Abstract
We have used the whole-cell patch-clamp technique to study the effects of 4-sulphonic-calixarenes and some other poly-sulphonic acid agents, such as suramin and basilen blue, on volume-regulated anion channel (VRAC) currents in cultured endothelial cells (CPAE cells).
The 4-sulphonic-calixarenes induced a fast inhibition at positive potentials but were ineffective at negative potentials. At small positive potentials, 4-sulphonic-calix[4]arene was a more effective inhibitor than 4-sulphonic-calix[6]arene and -calix[8]arene, which became more effective at more positive potentials.
Also suramin and basilen blue induced a voltage dependent current inhibition, reaching a maximum around +40 mV and declining at more positive potentials.
The voltage dependence of inhibition was modelled by assuming that these negatively charged molecules bind to a site inside VRAC that senses a fraction δ of the applied electrical field, ranging beween 0.16 to 0.32. 4-Sulphonic-calix[4]arene, suramin and basilen blue bind and occlude VRAC at moderate potentials, but permeate the channel at more positive potentials. 4-Sulphonic-calix[6]arene and -calix[8]arene however do not permeate the channel. From the structural information of the calixarenes, we estimate a lower and upper limit of 11*12 and 17*12 Å2 respectively for the cross-sectional area of the pore.
Keywords: 4-Sulphonic-calixarenes, suramin, basilen blue, voltage dependent block, open channel properties
Introduction
The control of various cell functions, as described in several reviews (Kirk, 1997; Nilius et al., 1994; 1996; Okada, 1997; Strange et al., 1996), has been attributed to volume-regulated anion channels (VRAC). However, the molecular structure of these channels, their activation and open pore properties remain largely unknown. Specific high-affinity ligands for VRAC are useful tools which might provide insight in its pore properties.
In a recent paper we have analysed the inhibitory action of 4-sulphonic-calix[4]arene on the volume-activated current ICl,swell through VRAC (Droogmans et al., 1998). This basket-shaped compound, made up of four para-substituted phenolic units meta-linked by methylene bridges, was shown to be an open pore blocker of VRAC. Its affinity for the channel was however rather low so that it is not really suited for biochemical purification of VRAC. The voltage-dependence of its inhibitory action, which could be described with a model in which the poly-anionic blocking particle binds at a site at an electrical distance of about 0.25 inside the membrane from where it can be released either to the outside or to the inside, revealed some interesting features of the pore region. Not only does the cross-sectional diameter of calix[4]arene provide a lower limit for the pore dimensions, but protonation of this putative membrane binding site by reducing extracellular pH enhances its affinity for 4-sulphonic-calix[4]arene.
In the present experiments we further explored the inhibitory action of 4-sulphonic-calix[4]arene and investigated the inhibitory action of some other higher-conjugated calixarene compounds and of some other poly-sulphonic acids agents, such as suramin and basilen blue, for which it was recently shown that they exert voltage-dependent inhibitory actions on ICl,swell (Galietta et al., 1997).
Methods
The methods used in the present experiments have been described in detail elsewhere (Nilius et al., 1994). Endothelial cells from an established cell line from bovine pulmonary artery (cell line CPAE, ATCC CCL-209) were grown in Dulbecco's modified Eagle's medium with 2 mM L-glutamine, 100 μg ml−1 streptomycin, 100 U ml−1 penicillin and 20% foetal bovine serum at 37°C in a fully humidified atmosphere of 10% CO2 in air. They were detached by exposure to 0.05% trypsin in a Ca2+- and Mg2+-free solution and plated on gelatine coated cover slips for electrophysiological experiments.
Whole-cell membrane currents were measured using an EPC-9 (Heka-Electronics, Lambrecht/Pfalz, Germany) patch clamp amplifier and were routinely sampled at 1 or 2 ms intervals (1024 points per record). The standard voltage protocol consisted of voltage steps ranging from −100 to +100 mV in increments of +10 mV which were applied every 6 s from a holding of −50 mV.
