Abstract
In the present study, we developed an experimental model of cystitis induced by cyclophosphamide (CYP). In order to characterize des-Arg9-BK-induced contraction on the urinary bladder (UB) during the development of inflammation and to quantify kinin B1 receptor gene expression using a quantitative RT–PCR technique.
In the presence of peptidase inhibitors captopril (10 μM), DL-thiorphan (1 μM) and DL-2-mercaptomethyl-3-guanidino-ethylthiopropanoic acid (MERGEPTA 5 μM), bradykinin (BK) (0.3–3,000 nM) evoked a concentration-dependent contraction of rat UB which was not different between the CYP- and vehicle-treated groups. Unlike BK, des-Arg9-BK (0.3–100,000 nM) did not contract UB from vehicle-treated rats but contracted vigorously bladder strips from CYP-treated rats 14, 24 and 168 h after treatment. In UB of 24 h treated rat, the pD2 value of des-Arg9-BK was 7.3±0.1.
The cyclo-oxygenase inhibitor indomethacin (3 μM) reduced by 30% the maximal response of des-Arg9-BK. Both the kinin B1 receptor antagonists des-Arg9-[Leu8]BK (10 μM) and des-Arg10-Hoe 140 (10 μM) produced a rightward shift of the concentration-response curve to des-Arg9-BK yielding pKB values of 6.8±0.2 and 7.2±0.1, respectively, whilst the kinin B2 receptor antagonist Hoe 140 (1 μM) had no effect.
After CYP treatment, mRNA coding for the kinin B1 receptor appeared predominantly in UB. In this organ, the induction was progressive, reaching a maximum 48 h after CYP treatment.
In conclusion, the present study provides strong evidence for an induction of kinin B1 receptors in UB of CYP-treated rats. This was associated at a molecular level with an increase in mRNA expression of the gene coding for the kinin B1 receptor. This kinin receptor displayed the whole features of a classical rat kinin B1 receptor.
Keywords: Rat, bradykinin B1 receptor, cystitis, cyclophosphamide, urinary bladder, receptor gene, sequence analysis, splice variants, quantitative polymerase chain reaction
Introduction
Haemorrhagic cystitis is a common side effect of cyclophosphamide chemotherapy that causes bleeding and pain (Stillwell & Benson, 1988). In spite of constant efforts that have been made to prevent the urotoxicity of this compound, haemorrhagic cystitis still occurs in 2–42% of patients receiving long-term treatment with CYP (Luce et al., 1988), and the mortality rate associated with the massive haemorrhagic form is about 75% (Droller et al., 1982). Thus, there is still a need for a better understanding of the pathophysiology of this disease to help for the development of a protective treatment of CYP-induced cystitis. In that way, the kallikrein-kinin system appears to be a promising target. BK and its C-terminal desarginated metabolite, des-Arg9-BK, are proinflammatory and algesic peptides which activate B2 and B1 receptors, respectively (Regoli & Barabe, 1980). Levels of these peptides are elevated in both plasma and peripheral tissues under inflammatory conditions like trauma or infection (Marceau et al., 1980). The B2 receptor is constitutively expressed whilst the B1 receptor is thought to be induced under stressful and inflammatory conditions (Pruneau et al., 1994; Marceau, 1995). The relative causative importance of the two kinin receptors in pain and inflammation is still a matter of debate. If most of the experimental studies have shown that B2 receptor activation is primarily involved in the acute phase of inflammation, there is increasing evidence to suggest that B1 receptors are expressed and become important during chronic inflammatory conditions (Dray, 1997).
The kallikrein-kinin system is present in the urinary tract. B2 receptors have been detected in the urinary bladder from rat and human origin (Figueroa et al., 1997) and BK which is continuously released in the UB (Saban et al., 1997a), has been shown to influence the excitatory motor innervation of this organ (Patra & Westfall, 1996). The participation of BK to the pathogenesis of experimental cystitis has also recently been suggested (Maggi et al., 1993; Saban et al., 1997b), and there are higher than normal levels of kinins in patients suffering from interstitial cystitis (Zuraw et al., 1994). On another hand, much less is known regarding a possible role of B1 receptors in the pathophysiology of the urinary tract although induction of this receptor in the UB of rat and rabbit has been demonstrated using various stimuli (Butt et al., 1995; Roslan et al., 1995; Meini et al., 1998).
In the present study, we developed a rat model of bladder inflammation resulting from the toxic effect of acrolein, the main metabolite of CYP, on the bladder wall (Cox, 1979). Using strips of UB, we characterized functionally B1 receptors which are gradually expressed for at least 1 week after CYP treatment. We also demonstrated increase in bladder B1 receptor gene expression after CYP treatment using quantitative reverse transcription polymerase chain reaction (RT–PCR).
Methods
Male Wistar rats (Iffa Credo, L'arbresles, France) weighing 350–450 g were used for all experiments.
Cyclophosphamide-induced haemorrhagic cystitis
Haemorrhagic cystitis was induced as previously described (Gray et al., 1986). Rats received intraperitoneal injections of 150 mg kg−1 of CYP or its corresponding vehicle (NaCl 0.9%). Rats were killed by CO2 intoxication at various times (1, 2, 4, 6, 14, 24, 48 or 168 h) after treatment with either CYP or its vehicle.
