Abstract
The expression of hepatocyte growth factor (HGF) is essential for normal placental development although its function is unknown. In this study we examined the effect of HGF on trophoblast cell motility and invasion of fibrin gels and investigated the possible role of nitric oxide (NO) in this process.
The human extravillous trophoblast cell line SGHPL-4 express both the constitutive and inducible isoforms of nitric oxide synthase (NOS).
HGF significantly stimulates cell motility in monolayer culture, the invasion of fibrin gels and the production of guanosine 3′:5′-cyclic monophosphate (cyclic GMP).
Invasion, motility and cyclic GMP production were inhibited by Ng-monomethyl-L-arginine (L-NMMA).
Cell motility was also significantly inhibited by the inducible NOS specific inhibitor 1400 W.
Neither 8 Br-cyclic GMP nor the NO donor spermine-NO had any significant effect on basal trophoblast cell motility.
The data presented in this study demonstrate a direct effect of trophoblast-derived NO synthesis on trophoblast cell function and support the idea that HGF is involved in the regulation of trophoblast invasion through mechanisms that involve the production of NO. However neither exogenous NO nor activation of cyclic GMP-dependent pathways alone are sufficient to stimulate trophoblast cell motility.
Keywords: Trophoblast, hepatocyte growth factor, nitric oxide, motility, invasion, placenta, pre-eclampsia, inducible nitric oxide synthase, constitutive nitric oxide synthase, asymmetric dimethylarginine
Introduction
There is considerable interest in the role of nitric oxide (NO) in pregnancy. Increased NO synthesis may be responsible for the fall in blood pressure observed in early pregnancy and is suggested to regulate blood flow and inhibit leukocyte adhesion in the developing placenta (Morris et al., 1996; Poston, 1997; Zarlingo et al., 1997).
Nitric oxide is formed from L-arginine by the enzyme nitric oxide synthase (NOS). This enzyme exists as three isoforms, two are constitutively expressed and respond to agonist stimulation by changes in activity, whereas the third isoform is induced in response to inflammatory cytokines. NO so produced is a potent inter- and intracellular messenger with a number of functions including the regulation of vascular tone, cellular aggregation, adhesion and angiogenesis (reviewed by Moncada, 1997). Many of these functions are important in establishing and maintaining a normal pregnancy.
The placenta is a rapidly growing, highly differentiated organ, consisting of many different cell types. During human placental development the cytotrophoblast stem cells either fuse to form multinucleate syncytial trophoblasts, or aggregate to form the cellular columns of anchoring villi. These villi subsequently give rise to a population of cells with an invasive phenotype, the extravillous trophoblasts. A sub-population of these extravillous trophoblasts invades the intradecidual segment of the uterine spiral arteries, destroying the elastic and muscular tissue of the vessel wall and replacing the endothelial cells. This creates a high flow, low resistance zone within the placental bed where maximal exchange of nutrients and waste products between the foetal and maternal circulation can occur (Robertson et al., 1973). Normal trophoblastic invasion requires stringent spatial and temporal regulation of processes such as adhesion to and degradation of components of the extracellular matrix, cellular proliferation and migration (Damsky et al., 1992; Zhou et al., 1997a). Although the mechanisms involved in the regulation of these processes are poorly understood, inadequate invasion has been implicated in several complications of pregnancy including miscarriage, pre-eclampsia and intrauterine growth restriction while excessive invasion is characteristic of choriocarcinoma (Lim et al., 1997; Zhou et al., 1993; 1997b).
Two isoforms of NOS have been identified enzymatically in the human placenta, the constitutively expressed endothelial isoform (cNOS) and the inducible isoform (iNOS; Morris et al., 1993) and immunocytochemical studies have localized them to trophoblasts (Buttery et al., 1994; Myatt et al., 1997). However little is known about the regulation of NO synthesis, or its possible function within these cells.
Nitric oxide synthase is competitively inhibited by guanidino-substituted arginine analogues including Ng, Ng-dimethylarginine (asymmetric dimethylarginine; ADMA) and N9-monomethyl-L-arginine (L-NMMA). Inhibition of NO synthesis in pregnant rats predictably results in increased blood pressure, however a reduction in trophoblast invasion is also observed which would suggest a specific NO-mediated effect on this process (Yallampalli & Garfield, 1993). In pre-eclampsia, a condition characterized by raised blood pressure and poor trophoblastic invasion, abnormal NO synthesis has been reported (Morris et al., 1996). This is supported by our data demonstrating that the concentration of ADMA in pregnancies complicated by pre-eclampsia was significantly elevated compared to normotensive controls (Fickling et al., 1993; Holden et al., 1998).
Hepatocyte growth factor (HGF) has a number of distinct biological functions. It is a potent mitogen, stimulates the dissociation and motility of epithelial cells and induces the formation of tube-like structures in vitro (Jiang & Hiscox, 1997; Tamagnone & Comoglio, 1997). HGF is present at high concentrations in the placenta (Wolf et al., 1991). Although its function has yet to be determined, disruption of the genes encoding HGF or its receptor, c-met, results in small placentae with poor trophoblastic development and is ultimately lethal (Uehara et al., 1995; Bladt et al., 1995). Evidence would also indicate that HGF stimulates NO production (Noiri et al., 1996).
It is therefore our hypothesis that HGF may play a role in trophoblast cell invasion and that NO may be one factor that regulates this process. However any study of primary human trophoblasts in culture is hampered by phenotypic instability in culture, paucity of material and contamination with other cell types. To overcome some of these problems we have developed a series of human trophoblast cell lines that have an extended lifespan and have retained many trophoblast-like functions detailed in this study and elsewhere (Choy & Manyonda, 1998). Using these cell lines we have investigated the effect of HGF on NO synthesis and examined the role of NO in the regulation of trophoblast cell motility and invasion.
Methods
Cytokines and antibodies
HGF and interleukin-1β (IL-1β, specific activity: 1.87×108 units mg−1) were obtained from R&D, U.K. and tumour necrosis factor α (TNFα, specific activity: >2×107 units mg−1) was obtained from Serotec, U.K. The antibodies used for flow cytometry were from the following companies: mouse anti-human integrin α3, α5 and β1 and rabbit anti-human integrin αVβ3 (Life Technologies, U.K.); mouse anti-human VLA-4 (R&D, U.K.); mouse anti-human α1, rat anti-human VLA-6 and mouse anti-human HLA-G (w6/32, Serotec, U.K.).