The standard extracellular solution was a Krebs solution, containing (in mM): NaCl 150, KCl 6, MgCl2 1, CaCl2 1.5, glucose 10, HEPES 10, triturated with NaOH to pH 7.4. The inwardly rectifying K+ current, which is present in CPAE cells, was inhibited by substituting K+ by Cs+ in the Krebs solution. The osmolarity, as measured with a vapour pressure osmometer (Wescor 5500, Schlag, Gladbach, Germany), was 320±5 mOsm.
After seal formation the normal Krebs solution was replaced with another isotonic solution that contained (in mM): NaCl 105, CsCl 6, MgCl2 1, CaCl2 1.5, glucose 10, HEPES 10, mannitol 90, titrated with NaOH to pH 7.4. VRAC was activated by omitting mannitol from this solution, resulting in a 25% hypotonicity.
Pipette solutions contained (in mM): CsCl 40, Cs-aspartate 100, MgCl2 1, EGTA 5, CaCl2 1.93, Na2ATP 4, HEPES 10 titrated with CsOH to pH 7.2. The calculated free Ca2+-concentration of this solution is 100 nM.
The various calixarenes were purchased from Acros Organic (New Jersey, U.S.A.), suramin and basilen blue from Sigma. All compounds were added to the bath. Their chemical structures are depicted in Figure 1.
Figure 1.
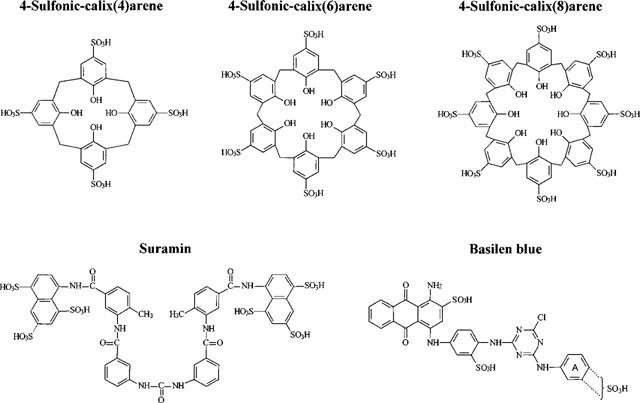
Chemical structures. The commercially available basilen blue is an A-ring isomer or a mixture of isomers.
All experiments were performed at room temperature (20–22°C). Data were analysed in Origin (MicroCal Software, Inc.). Pooled data are given as mean±s.e.mean.
Structural information on the calixarene molecules was extracted from the Cambridge Structural Database System (CSD System, Cambridge Crystallographic Data Centre, Lensfield Road, Cambridge, U.K.). Visualisation and size estimation was done by means of RasMol V2.4 (Molecular Visualisation Program, Roger Sayle, Glaxo Research and Development, Greenford, U.K.).
Results
Effect of calixarenes on ICl,swell
We have recently described the open pore block of VRAC by calix[4]arenes (Droogmans et al., 1998) and found that their open pore blocking characteristics might be useful for assessing the pore dimensions of VRAC. We have therefore compared the effects of three 4-sulphonic-calixarenes consisting of 4, 6 or 8 phenolic units. Since the blocking action of 4-sulphonic-calix[4]arene was found to be more pronounced at acidic pH, probably due to protonation of its presumed binding site, we performed the present experiments at pH 6.
Figure 2A illustrates the effect of the different calixarenes on current responses recorded from voltage steps ranging between −10 and +100 mV applied from a holding potential of −30 mV after activation of ICl,swell by applying a 25% hypotonic solution. Note that the inactivation of the current in control conditions at strong positive potentials is more pronounced at this acidic pH compared to that at pH 7.4 (Nilius et al., 1998). At intermediate positive voltages, 30 μM 4-sulphonic-calix[4]arene induces a fairly rapid and partial decline of the current, which is less prominent at more positive potentials. The time course of current decay in the presence of both 4-sulphonic-calix[6]arene or -calix[8]arene is also significantly slower than in the presence of 4-sulphonic-calix[4]arene. Finally the current traces at the right show that these effects are fully reversible. Also note that this time-dependent current inhibition occurs at positive potentials, but is absent at negative potentials.