Isolated organs experiments
The abdomen was opened and the entire UB was rapidly removed and placed in Krebs solution of the following composition (mM): NaCl 119, KCl 4.7, MgSO4 1.5, CaCl2 2.5, KH2PO4 1.2, NaHCO3 25, glucose 11 and EDTA 0.026. Intact bladder strips (3×10×1 mm) from the bladder neck to the dome were set up in 8 ml-jacketed organ baths containing Krebs solution maintained at 37°C and bubbled with 95% O2 and 5% CO2. Strips were left unstretched for 15 min, during which the bath fluid was changed every 5 min with fresh solution. Strips were then stretched in a stepwise fashion by 500 mg tension increments up to 2 g. After a further 15 min resting period, carbachol (10 μM) was injected in order to assess the contractile capacity of the tissue. After washing twice with normal Krebs solution and return to the baseline, captopril (10 μM), and DL-thiorphan (1 μM) were added into the organ bath in order to prevent the degradation of BK by angiotensin converting enzyme and neutral endopeptidase, respectively. The carboxypeptidase inhibitor (MERGETPA 5 μM), was also used in experiments involving BK. Thirty minutes later concentration-response curves to des-Arg9-BK, des-Arg10-kallidin (des-Arg10-KD) or BK were obtained. Each strip was used for a single concentration-response curve. In another series of experiments, responses to agonists were obtained in the presence or the absence of Hoe 140, des-Arg9-[Leu8]BK or des-Arg10-Hoe 140 added 15 min before. At the end of the experiments, after washing and return to the baseline level, the maximal contraction of each strip was obtained by adding carbachol (CBCL 10 μM). The contractile responses to agonists were expressed as percentage of the final contraction to CBCL. Emax was the maximal effect of kinin agonists.
Quantitative RT–PCR analysis of the rat B1 receptor mRNA
Rat B1 mRNA has previously been shown to be alternatively spliced (Bélichard et al., 1998; Ni et al., 1998). In order to quantitate both splice variants, we constructed two RNA standards corresponding to each of the splice alternants. Total RNA was prepared from rat UB that was removed under sterile conditions, quickly frozen in liquid nitrogen, disrupted using nitrogen-cooled mortar and pestle, and ground to a fine powder under liquid nitrogen. Samples were then homogenized in lysis buffer using a syringe and a 20-G needle. Total RNA was extracted using the RNeasy kit (Quiagen, Courtaboeuf, France). To assess RNA integrity, the fluorescence of 28S/18S ribosomal RNAs were determined using 50% (v v−1) formamide and heat treatment for RNA denaturation followed by electrophoresis in an agarose-formaldehyde gel. Three hundred ng of total RNA were reverse-transcribed using oligo dT 10 (Boehringer Mannheim, Meylan, France) as primer and 200 U of M-MLV reverse transcriptase (Promega, Charbonnières, France) in the presence of 30 U of RNase inhibitor (Promega, Charbonnières, France). Controls without RT and without RNA were included to check for DNA contaminations. Two RNA standards were constructed by introducing a 63 bp-deletion into each of the splice alternants cDNAs. In the sense primer of which sequence is Q7 : 5′- CTT AAT ACG ACT CAC TAT AGG GCA CAG GTG AAG CTG TGA GCT C-3′, the underlined part of this primer is an optimal T7 polymerase sequence (Baklanov et al., 1996). Q7 is located within exon 1 and identical to the mRNA strand. The antisense primer Q8 : 5′- GAT GCA GGC AGA GAC GTT CAG ATC GAT GAG CAG GCA CGT AGC TTG-3′ is located within exon 2 and is complementary to the mRNA strand. The first 25 bases of this primer, starting from the 5′ end and 63 bases downstream on the mRNA was in relation to the next 20 bases at the 3′ end of the primer. It produced the 63-bases deletion in the final PCR products. PCR amplification was performed with the Quiagen Taq DNA polymerase in a final volume of 20 μl, containing 1 μl of the RT solution, 20 pmol of primers Q7 and Q8, 1X Taq buffer (mM): Tris-HCl [pH 8.3] 10, KCl 50 and MgCl2 1.5, 1×Q solution, 0.125 mM dNTPs, and 0.1 U of Taq DNA polymerase (Quiagen, Courtaboeuf, France). Samples were subjected to 35 cycles of 30 s at 94°C, 30 s at 60°C, and 1 min at 72°C, followed by a final extension step of 5 min at 72°C.
The two amplified fragments corresponding to the two splice variants internal standards were separated by excision on a 2% agarose gel and purified with the Qiaquick gel purification kit (Qiagen, Courtaboeuf, France). One μg of each cDNA was then converted to cRNA by using the MEGAscript T7 in vitro transcription kit (Ambion, TX, U.S.A.) followed by a standard DNAse I treatment. cRNAs were extracted by phenol-chlorophorm, precipitated and each standard concentration quantitated by u.v. spectrophotometry.