Cell culture
SGHPL-4 cells, derived from primary extravillous trophoblasts, were cultured in Hams F10 containing 2 mM L-glutamine, 0.12% (wv−1) sodium bicarbonate, 16 mg ml−1 gentamicin and supplemented with 10% (vv−1) foetal calf serum (FCS). SGHPL-4 cells were formerly known as MC4 (Choy & Manyonda, 1998).
Flowcytometry
SGHPL-4 cells were grown to confluence on 9 cm petri dishes before being detached by treatment with 5 mM EDTA, 5 mM EGTA (pH 7.6) in Ca2+/Mg2+ free phosphate buffered saline (PBS). The cells were then washed three times with PBS containing 0.5% (wv−1) bovine serum albumin (BSA) and 0.1% (wv−1) sodium azide. Aliquots of cells were incubated with the appropriate primary antibody at the following dilutions: anti-α1, 1 : 20, anti-α3, 1 : 50, anti-α5, 1 : 50; anti-β1, 1 : 50; anti-αVβ3, 1 : 50; anti-VLA-4, 1 : 200, anti-VLA-6, 1 : 50; anti-HLA-G, 1 : 50 or mouse isotype or rabbit Ig controls as appropriate at room temperature for 30 min. Following three washes with PBS/BSA/azide the cells were incubated with fluorescein-labelled goat anti-mouse (Sigma, 1 : 200 dilution), anti-rabbit (Sigma, 1 : 100 dilution) or anti-rat (Biogenesis, 1 :50 dilution) for 30 min at room temperature in the dark. After a further three washes, cells were resuspended and fixed in 2% (wv−1) paraformaldehyde in PBS. Expression of cell surface antigens was analysed on a FACScan flow cytofluorimeter (Becton Dickinson).
Immunocytochemistry
SGHPL-4 cells (2×103 cells per well) were cultured for 24 h on chamber slides (Nalge Nunc) previously coated with human fibronectin (Sigma). Medium was replaced with Hams F10 containing 0.5% (vv−1) FCS for a further 24 h. The cells were then incubated in the presence or absence of HGF (10 ng ml−1) for 24 h. The cells were washed with PBS, fixed with cold acetone for 2 min and air dried. Following three further washes with PBS the cells were incubated with 10% goat serum in PBS for 20 min to suppress non-specific binding of IgG. After a further wash with PBS cells were incubated with rabbit anti-iNOS (C-19, Santa Cruz) or anti-cNOS (C-20, Santa Cruz) or negative control of normal rabbit immunoglobulin (DAKO, X0903) at 2 μg ml−1 in PBS with 1.5% goat serum for 1 h. The slides were washed three times with PBS and incubated with biotin-conjugated goat anti-rabbit IgG (Vector Laboratories) at 5 μg ml−1 for 45 min. Following a further three washes with PBS the slides were incubated with streptavidin-fluorescein (Vector Laboratories) at 15 μg ml−1 for 15 min in a dark chamber. After extensive washing the slides were mounted using Vectashield mounting medium and examined using a fluorescence microscope.
Total RNA isolation and analysis
Total RNA was isolated from confluent 9 cm plates of SGHPL-4 cells (approximately 2.5×106 cells) using RNAzol B (Biogenesis Ltd., U.K.) according to the manufacturer's instructions. Each well was loaded with 10 μg of RNA. Samples were then subjected to electrophoresis through 1.5% (wv−1) agarose-formaldehyde gels and transferred to a Hybond-N+ membrane (Amersham Life Sciences, U.K.) according to the manufacturer's instructions. Transfer was by diffusion overnight with 20× SSPE as buffer (3.6 M sodium chloride, 0.2 M sodium phosphate (pH 7.7) and 0.02 M EDTA). The RNA was fixed to the membrane by placing it on a pad of absorbent filter paper soaked in 0.05 M sodium hydroxide. The cDNA probe for iNOS was labelled with α-32P-dCTP (3000 Ci mmol−1) using a multiprime DNA labelling system (Pharmacia) according to the manufacturer's instructions. Prehybridization solution was prepared as follows: 5×SSPE, 50% (vv−1) deionized formamide, 5×Denhardt's solution (0.1% (wv−1) Ficoll, 0.1% (wv−1) polyvinylpyrrolidone, 0.1% (wv−1) BSA), 0.1% (wv−1) SDS supplemented with 20 μg ml−1 denatured herring sperm DNA and incubated with the membranes for 1 h at 42°C. For hybridization, membranes were incubated for 12 h at 42°C in the above buffer with the additional of denatured 32P-labelled iNOS probe. Hybridization solution was removed and the filters were washed once with 50 ml of 2× SSPE 0.1% (wv−1) SDS for 15 min at room temperature, twice with 50 ml of 2×SSPE 0.1% (wv−1) SDS for 30 min at 65°C and once with 50 ml of 1×SSPE 0.1% (wv−1) SDS for 30 min at 65°C. The membranes were autoradiographed with an intensifying screen at −70°C. Re-probing of the blot for a housekeeping gene was felt inappropriate as evidence indicates that expression of these genes is altered by NO (Bereta & Bereta, 1995). Equal loading was confirmed by densitometry of ribosomal RNA.
Determination of cyclic GMP production by SGHPL-4 cells
SGHPL-4 cells (5×105 cells ml−1, 500 μl per well, 24 well plate) were cultured for 24 h in medium. The cell monolayers were washed once with PBS and the medium replaced with 250 μl per well of Hams-F10 containing 0.5% (wv−1) BSA, 0.3 mM isobutyl-methylxanthine and an appropriate dose of recombinant HGF (0–100 ng ml−1) and incubated for 6 h at 37°C in a humidified atmosphere of 5% CO2. Cyclic GMP was determined in the incubation medium by radioimmunoassay using the method previously reported (Millatt et al., 1993).
Measurement of dimethylarginines
SGHPL-4 cells (1×106 cells ml−1, 10 ml per 9 cm culture dish) were cultured overnight. The medium was removed and the cell monolayer washed once with PBS. Medium (5 ml) containing HGF (0–100 ng ml−1) was added to the cells and they were incubated for a further 24 h. Quantitation of dimethylarginines was achieved by HPLC (Holden et al., 1998). In brief, dimethylarginines were partially purified by extraction with Bond Elut SCX columns (Jones Chromatography Ltd., U.K.) and elution with ammonia/methanol. Separation of ADMA and SDMA was achieved with an ODS C18 analytical HPLC column (Phase Separation, U.K.) on a Beckman System Gold apparatus using an ion-pair based mobile phase (25 mM phosphoric acid containing 10 mM hexane sulphonic acid and 1% (vv−1) acetonitrile, pH 5.0). Flow was maintained at 1 ml min−1 and absorbance monitored at 200 nm. Concentrations of ADMA in the samples were determined by comparison with synthetic standards (ADMA hydrochloride, Sigma). Extraction efficiency was determined by the addition of 10 μg Ng-monomethyl-L-arginine (L-NMMA, Sigma, U.K.) to each sample prior to extraction.