Figure 2.
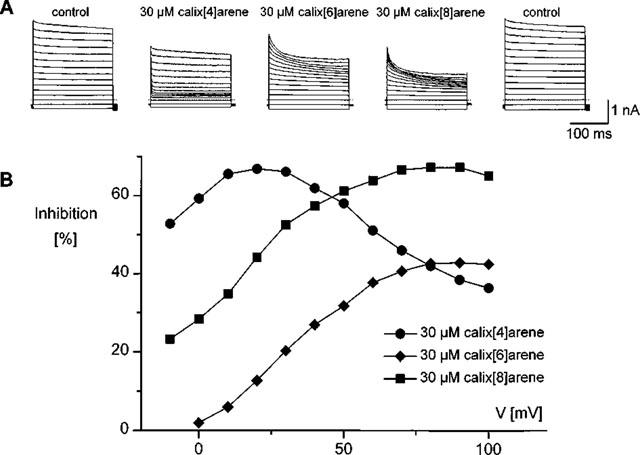
Effects of calixarenes on IC1,swell. (A) Current traces in response to voltage steps to potentials between −10 and +110 mV (step increment is 10 mV) applied from a holding potential of −30 mV. Traces were recorded after full activation of IC1, swell by applying a 25% hypotonic solution under control conditions, and in the presence of 30 μM of either 4-sulphonic-calix[4]arene, -calix[6] arene or -calix[8]arene. The traces at the right were obtained after washout and show the reversibility of the effects. (B) Inhibitions, expressed as a percentage reduction of the currents at the end of the voltage steps, as a function of the applied voltage calculated from the traces in (A). The inhibition of the current by 4-sulphonic-calix[4]arene is most prominent at small positive potentials, but declines at more positive potentials. The effect is much less pronounced for the other two calixarenes.
To assess the inhibitory effects of these compounds, we have calculated the fraction of the current at the end of the voltage step that is inhibited by each calixarene compound. These calculations were limited to potentials positive to the Cl-reversal potential (ECl) because the currents as well as the calixarene effects were small at potentials more negative than ECl making it difficult to obtain reliable estimates of the inhibition at these potentials. The results are summarized in Figure 2B, which shows the ‘apparent' inhibition as a function of voltage. We call it apparent because these calculations do not take into account the contribution of the background currents and therefore underestimate the inhibitory effect, especially at inhibitions close to 100%. Since the contribution of other current components to the total membrane current is much smaller than the amplitude of ICl,swell at positive potentials, these apparent values provide fairly good estimates of the real inhibition, except at strong inhibition levels. It is obvious that 4-sulphonic-calix[4]arene is the most effective inhibitor at small positive potentials, and that its effectiveness strongly decreases at more positive potentials. Both this decrease in blocking efficiency and the sub-maximal inhibition have been attributed to a relief of block at strong positive potentials, which promotes the release of the negatively charged calix[4]arene from its binding site in the pore into the cytosol.
The other two compounds, 4-sulphonic-calix[6]arene and 4-sulphonic-calix[8]arene, are less effective than 4-sulphonic-calix[4]arene at moderate positive potentials, but become more efficient at large positive potentials. It is a rather peculiar observation that 4-sulphonic-calix[6]arene is a less efficient blocker than 4-sulphonic-calix[8]arene, but this might be explained by its less flexible structure (flexibility induced by the methylene bridges). The blocking effect of these two calixarenes does not significantly decrease at strong positive potentials, at least not in the voltage range used in our experiments, an observation that is consistent with a negligible permeability of these compounds. However, the block does not reach 100% at strong positive potentials, as expected for a non-permeating open pore blocker. This discrepancy cannot be fully explained by a contamination of the swelling activated current with calixarene insensitive background currents, such as the non selective cation current, since their contribution to the total current at large positive potentials is less than 5%. An alternative explanation might be that the access of the larger calixarene compounds to the pore is sterically obstructed, which would be equivalent with a low affinity, voltage-independent binding site outside the pore in series with the voltage-dependent binding site inside the pore region. Such a model predicts a concentration dependence of inhibition that is more pronounced than its dependence on membrane potential.