BK B1 receptor mRNA was quantified by coamplifying a constant amount (300 ng) of total RNA with decreasing concentrations of internal standard cRNA (50–0.014 amol). All RNAs (internal standards at each concentration and total RNA) were reverse-transcribed as previously described and in the same reaction tube to avoid variations in the RT efficiency. PCR amplification was performed as described above except that we add 2 μCi of [α-33P]-dCTP (NEN, Paris, France) in the PCR mix for products visualization, and that we used 20 pmol of primer Q9 (5′- CAG GTG AAG CTG TGA GCT C-3′, i.e. primer Q7 without the T7 sequence) and 20 pmol of primer Q10 (5′- GAT GCA GGC AGA GAC GTT CAG ATC G-3′, i.e. 3′ portion of primer Q8, downstream from the deletion site). Samples were subjected to the same cycling conditions as described above. A negative control was used for each set of samples to check the RT and the PCR amplification reagents for any contamination.
Samples were run in a denaturating 6% polyacrylamide/7 M urea gel at 60 W for 120 min in a sequencing gel apparatus. Before loading, samples were incubated 5 min at 95°C in 22% formamide, 0.08% EDTA-pH 7.0, 0.07% bromophenol blue and 0.07% xylene cyanol FF. Gels were then dried and exposed to phosphorimaging screens for 24 h. Screens were visualized with a Storm PhosphorImager (Molecular Dynamics, CA, U.S.A.). Densitometric analysis was done using the ImageQuant software (Molecular Dynamics, CA, U.S.A.). An equimolar point was determined where the starting number of standard RNA transcripts is equal to the starting number of cellular target RNA transcripts. To determine this point, the ratios of the band intensities of the PCR products from the internal standard RNA and the target RNA were plotted against the starting molarity of internal standard molecules. Quantitative RT–PCR measurements of rat B1 mRNA were then done starting from 300 ng of total RNA in the same conditions as described above, firstly in various rat organs (liver, spleen, brain, heart, bladder, prostate, kidney, ileum, stomach and testis) 24 h after CYP administration and specifically in urinary bladder at various times (1, 4, 24, 48 or 168 h) after CYP administration.
Data analysis
In functional assays, EC50 was the concentration of agonist needed to reach 50% of the maximal response and was calculated using least-square analysis (Tallarida & Murray, 1981). pKB value (−log KB) was obtained according to the following equation: KB=[A]/(concentration ratio −1) where [A] is the concentration of the antagonist and concentration ratio is the EC50 in the presence of the antagonist divided by the EC50 in the absence of antagonist. The potency of the agonists is expressed as a pD2 value representing log (EC50).
Statistical analysis were performed using Statview (Abacus Concept, CA, U.S.A.). A one-way analysis of variance followed by a Student's t-test was used to establish significant differences between KB values. A P value less than 0.05 was considered as statistically significant.
All RNA concentrations (amol μg−1 of total RNA) are given as mean±s.e.mean. The significance of comparison of mean values was determined by means of a one-way analysis of variance followed by a Student's t-test using a significance level of P<0.05.
Drugs
Agents were obtained as follows: Hoe 140 (Neosystem, Strasbourg, France), MERGETPA (DL-2-mercaptomethyl-3-guanidinoethylthiopropanoic acid) (Calbiochem, CA, U.S.A.), BK, des-Arg9-BK and des-Arg10-KD (Bachem, Bubendorf, Switzerland). Other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A).
All agents were solubilized in distilled water except MERGETPA and indomethacin in dimethylsulphoxide.
Results
Effects of BK on rat UB strips
In the presence of the peptidase inhibitors MERGETPA (5 μM), captopril (10 μM) and DL-thiorphan (1 μM), BK (0.3–3,000 nM) contracted the rat UB similarly in CYP- and vehicle-treated rats whatever the duration of treatment (data not shown). Twenty-four hours after treatment, the pD2 values of BK were 8.13±0.15 and 8.22±0.10 for vehicle- and CYP-treated rats, respectively. Contractile responses to BK were similarly inhibited by the selective B2 receptor antagonist, Hoe140 (100 nM), giving a pKB value of 9.13±0.21.
Effects of des-Arg9-BK and des-Arg10-KD on UB strips
Unlike BK, des-Arg9-BK (0.3–100,000 nM) did not contract UB from vehicle-treated rats. In contrast, it induced a vigorous contraction of UB from CYP-treated rats at 14, 24, 48 and 168 h after treatment (Figure 1). In the presence of the peptidase inhibitors captopril and DL-thiorphan but not of MERGETPA, the sensitivity of the concentration-response curve to des-Arg9-BK was increased. The pD2 and Emax of des-Arg9-BK on UB from vehicle and CYP-treated rats are resumed in the Table 1. The cyclo-oxygenase inhibitor, indomethacin (3 μM), reduced by 30% the response to des-Arg9-BK. Des-Arg10-KD (0.3–30,000 nM) contracted UB of 24 h-CYP-treated rats with a similar potency than des-Arg9-BK, giving pD2 values of 7.4±0.2 and 7.3±0.1, respectively.
Figure 1.