Proliferation of SGHPL-4 cells
SGHPL-4 cells (2×104 cells ml−1, 500 μl per well, 24 well plates) were allowed to adhere for 6 h and the medium was then replaced with Hams F10 containing 0.5% (vv−1) FCS in the presence or absence of HGF at the stated concentrations. Cells were incubated for a further 24, 48 or 72 h, washed with PBS and the cell number determined using a haemocytometer following erythrosin B staining.
Cell motility
SGHPL-4 cells (2–13×103 cells per 35 mm plate) were allowed to adhere overnight in Hams F10 containing 10% (vv−1) FCS and the medium was then replaced with Hams F10 containing 0.5% (vv−1) FCS for a further 24 h. At the end of this period cell motility was determined in response to the stated concentration of HGF and/or NOS inhibitors L-NMMA or 1400 W (Garvey et al., 1997; Alexis, U.K.) at 37°C in an atmosphere of 5% CO2 in air. Images were captured every 15 min over a 20 h period using a JVC TK-C1360E CCD camera connected to an Olympus IX 50 phase contrast microscope driven by a SNP-8 PCI-BIB image acquisition board. At least ten cells in a field of view were chosen at random and the distance moved was quantified using Image Pro-Plus software (Media Cybernetics).
Invasion of fibrin gels
Gelatin coated cytodex-3 microcarrier beads (MCs, Sigma) were prepared according to the manufacturer's recommendations. Cells were removed from the culture vessel using trypsin/EDTA, washed once with PBS and resuspended at a density of 5×105 cells ml−1 with 1500 MC ml−1. Following incubation at 37°C for 1 h the cells were diluted to 30 ml with HEPES buffered DMEM containing 10% (vv−1) FCS and incubated on a roller at 37°C to prevent aggregation. After 48 h the medium was replaced with HEPES buffered DMEM containing 0.5% (vv−1) FCS and incubated on the roller for a further 24 h. Fibrin gels were prepared as detailed (Montesano et al., 1987). In brief, bovine fibrinogen (>95% of protein clottable, Sigma) was dissolved in PBS at a concentration of 2.5 mg ml−1 and 200 U ml−1 aprotinin (Trasylol, Bayer, Germany) added in a 35 mm plate. Cell-coated microcarrier beads were added at a density of 150 MCs ml−1 and clotting was induced by the addition of thrombin (0.625 U ml−1). After clotting the gels were equilibrated with culture medium supplemented with 0.5% (vv−1) FCS for 1 h. The medium was replaced with fresh medium (1 : 1 volume with the gel) and the cells incubated at 37°C in a humidified atmosphere of 5% CO2. After 24 h, the medium was replaced with medium containing the stimulus and/or the inhibitor as indicated. After 3 days at least ten MCs from each plate were chosen at random and invasion was observed using an Olympus IX 50 phase contrast microscope. Images were captured as above and the number and length of the processes formed were determined using Image Pro-Plus software (Media Cybernetics). Invasion was determined as any process greater than the average radius of a MC.
Statistics
Results were expressed as mean±standard error of the mean (s.e.mean) and were analysed using the non-parametric Mann-Whitney U test. Statistical significance was assumed at the 0.05 level. Experimenters blind to the treatment used carried out all analyses of trophoblast cell motility and invasion.
Results
Expression of integrins and extravillous trophoblast cell markers
The SV40 transfected human trophoblast cell line SGHPL-4 has retained a number of trophoblast-like characteristics including expression of HLA-G and integrins such as α3, αVβ3, β1, VLA-4 and VLA-6 (Figure 1). The cells were also weakly positive for α1 and α5 (data not shown).
Figure 1.
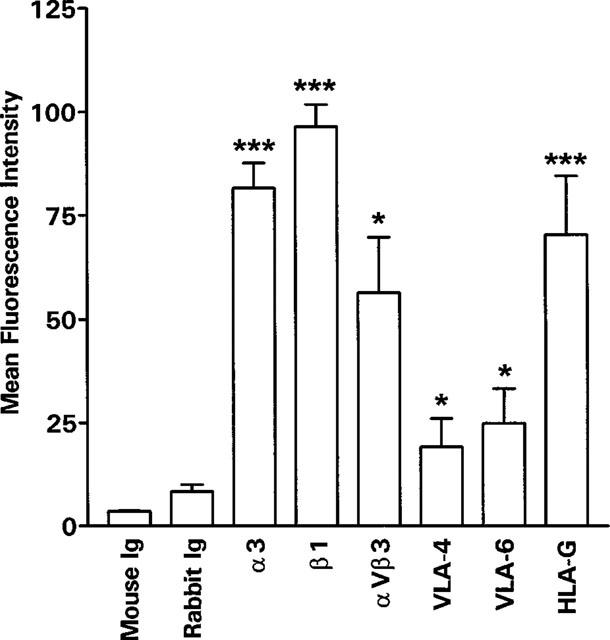
Flow cytofluorimetic analysis of integrin expression on SGHPL-4 cells. SGHPL-4 cells were removed from the plates and incubated with the indicated primary antibody. Binding of the antibody was analysed by flow cytometry after reaction with fluorescein-conjugated secondary antibody. Results shown are mean fluorescence intensity +s.e.mean for three experiments, each in duplicate. *P<0.05, ***P<0.0001 compared to mouse or rabbit Ig.
Expression of NOS mRNA and protein
Under basal conditions low levels of iNOS (Figure 2) and endothelial cNOS mRNA (data not shown) were detected in SGHPL-4 cells. In response to stimulation with HGF or a combination of IL-1β and TNFα for 6 h there was an increase in the expression of iNOS (Figure 2). Immunocytochemical analysis confirmed the subsequent expression of both isoforms of NOS (Figure 3). The cellular distribution of both cNOS and iNOS largely followed the reported patterns of expression for these two proteins, however preliminary analysis using confocal microscopy also indicated iNOS immunoreactive staining within the nucleus (data not shown). Controls using an irrelevant primary antibody at the appropriate protein concentration for each antibody were negative.
