W. Effects of Δ9-tetrahydrocannabinol on neurotransmitter accumulation and release mechanisms in rat forebrain synaptosomes. Life Sci. 1976;19:347–356. doi: 10.1016/0024-3205(76)90038-2. [DOI] [PubMed] [Google Scholar]
- LANDSMAN R.S., BURKEY T.H., CONSROE P., ROESKE W.R., YAMAMURA H.I. SR 141716A is an inverse agonist at the human cannabinoid CB1 receptors. Eur. J. Pharmacol. 1997;334:R1–2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- MACLENNAN S.J., REYNEN P.H., KWAN J., BONHAUS D.W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALONE D.T., TAYLOR D.A. 4th IUPHAR Satellite Meeting on Serotonin. Rotterdam; 1998a. Modulation by endogenous serotonin of striatal dopamine release following Δ9-tetrahydrocannabinol; p. 117. [Google Scholar]
- MALONE D.T., TAYLOR D.A. Modulation of Δ9-tetrahydrocannabinol-induced hypothermia by fluoxetine in the rat. Br. J. Pharmacol. 1998b;124:1419–1424. doi: 10.1038/sj.bjp.0701980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMOTO M., YOSHIOKA M., TOGASHI H., TOCHIHARA M., IKEDA T., SAITO H. Modulation of norepinephrine release by serotonergic receptors in the rat hippocampus as measured by in vivo microdialysis. J. Pharmacol. Exp. Ther. 1995;272:1044–1051. [PubMed] [Google Scholar]
- NAVARRO M., RODRIGUEZ DE FONSECA F., HERNANDEZ M.L., RAMOS J.A., FERNANDEZ RUIZ J.J. Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmacol. Biochem. Behav. 1994;47:47–58. doi: 10.1016/0091-3057(94)90110-4. [DOI] [PubMed] [Google Scholar]
- NEDERGAARD S., BOLAM J.P., GREENFIELD S.A. Facilitation of a dendritic calcium conductance by 5-hydroxytryptamine in the substantia nigra. Nature. 1988;333:174–177. doi: 10.1038/333174a0. [DOI] [PubMed] [Google Scholar]
- NG CHEONG TON J.M., GERARDT G.A., FRIEDMANN M., ETGEN A.M., ROSE G.M., SHARPLESS N.S., GARDNER E.L. The effects of Δ9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Co-ordinates. Sydney: Academic Press; 1986. [Google Scholar]
- PERRY K.W., FULLER R.W. Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci. 1992;50:1683–1690. doi: 10.1016/0024-3205(92)90423-m. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R. Evidence for the presence of cannabinoid CB1 receptors in mouse urinary bladder. Br. J. Pharmacol. 1996;118:2053–2058. doi: 10.1111/j.1476-5381.1996.tb15643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULLAR I.A., FINDLAY J.D. Effect of voltage-sensitive calcium channel antagonists on the release of 5-hydroxytryptamine from rat hippocampus in vivo. J. Neurochem. 1992;59:553–559. doi: 10.1111/j.1471-4159.1992.tb09405.x. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J.D., AANONSEN L., HARGREAVES K.M. SR 141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated mice. Eur. J. Pharmacol. 1997;319:R3–4. doi: 10.1016/s0014-2999(96)00952-1. [DOI] [PubMed] [Google Scholar]
- RUTTER J.J., AUERBACH S.B. Acute uptake inhibition increases extracellular serotonin in the rat forebrain. J. Pharmacol. Exp. Ther. 1993;265:1319–1324. [PubMed] [Google Scholar]
- SAKURAI YAMASHITA Y., KATAOKA Y., FUJIWARA M., MINE K., UEKI S. Δ9-tetrahydrocannabinol facilitates striatal dopaminergic transmission. Pharmacol. Biochem. Behav. 1989;33:397–400. doi: 10.1016/0091-3057(89)90521-2. [DOI] [PubMed] [Google Scholar]
- SCHMIDT C.J., FADAYEL G.M., SULLIVAN C.K., TAYLOR V.L. 5-HT2 receptors exert a state-dependent regulation of dopaminergic function: studies with MDL 100,907 and the amphetamine analogue, 3,4-methylenedioxymethamphetamine. Eur. J. Pharmacol. 1992;223:65–74. doi: 10.1016/0014-2999(92)90819-p. [DOI] [PubMed] [Google Scholar]
- SINTON C.M., FALLON S.L. Electrophysiological evidence for a functional differentiation between subtypes of the 5-HT1 receptor. Eur. J. Pharmacol. 1988;157:173–181. doi: 10.1016/0014-2999(88)90380-9. [DOI] [PubMed] [Google Scholar]
- SOFIA R., DIXIT B., BARRY H., III The effect of Δ1-tetrahydrocannabinol on serotonin metabolism in the rat brain. Life Sci. 1971;10:425–436. doi: 10.1016/0024-3205(71)90107-x. [DOI] [PubMed] [Google Scholar]
- TANDA G., PONTIERI F.E., DI CHIARA G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- TAYLOR D.A., FENNESSY M.R. ‘Antagonist'-precipitated withdrawal in the rat after chronic Δ9-tetrahydrocannabinol treatment. J. Pharm. Pharmacol. 1978;30:654–656. doi: 10.1111/j.2042-7158.1978.tb13353.x. [DOI] [PubMed] [Google Scholar]
- VERBERNE A.J.M., TAYLOR D.A., FENNESSY M.R. Withdrawal-like behaviour induced by inhibitors of biogenic amine reuptake in rats treated chronically with Δ9-tetrahydrocannabinol. Psychopharmacology. 1980;68:261–267. doi: 10.1007/BF00428113. [DOI] [PubMed] [Google Scholar]
- YADID G., PACAK K., KOPIN I.J., GOLDSTEIN D.S. Endogenous serotonin stimulates striatal dopamine release in conscious rats. J. Pharmacol. Exp. Ther. 1994;270:1158–1165. [PubMed] [Google Scholar]