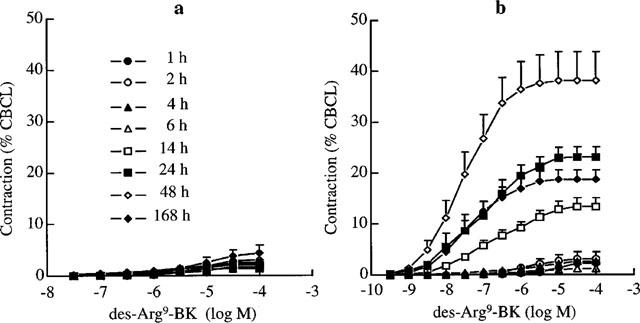
Concentration-related contractile response to des-Arg9-BK in the rat urinary bladder at different times following vehicle (a) or CYP treatment (b). Values are means±s.e.mean of 4–6 experiments.
Table 1.
Contractile potency of des-Arg9-BK in the rat urinary bladder following vehicle or CYP treatment
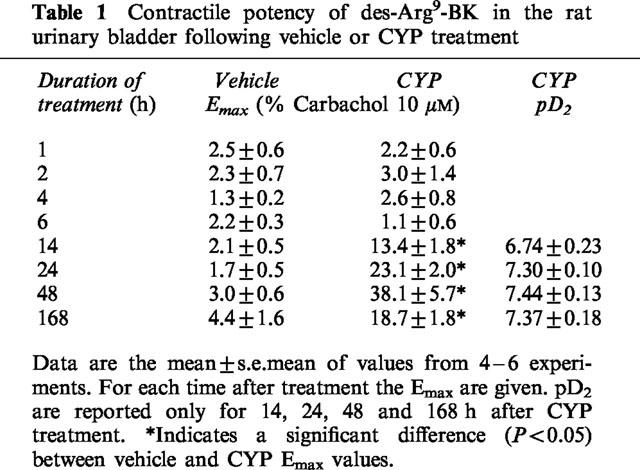
In order to assess the influence of the urothelium on the contraction to des-Arg9-BK, concentration-response curves were obtained in UB in presence or in absence of urothelium from 24 h-vehicle- or CYP-treated rat UB. Urothelium removal did not affect the concentration-response curves neither for vehicle- nor for CYP-treated animals (Figure 2).
Figure 2.
Concentration-related contractile response to des-Arg9-BK in the rat urinary bladder with or without urothelium from vehicle or 24 h-CYP-treated rats. Values are means±s.e.mean of six experiments.
Effects of kinin receptor antagonists on bladder contractility
In UB of 24 h-CYP-treated rats, both B1 receptor antagonists, des-Arg9-[Leu8]BK and des-Arg10-Hoe140 at 10 μM behaved as weak and erratic partial agonists. As expected, they also produced a rightward shift of the concentration-response curve to des-Arg9-BK without depression of maximal response yielding pKB values of 6.8±0.2 and 7.2±0.1, respectively (Figure 3). The B2 receptor-selective antagonist, Hoe140 at 1 μM had no significant effect on the concentration response curve to des-Arg9-BK (Figure 3).
Figure 3.
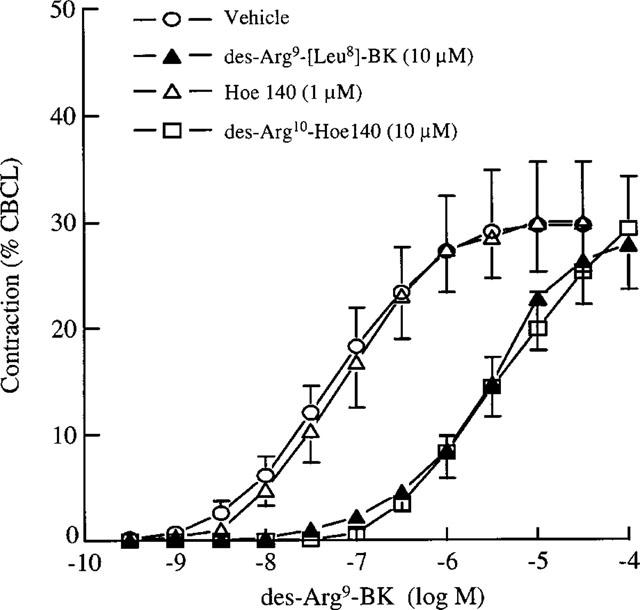
Effect of Hoe 140, des-Arg9-[Leu8]-BK and des-Arg10-Hoe 140 on concentration-related contractile response to des-Arg9-BK in the rat urinary bladder 24 h after CYP administration. Values are means±s.e.mean of six experiments.