Figure 2.
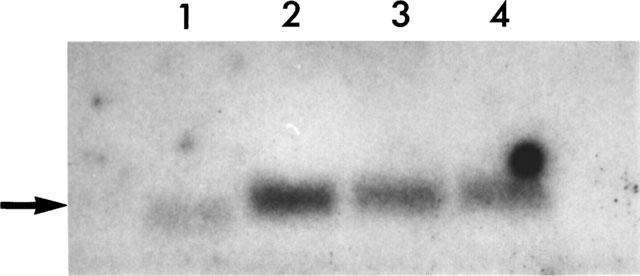
Northern blot analysis of SGHPL-4 cells. RNA was isolated from SGHPL-4 cells following a 6 h incubation in the absence (lane 1) or presence of IL-1β and TNFα (each at 5 ng ml−1; lane 2) or HGF (10 ng ml−1; lane 3; 100 ng ml−1; lane 4). After separation by electrophoresis through a 1.5% agarose gel the RNA was transferred to Hybond N+ membrane and a 3.6 kb band (shown by arrow) detected following hybridization with a 32P-labelled probe for human iNOS.
Figure 3.
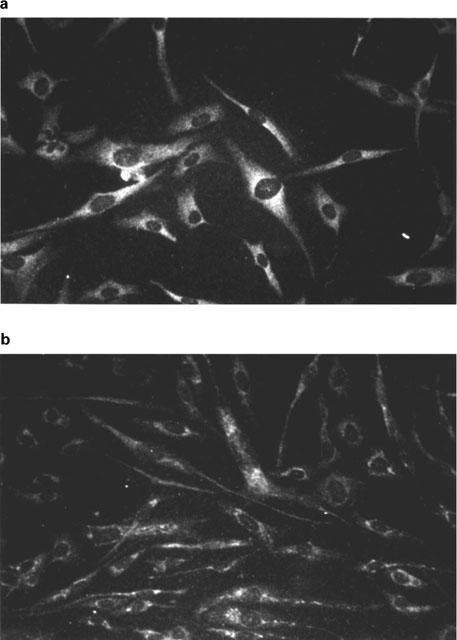
Immunocytochemical analysis of iNOS and cNOS expression by SGHPL-4 cells. SGHPL-4 cells (2×103 cells per well) were cultured on fibronectin-coated chamber slides for 24 h. Medium was replaced with Hams F10 containing 0.5% (vv−1) FCS for 24 h then the cells were incubated in the presence of HGF (10 ng ml−1) for a further 24 h. The cells were incubated with antibodies to iNOS (a) or cNOS (b). Binding of the antibody was analysed by fluoresence microscopy following reaction with biotin-conjugated secondary antibody and streptavidin-fluorescein.
Synthesis of cyclic GMP
Many of the actions of NO are mediated through the production of cyclic GMP via the activation of a soluble isoform of guanylate cyclase. In some cell types the NO produced can act in an autocrine manner on this cyclase. Stimulation of trophoblasts with 1 ng ml−1 HGF resulted in greater than 2 fold induction of cyclic GMP synthesis. Stimulation with 10 and 100 ng ml−1 HGF had no additional effect on the cyclic GMP production. The production of cyclic GMP was inhibited by incubation of the cells with L-NMMA at all the doses of HGF tested (Figure 4).
Figure 4.
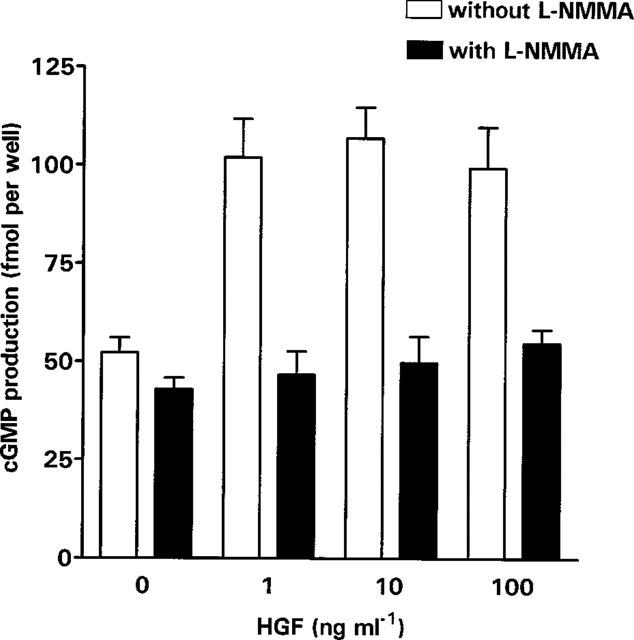
Cyclic GMP production by SGHPL-4 cells. SGHPL-4 cells were seeded at a density of 5×105 cells ml−1, 500 μl per well in 24 well plates. After 24 h the medium was removed and replaced with medium containing 0.3 mM isobutyl-methylxanthine and an appropriate dose of HGF (0–100 ng ml−1) plus or minus 3 mM L-NMMA for 6 h at 37°C in a humidified atmosphere of 5% CO2. Cyclic-GMP production was determined and the results presented are the mean+s.e.mean of three pooled experiments, each carried out in quadruplicate.
Synthesis of dimethylarginine
There was an accumulation of ADMA in the medium of SGHPL-4 cells over a 6 day period (Figure 5a). After the first 24 h the concentration of ADMA was 0.2 μg ml−1 mg−1 of protein and the concentration of SDMA, the physiologically inactive isomer of ADMA, was 0.04 μg ml−1 mg−1 of protein. After stimulation of SGHPL-4 cells with 1 ng ml−1 HGF for 24 h there was a statistically significant fall in the accumulation of ADMA (Figure 5b, P=0.029). However the accumulation of SDMA was unaffected by this treatment. The concentration of ADMA in the medium of cells stimulated with 10 and 100 ng ml−1 HGF was not significantly different from controls.
Figure 5.