Concentration dependence of the calix[6]arene inhibition
We have therefore investigated the concentration dependence of the 4-sulphonic-calix[6]arene induced block. Data of a representative experiment are shown in Figure 3. It is obvious from the traces in Figure 3A that the rate and extent of block increase with increasing concentration. From the voltage-dependence of block at different 4-sulphonic-calix[6]arene concentrations, shown in Figure 3B, it is clear that higher concentrations shift the voltage-dependence of the block to lower voltages. In addition, the ‘apparent' inhibition at 100 μM reaches a maximum of about 90% around +40 mV and slightly decreases at higher voltages. This decline might support a restricted permeability of VRAC for this compound, but could also be due to a contamination of ICl,swell with a background calixarene insensitive non selection cation current. This nearly 100% inhibition at high concentrations is in striking contrast with the sub-maximal inhibition at strong positive potentials, and is consistent with the above mentioned restricted access model.
Figure 3.
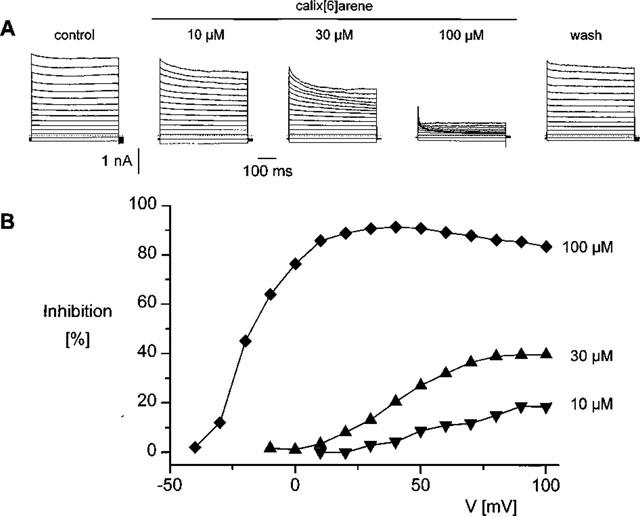
Concentration-dependence of the current inhibition by 4-sulphonic-calix[6]arene. (A) Current traces in the absence and presence of 10, 30 or 100 μM 4-sulphonic-calix[6]arene. The voltage protocol is the same as in Figure 1A. (B) Current inhibition expressed as a fraction of the current in control solution at each concentration obtained from the traces in A.
Time- and voltage-dependence of the block
We have therefore restricted our further analysis to the time-dependent fraction of the block, and analysed the inhibitory effects of the various calixarene compounds in terms of a model that we have described previously (Droogmans et al., 1998) for the 4-sulphonic-calix[4]arene block. We have first analysed the time-dependence of the calixarene sensitive current, obtained as the difference between the currents before and after applying these compounds. The traces corresponding to the current records in Figure 2A are shown in Figure 4A. The reversal potential of these calixarene-sensitive currents, obtained from the relation between the steady-state levels of these currents and voltage (not shown), is around −20 mV, indicating that they mainly represent currents through Cl− channels. The time course of the calixarene-sensitive current components could be approximated by a single exponential, the time constant of which represents the rate of onset of the block. These time-constants are shown as a function of voltage in Figure 4B.
Figure 4.