Quantification of B1 mRNAs expression in various rat tissues 24 h after CYP or saline administration
Levels of the two mRNA splice variants of B1 receptors were detected using RT–PCR including an internal standard (Figure 4). Quantitative results of both splice variants were obtained in ten distinct tissues originating from six animals (three saline- and three CYP-treated rats) and are summarized in Figure 5. When considering the full length mRNA (longest alternative splice form), in saline-treated rats and amongst the tested organs, levels of B1 receptor mRNA were almost undetectable (<0.1 amol μg−1 of total RNA) in liver, brain, heart, kidney and testis. Spleen, UB and prostate had values of 0.11±0.04, 0.51±0.10, and 0.09±0.03 amol μg−1 of total RNA, respectively. Highest expressions were detected in the two tissues originating from the gastrointestinal tract i.e. ileum (1.51±0.33 amol μg−1 of total RNA) and stomach (3.03±1.41 amol μg−1 of total RNA). Twenty-four hours after CYP treatment, B1 receptor mRNA level was not changed in either liver, brain, heart, kidney nor testis. In the stomach and ileum there was an unsignificant increase of B1 receptor mRNA, whereas in UB there was a significant (P<0.001) elevation in B1 receptor mRNA expression. Basal levels and CYP-induced variations of the shortest transcript (spliced form of the mRNA lacking 41 bp) were not different from those of the full length mRNA (Figure 6).
Figure 4.
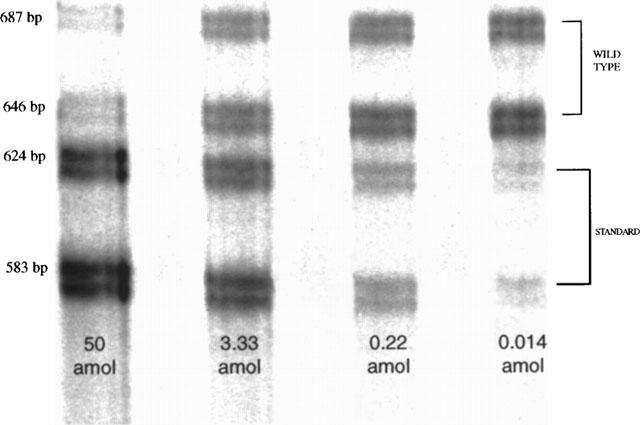
Quantification of B1 mRNAs from urinary bladder by quantitative RT–PCR. A representative gel image of polyacrylamide electrophoretic separation of PCR products is shown. The two upper bands are respectively the longest (687 bp) and the shortest alternative splice form (646 bp) of the B1 mRNA. The two lower bands correspond to the longest and shortest alternative splice form of respective competitor transcripts of the 63 bp-deleted RNA standards. Each lane corresponds to a different concentration of RNA standard used in the RT–PCR reaction. In this representative experiment, increasing concentrations of standard RNA from 0.014 amol to 50 amol were used in a 35-cycles competitive RT–PCR.
Figure 5.
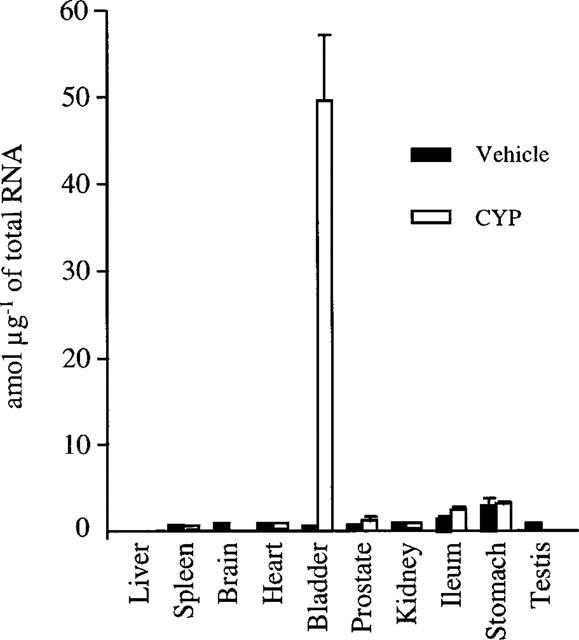
Quantitative expression of kinin B1 mRNA in various rat tissues, 24 h after vehicle or CYP administration. Values are means±s.e.mean of three experiments.
Figure 6.
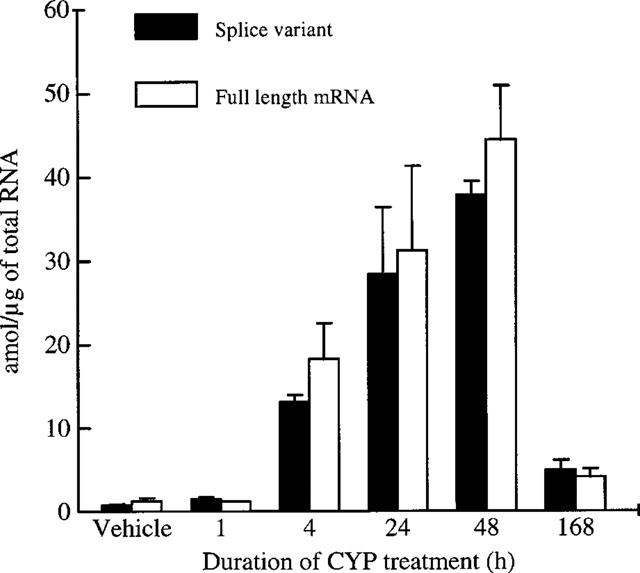
Kinetic of kinin B1 mRNA expression in rat urinary bladder following CYP administration. Values are means±s.e.mean of three experiments.