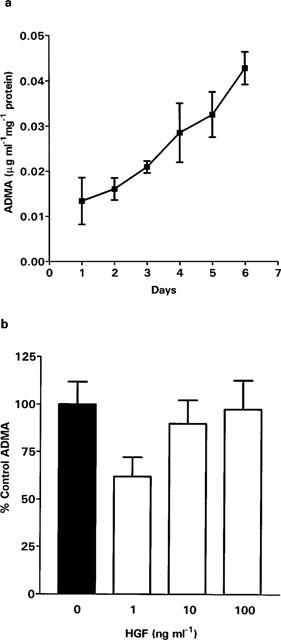
Production of dimethylarginine by SGHPL-4 cells. SGHPL-4 cells (106 cells ml−1, 10 ml per 9 cm culture dish) were cultured overnight. The medium was removed and replaced with medium (5 ml) containing HGF (0–100 ng ml−1) and the cells incubated for a further 24 h or the stated number of days. Quantitation of dimethylarginines was determined by HPLC analysis by comparison with synthetic standards. (a) represents ADMA accumulation over 6 days. The results are mean±s.e.mean of three pooled experiments. (b) represents the effect of HGF on ADMA accumulation over 24 h. The results presented are the mean +s.e.mean of three pooled experiments, each carried out in triplicate.
Cell motility
HGF stimulated the motility of SGHPL-4 cells in culture. Experiments carried out over the dose range of 1 to 100 ng ml−1 HGF, showed a maximal response at 10 ng ml−1. This dose was therefore chosen to examine the effect of inhibiting NO synthesis using two inhibitors of NOS, the non-isoform specific competitive inhibitor L-NMMA and the iNOS specific inhibitor 1400 W. Following the simultaneous addition of 10 ng ml−1 HGF and either 3 mM L-NMMA or 10 μM 1400 W, cell motility was monitored for 20 h. Motility was analysed between 6 and 15 h. There was a significant reduction in trophoblast cell motility with both inhibitors compared to HGF alone (Figure 6a, L-NMMA P<0.0001; Figure 6b, 1400 W P=0.0156). There was no significant difference between control cells and cells incubated with 10 ng ml−1 HGF and L-NMMA or 1400 W. Neither L-NMMA nor 1400 W had any effect on basal motility. The controlled release of NO into the medium of unstimulated cells by the addition of the NO donor spermine-NO in the range 10 nM to 100 μM had no significant effect on the motility of SGHPL-4 cells (control, mean distance moved ±s.e.mean, 54±4, n=91; 10 nM spermine-NO, 53±3, n=95; 10 μM spermine-NO, 51±5, n=20; 100 μM spermine-NO, 61±6, n=37). The addition of 1 mM 8-Bromo-cyclic GMP, the membrane permeable hydrolysis-resistant analogue of cyclic GMP, also had no significant stimulatory effect on motility (control, mean distance moved±s.e.mean, 54±4, n=91; 8-Bromo-cyclic GMP, 51±3, n=120).
Figure 6.
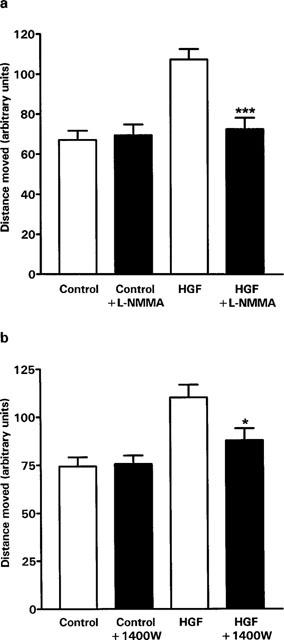
Effect of HGF and NOS inhibitors on SGHPL-4 cell motility. SGHPL-4 cells (2–13×103 cells per 35 mm plate) were allowed to adhere overnight in Hams F10 containing 10% (vv−1) FCS. The medium was replaced with Hams F10 containing 0.5% (vv−1) FCS for a further 24 h. At the end of this period, cell motility was determined in response to HGF (10 ng ml−1) and/or 3 mM L-NMMA or 10 μM 1400 W in Hams F10 containing 0.5% (vv−1) FCS at 37°C in an atmosphere of 5% CO2 in air. A time lapse sequence of images were captured over 20 h. Cells in a field of view were chosen at random and the distance moved was quantified between 6 and 15 h. The results presented are the mean+s.e.mean of at least three experiments, each consisting of the analysis of at least ten cells. *P=0.0156, **P<0.0001, significantly different from cells treated with HGF alone. (a) shows the effect of L-NMMA on motility. (b) shows the effect of 1400 W on motility.
Cell proliferation
In control cultures the cell number increased over 72 h. HGF did not increase the growth of SGHPL-4 cells (Figure 7). There was a trend towards inhibition of growth at 72 h.
Figure 7.
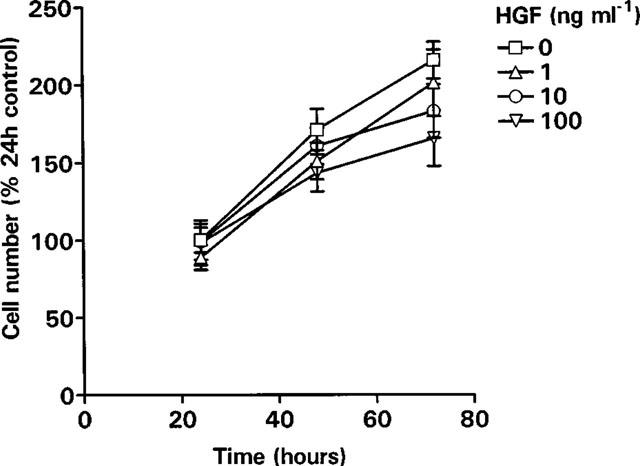
Effect of HGF on cell proliferation. SGHPL-4 cells were seeded at 2×104 cells ml−1, 500 μl per well in 24 well plates. Cells were allowed to adhere for 6 h and then the medium was replaced with Hams F10 (0.5% FCS) in the presence or absence of HGF (0–100 ng ml−1). Following incubation for 24, 48 or 72 h, the cells were washed with PBS, trypsinized, stained with erythrosin B and counted using a haemocytometer. The results presented are the mean±s.e.mean of three pooled experiments, each carried out in triplicate.
Invasion of fibrin gels
Trophoblast cells grown on gelatin coated microcarrier beads and embedded in fibrin gels invade the matrix by producing finger-like processes. These began to appear after approximately 24 h in culture and were present in both the control and HGF stimulated cultures (Figure 8). After 3 days in culture there was a significant increase (P<0.006) in the number of processes per MC bead in cultures stimulated with 10 ng ml−1 HGF compared with control cultures (Figure 9a). There was also a small (27%) but highly significant (P<0.0001), increase in the length of the processes following treatment with HGF (Figure 9b). In the presence of the NOS inhibitor L-NMMA both the number of processes and the length of the processes formed in the presence of HGF were significantly reduced. However L-NMMA did not have any significant effect on formation or length of these processes in the absence of HGF.