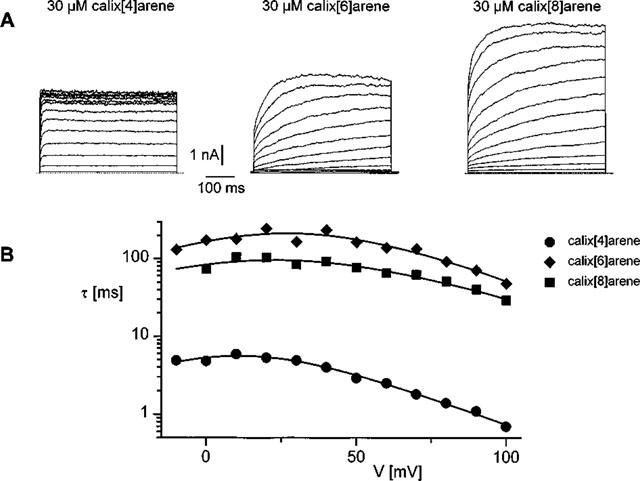
(A) Current traces representing the difference currents between control current and the currents blocked by 30 μM 4-sulphonic-calix[4]arene, -calix[6]arene and -calix[8]arene obtained from the data shown in Figure 1A. (B) Time constants of the difference currents, shown in A, as a function of voltage. The solid lines represent the fits of the data points to equation [2]. Best guesses for the parameters Kd(0), δ and k10(0) were 59 μM, 0.31 and 12.5 s−1 for 4-sulphonic-calix[4]arene, 77 μM, 0.23 and 4.8 s−1 for 4-sulphonic-calix[6]arene and 86 μM, 0.17 and 4.9 s−1 for 4-sulphonic-calix[8]arene. The value of the ratio k12(0)/k10(0) in the presence of calix[4]arene was 0.008, whereas it converged to zero in the presence of the other two compounds.
The voltage dependence of inhibition can be described, as shown previously (Droogmans et al., 1998), by binding of the negatively charged calixarenes to a site inside VRAC that senses a fraction δ of the applied electrical field. This binding occludes the channel, while its release from this site either to the outside or to the inside relieves the block. Large positive potentials favour the release of calixarene to the inside and therefore reduce the efficacy of the calixarene block. This model can be represented by the kinetic scheme
 |
in which VRAC and C-VRAC represent the fractions of the conducting and blocked channels. C0 and Ci correspond to the extra- and intracellular calixarene concentrations, the latter is assumed to be zero. Because the 4-sulphonic-calixarenes are negatively charged, the rate constants for the various transitions are voltage-dependent, and given by: k01(V)= k01(0)·exp(−zFδV/2RT), k10(V)=k10(0)·exp(zFδV/2RT), and k12(V)=k12(0)·exp(−zF(1−δ)V/2RT; k01(0), k10(0) and k12(0) represent the rate constants at 0 mV, V the membrane potential, z the charge of the blocking particle (approximately −4, −6 and −8 for the various calixarenes) and δ the electrical distance of the binding site from the external membrane side. R, T and F have their usual meaning.
The voltage dependence of the time constant of block or unblock as predicted by the model is given by
The solid lines in Figure 4B represent the best fit of the voltage-dependence of the time constants of inhibition to this equation. The ratio k12(0)/k10(0) for calix[6] and calix[8]arene converged to extremely small values. This is not unusual, because the release of bound calixarene to the inside of the cell will be very small for low values of k12(0) and therefore not contribute significantly to the time dependence of the time constants of inhibition by varying the ratio k12(0)/k10(0) between 0 and 0.001). Also the fit to calix[4]arene data sometimes converged to zero. We have therefore fixed this parameter at 0 to analyse the calixarene data.
The analysis of these difference currents is only valid if there is no current run-down. We have therefore for most experiments directly fitted the original current traces in the presence of the different calixarene compounds either to a single exponential or at strong positive potentials to a sum of two exponentials to take into account the inactivation of the current at these potentials. In the latter case we have used the time constant of the fast exponential component to assess the time-dependence of inhibition. The time constants of inhibition for each cell and each concentration of calixarene were again fitted to equation [2] with the ratio k12(0)/k10(0) fixed at zero. The averaged fitted parameters from all cells obtained at concentrations of 10, 30 or 100 μM of the 4-sulphonic-calixarenes are summarized in Table 1.
Table 1.