Quantification of B1 receptor mRNAs expression in rat UB at different times after CYP or saline administration
As shown in Figure 6, we confirmed a basal expression of B1 receptor mRNA in UB. After CYP treatment, we observed a progressive increase in B1 receptor mRNA expression that became significant from 4 h and onwards after drug administration reaching a maximum at 48 h. One week after CYP treatment, B1 receptor mRNA values were not returned to baseline. We also observed that the amount of each splice variant was not different between CYP- and vehicle-treated rats at any time.
Discussion
In the present study, we found a marked expression of kinin B1 receptors in the bladder wall of CYP-treated rats. One of the main metabolites of CYP is acrolein which is eliminated through the urine and therefore produces severe lesions in the bladder (Foad & Hess, 1976; Cox, 1979) characterized at an histological level by moderate to severe oedema, transmural hemorrhage, mucosal erosion and ulceration (Phillips et al., 1961). Whilst there was no functional evidence for the presence of B1 receptors in the bladder of untreated normal rats, contractions to des-Arg9-BK were obtained at 14 h and onwards after treatment with CYP. This was typical of an inducible pattern as previously demonstrated with the B1 receptor under various stressful conditions (Deblois et al., 1988; Pruneau et al., 1994). The pharmacological profile of the B1 receptor expressed in the bladder following CYP was similar to the one previously reported in other rat preparations including the duodenum (Paiva et al., 1989) and the oesophagus (Boxall et al., 1995). In this respect, contractions to des-Arg9-BK were antagonized without depression of maximal response by des-Arg9-[Leu8]BK, des-Arg10-Hoe 140, but not by Hoe 140. Values of pKB for the B1 receptor antagonists were comparable to the ones previously reported in the rat UB (Roslan et al., 1995), ileum (Meini et al., 1996), and oesophagus (Boxall et al, 1995). Interestingly, des-Arg9-[Leu8]BK displayed a weak partial agonist activity contracting the UB to 10% of the maximal response to des-Arg9-BK. This is much less than in the rat duodenum where the contraction to des-Arg9-[Leu8]BK represented 40% of the maximum response to des-Arg9-BK (Paiva et al., 1989) and the rat ileum where it behaved as a full agonist (Meini et al., 1996). As suggested by Kenakin (1987), these differences could be tentatively explained by a variable density of spare receptors amongst tissues.
We demonstrated that the cyclo-oxygenase inhibitor indomethacin, partially abolished the contractile response to des-Arg9-BK in agreement with the recently reported data from Meini et al. (1998). Since the urothelium may potentially modulate the response to contractile agents, experiments in which the urothelium was mechanically removed in both vehicle- and CYP-treated rats UB, were conducted. In both cases, the response to des-Arg9-BK was unaffected indicating either a lack of B1 receptors on epithelial cells or no obvious role for these receptors in the contraction.
We also examined the amount of rat B1 receptor mRNA using of quantitative RT–PCR technique in various tissues including UB of both CYP- and vehicle-treated rats. The use of RT–PCR to quantify specific rare mRNA transcripts is gaining favour over Northern blot or RNase Protection Assay due to its convenience, speed and sensitivity (Zimmermann & Mannhalter, 1996). The system described here used a synthetic RNA generated by in vitro transcription from the cDNA of rat B1 receptor, with a small deletion in the coding region. Using identical primers, the test sample and internal control amplified competitively in each reaction tube. This strategy avoids problems due to tube-to-tube variation, unequal amplification efficiency, and the plateau effect that arises with external controls or unrelated internal controls. Our results revealed a basal expression of mRNA coding for the B1 receptor in the stomach, ileum and UB and, to a lower extent, in the spleen and prostate. In the liver, brain, heart, kidney, and testis basal level of mRNA coding for the B1 receptor was almost undetectable. It is not known yet if the presence of B1 receptors mRNA has a functional significance for the concerned organs. The basal steady-state expression of B1 receptor mRNA in the UB of naive rats was not associated with a functional response to des-Arg9-BK, therefore suggesting that the receptor protein was not expressed on cell membranes. Although UB strips obtained 6 h after CYP administration were still unresponsive to des-Arg9-BK, corresponding mRNA level was increased by approximately 20 times compared to basal level.
Such a delayed functional response was likely due to the time necessary for mRNA translation, protein synthesis and receptor maturation. Twenty-four hours after CYP treatment, levels of B1 receptor mRNA were significantly increased in UB and not in other tested organs, indicating that, in this model, B1 receptor induction was restricted to the UB which is the target organ of CYP and its main metabolite acrolein.