Figure 8.
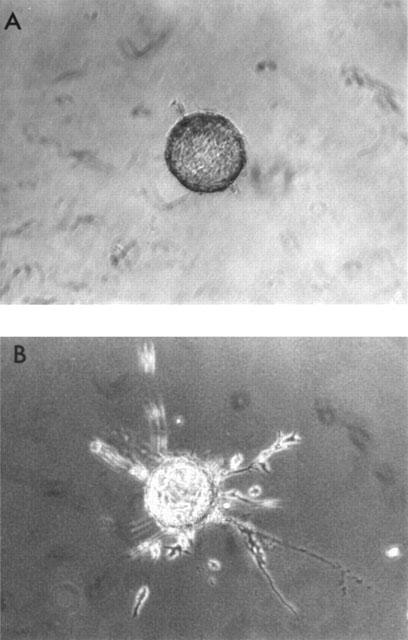
Invasion of fibrin gels by SGHPL-4 cells. Gelatin coated cytodex-3 microcarrier beads (MCs) were suspended with 5×105 cells ml−1 at 37°C for 1 h. The cells were diluted with HEPES buffered DMEM containing 10% (vv−1) FCS and incubated on a roller at 37°C to prevent aggregation. After 48 h the medium was replaced with HEPES buffered DMEM containing 0.5% (vv−1) FCS and incubated on the roller for a further 24 h prior to embedding in fibrin gels. (a) is a representative SGHPL-4 cell coated MC before incubation with HGF. (b) is a representative SGHPL-4 cell coated MC incubated with 10 ng ml−1 HGF for 72 h.
Figure 9.
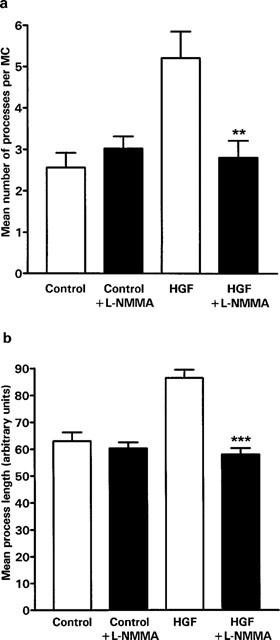
Effect of HGF and L-NMMA on SGHPL-4 cell invasion of fibrin gels. Gelatin coated cytodex-3 microcarrier beads (MCs) were suspended with 5×105 cells per ml at 37°C for 1 h. The cells were diluted with HEPES buffered DMEM containing 10% (vv−1) FCS and incubated on a roller at 37°C to prevent aggregation. After 48 h the medium was replaced with HEPES buffered DMEM containing 0.5% (vv−1) FCS and incubated on the roller for a further 24 h prior to embedding in fibrin gels. The gels were incubated in the presence or absence of HGF (10 ng ml−1 and L-NMMA (3 mM) as indicated for 72 h. Invasive processes longer than the mean bead radius were counted and measured following random selection of at least ten coated beads for each treatment. Experiments were performed in duplicate and results are presented as mean±s.e.mean. of three pooled experiments. **P=0.006, ***P<0.0001, significantly different from cells treated with HGF alone. (a) Shows the mean number of processes per MC, (b) shows the mean process length.
Discussion
In this study we demonstrate that the human trophoblast cell line SGHPL-4, in addition to maintaining characteristics of extravillous trophoblasts (Choy & Manyonda, 1998), express a number of integrins including α3, α5, αVβ3 and β1 associated with the invasive phenotype (Zhou et al., 1997a). We have used these cells as a model to study the regulation of trophoblast cell motility and invasion in vitro by HGF and to identify a possible role for NO in this process. In this study we present data that HGF stimulates trophoblast cell motility, invasion into fibrin gels and that NO produced by the inducible isoform of NOS is involved.
We have explored the NO pathway in SGHPL-4 cells and demonstrate by Northern blot and immunocytochemical analysis that these cells express both iNOS and cNOS (Figures 2 and 3). Stimulation of SGHPL-4 with HGF results in the apparent induction of expression of mRNA for iNOS. This was accompanied by the activation of guanylate cyclase and the production of cyclic GMP, which was used as a measure of NO synthesis. The NOS inhibitor L-NMMA inhibited the production of cyclic GMP by SGHPL-4 cells.
We have previously shown a fall in the release of ADMA in response to hormonal stimulation (Holden et al., 1997). Data from separate in vivo studies indicates a fall in the circulating concentration of HGF and a rise in ADMA in women suffering from pre-eclampsia (Furugori et al., 1997; Holden et al., 1998). We therefore examined whether HGF alters NO synthesis by reducing trophoblast ADMA. Although we could demonstrate a time-dependent increase in the release of ADMA by trophoblasts, this was unaffected by HGF.
Factors that stimulate iNOS expression and NO production in endothelial cells such as HGF, IL-1β and TNFα also stimulate cell motility (Bussolino et al., 1992; Rosen et al., 1993; Szekanecz et al., 1994; Radomski et al., 1993; Szabo, 1995). More recently, HGF-induced expression of iNOS in epithelial cells was reported to act as an essential switch for the initiation of cell motility (Noiri et al., 1996). Conversely transforming growth factor-β inhibits iNOS expression and cell motility in endothelial cells and vascular smooth muscle cells (Koyama et al., 1990; Merwin et al., 1991). Collectively these results suggest a role for NO synthesis in the regulation of cell motility. However, contrary to these findings, exogenously added NO donors inhibit endothelial and vascular smooth muscle cell motility (Dubey et al., 1995; Lau & Ma, 1996). Such conflict may suggest that the time of exposure, the concentration of NO to which the cells are exposed or cellular location of the exposure to NO may be important in defining the end result. This may also indicate that NO derived from iNOS (large amounts of NO) has different effects on cell motility than NO derived from cNOS (comparatively low concentration of NO). This may be particularly important in regard to trophoblasts, which express both iNOS and cNOS. In our experiments HGF at concentrations that stimulate NO production in trophoblasts also stimulated cell motility. Using the isoform specific inhibitor 1400 W we have demonstrated that HGF induced trophoblast cell motility is mediated by iNOS. The lack of effect of either L-NMMA or 1400 W to inhibit motility in control cells would suggest that NO is not responsible for basal cell motility. To investigate the roles of NO and the production of cyclic GMP further we examined the effect of the NO donor spermine-NO and the non-hydrolysable analogue of cyclic GMP, 8-Bromo cyclic GMP, on unstimulated trophoblast cell motility. The lack of effect observed in these experiments indicate that neither NO or the activation of G-kinase by cyclic GMP were sufficient to stimulate motility in these cells. The interaction of the NO-pathway with other pathways activated by stimulation with HGF warrant further examination.