Fitted parameters for the various calixarene compounds
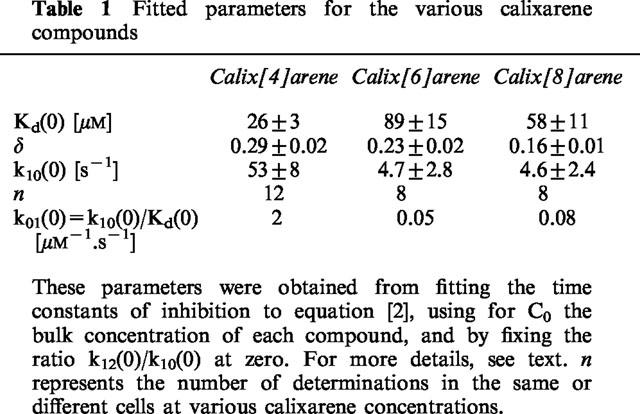
The main difference with the parameters for calix[4]arene at pH 7.4 reported previously is a pronounced decrease of Kd(0) from 220 μM at pH 7.4 to 26 μM at pH 6, suggesting that protonation of the putative binding site enhances its affinity for 4-sulphonic-calix[4]arene.
Effects of suramin and basilen blue on ICl,swell
Galietta et al. (1997) demonstrated voltage-dependent effects of suramin and basilen blue on volume-sensitive Cl− channels in human tracheal cells. Figure 5 illustrates the effect of suramin on the volume-regulated anion current activated by a hypotonic extracellular solution. The top panel shows current traces recorded in the same cell after full activation of the current during voltage steps to potentials of 20, 40 ... 140 mV applied from a holding potential of −50 mV under control conditions and in the presence of 1, 3 or 10 μM suramin respectively. It is obvious that suramin induces a time-dependent reduction of the current at potentials above +20 mV. This effect is faster and more pronounced at higher suramin concentrations. In analogy with the effects of 4-sulphonic-calix[4]arenes, we have attributed this inhibition to an open channel block by suramin. Fractional inhibition was calculated at the end of the voltage step and the time constants of onset of block were obtained from a single or double exponential fit of the time course of current decay, as described for the calixarenes. These quantities are represented as a function of voltage in the bottom two panels for the three different suramin concentrations. Current inhibition is voltage-dependent, as expected for an open channel blocker. Moreover, it reaches a maximum around +40 mV and declines at more positive potentials. These findings are similar to those observed with 4-sulphonic-calix[4]arene, and are consistent with a permeation of suramin at large positive potentials. The fits of the voltage dependence of the time constants to equation [2], using z=−6 for suramin, is superimposed on the data points. From 11 determinations in five cells at suramin concentrations ranging between 1 and 10 μM we obtained the following values of the parameters of the fit: Kd(0)=49±7 μM, δ=0.24±0.02, k12(0)/k10(0)=0.046±0.008 and k10(0)= 58±11 s−1. The calculated values for the remaining two parameters of the model, i.e. k01(0) and k12(0), are 1.24 μM−1.s−1 and 2.8 s−1 respectively.
Figure 5.
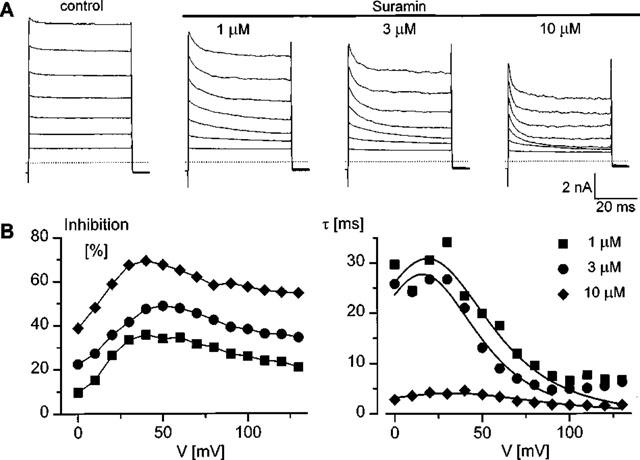
Voltage-dependence of the suramin induced inhibition of IC1,swell. (A) Current traces in response to voltage steps ranging from +20 to +140 mV (increment is 20 mV) from a holding potential of −50 mV after full activation of IC1,swell by applying a 25% hypotonic stimulus in control solutions and in the presence of 1, 3 or 10 μM suramin (upper panels from left to right). Note the time-dependent decay of the current in the presence of suramin. (B) Voltage-dependence of inhibition, expressed as a percentage of the total current, and time constant of inhibition. Note the peak in the voltage-dependence of inhibition around +40 mV. The solid lines in the right panel represent the fits of the data points to equation [2].