As recently described by Ni et al. (1998), we also identified by RT–PCR, two differentially spliced B1 receptor mRNA differing in size by the first 41 bases of exon 2. These splice variants were found in all the tested tissues expressing under basal conditions the B1 receptor mRNA. As previously described for the mouse CXCR-4 receptor (Heesen et al., 1997), the alternatively spliced region of B1 receptor gene cannot be defined by itself as an exon since there was no intron between this region and the rest of gene. Concerning the physiological significance of this splicing phenomenon, the location of the spliced sequence into the 5′-untranslated leader region (5′-UTR) of the rat B1 receptor gene does not preclude any modification at the receptor protein sequence level. However, these divergent mRNA leaders might play a role in the translational regulation of gene expression (Gayen & Peffley, 1995). If B1 receptor gene expression has been shown to be highly regulated at a transcriptional level (Marceau et al., 1998), we cannot rule out a regulation of the translation of mRNA coding for the B1 receptor. Translational regulation allows the cell to respond to environmental changes more rapidly than by changing the rate of transcription and this could be of particular importance for the regulation of B1 receptor protein expression which is highly altered by environmental changes. Nevertheless, the lack of conservation of such a structure in the B1 receptor between species, and the fact that an equimolar ratio of both transcripts was obtained in all tissues and not changed following receptor induction in UB at any time, raised doubts about the functional significance of the alternative splicing pattern.
In conclusion, we demonstrated by using both pharmacological and molecular biology approaches, that kinin B1 receptors are specifically induced in the UB wall of CYP-treated rats. Although we could not determine precisely which cell type expressed the B1 receptor, we showed that its expression was restricted to the UB and matched the time-course of inflammatory lesions developing in this organ after CYP. Since there is an increasing evidence in the literature for a role of B1 receptors in inflammatory pain, we anticipate that, a pharmacological treatment aiming at blocking kinin B1 receptors might certainly be of interest to evaluate their role in the development of haemorrhagic cystitis following CYP administration.
Acknowledgments
The authors gratefully acknowledge Anne Michelet-Prigent for her secretarial assistance.
Abbreviations
- BK
bradykinin
- CBCL
carbachol
- CYP
cyclophosphamide
- KD
kallidin
- MERGETPA
DL-2-mercaptomethyl-3-guanidino-ethylthiopropanoic acid
- RT–PCR
reverse transcription polymerase chain reaction
- UB
urinary bladder
References
- BAKLANOV M.M., GOLIKOVA L.N., MALYGIN E.G. Effect on DNA transcription of nucleotide sequences upstream to T7 promoter. Nucleic Acids Res. 1996;24:3659–3660. doi: 10.1093/nar/24.18.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÉLICHARD P., FAYE P., LUCCARINI J.-M., PAQUET J.-L., PRUNEAU D. The Fifteenth International Conference on Kinins. Nara, Japan; 1998. Molecular cloning of rat bradykinin B1 receptor gene: evidence for variable issue expression and alternative splicing. [Google Scholar]
- BOXALL S.J., WHEELDON A., BIRCH P.J. Characterisation of the bradykinin receptors that mediate contraction of the rat oesophagus in vitro. Br. J. Pharmacol. 1995;114:225P. [Google Scholar]
- BUTT S.K., DAWSON L.G., HALL J.M. Bradykinin B1 receptors in the rabbit urinary bladder: induction of responses, smooth muscle contraction, and phosphatidylinositol hydrolysis. Br. J. Pharmacol. 1995;114:612–617. doi: 10.1111/j.1476-5381.1995.tb17183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX P.J. Cyclophosphamide cystitis identification of acrolein as the causative agent. Biochem. Pharmacol. 1979;28:2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- DEBLOIS D., BOUTHILLIER J., MARCEAU F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit isolated aorta to des-Arg9-bradykinin. Br. J. Pharmacol. 1988;93:969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAY A. Kinins and their receptors in hyperalgesia. Can. J. Physiol. Pharmacol. 1997;75:704–712. [PubMed] [Google Scholar]
- DROLLER M.J., SARAL R., SANTOS G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology. 1982;20:256–258. doi: 10.1016/0090-4295(82)90633-1. [DOI] [PubMed] [Google Scholar]
- FIGUEROA C.D., NOVOA U., VALDES G., CORTHORN J., MÜLLER-ESTERL W. Localization of the bradykinin B2 receptor in uterus, bladder and Madin-Darby canine kidney cells. Immunopharmacology. 1997;36:127–133. doi: 10.1016/s0162-3109(97)00011-8. [DOI] [PubMed] [Google Scholar]
- FOAD B.S., HESS E.V. Urinary bladder complications with cyclophosphamide therapy. Arch. Intern. Med. 1976;136:616–619. [PubMed] [Google Scholar]