Invasion of the uterine decidua by extravillous trophoblasts in vivo and the fibrin gels used above are likely to involve the co-ordinated regulation of a number of distinct processes including proliferation, cell motility and degradation of the extracellular matrix. In the experiments presented above we can exclude proliferation as a major contributing factor in the invasion of fibrin gels stimulated by HGF. Although the trophoblasts did proliferate under the conditions used (0.5% serum) over the period of the assay, this was not enhanced by HGF (Figure 7). We have shown that HGF stimulated NO production has a role to play in trophoblast invasion. However it is likely that extracellular matrix degradation by trophoblast derived metalloproteases is also important as both NO donors and inflammatory cytokines increase the activity of collagenase and stromelysin, two metalloproteases present in human and bovine articular cartilage (Murrell et al., 1995).
In conclusion our study indicates that HGF stimulates NOS mRNA expression, NO synthesis and trophoblast invasion of fibrin gels. HGF also increases trophoblast cell motility. The competitive inhibitor of NOS enzymatic activity, L-NMMA, significantly inhibited HGF stimulated invasion, motility and NO production by human trophoblasts. Using the iNOS specific inhibitor 1400 W we have demonstrated that HGF mediated motility is dependent on iNOS activity. However the elevation of NO and/or activation of G-kinase alone are insufficient to stimulate trophoblast cell motility. The data presented in this study demonstrates for the first time a direct effect of trophoblast derived NO synthesis on trophoblast cell function and supports our hypothesis that HGF is involved in the regulation of trophoblast invasion through mechanisms that involve NO.
Acknowledgments
This work was supported by the Wellcome Trust, the Tommy's Campaign and WellBeing.
Abbreviations
- ADMA
Ng Ng-dimethylarginine
- cyclic GMP
guanosine 3′:5′-cyclic monophosphate
- cNOS
constitutive nitric oxide synthase
- HGF
hepatocyte growth factor
- iNOS
inducible nitric oxidase synthase
- L-NMMA
Ng-monomethyl-L-arginine
- MC
microcarrier bead
- NO
nitric oxide
- NOS
nitric oxide synthase
References
- BERETA J., BERETA M. Stimulation of glyceraldehyde-3-phospate dehydrogenase mRNA levels by endogenous nitric oxide in cytokine-activated endothelium. Biochem. Biophys. Res. Commun. 1995;217:363–369. doi: 10.1006/bbrc.1995.2785. [DOI] [PubMed] [Google Scholar]
- BLADT F., RIETHMACHER D., ISENMANN S., AGUZZI A., BIRCHMEIER C. Essential role for c-met receptor in the migration of myogenic precursor cells into limb buds. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- BUSSOLINO F., DI RENZO M.F., ZICHE M., BOCCHIETTO E., OLIVERO M., NALDINI L., GAUDINO G., TAMAGNONE L., COFFER A., COMOGLIO P.M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell. Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTERY L.D., MCCARTHY A., SPRINGALL D.R., SULLIVAN M.H., ELDER M.G., MICHEL T., POLAK J.M. Endothelial nitric oxide synthase in the human placenta: regional distribution and proposed regulatory role at the feto-maternal interface. Placenta. 1994;15:257–265. doi: 10.1016/0143-4004(94)90017-5. [DOI] [PubMed] [Google Scholar]
- CHOY M.Y., MANYONDA I.T. The phagocytic activity of human first trimester extravillous trophoblasts. Human Repro. 1998;13:2941–2949. doi: 10.1093/humrep/13.10.2941. [DOI] [PubMed] [Google Scholar]
- DAMSKY C.H., FITZGERALD M.L., FISHER S.J. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway in vivo. J. Clin. Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBEY R.K., JACKSON E.K., LUSCHER T.F. Nitric oxide inhibits angiotensin-II induced migration of rat aortic smooth muscle cells. Role of cyclic nucleotides and angiotensin I receptors. J. Clin. Invest. 1995;96:141–149. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FICKLING S.A., WILLIAMS D., VALLANCE P., NUSSEY S.S., WHITLEY G.STJ. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet. 1993;342:242–243. doi: 10.1016/0140-6736(93)92335-q. [DOI] [PubMed] [Google Scholar]
- FURUGORI K., KURAUCHI O., ITAKURA A., KANOU Y., MURATA Y., MIZUTANI S., SEO H., TOMODA Y., NAKAMURA T. Levels of hepatocyte growth factor and its messenger ribonucleic acid in uncomplicated pregnancies and those complicated by preeclampsia. J. Clin. Endocrinol. Metabol. 1997;82:2726–2730. doi: 10.1210/jcem.82.8.4176. [DOI] [PubMed] [Google Scholar]
- GARVEY E.P., OPLINGER J.A., FURFINE E.S., KIFF R.J., LASZLO F., WHITTLE B.J., KNOWLES R.G. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric oxide synthase in vitro and in vivo. J. Biol. Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- HOLDEN D.P., FICKLING S.A., WHITLEY G.STJ., NUSSEY S.S. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and pre-eclampsia. Am. J. Obs. Gynae. 1998;178:551–556. doi: 10.1016/s0002-9378(98)70437-5. [DOI] [PubMed] [Google Scholar]
- HOLDEN D.P., NUSSEY S.S., WHITLEY G.STJ. A new mechanism by which oestrogen may enhance endothelial cell nitric oxide production. J. Endocrinol. 1997;155:P70. [Google Scholar]
- JIANG W.G., HISCOX S. Hepatocyte growth factor/scatter factor, a cytokine playing multiple and converse roles. Histol. Histopath. 1997;12:537–555. [PubMed] [Google Scholar]
- KOYAMA N., KOSHIKAWA T., MORISAKI N., SAITO Y., YOSHIDA S. Bifunctional effects of transforming growth factor-β on migration of cultured rat aortic smooth muscle cells. Biochem. Biophys. Res. Comm. 1990;169:725–729. doi: 10.1016/0006-291x(90)90391-y. [DOI] [PubMed] [Google Scholar]