Similar results were obtained in the presence of basilen blue, which has a valence of −3 (Figure 6). The traces in the top panel were all obtained from the same cell after activating ICl,swell by a 25% hypotonic solution in the absence and in the presence of various concentrations of basilen blue. The quantified results, together with the corresponding fits to the model equations, are shown in the bottom two panels, clearly illustrating that our model is also able to explain the observed voltage dependence of inhibition. The averaged parameters of the fits obtained from five cells and at concentrations of 1, 3 or 10 μM were: Kd(0)=6.9±1.0 μM (n=15), δ=0.32±0.02 (n=15), k12(0)/k10(0)=0.26±0.04 (n=15) and k10(0)= 13.4±2.9 s−1 (n=8). The calculated values for k01(0) and k12(0) are respectively 1.9 μM−1.s−1 and 3.5 s−1.
Figure 6.

Voltage-dependence of the basilen blue induced inhibition of IC1,swell. (A) Current traces in response to voltage steps ranging from +20 to +140 mV (increment is 20 mV) from a holding potential of −50 mV after full activation of IC1,swell by applying a 25% hypotonic stimulus in control solutions and in the presence of 1, 3 or 10 μM basilen blue (upper panels from left to right). Note the time-dependent decay of the current in the presence of the inhibitor. (B) Voltage-dependence of inhibition, expressed as a percentage of total current, and time constant of inhibition calculated from the traces in A. The solid lines represent the fits of the data points to equation [2].
Discussion
Our data reveal some interesting properties of the VRAC pore region. A first interesting observation is that protonation of the putative binding site of 4-sulphonic-calix[4]arene inside the membrane at acidic cellular pH drastically increases the affinity for VRAC and thereby enhances its blocking efficacy. It is highly unlikely that the observed pH effect is due to a change in polarization of the poly-sulphonic acid compounds because of the low pKa of these groups and because protonation would decrease the anionic fraction of the compounds and hence their voltage-dependent blocking potency. It is also unlikely that the observed pH effects are due to changes in ionization of the −OH group of the phenolic unit, since we recently showed that there are no significant differences between 4-sulphonic-calix[4]arene and the methylated (−OMe) form 5,11,17,23-tetrasulfonato-25,26,27,28-tetramethoxy-calix[4]arene (Droogmans et al., 1998). We therefore propose that protonation of the putative binding site, probably at histidine residues (Droogmans et al., 1998), enhances its affinity for 4-sulphonic-calix[4]arenes.
The electrical distance δ obtained for the different calixarenes, suramin and basilen blue are fairly similar (0.16–0.32 from the outside), indicating that they probably bind to the same site in the membrane. This finding also gives further support to the proposed model to describe the voltage-dependence of the inhibition. The observation that 4-sulphonic-calix[6]arene and -calix[8]arene do not permeate the membrane (k12≈0) is consistent with their larger dimensions, and provides an upper limit for the pore diameter. The cross-sectional area of 4-sulphonic-calix[4]arene and 4-sulphonic-calix[6]arene, as estimated by the shortest and longest distances between opposite oxygens are approximately ∼11*12 and 17*12 Å2 respectively, and represent approximate lower and upper limits for the pore dimension. No structural data were available for 4-sulphonic-calix[8]arene, but we found a value of approximately ∼19*20 Å2 (as measured at the −CH3 group of the tertiary butyl) for the related 4-tert-butylcalix[8]arene. These values are comparable to those obtained from the relation between the relative permeabilities of various anions and their Stoke's diameter, using the ‘excluded volume effect' model (Dwyer et al., 1980), i.e. 11.5 Å or 7.3 Å depending on whether or not frictional forces are taken into account (Nilius et al., 1999).