- GAYEN A.K., PEFFLEY D.M. The length of 5′-untranslated leader sequences influences distribution of 3-hydroxy-3-methylglutaryl-coenzyme A reductase mRNA in polysomes: effects of lovastatin, oxysterols, and mevalonate. Arch. Biochem. Biophys. 1995;322:475–485. doi: 10.1006/abbi.1995.1491. [DOI] [PubMed] [Google Scholar]
- GRAY K.J., ENGELMANN U.H., JOHNSON E.H., FISHMAN I.J. Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (Cytoxan) therapy. J. Urol. 1986;136:497–500. doi: 10.1016/s0022-5347(17)44929-9. [DOI] [PubMed] [Google Scholar]
- HEESEN M., BERMAN M.A., HOPKEN U.E., GERARD N.P., DORF M.E. Alternate splicing of mouse fusin/CXC chemokine receptor-4: stromal cell-derived factor-lalpha is a ligand for both CXC chemokine receptor-4 isoforms. J. Immunol. 1997;158:3561–3564. [PubMed] [Google Scholar]
- KENAKIN T.Stimulus-response curves and ‘spare receptors' Pharmacologic Analysis of Drug-Receptor Interaction 1987Raven Press: New York; 37–39.In: Kenakin T (ed) [Google Scholar]
- LUCE J.K., SIMONS J.A., STILLWELL T.J., BENSON R.C., JR Efficacy of mesna in preventing further cyclophosphamide-induced hemorrhagic cystitis. Med. Ped. Oncol. 1988;16:372–374. doi: 10.1002/mpo.2950160603. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., DEL BIANCO E., LECCI A., GULIANI S. Evidence for the involvement of bradykinin in chemically-evoked cystitis in anaesthetized rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 1993;347:432–437. doi: 10.1007/BF00165395. [DOI] [PubMed] [Google Scholar]
- MARCEAU F. Kinin B1 receptors: a review. Immunopharmacology. 1995;30:1–26. doi: 10.1016/0162-3109(95)00011-h. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., BARABE J., ST-PIERRE S., REGOLI D. Kinin receptors in experimental inflammation. Can. J. Physiol. Pharmacol. 1980;58:536–542. doi: 10.1139/y80-088. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MEINI S., LECCI A., CUCCHI P., CATALIOTO R.M., CRISCUOLI M., MAGGI C.A. Inflammation modifies the role of cyclooxygenases in the contractile responses of the rat detrusor smooth muscle to kinin agonists. J. Pharmacol. Exp. Ther. 1998;287:137–143. [PubMed] [Google Scholar]
- MEINI S., LECCI A., MAGGI C.A. The longitudinal muscle of rat ileum as a sensitive monoreceptor assay for bradykinin B1 receptors. Br. J. Pharmacol. 1996;117:1619–1624. doi: 10.1111/j.1476-5381.1996.tb15331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NI A., CHAO L., CHAO J. Transcription factor nuclear factor kappa B regulates the inducible expression of the human B1 receptor gene in inflammation. J. Biol. Chem. 1998;273:2784–2791. doi: 10.1074/jbc.273.5.2784. [DOI] [PubMed] [Google Scholar]
- PAIVA A.C.M., PAIVA T.B., PEREIRA C.C., SHIMUTA S.I. Selectivity of bradykinin analogues for receptors mediating contraction and relaxation of the rat duodenum. Br. J. Pharmacol. 1989;98:206–210. doi: 10.1111/j.1476-5381.1989.tb16883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRA P.B., WESTFALL D.P. Potentiation by bradykinin and substance P of purinergic neurotransmission in urinary bladder. J. Urol. 1996;156:532–535. doi: 10.1097/00005392-199608000-00077. [DOI] [PubMed] [Google Scholar]
- PHILLIPS F.S., STERNBERG S.S., CRONIN A.P., VIDAL P.M. Cyclophosphamide and urinary bladder toxicity. Cancer Res. 1961;21:1577–1581. [PubMed] [Google Scholar]
- PRUNEAU D., LUCCARINI J.M., ROBERT C., BELICHARD P. Induction of kinin B1 receptor-dependent vasoconstriction following balloon catheter injury to the rabbit carotid artery. Br. J. Pharmacol. 1994;111:1029–1034. doi: 10.1111/j.1476-5381.1994.tb14847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGOLI D., BARABE J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- ROSLAN R., CAMPBELL E.A., DRAY A. The induction of bradykinin B1 receptors in the non-inflamed and inflamed rat urinary bladder. Br. J. Pharmacol. 1995;114:228P. [Google Scholar]
- SABAN M.R., SABAN R., BJORLING D.E. Kinetics of peptide-induced release of inflammatory mediators by the urinary bladder. Br. J. Urol. 1997a;80:742–747. doi: 10.1046/j.1464-410x.1997.00415.x. [DOI] [PubMed] [Google Scholar]
- SABAN R., FRANZ J., BJORLING D.E. Spontaneously released substance P and bradykinin from isolated guinea-pig bladder. Br. J. Urol. 1997b;79:516–524. doi: 10.1046/j.1464-410x.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- STILLWELL T.J., BENSON R.C., JR Cyclophosphamide-induced hemorrhagic cystitis. A review of 100 patients. Cancer. 1988;61:451–457. doi: 10.1002/1097-0142(19880201)61:3<451::aid-cncr2820610308>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., MURRAY R.B. Manual of Pharmacologic Calculations with Computer Programs. Springer-Verlag: New York; 1981. [Google Scholar]
- ZIMMERMANN K., MANNHALTER J.W. Technical aspects of quantitative competitive PCR. Biotechniques. 1996;21:268–272. doi: 10.2144/96212rv01. [DOI] [PubMed] [Google Scholar]
- ZURAW B.L., SUGIMOTO S., PARSONS C.L., HUGLI T., LOTZ M., KOZIOL J. Activation of urinary kallikrein in patients with interstitial cystitis. J. Urol. 1994;152:874–878. doi: 10.1016/s0022-5347(17)32595-8. [DOI] [PubMed] [Google Scholar]