- LAU Y.T., MA W.C. Nitric oxide inhibits migration of cultured endothelial cells. Biochem. Biophys. Res. Comm. 1996;221:670–674. doi: 10.1006/bbrc.1996.0654. [DOI] [PubMed] [Google Scholar]
- LIM K.H., ZHOU Y., JANATPOUR M., McMASTER M., BASS K., CHUN S.H., FISHER S.J. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 1997;151:1809–1818. [PMC free article] [PubMed] [Google Scholar]
- MERWIN J.R., NEWMAN W., BEALL L.D., TUCKER A., MADRI J. Vascular cells respond differentially to transforming growth factors β1 and β2 in vitro. Am. J. Path. 1991;138:37–51. [PMC free article] [PubMed] [Google Scholar]
- MILLATT L.J., JACKSON R., WILLIAMS B.C., WHITLEY G.STJ. Nitric oxide stimulated cGMP in human thyrocytes. J. Mol. Endocrinol. 1993;10:163–169. doi: 10.1677/jme.0.0100163. [DOI] [PubMed] [Google Scholar]
- MONCADA S. Nitric oxide in the vasculature: physiology and pathophysiology. Ann. N.Y. Acad. Sci. 1997;811:60–67. doi: 10.1111/j.1749-6632.1997.tb51989.x. [DOI] [PubMed] [Google Scholar]
- MONTESANO R., PEPPER M.S., VASSALLI J.D., ORCI L. Phorbol ester induces cultured endothelial cells to invade a fibrin matrix in the presence of fibrinolytic inhibitors. J. Cell Physiol. 1987;132:509–516. doi: 10.1002/jcp.1041320313. [DOI] [PubMed] [Google Scholar]
- MORRIS N.H., EATON B.M., DEKKER G. Nitric oxide, the endothelium, pregnancy and pre-eclampsia. Br. J. Obs. Gynae. 1996;103:4–15. doi: 10.1111/j.1471-0528.1996.tb09508.x. [DOI] [PubMed] [Google Scholar]
- MORRIS N.H., EATON B.M., SOORANNA S.R., STEER P.J. NO synthase activity in placental bed and tissues from normotensive pregnant women. Lancet. 1993;342:679–680. [PubMed] [Google Scholar]
- MURRELL G.A., JANG D., WILLIAMS R.J. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem. Biophys. Res. Comm. 1995;206:15–21. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- MYATT L., EIS A.L., BROCKMAN D.E., GREER I.A., LYALL F. Endothelial nitric oxide synthase in placental villous tissue from normal pre-eclamptic and intrauterine growth restricted pregnancies. Human Reprod. 1997;12:167–172. doi: 10.1093/humrep/12.1.167. [DOI] [PubMed] [Google Scholar]
- NOIRI E., PERESLENI T., SRIVASTAVA N., WEBER P., BAHOU W.F., PEUNOVA N., GOLIGORSKY M.S. Nitric oxide is necessary for a switch from stationary to locomoting phenotype in epithelial cells. Am. J. Physiol. 1996;270:C794–802. doi: 10.1152/ajpcell.1996.270.3.C794. [DOI] [PubMed] [Google Scholar]
- POSTON L. The control of blood flow to the placenta. Exp. Physiol. 1997;82:377–387. doi: 10.1113/expphysiol.1997.sp004033. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., VALLANCE P., WHITLEY G., FOXWELL N., MONCADA S. Platelet adhesion to human vascular endothelium is modulated by constitutive and cytokine induced nitric oxide. Cardiovasc. Res. 1993;27:1380–1382. doi: 10.1093/cvr/27.7.1380. [DOI] [PubMed] [Google Scholar]
- ROBERTSON W.B., BROSENS I.A., DIXON H.G. Placental bed vessels. Am. J. Obstet. Gynecol. 1973;117:294–295. doi: 10.1016/0002-9378(73)90655-8. [DOI] [PubMed] [Google Scholar]
- ROSEN E.M., ZITNIK R.J., ELIAS J.A., BHARGAVA M.M., WINES J., GOLDBERG I.D. The interaction of HGF-SF with other cytokines in tumor invasion and angiogenesis. EXS. 1993;65:301–310. [PubMed] [Google Scholar]
- SZABO C. Alterations in nitric oxide production in various forms of circulatory shock. New Horizons. 1995;3:2–32. [PubMed] [Google Scholar]
- SZEKANECZ Z., SHAH M.R., HARLOW L.A., PEARCE W.H., KOCH A.E. Interleukin-8 and tumor necrosis factor α are involved in human aortic endothelial cell migration. The possible role of these factors in human aortic aneurysmal blood vessel growth. Pathobiol. 1994;62:134–139. doi: 10.1159/000163891. [DOI] [PubMed] [Google Scholar]
- TAMAGNONE L., COMOGLIO P.M. Control of invasive growth by hepatocyte growth factor (HGF) and related scatter factors. Cytokine and Growth Factor Reviews. 1997;8:129–142. doi: 10.1016/s1359-6101(97)00007-5. [DOI] [PubMed] [Google Scholar]
- UEHARA Y., MINOWA O., MORI C., SHIOTA K., KUNO J., NODA T., KITAMURA N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- WOLF H.K., ZARNEGAR R., OLIVER L., MICHALOPOULOS G.K. Hepatocyte growth factor in human placenta and trophoblastic disease. Am. J. Pathol. 1991;138:1035–1043. [PMC free article] [PubMed] [Google Scholar]
- YALLAMPALLI C., GARFIELD R.E. Inhibition of nitric oxide synthase in rats during pregnancy produces signs similar to those of pre-eclampsia. Am. J. Obs. Gynae. 1993;169:1316–1320. doi: 10.1016/0002-9378(93)90299-x. [DOI] [PubMed] [Google Scholar]
- ZARLINGO T.J., EIS A.L., BROCKMAN D.E., KOSSENJANS W., MYATT L. Comparative localisation of endothelial and inducible nitric oxide synthase isoforms in haemochorial and epitheliochorial placentae. Placenta. 1997;18:511–520. doi: 10.1016/0143-4004(77)90004-2. [DOI] [PubMed] [Google Scholar]
- ZHOU Y., DAMSKY C.H., CHIU K., ROBERTS J.M., FISHER S.J. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J. Clin. Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU Y., DAMSKY C.H., FISHER S.J. Pre-eclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. J. Clin. Invest. 1997b;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU Y., FISHER S.J., JANATPOUR M., GENBACEV O., DEJANA E., WHEELOCK M., DAMSKY C.H. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. J. Clin. Invest. 1997a;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]