In summary, we have been able to demonstrate that poly-anionic blockers of VRAC, such as 4-sulphonic-calix[4]arene, suramin and basilen blue, are able to permeate VRAC at positive potentials. The same approach might also be useful to demonstrate a possible permeation of other voltage-dependent anionic blockers of VRAC, including nucleosides and nucleotides (Nilius et al., 1996). Since information on the putative channel structure is lacking and its membership of the CLC-family is controversial (Jentsch et al., 1999), it is not possible to speculate as to which sequences may represent the pore region.
Acknowledgments
We thank Dr N. Blaton for the structural information about the calixarenes and Dr C. Pannecouque for helpful discussions. We are also indebted to Dr Ravshan Sabirov for critical discussion of the work during his stay in Leuven made possible by INTAS. Chantal Maertens is a Research Assistant of the Flemish Fund for Scientific Research (F.W.O.-Vlaanderen). This research was supported by European Union grant BMH4-CT96-0602 and grant G0237.95.N of the F.W.O. from the Flemish Government and INTAS-94-0241.
Abbreviations
- CPAE
cow pulmonary endothelial cell
- ECl
chloride equilibrium potential
- ICl,swell
swelling-activated Cl− current
- VRAC
volume-regulation anion channel
References
- DROOGMANS G., PRENEN J., EGGERMONT J., VOETS T., NILIUS B. Voltage-dependent block of endothelial volume-regulated anion channels by calix[4]arenes. Am. J. Physiol. 1998;275:C646–C652. doi: 10.1152/ajpcell.1998.275.3.C646. [DOI] [PubMed] [Google Scholar]
- DWYER T.M., ADAMS D.J., HILLE B. The permeability of the endplate channel to organic cations in frog muscle. J. Gen. Physiol. 1980;75:469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENTSCH T.J., FRIEDRICH T., SCHRIEVER A., YAMADA H. The CLC chloride channel family. Pflügers Arch. Eur. J. Physiol. 1999;437:783–795. doi: 10.1007/s004240050847. [DOI] [PubMed] [Google Scholar]
- GALIETTA L.J.V., FALZONI S., DI VIRGILIO F., ROMEO G., ZEGARRA-MORAN O. Characterisation of volume-sensitive taurine- and Cl-permeable channels. Am. J. Physiol. 1997;273:C57–C66. doi: 10.1152/ajpcell.1997.273.1.C57. [DOI] [PubMed] [Google Scholar]
- KIRK K. Swelling-activated organic Osmolyte Channels. J. Membr. Biol. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- NILIUS B., EGGERMONT J., VOETS T., DROOGMANS G. Volume-activated Cl-channels. Gen. Pharmacol. 1996;27:1131–1140. doi: 10.1016/s0306-3623(96)00061-4. [DOI] [PubMed] [Google Scholar]
- NILIUS B., OIKE M., ZAHRADNIK I., DROOGMANS G. Activation of a Cl− current by hypotonic volume increase in human endothelial cells. J. Gen. Physiol. 1994;103:787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., DROOGMANS G. Modulation of volume-regulated anion channels by extra- and intracellular pH. Pflügers Arch. Eur. J. Physiol. 1998;436:742–748. doi: 10.1007/s004240050697. [DOI] [PubMed] [Google Scholar]
- NILIUS B., VOETS T., EGGERMONT J., DROOGMANS G.VRAC: A multifunctional volume regulated anion channel in vascular endothelium Chloride Channels 1999Oxford (England): Isis Medical Media Limited; ed. R. Kozlowski [Google Scholar]
- OKADA Y. Volume expansion-sensing outward-rectifier Cl-channel: fresh start to the molecular identity and volume sensor. Am. J. Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- STRANGE K., EMMA F., JACKSON P.S. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]


